ABSTRACT
Toheroa (Paphies ventricosa) were formerly abundant on west and south-facing New Zealand surf beaches. Harvesting of this surf clam was intense during the early to mid-1900s, and populations declined to levels where harvesting was no longer viable. Despite having now been protected for 35–45 years, toheroa have failed to recover. This paper reviews the history of human interactions with toheroa and our understanding of their ecology, with a view to identifying knowledge requirements for management and restoration. Historical and legal documents pertaining to the use of marine resources are reviewed and scientific understanding compared with customary and local knowledge. We consider the factors that may be preventing toheroa recovery and make recommendations for research into life history, habitat requirements and the ecological consequences of changing land use. Management options are suggested to address the effects of vehicle use on beaches, poaching and the failings of the customary harvest system.
Introduction
Toheroa (Paphies ventricosa) are a species of large intertidal surf clam endemic to New Zealand. At the start of the twentieth century, extensive toheroa populations were present on the exposed west-facing surf beaches of Taitokerau (Northland) and the Kāpiti-Horowhenua coast, and on the south coast of Murihiku (Southland; ; Redfearn Citation1974). Toheroa were a staple food for Māori in these areas and began to be harvested more extensively by pakeha (New Zealanders of European descent) from the late 1800s (Redfearn Citation1974; Murton Citation2006 and references therein). The popularity of toheroa as a recreational harvest grew quickly and commercial operations were soon established, primarily for the export of canned toheroa (Williams, Sim-Smith, et al. Citation2013). Toheroa populations fluctuated in size as is typical of surf clams (Coe Citation1955; de Villiers Citation1974; Arntz et al. Citation1988; Fiori et al. Citation2004; McLachlan and Brown Citation2006). However, concerns soon developed that the combined harvest of the commercial fishery and the largely unregulated recreational fishery were depleting the resource (Murton Citation2006). Fisheries regulations were introduced incrementally from 1913 (Murton Citation2006; Miskelly Citation2016), but ultimately failed to halt the decline of the fishery. By the mid-1900s toheroa populations declined to levels where their harvest was no longer viable (Redfearn Citation1974; Stace Citation1991; Murton Citation2006). All commercial harvest ceased by 1969 and regional recreational fishery closures occurred between 1971 and 1980 (Williams, Sim-Smith, et al. Citation2013). Since that time, toheroa harvesting has been restricted to customary take by Māori (for which an authorisation to take for customary purposes is required), largely for hui (meetings) or tangi (funerals), and a number of recreational open days in Murihiku, the last of which took place at Oreti Beach in 1993 (Miskelly Citation2016). Despite having been protected for 40+ years, toheroa populations nationwide have, for unknown reasons, failed to recover, with some populations continuing to decline (Williams, Sim-Smith, et al. Citation2013).
Figure 1. Distribution of toheroa (Paphies ventricosa) in New Zealand. Major populations are underlined. Figure reproduced from Redfearn (Citation1974).
Knowledge of the biology and ecology of toheroa, and of New Zealand surf clams in general, is surprisingly limited compared to other iconic New Zealand kai moana (seafood) species. This is in spite of the cultural significance of toheroa, their status as a New Zealand culinary icon, their potential commercial value and the mystery surrounding the reasons for their collapse and lack of recovery. Systematic keyword searches in the Scopus online journal database using the terms ‘toheroa’, Paphies ventricosa and this species’ previous names Amphidesma ventricosa or Mesodesma ventricosa produced only 21 peer-reviewed scientific journal articles, including a 1928 summary in Nature of Malcolm’s (Citation1928) work on the nutritional value of toheroa soup. By comparison, searches for the similarly iconic and recreationally and/or commercially important New Zealand kai moana species: cockles (Chione stutchburyi or Austrovenus stutchburyi); snapper (Pagrus auratus or Chrysophrys auratus); crayfish (Jasus edwardsii); and mussels (Perna canaliculus), produced 139, 562, 384 and 322 publications, respectively. These search results are not necessarily a fair indication of the research effort that has been directed at toheroa. Much of the toheroa science conducted to date relates to population surveys and stock assessment rather than ecological research. For example, 30 toheroa surveys were conducted at Oreti Beach, in Murihiku, between 1969 and 2005 (Beentjes and Gilbert Citation2006b). This body of stock assessment work resides largely in the grey literature in the form of reports to government agencies (Ministry for Primary Industries and its precursors) and is not easily discoverable through journal database searches. The heyday of toheroa research was the 1950s to 1970s period when researchers such as Cassie (Citation1951, Citation1955), Rapson (Citation1952, Citation1954) and Redfearn (Citation1974, Citation1982) produced detailed accounts of toheroa natural history and developed ideas about the ecology of toheroa that remain largely unchanged today. In recent times, toheroa research has occurred only sporadically, in accordance with the interests and resources of individual researchers. There has been no coordinated national approach to acquiring knowledge that may be needed to support the restoration and management of this taonga (treasured) species.
Increasingly, Māori are able to take a leading role in the management of their rohe (territory) (Moller, Lyver, et al. Citation2009; Taiapa et al. Citation2014). Environmental restoration, particularly of ecosystems that once supported the provision of food, water and other resources to tangata whenua (people of the land – Māori), is of particular importance to Māori (Tipa and Teirney Citation2006; Smith et al. Citation2011; Taiapa et al. Citation2014). Ecological information, both western science and mātauranga Māori (Māori knowledge), is being sought to support restoration and management efforts for a range of species and environments (Smith et al. Citation2011; Robb Citation2014; Taiapa et al. Citation2014). For Taitokerau, Kāpiti-Horowhenua and Murihiku, there are aspirations that toheroa will recover to the point where they can once again be a sustainably harvested resource (customary, recreational and commercial). Some groups also hope that toheroa may one day be cultured commercially, to both assist with restoration activities and to provide new sources of revenue and employment for Māori (Newcombe et al. Citation2015).
Given the current level of interest in toheroa from the communities living at these beaches, the resource users and managers, and the scientific community, now is an appropriate time to consider the existing knowledge of the biology and ecology of toheroa with a view to identifying information requirements for future management and restoration. As a contribution to this process, this paper combines the knowledge of experts from a diversity of backgrounds with a view to integrating both ‘western scientific’ and mātauranga Māori perspectives. By taking this approach to the review of human interactions with toheroa, and by comparing our current scientific understanding of toheroa against the knowledge held by local experts, we provide a comprehensive summary of toheroa-related knowledge (and knowledge gaps) and context to the present ecological challenges and uncertainty. While the local experts, whose knowledge has been incorporated into this review, would traditionally be thought of as laypeople or amateur naturalists, in this instance many are kaitiaki (guardians) or tohunga (experts). These are formal titles or positions bestowed within Māori culture, and the practitioners are holders of a considerable body of personally acquired and intergenerationally transferred environmental knowledge. By assembling this diverse body of knowledge and identifying factors that may be preventing the recovery of toheroa and possible management actions, this review provides a resource for anyone with an interest in toheroa or their management and a starting point for future investigations into this species. This review does not attempt to replicate or extend the critical analyses of trends in toheroa population abundance and size structure that have been conducted on numerous occasions over the last 50+ years (see Beentjes and Gilbert Citation2006b; Williams, Sim-Smith, et al. Citation2013; Williams, Ferguson, et al. Citation2013 and references therein). This contemporary approach to fisheries research and management has been unsuccessful in informing or facilitating the restoration of toheroa and as such there is a need to understand the failings of the current management system and explore alternative approaches.
Methods
The available published and unpublished literature on toheroa was reviewed, including scientific research papers, graduate research theses and reports to the Ministry for Primary Industries (and its precursors). The task of collating and synthesising the literature was somewhat simplified by the fact that several reports, focussing on the factors that may be preventing the recovery of toheroa, have been recently produced (Heasman et al. Citation2012; Williams, Sim-Smith, et al. Citation2013). In addition to the scientific data sources traditionally examined when conducting ecological reviews of this nature, we also searched newspaper archives and legal and historical data sources, including university theses and documents pertaining to Māori use of marine resources and Treaty of Waitangi claims.
Once the available ecological literature had been reviewed, meetings were held with local experts in Taitokerau, Kāpiti-Horowhenua and Murihiku. The scientific understanding of toheroa ecology was discussed and local experts were able to provide their own perspectives. For the most part, these meetings took place on or near the toheroa beaches. Where scientific and local knowledge differed it was sometimes possible to examine toheroa beds or the surrounding environment to validate or challenge the scientific or local understanding. In other cases, scientific and local knowledge were aligned but the understanding of the mechanisms behind observations differed. In these cases, alternative observations or interpretations of observations were shared and recorded.
The early history of toheroa
Toheroa were once abundant on Te Oneroa-a-Tōhē (Ninety Mile Beach), Mitimiti, Ripiro (Dargaville / North Kaipara Beach) and Te Oneone Rangatira (Muriwai) beaches in Taitokerau, on the Kāpiti-Horowhenua coast from the Rangitikei River to Waikanae Beach, and in Murihiku at Oreti, Bluecliffs and Orepuki Beaches (). Small populations also existed in the North Island at Spirits Bay, Tom Bowling Bay, Tokerau, Te Arai, Whangape, Pollok, Piha, Ohope, Opotiki, and in the South Island at Hampden, Waikouiti and Long Beach, although only single specimens have been found at the latter two beaches (Hoby Citation1933; Cassie Citation1955; Street Citation1971; Redfearn Citation1974). For Māori in Taitokerau, Kāpiti-Horowhenua and Murihiku, toheroa had long been a staple food (Murton Citation2006 and references therein). The toheroa beds on the West Coast of Taitokerau are said to have been particularly valuable, with early newspaper articles suggesting that attempts to secure the possession of these beds may have given rise to some warfare among Māori (Stallworthy Citation1916). Toheroa were largely dried or smoked and were probably a traded commodity (Stace Citation1991). Toheroa, also known in some regions as taiwhatiwhati roroa, moeone, tupehokura, roroa and tohemanga, are a taonga (treasure), a prestigious kai moana and are a desirable dish to be served at hui or tangi for coastal peoples (Murton Citation2006). Toheroa are linked to the Māori people through whakapapa (genealogy). They are given the same respect as the family, or tribal entity, provoking a fierce ethic of stewardship, or kaitiakitanga (Smith Citation2013). The provision of toheroa to visitors is also an important component of manaakitanga (the act of giving mana, or utmost respect, to another through the expression of hospitality and generosity), a tikanga (custom) that has been compromised by the mismanagement and collapse of the fishery.
The whakapapa of the toheroa is that it was held in such high esteem that it was brought to New Zealand from Hawaiki (the traditional Māori place of origin where Io, the supreme being, created the world and its first people) by the high chief Mareao who seeded toheroa on the west coast of the North Island (Wai27 Citation1988). A second origin story recalls an incident in which a group of Te Rarawa men from Ahipara (Taitokerau) were caught poaching kukupa (New Zealand wood pigeon; Hemiphaga novaeseelandiae) in the Hokianga area. Forced to flee for their lives back up to Te Oneroa-a-Tōhē, they discarded all their food and equipment in order to outrun their pursuers. After many hours, famished and nearly spent, they pleaded with their chief to intercede with the spirits. Mounting a rocky outcrop and facing the beach, the chief uttered a karakia (prayer), beseeching his atua (god) to save them. Immediately a whirlwind appeared and the chief was told to continue his journey and the way would be made clear. Shortly afterwards the men noticed slit-like holes in the sand. Thinking that shellfish might be lurking beneath, they dug but found nothing. Dejected, they were at the point of giving up when the whirlwind appeared again, giving the message ‘Tohe roa, tohe roa!’ (‘Persist a long time!’). Digging deeper, they eventually found the shellfish, eased their hunger and got home safely. In giving thanks for their narrow escape, the chief declared that the life-giving shellfish should be known as toheroa (Stace Citation1991).
Māori would often make hazardous expeditions, sometimes fatal, to the long beaches of the west coast of Taitokerau to collect toheroa (Stallworthy Citation1916, Stace Citation1991). These journeys were not just for the purposes of food harvest. The translocation of toheroa to new locations and their cultivation may have been common practise for Māori (J. Williams Citation2004, Citation2012). Translocations are thought to have been for the purpose of establishing new populations in areas where they could be more easily accessed. Live toheroa may have even been a traded commodity. Rakiihia Tau (Ngāi tahu), in his testimony to the Waitangi Tribunal (Wai27 Citation1988), describes the translocation of toheroa in the South Island, stretching back at least four or five generations, to Kahurangi Point (north of Karamea) and to the beaches of Canterbury, Otago and Murihiku. New Zealand Government agencies (New Zealand Marine Department) were also involved in transplants during the 1920s and 1930s, moving thousands of toheroa between North Island beaches (New Zealand Herald Citation1926, Citation1934; Auckland Star Citation1930). Historical documents including newspaper and magazine articles describe how ‘many experiments have been made to transplant the toheroa to other beaches, apparently of similar nature to its native haunts’ (Samuel Citation1936).
