ABSTRACT
We employed flow cytometry to assess plasma membrane viability of sperm cells in farmed giant kokopu Galaxias argenteus, an endemic amphidromous New Zealand fish. Specifically, the variables of fish size (TL mm) and weight (grams) were assessed against sperm viability. We found no significant difference (P < .05) in sperm viability when compared across size and weight distribution of G. argenteus. This study shows that flow cytometry is an effective, accurate and rapid method for analysing sperm viability in G. argenteus.
Introduction
Gamete quality is an important factor in aquaculture, as high-quality gametes have a better chance of producing viable offspring that will reach adult size in better condition (Mylonas et al. Citation2003; Trippel Citation2003; Rurangwa et al. Citation2004; Bobe and Labbé Citation2010; Valdebenito et al. Citation2013; Cabrita et al. Citation2014). Gamete quality is an important parameter in fish aquaculture and studies on gamete viability provide valuable information for the management of reproduction (Trippel Citation2003; Bobe and Labbé Citation2010; Richie and Marshall Citation2013; Cabrita et al. Citation2014; Hickford and Schiel Citation2014). As a result, identifying quality brood stock has become an important strategy in influencing reproductive success. While ova lipidomics research using sophisticated chromatography techniques have been widely studied in female teleosts (eg Salze et al. Citation2005; Grote et al. Citation2011), advanced analytical research on sperm quality parameters is less frequent. Historically, sperm quality investigations have employed methods such as light microscopy to ascertain concentration, dark field microscopy to assess motility or computer-assisted sperm analysis to measure motility (Ciereszko and Dabrowski Citation1993; Tripple Citation2003; Cabrita et al. Citation2009; Bobe and Labbé Citation2010; Fauvel et al. Citation2010; Valdebenito et al. Citation2013). For the few studies that have investigated specific sperm parameters in fish, flow cytometry is being advocated as a more accurate methodology compared to older microscopy methods (eg Brito et al. Citation2016; Ciereszko et al. Citation2016; Nynca et al. Citation2016; Yang et al. Citation2016; Zilli et al. Citation2016).
Flow cytometry provides a more targeted and sophisticated method with which to assess parameters such as sperm membrane viability, motility, Reactive Oxygen Species (ROS), spermatocrit density and composition of seminal plasma, sperm apoptosis, mitochondrial potential and DNA fragmentation (Petrunkina and Harrison Citation2010; Hossain et al. Citation2011; Acosta et al. Citation2016; Brito et al. Citation2016; Nynca et al. Citation2016; Torres and Tiersch Citation2016; Yang et al. Citation2016). For example, flow cytometry allows very large numbers of sperm (up to 10,000) to be counted and analysed quickly in just one sample, which offers an advantage over older more laborious methods such as manual counting under light microscopy. The specificity of flow cytometry involves its technique where a combination of fluorescent dyes are used to stain specific cell components or organelles that are subsequently detected using laser beams, which can then measure fluorescence intensity of these bio-marked regions.
The aim of this study was to apply flow cytometry for analysis of sperm plasma membrane viability in the farmed giant kokopu Galaxias argenteus. The juveniles of G. argenteus are one of the five galaxiid species that make up what is known in New Zealand as whitebait, an iconic food source and delicacy of high economic value (Cadwallader Citation1976; McDowall Citation2010); the other four being Galaxias brevipinnis (kōaro), Galaxias fasciatus (banded kokopu), Galaxias maculatus (inanga) and Galaxias postvectis (shortjaw kokopu). G. argenteus are classed as ‘at risk-declining’ by the Department of Conservation (Goodman et al. Citation2014), and the largest and rarest endemic galaxiid in New Zealand (Bonnett and Lambert Citation2002; Bonnett and Sykes Citation2002; Hansen et al. Citation2004; Baker and Smith Citation2007; Franklin et al. Citation2015).
In order to investigate sperm viability across different somatic parameters, the relationship between body size (mm) and weight (grams) was measured. To the best of our knowledge, no research on any aspect of sperm quality has been conducted to date on G. argenteus, or any other New Zealand galaxiid. Therefore, a flow cytometry protocol will be extremely important to the G. argenteus aquaculture industry where knowledge on semen quality parameters is important for identifying high-quality parental stocks.
Materials and methods
Specimens
Adult G. argenteus were sourced from New Zealand Premium Whitebait Limited in Warkworth, a company that is developing the whitebait aquaculture industry in New Zealand. Specimens were hatched from captive parental stocks, F1 and wild caught, and were 4 years old at the time of this study. All specimens were reared and maintained in the same Recirculating Aquaculture System (RAS).
Flow cytometry
To the best of our knowledge, there are no fish-specific sperm cytometry kits available. We used the Thermo Fisher LIVE–DEAD Sperm Viability Kit (L-7011), which is commonly used for terrestrial animals, but also advertised as suitable for fish. This kit contains fluorescent dyes propidium iodide (PI) and SYBR-14 to bio-mark sperm cells. PI penetrates cells with damaged or disrupted plasma membrane staining the nuclear region red, while SYBR-14 penetrate cells with intact or complete plasma membranes staining the nuclear region green. SYBR-14 produces green fluorescence at 525 nm while PI produces red fluorescence at 610 nm. This is the preferred combination of stains for analysing sperm membrane viability as both target DNA and have been successfully used on a range of species including humans, cattle, birds and insects (Hossain et al. Citation2011).