The practise of translocating toheroa has persisted into more recent times. Kaitiaki have shared their knowledge of toheroa translocation from Taitokerau and Horowhenua to the eastern beaches of Taitokerau, the Bay of Plenty, Horowhenua and Hawke’s Bay (various, personal communication). Toheroa were also translocated from Bluecliffs Beach to Orepuki in Te Waewae Bay (Futter Citation2011). Toheroa translocation appears to have been a common practice at least up until the 1970s when the toheroa fisheries closed. This is perhaps not surprising as the translocation of toheroa, and other shellfish species including cockles (Austrovenus stutchburyi), scallops (Pecten novaezelandiae), tuatua (Paphies australis) and paua (Haliotis iris) was probably an important component of Māori marine resource management stretching back several hundred years (Wai27 Citation1988; Williams Citation2004, Citation2012).
Despite what appears to be widespread and repeated translocation activity, it would seem that in most locations, transplanted toheroa have failed to thrive or persist. This is probably due to the specific habitat requirements of toheroa and the necessity for new populations to be sustained through self-recruitment (of pelagic larvae) when transplanted outside their natural geographical distribution. Due to the 20–40 day larval period of toheroa (Redfearn Citation1974, Citation1982; Gadomski et al. Citation2015), the hydrodynamic conditions resulting in the retention of larvae would be required for the necessary self-recruitment to occur (Cowen et al. Citation2007 and references therein). Research, incorporating genetics, archaeology and oral history, is currently underway to ascertain whether any of the present day toheroa populations result from historical transplants (Ross unpublished data). Should the translocation hypothesis be correct, this traditional practise may explain the peculiar geographical distribution of the toheroa which spans biogeographical boundaries and is unlike that of any other New Zealand marine organism (; Ross et al. Citation2009; Shears et al. Citation2008).
In Taitokerau (but not in Murihiku), the story of toheroa is closely tied to that of the dune grass pingao (Ficinia spiralis), a New Zealand endemic sedge that grows on active sand dunes (Stace Citation1991; Te Tuhi & Gregory Citation2008). The whakapapa of pingao is that she was put on the dunes by her father, Tangaroa (the god of the sea), to nurture her whanau (family), the toheroa. The authors have heard two versions of Māori lore regarding the reproduction of toheroa. One is that toheroa spat are born in the pingao (whose seeds are shaped like toheroa spat) and in the native dune grass spinifex (Spinifex sericeus; also known in Te Reo Māori as matihetihe, kowharawhara, raumoa and turikakoa). The other is that spat are carried to the dunes on the foaming surf at the highest of tides and are deposited on the sand dunes and into the pingao and spinifex where they are reared within the structures of these grasses. Transportation from the dune grasses to the juvenile beds in the upper intertidal occurs in spring. The spat held by the pingao are blown onto the upper beach, while the tumbleweed-like seed heads of the spinifex transport their toheroa pēpi (babies) along the beach through the action of cross-shore winds. The spat are then washed from the tumbling seed heads as they traverse the numerous streams and seeps running down the northern beaches. These streams are where the spat settle thus explaining the stream-associated distribution of toheroa in Taitokerau. At times when the transference of spat to the beach was considered to be taking place, children were not allowed to play their game of waiwatai, in which seed heads were chased as they were blown over the sand (Rapson Citation1952). This tikanga provided protection for both the nursery habitat and the vulnerable early life stages of toheroa.
Traditional Māori fisheries management practices were not restricted to translocation and nursery protection (). The setting aside of reserves (rāhui) and spawning areas was something that Māori had long practised (Minister of Marine Citation1933; Parore Citation1933). There was also a cessation of harvesting during the main spawning time which was indicated by the annual flowering of kumarahou (Pomaderris kumarahou) and the full moon (Smith Citation2003). It has also been suggested that Māori may have preferentially harvested middle-sized toheroa, possibly as a consequence of processing, preservation and usage practices, leaving larger individuals in place as broodstock to sustain the resource (J. Te Tuhi, personal communication). It is possible the practise of not harvesting large toheroa may have led to some degree of inaccuracy in archaeological midden records, as small toheroa and large tuatua can be hard to differentiate without knowledge of diagnostic shell characteristics (Cassie Citation1955). As a consequence, the historical geographic distribution of toheroa and their importance in the diet of Māori may have been underestimated in some locations (Ross, unpublished data).
Table 1. Summary of factors considered to potentially be preventing the recovery of toheroa, the mechanisms by which these factors might affect toheroa recovery, possible management actions to address factors and relevant toheroa literature.
The recent history of toheroa
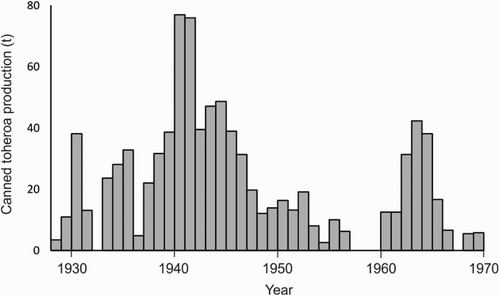
When pakeha first began taking notice of toheroa, they appeared to be so abundant as to be considered an almost inexhaustible resource (Samuel Citation1936). By the late nineteenth century, the pakeha residents of Taitokerau towns, such as Te Kopuru, Aratapu and Dargaville, began spending time at the toheroa beaches over summer and rapidly came to appreciate toheroa, both as a food item and as bait for fishing (Murton Citation2006). The first toheroa cannery was established at Mahuta Gap, Ripiro Beach, in the 1890s (Stace Citation1991), and before long four factories operated on that stretch of Kaipara coastline and a fifth at Te Oneroa-a-Tōhē (; Redfearn Citation1974; Stace Citation1991). The total commercial production for Taitokerau beaches from 1928 to 1969 was typically around 20 tonnes of canned product per annum, with record production of 77 tonnes in 1940 (; Redfearn Citation1974). At various times and for brief periods toheroa were also canned at Muriwai, on the Kāpiti-Horowhenua coast and at Te Waewae Bay (Williams, Sim-Smith, et al. Citation2013).
Figure 2. (Clockwise from top left) Toheroa collected from Hokio Beach during the September open day in 1977 (EP/1077/3679/36-F. Alexander Turnbull Library (ATL)); Three women from Northland, photographed ca. 1910–1930s, shelling toheroa meat into tin cans for a toheroa cannery. Their kete (flax bags) are full of shellfish, and they are surrounded by empty shells (1/1-026522-G. ATL); Harvesting toheroa on a Northland beach, ca. 1920s–1930s (1/1-010575-G. ATL); Toheroa being dug from trenches on Muriwai Beach, 1962. (AAQT 6539, A70987. Archives New Zealand, The Department of Internal Affairs, Te Tari Taiwhenua).

Figure 3. Total commercial production of toheroa (Paphies ventricosa; tonnes of canned toheroa product) from canneries at Northland Beaches (Te Oneroa-a-Tōhē, Ripiro and Te Rangatira) from 1928 to 1969. Data from Marine Department Annual records for 1928–1940 and 1943–1948 tabulated by Cassie (Citation1955) and for 1941–1942 and 1949–1969 graphed by Redfearn (Citation1974). Figure reproduced from Williams, Sim-Smith, et al. (Citation2013).
The emergence of toheroa as one of ‘New Zealand’s great contributions to the epicurean world’ had to wait until the visit of the Prince of Wales in 1921. Prince Edward asked for a second helping of toheroa soup at a banquet, a request that broke with royal protocol and was reported throughout the Empire (Stace Citation1991). After this event, no self-respecting New Zealand hotel was without toheroa soup on the menu, and the collecting and eating of toheroa became a national pastime (Murton Citation2006). Toheroa, ‘a delicacy highly esteemed by the most fastidious gourmet’ was considered ‘a gift of nature which is a remarkable commercial asset, and although a mere shellfish, has done much to advertise the Dominion all over the world’ (Samuel Citation1936). From the 1920s, roads improved and cars became more common, making the toheroa beaches accessible to people living further away in towns such as Auckland, Whangarei and Wellington.
The harvesting of toheroa escalated quickly and it was not long before toheroa numbers began declining (Murton Citation2006; Williams, Ferguson, et al. Citation2013). At Ripiro, there was soon friction between the various groups of toheroa harvesters including local and visiting Māori, local pakeha and those residing at beach campgrounds and settlements during the summer, hawkers who dug and sold toheroa, and the canneries (Murton Citation2006). At Ripiro, a reserve for ‘camper’ harvesting of toheroa was established in 1913. In 1915, lease areas for commercial harvesting were established. Recreational harvesting regulations were first introduced in 1932 in response to dwindling numbers and mass mortality events in northern populations (Williams, Sim-Smith, et al. Citation2013). The Marine Department established a two-month closed season during spawning (October to November), introduced a minimum size (3 in./76 mm), banned certain digging implements for non-commercial harvesters and introduced a quota for pakeha (50 toheroa per person per day), but not Māori. A quota for Māori was introduced in 1941 (Redfearn Citation1974).
After commercial harvesting began, Māori expressed dismay over wasteful methods and the depletion they observed (Murton Citation2006). They opposed commercial toheroa digging in traditional harvesting areas and from 1915, Māori at Ripiro lobbied for the establishment of ‘Māori toheroa reserves’ (similar to the camper reserve established in 1913). However, the Chief Inspector of Fisheries (Citation1939) commented that toheroa were a ‘national possession’ that belonged to everyone, and just because some families lived near them he did not think that they alone should be privileged. Māori requests and complaints were largely ignored as was the fact that toheroa were a traditional and staple Māori food, but ‘only relish as far as Europeans are concerned’ (Under Secretary Maori Affairs Citation1950). By the 1940s and 1950s it had become fashionable for urban pakeha to make the trip to the collect toheroa (). In 1957, the occupants of 2000–3000 cars visited Glinks Gully (Ripiro) over a weekend to harvest toheroa (Auckland Star Citation1957). In 1966 an estimated 12,000 cars and 50,000 people visited Ripiro Beach in one weekend, harvesting an estimated 1,000,000 toheroa (Murton Citation2006 and references therein).
Unfortunately, there is only limited population data from the early days of the toheroa fishery (; Williams, Ferguson, et al. Citation2013). No one knows how many toheroa were present on the beaches at the start of the twentieth century but it is likely that population sizes did fluctuate, as is typical for surf clams (Coe Citation1955; de Villiers Citation1974; Arntz et al. Citation1988; McLachlan et al. Citation1996; Fiori et al. Citation2004; McLachlan and Brown Citation2006). The commercial fishery has traditionally received much of the blame for the decline of toheroa. Based on what we now know, however, it appears likely that non-commercial harvesting was of at least equal importance in contributing to the collapse of toheroa populations. Commercial harvesting practices varied between locations (B. Searle, personal communication). At Ripiro Beach, commercial harvesting was restricted to lease areas from 1915 (Murton Citation2006). Accounts from those involved in the later periods of the commercial fishery indicate that individual toheroa beds were only partially harvested and the harvest limited to mid-sized toheroa (B. Searle, personal communication). Smaller toheroa were returned to the trenches from which they were dug and larger specimens either returned to the trenches or transplanted to the southern end of Pouto Peninsula, to act as a source of recruits to beds to the north (). Similar harvesting practises were not necessarily used at other beaches. Differences in harvesting practices may to some extent explain the persistence of adult toheroa beds at Ripiro, while at Te Oneroa-a-Tōhē and the Kāpiti-Horowhenua Beaches toheroa have all but disappeared. In contrast to the commercial fishery, the recreational fishery was not managed spatially and there was no maximum size limit in place to ensure that the reproductive capacity of the beds was maintained. There are also accounts of recreational fishers following behind the commercial harvesters and ‘cleaning out’ sections of toheroa beds purposefully left undug by the canneries (B. Searle, personal communication). To manage the recreational harvest, bag limits (50 for pakeha in 1932; 80 for Māori in 1939; 20 per person regardless of ethnicity in 1950), a minimum size and seasonal harvesting restrictions were incrementally introduced (Murton Citation2006; Miskelly Citation2016). However, these regulations probably became less effective at controlling total catch as the numbers of recreational harvesters grew.
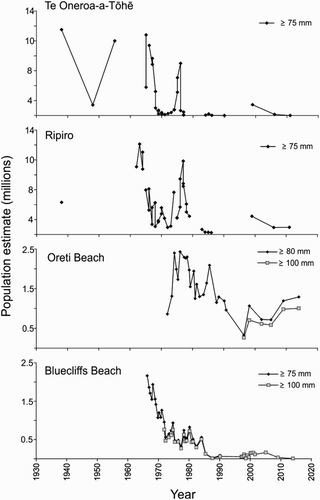
Figure 4. Population estimates of large adult toheroa (Paphies ventricosa) at Te Oneroa-a-Tōhē (≥75 mm; 1933–2010), Ripiro (≥ 75 mm; 1938–2011), Oreti (≥80 mm and ≥100 mm; 1972–2009) and Bluecliffs Beach (≥75 mm and ≥ 100 mm; 1966–2009) from 1930 to 2015. Figures reproduced from Williams, Ferguson, et al. (Citation2013), Beentjes (Citation2010a, Citation2010b) and Berkenbusch et al. (Citation2015).
Once population surveys began in earnest in the 1960s, large fluctuations in abundances were recorded (Beentjes Citation2010a, Citation2010b; Williams, Sim-Smith, et al. Citation2013; ). Mass mortalities were reported at various locations in 1888, 1900, 1917, 1932, 1938, 1956–1959, 1970–1971, 2001 and 2013, contributing to this variability in biomass, as did high levels of recreational and commercial harvesting (Rapson Citation1954; Cassie Citation1955; Williams, Sim-Smith, et al. Citation2013). In 1955, the North Island closed season for both commercial and recreational fisheries was extended to 10 months and quotas reduced. By 1966 the total commercial harvest of toheroa had dropped to less than 10 tonnes of canned product per annum (), and all commercial harvesting ceased in 1969. Toheroa populations continued to decline and recreational harvesting was eventually closed at Te Oneroa-a-Tōhē (1971), Muriwai (1976), Kāpiti-Horowhenua (1978) and Ripiro (1980). Oreti and Bluecliffs beaches in Murihiku were opened sporadically for harvesting from 1972, with the last open days at Bluecliffs and Oreti held in 1980 and 1993, respectively (Stace Citation1991; Miskelly Citation2016). Since the fishery closures, harvesting has been restricted to a limited take for customary purposes. Illegal harvesting of toheroa occurs at most locations (various, personal communication) but has not been quantified (Williams, Sim-Smith, et al. Citation2013). Despite having been protected for 40 + years, toheroa populations have, for unknown reasons, failed to recover (Williams, Ferguson, et al. Citation2013; Berkenbusch et al. Citation2015).
The biology and ecology of toheroa
Toheroa are suspension-feeding surf clams in the family Mesodesmatidae. This family also includes three other bivalves of the New Zealand endemic genus Paphies. These clams are pipi (P. australis), tuatua (P. subtriangulata) and the deep water or southern tuatua (P. donacina). Toheroa are the largest of these four Paphies species, and the largest of any clam species found in New Zealand. In Murihiku, toheroa commonly grow to 100–145 mm and are sometimes recorded up to 150 mm (Beentjes Citation2010a, Citation2010b). In Taitokerau, subfossil toheroa shells commonly exceed 150 mm in length, but living specimens rarely exceed 100 mm (Cook Citation2010; Williams, Ferguson, et al. Citation2013; P. Ross, personal communication). Paphies australis may grow to 100 mm, P. subtriangulata to 80 mm and P. donacina to 110 mm (Cook Citation2010). The morphology of juvenile and adult toheroa was described by Rapson (; Citation1954). Their shell is solid and ovately shaped with valves that do not completely close (). The gaps between the valves are covered by folds of the mantle, which can appear pink in some individuals (S. Smith, personal communication). The toheroa has two long extendable siphons that protrude from the sand when feeding (). The siphons are separate, long (relative to other Paphies spp.) and are highly contractile. The outer aperture of the inhalant siphon is encircled by a complex of tentacles which serve to prevent the passage of large particles into the mantle cavity (Rapson Citation1952). The foot is large and triangular () and enables the animal to burrow rapidly into the sand, with large individuals able to burrow to depths of greater than 20 cm (Kondo & Stace Citation1995).
Figure 5. Internal anatomy of toheroa (Paphies ventricosa), with left valve and mantle removed. Figure reproduced from Rapson (Citation1952).
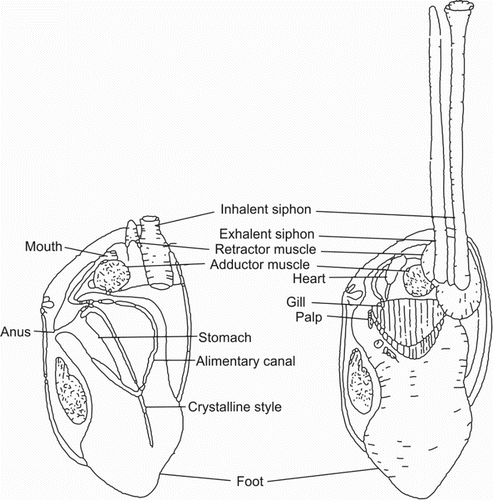
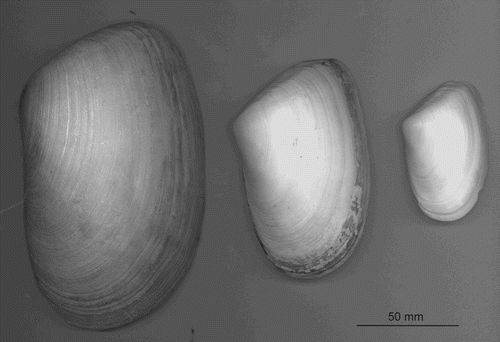
Figure 6. Left valves of toheroa (Paphies ventricosa) shells collected at Ripiro (153 mm), Oreti (108 mm) and Mt Maunganui (70 mm) beaches.
Reproduction and larval development
Toheroa are gonochoristic (separate sexes in different individuals and sex does not change over an individual’s lifetime), although hermaphroditic individuals are very occasionally observed (Hoby Citation1933, Smith Citation2003). Smith (Citation2003), using histology, recorded a 1:1 male to female sex ratio for toheroa at Ripiro. In contrast, the mātauranga in Taitokerau is that male toheroa are identifiable by their pink mantle colouration. These pink individuals are relatively rare and beds are assumed to be female dominated with a female to male sex ratio of approximately 80:1 (B. Searle and J. Te Tuhi, personal communication). The mātauranga is at odds to the observations of Smith (Citation2003) who found no relationship between the sex of toheroa and their mantle colouration.
Estimations of the age and size at which toheroa reach sexual maturity vary by region. Redfearn (Citation1974), studying northern toheroa, found that the majority of toheroa were reproductive at a length of 32 mm (<1 year) and all were mature by 47 mm. In Murihiku, Beentjes and Gilbert (Citation2006b) found that toheroa reached sexual maturity by about 2 years (c. 76 mm). Toheroa reproduce by broadcast-spawning, releasing their gametes into the seawater for external fertilisation. In northern toheroa, primary gametogenesis occurs during autumn and winter, culminating in a major spawning event in early spring (Redfearn Citation1974; Smith Citation2003). Additional major spawning events may occur in summer (December–January) and autumn (March) (Redfearn Citation1974; Smith Citation2003). When conditions permit, northern toheroa appear able to spawn continuously over the entire year (B. Searle, personal communication). Like for many other temperate bivalves, food abundance and changes in water temperature are thought to primarily influence the onset and duration of spawning. Southern toheroa, which experience much cooler water and atmospheric temperatures, have two main spawning periods, the first in spring and a second in the late summer (Gadomski et al. Citation2015).
A high degree of synchrony in gametogenesis and spawning has been observed for both sexes. Although it appears that male and female gametes may mature at differing times, the shedding of gametes is well synchronised, a behaviour likely to increase fertilisation success in a turbulent surf zone (Smith Citation2003; Gadomski and Lamare Citation2015). The timing of spawning appears to follow environmental cues, with Smith (Citation2003) recording major peaks in spawning activity (both in situ and in vitro) during new and full moon phases. Toheroa have been observed spawning in situ at Ripiro. In one instance, spawning was observed for a period of 10 minutes at night on an incoming tide when between 80 and 100 adult toheroa emerged from the sand with their siphons extended, releasing gametes in a stream from their exhalent siphon into the incoming waves (Akroyd Citation2002; Smith Citation2003). Adult females can release 15–20 million eggs during a single spawning event (Hoby Citation1933; Redfearn Citation1982). While the exact size-fecundity relationship is unknown, fecundity of female toheroa increases with size (Hoby Citation1933; Smith Citation2003).
The larvae of toheroa are planktonic. For northern toheroa, the pelagic larval duration is about 3 weeks (Redfearn Citation1982). For southern toheroa, the pelagic period may be closer to 6 or 7 weeks (Gadomski et al. Citation2015). Gadomski et al. (Citation2015) found that the growth and development of larvae was temperature dependent with faster growth in warmer waters (20° vs. 12° or 16°C). Following a pelagic period, the larvae that are able to reach a suitable beach habitat and then settle out of the water column and into the surf zone, metamorphosing into juvenile toheroa (spat) with a length at settlement of 2 mm or less (Redfearn Citation1974). The morphology of embryonic and larval toheroa stages is typical of related bivalves (Booth Citation1977) and is described by Redfearn (Citation1982) and Gadomski et al. (Citation2015).
Settlement and distribution
At settlement, spat are collected in wave fronts and may be carried up the beach at any stage of the tide. During the interval of slack water just before each wave recedes from the beach, spat are able to dig themselves into the sand to a depth of 10–20 mm (Redfearn Citation1974). Initially, spat appear to be unable to retain good purchase in the substrate and are frequently resuspended out of the sediment. This cycle of passive transport through repeated settlement and resuspension over successive waves, tides and days gradually moves juveniles to the upper shore where they form a band just below the level of the high water mark (Redfearn Citation1974; Smith Citation2003; Beentjes Citation2010b; Williams, Sim-Smith, et al. Citation2013). Redfearn (Citation1974) reported that juveniles are separated from the rest of the population, for the first 18 months after spatfall, either because they occupy different levels of the beach or because they form single cohort beds. However, these observations are in conflict with those made more recently at Ripiro (Williams, Ferguson, et al. Citation2013; P. Ross, personal communication) of mid-tide adult beds containing toheroa of all size classes.
Although it is not known whether toheroa use chemical, biological or physical cues, or a combination of these, to guide settlement, at Ripiro beach Smith (Citation2003) found that densities of juvenile toheroa (less than 32 mm) on the upper shore were higher in areas directly above (upshore of) adult beds, compared to areas where no adult beds were present. This putative juvenile–adult association could be the result of: larval attraction to adult toheroa; hydrodynamic conditions that regularly deposit planktonic larvae, or post-settlement toheroa, at the same locations along the beach; or favourable environmental conditions that lead to higher recruitment or survival rates in certain areas. In contrast, at Oreti, toheroa of all sizes are spread along the entire length of the beach and there does not appear to be any clear relationship between juvenile and adult distribution (Beentjes and Gilbert Citation2006a; Beentjes Citation2010b). Adults, however, tend to be found at the highest densities at the southeast end of Oreti Beach where in 2009, 90% of adults were found in the first 3 km of the 17 km beach.
At both Ripiro and at Oreti beaches (but not Kāpiti-Horowhenua beaches; J. Tamihana, personal communication), larger toheroa tend to be found further down the shore, which may suggest that the degree of submergence experienced at this elevation provides the optimal feeding regime. While this distribution may expose them to greater wave forces, the growing toheroa, now with longer siphons, appear better able to maintain their position on the beach by burrowing to greater depths. It is possible that the involuntary dislodging of smaller and intermediate size toheroa by wave action may be responsible for the more variable positioning of these size classes across the intertidal zone. Where spat or juveniles become stranded above a receding tide due to cyclic changes in tidal height (lunar cycle), changes in atmospheric pressure (affecting sea level) or by waves of larger than normal amplitude, mortality due to desiccation or predation by birds (black-backed gulls, Larus dominicanus; red-billed gulls, L. novaehollandiae scopulinus; pied oyster catchers, Haematopus finschi) can be high (B. Searle, personal communication). Stranded toheroa are often unable to burrow into dry hard packed sand (P. Ross, personal communication), but may survive a tidal cycle (or more) unburied if atmospheric conditions are not too hot or dry (Cassie Citation1955; B. Searle, personal communication).
In Taitokerau, adult toheroa beds are most commonly found in areas subject to freshwater inputs, either near streams (possibly better described as ephemeral overland flows) and seeps, or where the water table lies close to the surface (Rapson Citation1954; Redfearn Citation1974; Akroyd Citation2002). Similarly, at Oreti, high density adult beds are most often found close to the Oreti River estuary (Beentjes Citation2010b). The mechanisms responsible for this freshwater association are unknown. Possible explanations include: that these areas remain moist and cool when the tide recedes (reducing the risk of desiccation); that freshwater inputs result in locally elevated concentrations of palatable phytoplankton; that the altered beach morphology associated with streams (embayments with reduced beach slope) aggregates phytoplankton and toheroa (both pre- and post-settlement); or that the freshwater inputs are modifying other physical or chemical properties of beach sediment making these areas more suitable for toheroa occupation. At present, stream associated beds at Ripiro tend to be small (5–50 m wide), dense (up to 1156 toheroa m−2; Williams, Ferguson, et al. Citation2013), contain a range of size classes and are stable over time. During the 1970s, a period when the toheroa fishery was being closed due to declining populations, individual toheroa beds associated with streams were much larger than they are today and could occupy several hectares of beach and be hundreds of metres wide (Redfearn Citation1974). Away from streams and seeps, toheroa beds at Ripiro are more diffuse (>100 m across), contain toheroa at much lower densities and may be composed of a single size class (Rapson Citation1952; Redfearn Citation1974). These beds are often talked about as being transient and not persisting at a location over time, although this is not always the case (B. Searle, personal communication). Adult beds typically occur down to approximately mid-tide level (Redfearn Citation1974), although individual shellfish can be found all the way down to low-tide. It has been suggested that toheroa may occur subtidally, both adjacent to intertidal populations and also at locations where intertidal populations do not occur (Cassie Citation1951, Citation1955; Waugh and Greenway Citation1967; Greenway Citation1969). Despite several attempts to find these beds, using diver (Street Citation1971) and dredge surveys (Redfearn Citation1974), the existence of sub-littoral toheroa has not been recorded by scientists. However, former commercial tuatua harvesters at Ripiro have provided accounts of large toheroa occurring subtidally and have indicated that these subtidal toheroa have been used on multiple occasions to restock depleted intertidal beds. Tuatua, which typically occupy the lower intertidal and subtidal zones, can be abundant on North Island toheroa beaches. Although the distributions of these two species seldom overlap, recent surveys (2010 and 2011) found tuatua had largely replaced toheroa at Te Oneroa-a-Tōhē, and whilst toheroa densities were greater than tuatua at Ripiro, tuatua densities at this location were highest within toheroa beds (Williams, Ferguson, et al. Citation2013).
Growth
Growth rates of toheroa have been quantified by Rapson (Citation1952), Cassie (Citation1951, Citation1955), Redfearn (Citation1974) and Beentjes and Gilbert (Citation2006b) using a combination of length-frequency cohort analysis (with measurement of macroscopic shell rings) and mark and recapture data (Redfearn Citation1974; Beentjes and Gilbert Citation2006b). Kaitiaki in Taitokerau suggest that major shell rings are laid down at around September and March each year, with minor rings laid down on each full and new moon (B. Searle, personal communication). Further validation of these observations, and of earlier work, may be required before shell reading can be confidently used to assess growth and longevity (Naylor et al. Citation2010). In northern populations, new recruits grow initially at about 3.3 mm per month, and may reach 43 mm after 1 year, 71 mm after 2 years and 100 mm after 4–5 years (Redfearn Citation1974). In Murihiku, the analysis of mark-recapture data indicates that toheroa grow very fast initially, attaining a length of about 70 mm within the first year and 100 mm within 4–5 years (Beentjes and Gilbert Citation2006b). This contrasts with the estimates based on shell ring counts by Cassie (Citation1955) who reported slower growth rates, with the minimum legal size (at that time; 76 mm) not attained until about 10 years. Neither dataset is capable of giving a categorical estimate of maximum age, but both are consistent with a maximum age of about 20 years as suggested by Cassie (Citation1955).
Feeding
Toheroa are generalist filter feeders, consuming phytoplankton and organic debris which are separated in the alimentary canal (Cassie Citation1955). Surf diatoms of the genus Chaetoceros are some of the most predominant phytoplankton in exposed inshore coastal waters (Cassie-Cooper Citation1996; McLachlan and Brown Citation2006), with Chaetoceros armatum accounting for up to 96% of the phytoplankton in the water at Ripiro during winter months (Rapson Citation1954). The high-energy surf beach environment, where toheroa occur, can support a high biomass of diatoms (McLachlan and Brown Citation2006). Cassie (Citation1955) observed dense phytoplankton blooms on the water surface at all beaches where toheroa were present. Heavy slicks of algae can be deposited onto the intertidal beach and at times are so thick and slippery that driving on the beach can be hazardous. Cassie (Citation1955) hypothesised that toheroa rely on these algal blooms to obtain sufficient nutrition for growth and reproduction. Anecdotal reports suggest toheroa condition markedly improves after the autumn rains commence, coincident with the dense phytoplankton blooms, visible as a ‘greenish-brown scum on the beach and in the water’ (Hefford Citation1931; Cassie Citation1955).
Post-settlement movement
Although toheroa beds associated with streams or seeps may persist at a location over time (Rapson Citation1954; Redfearn Citation1974), bed position within a location can be quite variable. The along shore relocation of entire beds by 30 m or more has been observed over a single night (Redfearn Citation1974). Although the triggers of mass toheroa relocation are unknown, there is evidence that a variety of factors may influence bed movement and stability. For example, bed relocation has been recorded following storms (Akroyd Citation2002), and kaitiaki report that toheroa beds must move in response to the discarding of harvest-damaged (dead or dying) toheroa within a bed. Consequently, many Māori disapprove of harvesting (or survey) practises that damage unharvested animals or involve the processing of toheroa, or discarding of their waste, on the beach or near a toheroa bed.
Tagging experiments have shown that while the majority of toheroa are fairly sedentary, some individuals are highly mobile, moving between beds and over several kilometers (Greenway and Allen Citation1962). There is, however, some uncertainty around the relative importance of passive versus active movement in regulating post-settlement movement. While there is little doubt that toheroa may be involuntarily dislodged and moved by heavy swells, they have also been observed using the swash of waves to move up and down the beach in what has been assumed to be an active behaviour (Mestayer Citation1921; Redfearn Citation1974; Ellers Citation1995a, Citation1995b). Kaitiaki in Taitokerau have suggested that beds may move as often as every tidal cycle to track the cyclic pattern of changing tidal heights. It is this tidally driven movement, coupled with variability in atmospheric pressure and wave climate, which has been implicated in generating mass stranding and mortality events. Toheroa are often observed, particularly on the incoming tide, emerging out of the sand, with their siphons extended just ahead of a swash front for forward movement, or after a swash front for backward movement. As the swash wave passes over, toheroa release their foot from the substrate and are moved in the direction of the flow. As the wave recedes, the toheroa rapidly burrow back into the sediment (Redfearn Citation1974). Mestayer in 1921 wrote
at one moment you will see the bare sand as the wave comes in, and immediately it starts to recede, simultaneously and in hundreds of thousands according to the size of the beds, the toheroa emerge from the sand end first, and go down with the receding water, and according to the set of the tide, either up or down the beach.
Sources of post-settlement mortality
Post-settlement mortality of toheroa is likely to be caused by numerous factors, both natural and anthropogenic (). Mass mortalities of toheroa populations appear to be relatively common and have usually occurred during summer months in northern populations (Williams, Sim-Smith, et al. Citation2013), and more frequently during winter months in the south (Eggleston and Hickman Citation1972). Although many mass mortalities have been observed (Cassie Citation1951; Redfearn Citation1974; Akroyd et al. Citation2002), few have been thoroughly investigated and there is in general only speculation as to the causes of individual events (Eggleston and Hickman Citation1972; Hine and Wesney Citation1997; Akroyd Citation2002; Ross et al. Citation2017). The most likely sources of post-settlement mortality were reviewed by Williams, Sim-Smith, et al. (Citation2013) and include desiccation of individuals stranded above the upper intertidal during periods of hot weather; winter southerly storms in Murihiku that may dislodge toheroa, with the extreme cold then slowing or reducing their ability to rebury, making them vulnerable to exposure (freezing) and predation; sediment instability resulting in smothering or exposure to predation; toxic algal blooms (TAB) causing mortality via smothering and anoxia (as a result of the biological oxygen demand of senescent cells); predation by birds, crabs, fish and rays; mortality caused by beach vehicle traffic; human harvesting (formerly commercial and recreational, presently customary and illegal) and disease. Until recently, no specific diseases or parasites were known to afflict toheroa (Hine and Wesney Citation1997). However, a recent examination of toheroa at Ripiro recorded incidences of both gas-bubble disease and Rickettsia-like organisms (RLOs; Ross et al. Citation2017), a group of bacteria frequently associated with shellfish mass mortality events (Malouf et al. Citation1972; Wu et al. Citation2003; Zhu et al. Citation2012; Carvalho et al. Citation2013). Agrichemicals have also been implicated in toheroa mortality. In the 1970s, the pesticide Dieldrin, which is considered highly toxic to fish and aquatic invertebrates (Johnson and Finley Citation1980; Hoke et al. Citation1995), was used to control black beetle (Heteronychus arator) in pine forest plantations. It has been reported that toheroa have not grown near areas that were sprayed or where the pesticide was stockpiled on the beach prior to application by top dressing (Smith Citation2013; B. Searle, personal communication). Kaitiaki have also observed the disappearance of toheroa beds from areas adjacent to commercial forestry operations. These losses were attributed to the degradation of water quality in the streams flowing onto the beaches near toheroa beds (Smith Citation2013).
The biology of toheroa, like that of other intertidal surf clams, makes them highly vulnerable to overexploitation and collapse. Being intertidal they are readily accessible to fishers and their harvest incurs very low operating costs (McLachlan et al. Citation1996). For toheroa, human harvesting was undoubtedly an ecologically significant source of mortality before commercial and recreational harvesting ceased and a major contributor to the collapse of the fishery. However, in the present day, the significance of human harvesting (customary and illegal) as a source of mortality relative to other factors has not been quantified (Williams, Sim-Smith, et al. Citation2013). Based on our observations and communications with kaitiaki, honorary fisheries officers and residents at Ripiro, and to a lesser extent at other locations, it would appear that the levels of human harvesting are significant. Illegal harvesting is common with poaching events ranging in size from residents or visitors ‘just getting a feed every now and then’, which may be once a year or once a week, to large-scale illegal harvesting for the black market. Current harvest levels (illegal and authorised customary take) are largely unquantified and it is likely that the importance of harvest-related mortality varies between locations. This may explain regional differences in population dynamics and the sequence of events leading up to regional fisheries closures.
Current population structure
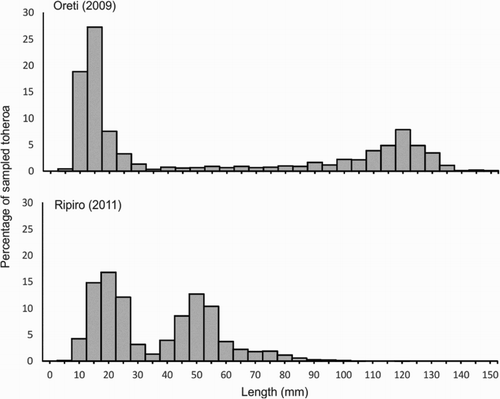
From the available time series data for the six main toheroa populations (Te Oneroa-a-Tōhē, Ripiro, Te Oneone Rangatira, Kāpiti-Horowhenua, Oreti and Bluecliffs), it is evident that there has been a general decline in the abundance of toheroa over time (; Beentjes and Gilbert Citation2006a, Citation2006b; Williams, Sim-Smith, et al. Citation2013, Williams, Ferguson, et al. Citation2013). There is a great deal of variation in estimates of abundance, and not all populations have followed the same fluctuation trends. This again suggests that there may be different local drivers acting on populations rather than a major overriding influence at a national level. The overall downturn observed has not been as marked in some populations as others. Ripiro appears to hold greater densities of juveniles and young adult toheroa than other beaches, suggesting that recruitment is more consistent there than elsewhere (). However, for unknown reasons very few large adult toheroa are found at Ripiro. During the 2010 survey, only 3% of the sampled population was larger than 75 mm (; Williams, Ferguson, et al. Citation2013). At Te Oneroa-a-Tōhē and on the Kāpiti-Horowhenua coast toheroa are now scarce (Williams, Sim-Smith, et al. Citation2013; Newcombe et al. Citation2014; J. Tamihana, personal communication). Strong recruitment events are routinely recorded at Te Oneroa-a-Tōhē (L. Austen, personal communication), but recruits rarely persist into adulthood and beds containing animals of a harvestable size are either largely absent, or are a closely guarded secret (L. Austen, personal communication). Conversely, the population structures at Oreti and Bluecliffs are characteristically bimodal, with a strong adult mode of toheroa greater than 90 mm in length, very few intermediate size toheroa, and a juvenile mode of variable strength (; Beentjes and Glibert Citation2006a, Citation2006b; Beentjes Citation2010a, Citation2010b). Beentjes (Citation2010b) suggested that the likely explanation for this is that mortality of juveniles is high and relatively few survive through to the sub-adult size (40–75 mm). Those that do survive grow rapidly and the strong mode between 100 and 140 mm represents the accumulation of multiple cohorts. At Bluecliffs Beach, in Te Waewae Bay, significant beach erosion and loss of sand since the mid-1980s has exposed underlying gravel and cobble substrates, significantly reducing the availability of habitat suitable for toheroa (Beentjes and Glibert Citation2006a; Beentjes Citation2010a).
Figure 7. Length-frequency distribution of toheroa sampled during the 2009 at Oreti (n = 1221; Beentjes Citation2010b) and in 2011 survey at Ripiro (n = 7578, Williams, Ferguson, et al. Citation2013).
Factors preventing the recovery of toheroa
Despite 40+ years of protection, toheroa populations across New Zealand have, for unknown reasons, failed to recover. Similar patterns have been observed in other large, long-lived and good tasting intertidal bivalves worldwide (McLachlan et al. Citation1996). Surf clam fisheries, which tend to be more recreational or artisanal than commercial, are notoriously difficult to manage since numbers of harvesters cannot usually be controlled and exploitation must be limited solely by size, bag limit, season or area restrictions. In comparison to rocky reef environments where there are numerous examples of population recovery following the introduction of harvesting restrictions (Costello Citation2014), the managed recovery of open coast beach species is rare (McLachlan et al. Citation1996; Ferguson et al. Citation2015). Where post-exploitation populations have failed to recover, continued overharvesting as well as a range of other threats including off-road vehicles, pollution, coastal engineering and coastal development have been implicated (McLachlan et al. Citation1996). Heasman et al. (Citation2012) and Williams, Sim-Smith, et al. (Citation2013) reviewed the factors considered most likely to be preventing the recovery of toheroa. Climate and weather, food availability, TABs, vehicle activity, water quality and changes in land use were identified as possible environmental factors (). Given the recent detection of gas-bubble disease and RLOs in toheroa (Ross et al. Citation2017), these ailments may be added to this list. A loss of stewardship ethic among Māori, the negative effects of preferential harvest of large toheroa and negative features of the customary harvest system were identified as possible human factors standing in the way of recovery (Smith Citation2013). Again, given our recent observations, it is likely that illegal harvesting may also be an important factor, particularly in Taitokerau where large toheroa are now uncommon. Of the factors mentioned above, the manner in which vehicles are driven on and have access to toheroa beaches, the way that lands adjacent to these beaches are used and levels of continued harvesting are seen as three areas where human impacts could be mitigated. Conversely, the effects of climate on food, larval supply or the occurrence of TABs are seemingly beyond immediate human control.
Vehicle effects
Studies in Australia, South Africa and the United States have documented the effects of vehicle activity on beach fauna. Impacts have included mortality in surf clams (Donax serra and D. deltoids) as a direct consequence of crushing (Van der Merwe and Van der Merwe Citation1991; Schlacher et al. Citation2008), reduced species richness and diversity of intertidal communities (MacLeod et al. Citation2009) and local extinctions and regional declines in vehicle sensitive species (Hubbard et al. Citation2014). Many of New Zealand’s beaches, including Te Oneroa-a-Tōhē, Ripiro, the Kāpiti-Horowhenua beaches and Oreti, are designated state highways and are subject to high levels of vehicle traffic. By law, speeds are limited to 100 km per hour and erratic driving (loss of traction and ‘doughnuts’) is forbidden, although the extent to which this is monitored and managed varies between beaches. There is strong evidence that beach traffic can cause toheroa mortality (Redfearn Citation1974, Brunton Citation1978, Hooker and Redfearn Citation1998, Moller, Moller, et al. Citation2009), either directly through crushing, or indirectly through exposure, which increases the risk of desiccation or predation by birds. Moller, Moller, et al. (Citation2009) found that low levels of vehicle activity do not cause significant mortality of adult toheroa, but even a single vehicle pass can cause significant mortality in juveniles, particularly those living high on the beach in soft sand. Consequently, beach events involving large numbers of vehicles, for example, beach fishing competitions or off-road vehicle races could result in high levels of juvenile mortality (Moller, Moller, et al. Citation2009).
Changes in land use
The effects of changing land use on coastal and nearshore ecosystems are well studied internationally and include disruption to the hydrologic cycle (Huber et al. Citation2008), accelerated soil erosion (Lohrer et al. Citation2004; Baptista Neto et al. Citation2013) and water quality deterioration (Leh et al. Citation2011; Ramos-Scharrón, et al. Citation2015; Seers and Shears Citation2015). Landscape modification has resulted in the degradation of ecosystems including coral reefs (Stender et al. Citation2014), estuaries (Pratt et al. Citation2014) and rocky reefs (Walker Citation2007; Pulfrich and Branch Citation2014). The effects of land use on surf beaches are less well studied (Schlacher et al. Citation2015).
In New Zealand, the land surrounding Te Oneroa-a-Tōhē, Ripiro and Te Oneone Rangatira was originally covered in native broadleaf forest (Smale et al. Citation1996). Much of this land was cleared by Māori 500–700 years ago (Coster Citation1989), with large areas of the remaining native vegetation removed by early European settlers (Cockayne Citation1911; Bacon Citation1976; McKelvey Citation1999). Large-scale planting of exotic marram grass (Ammophila arenaria) and tree lupin (Lupinus arboreus) was instigated in the early 1900s in an effort to stabilise sand dunes. Marram grass in particular altered the morphology of sand dunes and beach hydrology (Esler Citation1970; Hesp Citation1999; Müller Citation2011). In recent years, efforts have been made, across much of New Zealand, to restore natural dune plant vegetation. Numerous restoration programmes have focused on planting spinifex, pingao and sand tussock (Poa billardierei) for cultural, aesthetic and recreational values as well as conservation and biodiversity considerations (J. Te Tuhi & B. Young, personal communication; Bergin and Kimberley Citation1999).
From the 1950s to 1970s, much of the land adjacent to Te Oneroa-a-Tōhē (Aupouri State Forest), Ripiro (Poutu Forest) and Te Oneone Rangatira (Woodhill Forest) in Taitokerau and Waitarere (Waitarere Forest) and Tangimoana Beaches (Tangi Moana Forest) on the Horowhenua coast was converted to pine forest (Pinus radiata). Williams, Sim-Smith, et al. (Citation2013) discuss in detail the consequences of afforestation, which include changes in groundwater chemistry (Staaf and Olsson Citation1994; Quinn et al. Citation1997), a reduction in soil moisture levels, water table height (Cromarty and Scott Citation1996; McKelvey Citation1999; Huber et al. Citation2008) and reduced freshwater seepage flowing onto Te Oneroa-a-Tōhē and Ripiro beaches. Ground water is also diverted away from coastal streams to support agriculture at some locations, further reducing freshwater inputs to the coast (P. Ross, personal communication). Many streams that formerly flowed onto the toheroa beaches of Taitokerau are no longer there (Williams, Sim-Smith, et al. Citation2013). Williams, Sim-Smith, et al. (Citation2013) compared the number of water courses on historical versus modern day topographic maps and showed a reduction of 64% (53 of 83) at Te Oneroa-a-Tōhē and 40% (6 of 15) at Ripiro. Given the clear relationship that exists between toheroa beds and points of freshwater input onto beaches in northern New Zealand (Rapson Citation1954; Redfearn Citation1974; Williams, Sim-Smith, et al. Citation2013; Williams, Ferguson, et al. Citation2013), it is conceivable that reductions in freshwater flow may have contributed to the decline of toheroa. However, as the specific mechanism(s) behind the relationship are unknown, there can be no certainty, without further research.
Human harvesting
Worldwide, many populations of marine species, including surf clams, continue to decline despite legislation providing protection, mainly due to poaching and/or accidental mortality (McLachlan et al. Citation1996). Examples of ‘protected’ species where illegal harvesting is preventing recovery include bivalves and gastropods (Katsanevakis et al. Citation2011), sea turtles (Koch et al. Citation2006), Atlantic bluefin tuna, sharks and other fish species (Agnew et al. Citation2009; Techera and Klein Citation2011). For toheroa, much of the customary harvest is undocumented and the illegal harvest unquantified (Heasman et al. Citation2012; Williams, Sim-Smith, et al. Citation2013). Based on our recent observations in Taitokerau, illegal harvesting of ‘protected’ toheroa is widespread, frequent and has in some cases resulted in the reduction and disappearance of adult toheroa beds (P. Ross and J. Cope, personal communication). In Murihiku, a recent estimate suggests that the combined customary and illegal harvest of toheroa could easily account for as much as 13–50% of the toheroa population each year (Heasman et al. Citation2012).
Knowledge gaps and future research
The fauna of ocean coast beaches are cryptic and mobile and easily overlooked by ecologists, thus surf clams are generally understudied (McLachlan et al. Citation1996). Recruitment (including the role of nearshore hydrodynamics in settlement processes), density-dependent processes (that may lead to variation in growth, mortality and recruitment) and incidental mortality and disturbance associated with harvesting are topics that have previously been identified as deserving special consideration for future surf clam research (McLachlan et al. Citation1996). Since the collapse and closure of the toheroa fisheries, much of the research effort relating to this species has been invested in abundance surveys and stock assessments (reviewed by Beentjes and Gilbert Citation2006a, Citation2006b; Williams, Sim-Smith, et al. Citation2013). Studies furthering our understanding of toheroa life history and the factors preventing their recovery have been limited. Where researchers have conducted empirical studies, the knowledge gained has contributed to subsequent assessments of the possible obstacles to recovery (Heasman et al. Citation2012; Williams, Sim-Smith, et al. Citation2013). The authors of these empirical studies have in some cases suggested management actions to support restoration, for example, traffic management (Moller, Moller, et al. Citation2009) or temporal management of cultural harvest (Smith Citation2003). These suggestions have not been implemented. Population surveys will continue to be important in assessing the effectiveness of future management regimes, particularly where survey methodologies are comparable in time and space (). However, on their own, surveys will not facilitate the recovery of toheroa or even necessarily provide the information needed to inform management and restoration efforts.
Survey data showing regional variation in population structure (; Williams, Sim-Smith, et al. Citation2013) indicate that there are probably different local drivers acting on different populations. Some populations appear to be recruit limited (Murihiku and Kāpiti-Horowhenua) while in others the obstacles to recovery appear to be acting at later life stages. Understanding where and how life history bottlenecks are acting will be key to developing area specific management plans to support toheroa restoration. The geographical range of toheroa is subtropical to subantarctic () implying a degree of flexibility to large-scale oceanographic and atmospheric variation. Conversely, toheroa distribution within and among beaches can be highly variable indicating very specific habitat requirements. Together, these macro and mesoscale patterns suggest that the physical beach environment might be a more important determinant of beach habitability than climate, or that habitat variability is interacting with climate to determine distribution patterns.
It is still not clear what makes for optimal toheroa habitat, particularly, why toheroa occur where they do, or why their distribution patterns and population structures vary between northern and southern beaches. There is clearly an association between toheroa and freshwater inputs in northern New Zealand. There is currently no understanding of whether this is a response to the freshwater itself, the effects of freshwater on environmental parameters (such as grain size or beach temperature) or the beach morphology associated with streams retaining toheroa and delivering their food. Additionally, the fact that there is still uncertainty around the existence of sub-littoral toheroa, more than half a century after the possibility was first raised by Cassie (Citation1951), is indicative of the paucity of our ecological knowledge of this species. The slow progress made in filling these gaps may result from the many difficulties associated with studying surf beach ecosystems (McLachan and Brown Citation2006). With a more complete understanding of what constitutes optimal toheroa habitat, and an appreciation of changing land use over time, it may be possible to determine if habitat components have been lost from areas where toheroa formerly thrived. With this knowledge, efforts could be made to adjust environmental management regimes to rehabilitate key habitat characteristics in support of restoration, for example, by altering the types of vegetation growing adjacent to key toheroa beds to restore the flow of groundwater to the coast.
The relative importance of self- versus external recruitment for the maintenance and recovery of toheroa populations is unknown. For species such as toheroa, which occur in fragmented populations (), the exchange of individuals (connectivity) between populations is considered critical for population stability (Cowen et al. Citation2007). For toheroa, connectivity occurs solely via a dispersive larval phase (Ross et al. Citation2009). A possible consequence of the population decline and range contraction observed in toheroa is a decrease in among-population connectivity (Jones et al. Citation2007). Estimating connectivity is extremely challenging because the nature of marine ecosystems generally precludes the direct measurement of larval exchange among populations (Cowen et al. Citation2007). Dispersal of toheroa larvae is likely to be at the scale of 10s to 100s of km rather than 1000s of km (Sutton and Bowen Citation2011). Consequently, connectivity among all toheroa populations would not be expected (). Having a better understanding of larval exchange among toheroa populations may help explain the observed population dynamics of toheroa, help with predicting their responses to environmental change and management regimes, and assist with designing conservation strategies to facilitate restoration through connectivity and larval recruitment.
The reseeding of toheroa populations with hatchery-reared spat (or juveniles) has been suggested as an alternative management approach for restoration (Newcombe et al. Citation2015). There is widespread interest in toheroa aquaculture, particularly from Māori groups interested in restoration of this taonga and in culturing toheroa as a commercial venture (various, personal communication). These two interests may ultimately be complimentary as the development of commercial toheroa aquaculture could facilitate the production of spat for the large-scale reseeding and enhancement of natural populations. Newcombe et al. (Citation2015) discuss the challenges associated with adapting existing culture techniques to suit species living in high-energy environments. Redfearn (Citation1982) and more recently Mandeno (Citation1999), Smith (Citation2003) and Gadomski et al. (Citation2015) developed spawning and larval rearing techniques, and culture through to post-settlement size (c. 30 mm) was achieved at the Mahanga Bay shellfish hatchery in Wellington in the 1980s (P. Redfearn personal communication). At that time, toheroa were not considered to be of commercial interest and development of the species for aquaculture was not pursued.
While toheroa aquaculture does appear viable, a good understanding of the environmental factors and life history bottlenecks preventing the recovery of wild populations is essential before assessing the utility of aquaculture for enhancement and restoration. For example, reseeding for restoration is unlikely to be effective at beaches where beach habitat is no longer suitable (Beentjes et al. Citation2006) or where recruitment rates are high but survival past later life stages is low (i.e. Ripiro and Te Oneroa-a-Tōhē; ). Conversely, at beaches where populations are recruitment limited, reseeding may overcome natural obstacles to recovery. More information on natural recruitment patterns, population structure and ecology is required before embarking on reseeding projects.
The discovery of RLOs and gas-bubble disease in toheroa (Ross et al. Citation2017) is of concern and may to some extent help explain contemporary population dynamics and mass mortalities that have been recorded in toheroa and tuatua in Taitokerau (Williams, Sim-Smith, et al. Citation2013). More work is required to understand the interactions between these two conditions and to determine their distribution across species, space and time. The detection of RLOs will likely have implications for toheroa aquaculture and for the translocation of toheroa, and other shellfish, between beaches for reseeding or enhancement purposes. The spread of pathogens to unaffected populations should be avoided if possible.
Current management options
While there is a lot we do not know about the ecology of toheroa and the major sources of toheroa mortality, there are steps that could be taken immediately to help with restoration and management efforts (). For example, beach traffic can cause toheroa mortality, particularly in juveniles. In South Africa, motorised vehicles were banned from most beaches between 2001 and 2002 (DEAT Citation2004), primarily for the protection of endangered species (birds and turtles). The ban was not well received by off-road enthusiasts, and some other beach users, but did result in measurable ecological benefits for intertidal beach invertebrates (Lucrezi et al. Citation2014).
Removing vehicles from New Zealand beaches may not be a realistic, popular or desirable option. However, modifying driver behaviour at certain times and on certain sections of beach probably is. Schlacher et al. (Citation2008) investigated the impacts of vehicle activity on the surf clam Donax deltoides in Australia and found that driving modes and patterns were key factors in determining clam mortality and physical habitat disturbance. Speed restrictions that alter driver behaviour could be introduced in key areas (adult beds and high recruitment areas) and beach users provided with recommendations for driving behaviour around important toheroa habitat (e.g. streams). Limiting the mass use of beaches by vehicles during the periods of high recruitment (late spring – early summer and early autumn; Redfearn Citation1974; Smith Citation2003; Gadomski et al. Citation2015), for example, in fishing contests, could reduce juvenile mortality and support recovery in recruit limited populations. Moller et al. (Citation2009b) discuss seasonal or spatial closures and the insertion of physical barriers as a way of discouraging vehicles from transiting along the upper intertidal zone where juvenile toheroa are most abundant. Providing a rationale for any driving recommendations or regulations will undoubtedly be key to attaining buy-in and compliance from beach users.
Despite their status as New Zealand’s most protected shellfish, illegal harvesting of toheroa is widespread and frequent. As toheroa are no longer a commercially harvested species, the motivation, or commercial pressure, to enforce fisheries regulations is probably less than in other frequently poached kai moana, for example, scallops, paua or crayfish. Toheroa also occur on isolated stretches of coastline and there is a reliance on honorary (volunteer) fisheries officers and local communities to police fisheries regulations. It is clear that the resourcing of toheroa fisheries management has been insufficient to prevent widespread illegal harvesting. Alternative management approaches involving education and community involvement may be more successful. Information panels (interpretive signs) at major beach access ways explaining how to differentiate between toheroa and tuatua should be a minimum first step and might reduce rates of accidental harvesting. Similarly, providing the rationale around why toheroa harvesting (without a customary permit) is prohibited may raise some awareness around the significance and plight of toheroa and reduce intentional illegal harvesting. Involving local communities in toheroa research and management may result in an increased stewardship ethic, a greater willingness to comply with harvesting regulations and more efficient enforcement through community peer pressure and the reporting of illegal activity. The communities (both Māori and Pakeha) at the toheroa beaches are for the most part enthusiastic about toheroa restoration but in some cases feel abandoned by fisheries managers and unable to participate or contribute to the restoration process (various, personal communication; Memon et al. Citation2003; Gnanalingam and Hepburn Citation2015).
As kaitiaki of the whenua (land), the moana (sea) and kai (food), and as issuers of customary permits for the collection of protected species, Māori have an important role to play in ensuring the sustainability of toheroa for future generations. This is somewhat ironic, given that Māori were largely excluded from the management process for the duration of the commercial and recreational fisheries (Murton Citation2006). It was only after the fisheries had collapsed and interest from other user groups diminished, that responsibility for toheroa management was passed back to tangata whenua, the traditional managers of this resource. During this post-fishery period, toheroa populations have not recovered and there is a perception from within Māoridom that in its present form, the customary permit system does not promote sustainable customary harvest (Smith Citation2013).
In Taitokerau, one key area of concern is that resolutions issued at the iwi (tribe) level aiming to protect the toheroa resource, for example by placing rāhui (temporary closures) on harvesting, do not necessarily preclude individual hapu (subtribe) or marae from within that iwi, or from another iwi elsewhere, continuing to issue permits for customary harvest. Consequently, permit issuers may have limited or no connection to the beach, have little knowledge of the current status of the resource and little regard for its sustainability (Smith Citation2013). An alternative system may be required where customary permits can only be issued and executed by kaitiaki with a good understanding of the condition of local toheroa populations. Financial resourcing of kaitiaki positions, collaboration with fisheries managers and science providers and better co-operation among and within iwi will be required for such a system to work. Kaitiaki would then be in a position to monitor toheroa beds and would have the mana (status and authority) to allocate and enforce levels of customary harvest that would not compromise sustainability. Rāhui could be declared if toheroa populations were in decline or thought to be particularly vulnerable (). In Murihiku, Māori, Regional Council, the Department of Conservation and Police all play a part in toheroa management (R. Trainor, personal communication). In the far north (Te Hiku o Te Ika), the recent formation of the Te Oneroa-a-Tōhē Beach Board, with equal iwi and local authority membership, may represent an opportunity to develop this concept of a more collaborative, centralised and innovative management strategy (Te Hiku Claims Settlement Bill Citation2015).
Conclusions
In recent times, the contribution of environmental managers and marine scientists to toheroa management has largely been to conduct surveys that monitor the status of the discrete populations. Toheroa have not recovered and it is clear that the current management regime is not geared towards restoration. Unfortunately, the research community has not been able to provide any clear direction for alternative management approaches. There are too many possible explanations for the continued demise of toheroa and too little research has been conducted to address the uncertainties outlined above. More research is needed. But first, efforts must be made to overcome the distrust that can exist between Māori, resource managers and the research community. Researchers have a history of exploiting Matāuranga Māori (Māori knowledge), failing to report back to communities on the findings of their research, and tend to focus on research outputs rather than environmental outcomes for impacted communities (Waitangi Tribunal Citation2011; Broughton et al. Citation2015). Conversely, it can be difficult for researchers to work with Māori as the process of engagement and relationship building, which is crucial to collaborative research, can be intimidating and rarely fits within the short timeframes available for formulating research proposals or conducting research. As a consequence it can be challenging for non-Māori researchers to work on culturally important species and for Māori to access scientific knowledge to support restoration and management in their rohe.
If efforts to restore toheroa are to be successful, it will undoubtedly require collaboration and a willingness by all parties to step outside their traditional comfort zones. A better flow of information among interested groups is needed and may eventually build trust and allow for the development of more effective working relationships. Researchers working alongside local experts and kaitiaki will lead to better research outcomes. Engagement with local communities and resource users will lead to a better understanding of the human components of this ecological problem; community ‘ownership’ of toheroa restoration; and better uptake and enforcement of regulations or management plans. Importantly, collaboration and engagement will build capacity and knowledge within these coastal communities. As a consequence, communities will be empowered to take a leading role in the management of their rohe and to continue collecting ecological information beyond the limited timeframes usually associated with academic research projects.
Acknowledgements
We would like to acknowledge the people of the toheroa beaches and the kaitiaki of the following iwi for their contributions to the research: Ngāi Tahu; Ngāti Raukawa; Ngāti Whātua; Ngāpuhi; Te Uri o Hau; Te Roroa; Te Rarawa; Te Aupouri; Ngāi Takoto and Ngāti Kuri.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agnew DJ, Pearce J, Pramod G, Peatman T, Watson R, Beddington JR, Pitcher TJ. 2009. Estimating the worldwide extent of illegal fishing. PLoS ONE. 4(2):e4570. doi:10.1371/journal.pone.0004570.
- Akroyd JM. 2002. Investigation into enhancement of toheroa (Paphies ventricosa) populations on a North Island West Coast Beach, New Zealand [unpublished MAppSci thesis]. Auckland: Auckland University of Technology. 72 p.
- Akroyd JM, Walshe KAR, Manly B, Te Tuhi J, Searle B, Searle, R. 2008. Distribution and abundance of toheroa (Paphies ventricosa) on Dargaville and Muriwai Beaches, 2006–2007. New Zealand Fisheries Assessment Report 2008/29. 28 p.
- Akroyd JM, Walshe KAR, Millar RB. 2002. Abundance, distribution, and size structure of toheroa (Paphies ventricosa) at Ripiro Beach, Dargaville, Northland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 36:547–553. doi: 10.1080/00288330.2002.9517110
- Arntz WE, Valdina E, Zeballos J. 1988. Impact of El niño 1982–83 on the commercially exploited invertebrates (moviscos) of the Peruvian shore. Meeresforsch. 32:3–22.
- Auckland Star. 1957 Jul 14. Glinks Gully toheroa beds thrashed.
- Auckland Star. 1930 May 12. Life of the toheroa.
- Bacon MR. 1976. Report on a coastal resource area, Kaipara South peninsula. 115 p.
- Baptista Neto JA, Barreto CF, Martins da Silva MA, Smith BJ, Mcallister JJ, Vilela CG. 2013. Nearshore sedimentation as a record of landuse change and erosion: Jurujuba Sound, Niterói, SE Brazil. Ocean and Coastal Management. 77:31–39. doi: 10.1016/j.ocecoaman.2012.04.022
- Beentjes MP. 2010a. Toheroa survey of Bluecliffs Beach, 2009, and review of historical surveys. New Zealand Fisheries Assessment Report 2010/7. 42 p.
- Beentjes MP. 2010b. Toheroa survey of Oreti Beach, 2009, and review of historical surveys. New Zealand Fisheries Assessment Report 2010/6. 40 p.
- Beentjes MP, Carbines GD, Willsman AP. 2006. Effects of beach erosion on abundance and distribution of toheroa (Paphies ventricosa) at Bluecliffs Beach, Southland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 40:439–453. doi: 10.1080/00288330.2006.9517434
- Beentjes MP, Gilbert DJ. 2006a. Bluecliffs Beach 2005 toheroa survey: yield per recruit and review of historical surveys. New Zealand Fisheries Assessment Report 2006/37. 48 p.
- Beentjes MP, Gilbert DJ. 2006b. Oreti Beach 2005 toheroa survey: yield per recruit and review of historical surveys. New Zealand Fisheries Assessment Report 2006/36. 47 p.
- Bergin DO, Kimberley MO. 1999. Rehabilitation of coastal foredunes in New Zealand using indigenous sand-binding species. Science for Conservation. Department of Conservation, Wellington. 52 p.
- Berkenbusch K, Abraham E, Neubauer P. 2015. Distribution and abundance of toheroa in Southland, 2013–14. New Zealand Fisheries Assessment Report 2015/17. 41 p.
- Booth JD. 1977. Common bivalve larvae from New Zealand: Mytilacea. New Zealand Journal of Marine and Freshwater Research. 11:407–440. doi: 10.1080/00288330.1977.9515687
- Broughton D, (Te Aitanga-a-Hauiti, Taranaki, Ngāti Porou, Ngāpuhi), McBreen K, (Waitaha, Kāti Māmoe, Ngāi Tahu). 2015. Mātauranga Māori, tino rangatiratanga and the future of New Zealand science. Journal of the Royal Society of New Zealand. 45:83–88. doi: 10.1080/03036758.2015.1011171
- Brunton PM. 1978. Toheroa predation by black-backed gulls on Dargaville Beach, North Auckland, New Zealand. Notornis. 25:128–140.
- Carvalho YBM, Poersch LH, Romano LA. 2013. Rickettsia-associated mortality of the yellow clam Mesodesma mactroides (Bivalvia: Mesodesmatidae) in southern Brazil. Malacologia. 56:301–307. doi: 10.4002/040.056.0217
- Cassie RM. 1951. A molluscan population with an unusual size frequency-distribution. Nature. 167:284–285. doi: 10.1038/167284a0
- Cassie RM. 1955. Population studies on the toheroa, Amphidesma ventricosum gray (Eulamellibranchiata). Marine and Freshwater Research. 6:348–391. doi: 10.1071/MF9550348
- Cassie-Cooper V. 1996. Microalgae: microscopic marvels. Hamilton: Riverside Books. 164 p.
- Chief Inspector of Fisheries. 1939. To Secretary, Marine Department, January 25. Marine Department, M1 2/12/9, Archives New Zealand, Wellington.
- Cockayne L. 1911. Report on the dune-areas of New Zealand, their geology, botany and reclamation. Appendix J. New Zealand House of Representatives. C 13:1–76.
- Coe WR. 1955. Ecology of the bean clam Donax gouldi on the coast of southern California. Ecology. 36:512–514. doi: 10.2307/1929590
- Cook S. 2010. Class Bivalvia. In: Cook SdC, editor. New Zealand coastal marine inverebrates, volume One. Christchurch: Canterbury University Press; p. 471–541.
- Costello M. 2014. Long live marine reserves: a review of experiences and benefits. Biological Conservation. 176:289–296. doi: 10.1016/j.biocon.2014.04.023
- Coster J. 1989. Dates from the dunes: a sequence for the Aupouri Peninsula, Northland, New Zealand. New Zealand Journal of Archaeology. 11:51–75.
- Cowen RK, Gawarkiewicz G, Pineda J, Thorrold SR, Werner FE. 2007. Population connectivity in marine systems: An overview. Oceanography. 20:14–21. doi: 10.5670/oceanog.2007.26
- Cromarty P, Scott DA. 1996. A directory of wetlands in New Zealand. Wellington: Department of Conservation. 395 p.
- De Villiers G. 1974. Growth, population dynamics, a mass mortality and arrangement of white sand mussels, Donax serra roding, on beaches in the south-western cape province. Investigational Report/Sea Fisheries Branch. Department of Industries. South Africa. 109:1–31.
- [DEAT] Department of Environmental Affairs and Tourism. 2004. Guidelines on the implementation of regulations pertaining to the control of vehicles in the coastal zone. Department of Environmental Affairs and Tourism, Pretoria, South Africa. https://www.environment.gov.za/sites/default/files/legislations/nema_coastalzone_vehicleuse_g27066rg8113gon1399.pdf
- Eggleston D, Hickman RW. 1972. Mass stranding of molluscs at Te Waewae bay, Southland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 6:379–382. doi: 10.1080/00288330.1972.9515432
- Ellers O. 1995a. Behavioural-control of swash-riding in the clam Donax variabilis. Biological Bulletin. 189:120–127. doi: 10.2307/1542462
- Ellers O. 1995b. Discrimination among wave-generated sounds by a swash-riding clam. Biological Bulletin. 189:128–137. doi: 10.2307/1542463
- Esler AE. 1970. Manawatu sand dune vegetation. Proceedings of the New Zealand Ecological Society. 17:41–46.
- Ferguson GJ, Ward TM, Gorman D. 2015. Recovery of a surf clam Donax deltoides population in Southern Australia: successful outcomes of fishery-independent surveys. North American Journal of Fisheries Management. 35:1185–1195. doi: 10.1080/02755947.2015.1091408
- Fiori S, Vidal-Martínez VM, Simá-Álvarez R, Rodríguez-Canul R, Aguirre-Macedo ML, Defeo O. 2004. Field and laboratory observations of the mass mortality of the yellow clam Mesodesma mactroides in South America: the case of Isla del Jabalí, Argentina. Journal of Shellfish Research. 23:451–455.
- Futter JM. 2011. An investigation into the Murihiku toheroa (Paphies ventricosa): matauranga, monitoring and management [unpublished MSc thesis]. University of Otago. Dunedin 140 p.
- Gadomski K, Lamare M. 2015. Spatial variation in reproduction in southern populations of the New Zealand bivalve Paphies ventricosa (Veneroida: Mesodesmatidae). Invertebrate Reproduction and Development. 59:81–95. doi: 10.1080/07924259.2015.1007216
- Gadomski K, Moller H, Beentjes M, Lamare M. 2015. Embryonic and larval development of the New Zealand bivalve Paphies ventricosa gray, 1843 (Veneroida: Mesodesmatidae) at a range of temperatures. Journal of Molluscan Studies. 81:356–364. doi: 10.1093/mollus/eyv001
- Gnanalingam G, Hepburn C. 2015. Flexibility in temporary fisheries closure legislation is required to maximise success. Journal of Marine Policy. 61:39–45. doi: 10.1016/j.marpol.2015.06.033
- Greenway JP. 1969. Population surveys of toheroa (Mollusca: Eulamellibranchiata) on northland beaches, 1962–67. New Zealand Journal of Marine and Freshwater Research. 3:318–338. doi: 10.1080/00288330.1969.9515300
- Greenway JP, Allen KR. 1962. Toheroa survey – Ninety Mile Beach October 1961. Special Fisheries Report. New Zealand Marine Department, Wellington, No. 13. 11 p.
- Heasman K, Keeley N, Sinner J. 2012. Factors affecting populations of Toheroa (Paphies ventricosa): a literature review. Manaaki Taha Moana Research Report No. 10. Cawthron Report No. 1997. 29 p. Plus appendices.
- Hefford AE. 1931. Marine Department Annual Report for 1930–1931. New Zealand Marine Department, Wellington. 15–27 p.
- Hesp PA. 1999. The beach backshore and beyond. In: Short AD, editor. Handbook of beach and shoreface morphodynamics. Chichester: John Wiley & Sons Ltd; p. 145–169.
- Hine PM, Wesney B. 1997. Virus-like particles associated with cytopathology in the digestive gland epithelium of scallops Pecten novaezelandiae and toheroa Paphies ventricosum. Diseases of Aquatic Organisms. 29:197–204. doi: 10.3354/dao029197
- Hoby KE. 1933. The anatomy of Amphidesma ventricosum (gray) [unpublished MSc thesis]. Wellington: Victoria University of Wellington. 86 p.
- Hoke RA, Kosian PA, Ankley GT, Cotter AM, Vandermeiden FM, Phipps GL, Durham EJ. 1995. Ceck studies with Hyalella azteca and Chironomus tentans in support of the development of a sediment quality criterion for dieldrin. Environmental Toxicology and Chemistry. 14:435–443. doi: 10.1002/etc.5620140313
- Hooker S, Redfearn P. 1998. Preliminary survey of toheroa (Paphies ventricosa) populations on Ninety Mile Beach and possible impacts of vehicle traffic. NIWA Client Report AK98042 for Northland Regional Council. 32 p.
- Hubbard DM, Duggan JE, Schooler NK, Viola SM. 2014. Local extirpations and regional declines of endemic upper beach invertebrates in southern California. Estuarine, Coastal and Shelf Science. 150:67–75. doi: 10.1016/j.ecss.2013.06.017
- Huber A, Iroumé A, Bathurst J. 2008. Effect of Pinus radiata plantations on water balance in Chile. Hydrological Processes. 22:142–148. doi: 10.1002/hyp.6582
- Johnson WW, Finley MT. 1980. Handbook of acute toxicity of chemicals to fish and aquatic invertebrates, resource publication 137. Washington, DC: US Department of Interior, Fish and Wildlife Service.
- Jones GP, Srinivasan M, Almany GR. 2007. Population connectivity and conservation of marine biodiversity. Oceanography. 20:100–111. doi: 10.5670/oceanog.2007.33
- Katsanevakis S, Poursanidis D, Issaris Y, Panou A, Petza D, Vassilopoulou V, Chaldaiou I, Sini M. 2011. “Protected” marine shelled molluscs: thriving in Greek seafood restaurants. Mediterranean Marine Science. 12:429–438. doi: 10.12681/mms.42
- Koch V, Nichols WJ, Peckham H, de la Toba V. 2006. Estimates of sea turtle mortality from poaching and bycatch in Bahia Magdalena, Baha California Sur, Mexico. Biological Conservation. 128:327–334. doi: 10.1016/j.biocon.2005.09.038
- Kondo Y, Stace G. 1995. Burrowing ability and life position of toheroa (Paphies ventricosa: Mesodesmatidae), an unusually large, deep-burrowing ocean beach bivalve endemic to New Zealand. Japanese Journal of Malacology. 45:67–76.
- Leh M, Bajwa S, Chaubey I. 2011. Impact of land use change on erosion risk: an integrated remote sensing, geographic information system and modelling methodology. Land Degradation and Development. 24:409–421.
- Lohrer AM, Thrush SF, Hewitt JE, Berkenbusch K, Ahrens M, Cummings VJ. 2004. Terrestrially derived sediment: response of marine macrobenthic communities to thin terrigenous deposits. Marine Ecology Progress Series. 273:121–138. doi: 10.3354/meps273121
- Lucrezi S, Saayman M, van der Merwe P. 2014. Impact of off-road vehicles (ORVs) on ghost crabs of sandy beaches with traffic restrictions: a case study of Sodwana Bay, South Africa. Environmental Management. 53:520–533. doi: 10.1007/s00267-013-0223-5
- MacLeod CK, Forbes SE, Shepherd CJ, Crawford C. 2009. Effects of oyster farming service vehicles on an intertidal sand flat. Aquaculture Research. 40:772–780. doi: 10.1111/j.1365-2109.2008.02156.x
- Malcolm J. 1928. Food values of New Zealand fish. Part 9: Tinned toheroa and toheroa soup. Transactions of the New Zealand Institute. 59:85–90. Cited in: Nature. 1928. 122:664.
- Malouf R, Maurer D, Keck R, Epifanio C. 1972. Occurrence of gas-bubble disease in three species of bivalve molluscs. Journal of the Fisheries Research Board of Canada. 29:588–589. doi: 10.1139/f72-098
- Mandeno M. 1999. Gonadal cycle and spawning induction in Toheroa (Paphies ventricosa) from southern New Zealand [unpublished MSc thesis]. Dunedin: University of Otago.
- McKelvey PJ. 1999. Sand forests: a historical perspective of the stabilisation and afforestation of coastal sands in New Zealand. Christchurch: Canterbury University Press. 168 p.
- McLachlan A, Brown AC. 2006. The ecology of sandy shores. Burlington, MA: Academic Press. 373 p.
- McLachlan A, Dugan JE, Defeo O, Ansell AD, Hubbard DM, Jarmillo E, Penchaszadeh PE. 1996. Beach clam fisheries. Oceanography and Marine Biology: an Annual Review. 34:163–232.
- Memon PA, Sheeran B, Ririnui T. 2003. Strategies for rebuilding closer links between local indigenous communities and their customary fisheries in Aotearoa/New Zealand. Local Environment. 8:205–219. doi: 10.1080/1354983032000048505
- Mestayer MK. 1921. Notes on the habits and uses of the toheroa. New Zealand Journal of Science and Technology. 4:84–85.
- Minister of Marine. 1933. To L. W. Parore, March 7. Marine Department, M1 2/12/9, Archives New Zealand, Wellington.
- Miskelly C. 2016. Legal protection of New Zealand’s indigenous aquatic fauna – an historical review. Tuhinga. 27:81–115.
- Moller H, Lyver PO, Bragg C, Newman J, Clucas R, Fletcher D, Kitson J, McKechnie S, Scott D, Rakiura Titi Island Administering Body. 2009. Guidelines for cross-cultural participatory action research partnerships: a case study of a customary seabird harvest in New Zealand. New Zealand Journal of Zoology. 36:211–241. doi: 10.1080/03014220909510152
- Moller JS, Moller SI, Futter JM, Moller JA, Harvey JP, White HA, Stirling F, Moller H. 200b. Potential impacts of vehicle traffic on recruitment of toheroa (Paphies ventricosa) on Oreti Beach, Southland, New Zealand. He Kōhinga Rangahau No. 5, University of Otago, Dunedin. 61 p.
- Müller J. 2011. Influence of vegetation cover on coastal aquifer fluctuations and sand transport on Matakana island [unpublished MSc thesis]. Hamilton: University of Waikato.
- Murton B. 2006. ‘Toheroa wars’: cultural politics and everyday resistance on a northern New Zealand beach. New Zealand Geographer. 62:25–38. doi: 10.1111/j.1745-7939.2006.00046.x
- Naylor R, Maxwell K, Maolagain CO, Fu D. 2010. Estimation of growth and age in Toheroa. Draft Final Research Report for Ministry of Fisheries project TOH200802. 11 p. (Unpublished report held by NIWA, Wellington.).
- New Zealand Herald. 1926 Aug 9. New toheroa bed.
- New Zealand Herald. 1934 Aug 13. Toheroa experiment.
- Newcombe E, Poutama M, Allen C, Smith H, Clark D, Atalah J, Spinks A, Ellis J, Sinner J. 2014. Kaimoana on beaches from Hōkio to Ōtaki, Horowhenua. Manaaki Taha Moana Research Report No. 22. Cawthron Report No.2564. 40 p. Plus appendices.
- Newcombe E, Sciascia P, Heasman K, Spinks A, Poutama M, Smith H, Sinner J. 2015. Towards revitalisation of toheroa in the Horowhenua. Manaaki Taha Moana Research Report No. 24. Cawthron Report No. 2756. 35 p.
- Parore LW. 1933. To Rt. Hon. J. G. Coates, February 13. Marine Department, M1 2/12/9, Archives New Zealand, Wellington.
- Pratt DR, Lohrer AM, Pilditch CA, Thrush SF. 2014. Changes in ecosystem function across sedimentary gradients in estuaries. Ecosystems. 17:182–194. doi: 10.1007/s10021-013-9716-6
- Pulfrich A, Branch GM. 2014. Effects of sediment discharge from Namibian diamond mines on intertidal and subtidal rocky-reef communities and the rock lobster Jasus lalandii. Estuarine, Coastal and Shelf Science. 150:179–191. doi: 10.1016/j.ecss.2013.10.017
- Quinn JM, Cooper AB, Davies-Colley RJ, Rutherford JC, Williamson RB. 1997. Land use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams. New Zealand Journal of Marine and Freshwater Research. 31:579–597. doi: 10.1080/00288330.1997.9516791
- Ramos-Scharron CE, Torres-Pulliza D, Hernández-Delgado EA. 2015. Watershed- and island wide-scale land cover changes in Puerto Rico (1930s–2004) and their potential effects on coral reef ecosystems. Science of the Total Environment. 506–507:241–251. doi: 10.1016/j.scitotenv.2014.11.016
- Rapson AM. 1952. The toheroa, Amphidesma ventricosum gray (Eulamellibranchiata), development and growth. Australian Journal of Marine and Freshwater Research. 3:170–198. doi: 10.1071/MF9520170
- Rapson AM. 1954. Feeding and control of toheroa (Amphidesma ventricosum gray) (Eulamellibranchiata) populations in New Zealand. Australian Journal of Marine and Freshwater Research. 5:486–512.
- Redfearn P. 1974. Biology and distribution of the toheroa, Paphies (Mesodesma) ventricosa (Gray). Fisheries Research Bulletin No. 11. New Zealand Ministry of Agriculture and Fisheries. 51 p.
- Redfearn P. 1982. Larval shell development of the toheroa, Paphies ventricosa (gray, 1843) (Mactracea, Mesodesmatidae). New Zealand Journal of Marine and Freshwater Research. 16:241–252. doi: 10.1080/00288330.1982.9515967
- Robb M. 2014. When two worlds collide: mātauranga māori, science and health of the Toreparu Wetland [unpublished MSc thesis]. Hamilton: University of Waikato.
- Ross PM, Hogg ID, Pilditch CA, Lundquist CJ. 2009. Phylogeography of New Zealand’s coastal benthos. New Zealand Journal of Marine and Freshwater Research. 43:1009–1027. doi: 10.1080/00288330.2009.9626525
- Ross PM, Pande A, Jones B, Cope J, Flowers G. 2017. First detection of gas bubble disease and Rickettsia-like organisms in Paphies ventricosa, a New Zealand surf clam. Journal of Fish Diseases. doi: 10.1111/jfd.12684
- Samuel E. 1936. The toheroa – New Zealand’s exclusive shellfish. The New Zealand Railways Magazine. 11:54–55.
- Schlacher TA, Thompson LMC, Walker SJ. 2008. Mortalities caused by off-road vehicles (ORVs) to a key member of sandy beach assemblages, the surf clam Donax deltoids. Hydrobiologia. 610:345–350. doi: 10.1007/s10750-008-9426-9
- Schlacher TA, Weston MA, Schoeman DS, Olds AD, Huijbers CM, Connolly RM. 2015. Golden opportunities: a horizon scan to expand sandy beach ecology. Estuarine, Coastal and Shelf Science. 157:1–6. doi: 10.1016/j.ecss.2015.02.002
- Seers BM, Shears NT. 2015. Spatio-temporal patterns in coastal turbidity – long-term trends and drivers of variation across an estuarine-open coast gradient. Estuarine, Coastal and Shelf Science. 154:137–151. doi: 10.1016/j.ecss.2014.12.018
- Shears NT, Smith F, Babcock RC, Duffy CAJ, Villouta E. 2008. Evaluation of biogeographic classification schemes for conservation planning: application to New Zealand’s coastal marine environment. Conservation Biology. 22:467–481. doi: 10.1111/j.1523-1739.2008.00882.x
- Smale MC, Hall GMJ, Gardner RO. 1996. Monitoring condition of sand dune kanuka forest at Woodhill. Science for Conservation No. 26. Department of Conservation, Wellington. 17 p.
- Smith H, Spinks A, Hoskins T, Poutama M. 2011. State of Ecological/Cultural Landscape Decline of Horowhenua Coastline Between Hokio and Waitohu Streams. Manaaki Taha Moana Research Report No. 2. Palmerston North: Massey University.
- Smith S. 2003. The reproduction and recruitment of toheroa (Paphies ventricosa) [unpublished MSc thesis]. Auckland: University of Auckland.
- Smith S. 2013. Factors influencing the abundance of Toheroa (Paphies ventricosa) on Northland beaches: Perspectives from the beach. In: Williams J, Sim-Smith C, Paterson C. 2013. Review of factors affecting the abundance of toheroa (Paphies ventricosa). New Zealand Aquatic Environment and Biodiversity Report No. 114. 76 p.
- Staaf H, Olsson BA. 1994. Effects of slash removal and stump harvesting on soil water chemistry in a clearcutting in SW Sweden. Scandinavian Journal of Forest Research. 9:305–310. doi: 10.1080/02827589409382844
- Stace G. 1991. The elusive toheroa. New Zealand Geographic Magazine. 9:18–34.
- Stallworthy J. 1916. Early Northern Wairoa. Dargaville: The Wairoa Bell and Advertiser.
- Stender Y, Jokiel PL, Rodgers KS. 2014. Thirty years of coral reef change in relation to coastal construction and increased sedimentation at Pelekane Bay, Hawaiʻi. PeerJ 2:e300. https://doi.org/10.7717/peerj.300
- Street RJ. 1971. Studies on toheroa at Te Waewae Bay, Southland. Fisheries Technical Report No. 70. Fisheries Division, Marine Department, Wellington. 22 p.
- Sutton PJH, Bowen MM. 2011. Currents off the west coast of Northland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 45:609–624. doi: 10.1080/00288330.2011.569729
- Taiapa C, Bedford-Rolleston A, Rameka W. 2014. Ko te Hekenga i te Tai a Kupe, a cultural review of the health of Te Awanui, Tauranga Harbour. Manaaki Taha Moana Research Report No 3. Palmerston North: Massey University. 93 p.
- Te Hiku Claims Settlement Bill. 2015. (No. 201-2).
- Te Tuhi J, Gregory R. 2008. Toheroa. Wellington: Huia Education. 32 p.
- Techera EJ, Klein N. 2011. Fragmented governance: reconciling legal strategies for shark conservation and management. Marine Policy. 35:73–78. doi: 10.1016/j.marpol.2010.08.003
- Tipa G, Teirney L. 2006. A cultural health index for streams and waterways: a tool for nationwide use. Wellington: Ministry for the Environment. 54 p.
- Under Secretary Maori Affairs. 1950. To Secretary, Marine Department, September 11. Marine Department, M1 2/12/574, Archives New Zealand, Wellington.
- Van der Merwe D, Van der Merwe D. 1991. Effects of off-road vehicles on the macrofauna of a sandy beach. South African Journal of Science. 87:210–213.
- Wai27. 1988. Ngäi Tahu claim. Folio J10 Evidence of David T. Higgins, Huata Holmes, Trevor Hape Howse and Henare Rakiihia Tau.
- Waitangi Tribunal. 2011. Ko Aotearoa tēnei: a report into claims concerning New Zealand law and policy affecting Māori culture and identity: Te Taumata Tuarua. Wellington: Legislation Direct. 787 p.
- Walker JW. 2007. Effects of fine sediments on settlement and survival of the sea urchin Evechinus chloroticus in northeastern New Zealand. Marine Ecology Progress Series. 331:109–118. doi: 10.3354/meps331109
- Waugh GD, Greenway JP. 1967. Further evidence for the existence of sub-littoral populations of toheroa, Amphidesma ventricosum gray (Eulamelli-branchiata), off the west coast of New Zealand. New Zealand Journal of Marine and Freshwater Research. 1:407–411. doi: 10.1080/00288330.1967.9515215
- Williams J. 2004. E pākihi hakinga a kai: an examination of pre-contact resource management practise in Southern Te Wāi Pounamu [unpublished PhD thesis]. Dunedin: University of Otago.
- Williams J. 2012. Ngai Tahu Kaitiakitanga. MAI Journal. 1:89–102.
- Williams JR, Ferguson H, Tuck I. 2013. Distribution and abundance of toheroa (Paphies ventricosa) and tuatua (P. subtriangulata) at Ninety Mile Beach in 2010 and Dargaville Beach in 2011. New Zealand Fisheries Assessment Report 2013/39. 52 p.
- Williams JR, Sim-Smith C, Paterson C. 2013. Review of factors affecting the abundance of toheroa (Paphies ventricosa). New Zealand Aquatic Environment and Biodiversity Report No. 114. 76 p.
- Wu X, Li D, Pan J, Jiang J. 2003. Studies on rickettsia-like organism (RLO) disease of tropical marine pearl oyster – epidemiological investigation of RLO disease in juvenile populations of maricultured Pinctada maxima. Acta Oceanologica Sinica. 22:421–435.
- Zhu ZW, Xu T, He ZY, Wu XZ, Wu LJ, Meng QG, Huang JQ. 2012. Rickettsia-like organism infection associated with mass mortalities of blood clam, Tegillarca granosa, in the Yueqing Bay in China. Acta Oceanologica Sinica. 31:106–115. doi: 10.1007/s13131-012-0182-3