There are two approaches to flow cytometry; using an exact sample volume known as true volume, or by setting an event limit, usually 10,000, and using fluorescent counting beads of a known concentration per µl to work out the actual concentration (Brito et al. Citation2016). The advantage of fluorescent beads is that it allows very small samples or samples that are viscous (as in this study) and hard to measure accurately to be diluted before analysing. However, for the fluorescent bead method to work properly, it is vitally important that the bead solution is correctly vortexed in order to mix and suspend the beads before use. To repeat this experiment, the following procedures should be followed:
Sperm extraction
All sperm samples were manually extracted by stripping on location at New Zealand Premium Whitebait Limited. Our samples were acquired in July, falling in the middle of the spawning season (June–August 2016) for G. argenteus. Specimens were chosen at random from tanks holding only males using a large net. In total, the sperms of 45 G. argenteus were extracted and immediately kept at 4°C at the laboratory. To ensure that the same fish was not sampled twice, specimens were striped and placed in a recovery bath until all sampling was completed. The recovery bath contained dilute Melafix, a natural antibacterial agent containing 9% Cajeput oil to avoid infection, before fish were released back into the major holding tanks. Sperm samples were extracted by gently pressing on the belly of each fish and massaging the sperm out of the genital pore. Care was taken to dry the genital pore of each fish before extraction to avoid collecting urine, or accidental activation of extracted sperm due to contact with water. Total length (TL mm) and total weight (g) measurements were recorded for each fish, before one drop of sperm was collected into a small petri dish.
Laboratory preparation and dilution
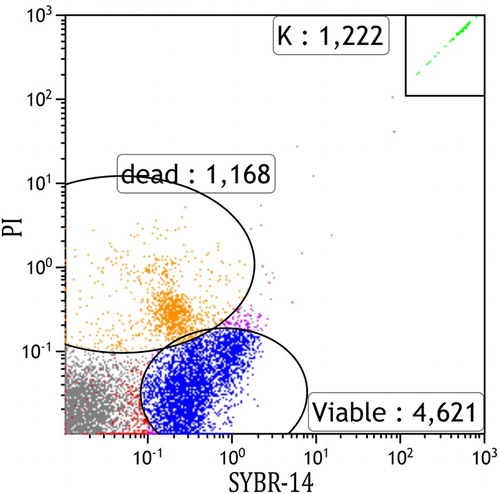
First, a HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) saline buffer solution was produced in order to dilute the samples; HEPES buffer (10 mM HEPES, 150 mM NaCl, 10% bovine serum albumin (BSA), pH 7.4). To prepare a HEPES buffer, the following ingredients were used: 100 mL of ultrapure water, 877 mg of NaCl, 238 mg HEPES and 10 g BSA. Next, the primary samples were diluted by pipetting 1 mL of buffer into the petri dish and using the pipette to pump the buffer fluid to mix with the primary sperm samples. This step is required as G. argenteus sperm samples are too viscous to be accurately pipetted. The HEPES buffer solution was stored at 4°C in the refrigerator when not being used but allowed to warm to room temperature before use. Primary sperm samples were diluted in sets of five to speed up the process, while remaining samples were kept on ice in a cooler until required. The samples were then diluted to 10× dilutions by pipetting 100 µl of sample into 900 µl of buffer in a 2.5 mL tube and vortexed to make a stock solution. These were then diluted again to 1000× dilution by pipetting 1 µl of the stock sample into 99 µl of buffer and again vortexed to mix. 1000× dilution was agreed on during trial runs as this dilution regularly produced repeatable results and allowed approximately 1000 micro beads to show in the majority of samples. The number of beads is an important factor in calculating the final concentration of cells and a minimum of 1000 beads is agreed on in literature and in the instructions provided in the Thermo Fisher kit (see ; micro bead number is represented as K).
Cell staining and counting
The fluorescent stains SYBR-14 and PI were added to the 1000× diluted samples in order to stain the cells with intact and compromised cell membranes, respectively. Each stain was kept frozen when not in use and allowed to defrost and warm to room temperature before use. SYBR-14 must also be diluted using dimethyl sulfoxide (DMSO) to 10× dilution and not be stored and reused. In order to make the reagents in the sperm viability kit fit, our sample size smaller quantities were used. Therefore, SYBR-14 was diluted to 50× by using 0.5 µl of SYBR-14 into 24.5 µl DMSO and this solution was vortexed to mix. 0.5 µl of dilute SYBR-14 was then added to each 100 µl sample along with 0.5 µl of PI and vortexed to mix. Samples were then incubated at 36°C for 10 min. During prior trial runs, samples were run with just SYBR-14 or PI rather than both stains so that there was no confusion as to which stain was showing up on the graphical flow cytometry output. Each area could then be gated to only pick up the final count of either live cells with intact cell membranes or dead cells with damaged membranes. After incubation, each sample was mixed 1:1 with 100 µl of fluorescent counting micro beads of a known concentration of per µl, vortexed to mix and then all 200 µl of the solution pipetted into flow cytometry tubes. The flow cytometer was limited to 10,000 events, and each sample was run twice and averaged. If a particularly high or low reading came out, then a third replicate sample was run.
Statistical analysis
The total number of sperm cells counted by the flow cytometer was recorded after which the actual concentration per µl was calculated by the equation A/B × C, where the total number of sperm cells counted by the flow cytometer (A) is divided by the concentration of beads per µl (B) times the number of beads counted by the flow cytometer (C). The same equation can calculate the number of cells with an intact cell membrane or with a damaged cell membrane. The total number of live and dead sperms (per µl) was used to calculate a live/dead percentage for each of the 45 specimens. All statistical analyses were conducted in R version 3. 2. 1 (R Development Core Team Citation2016). Following exploratory correlation analysis (data not shown), we applied more flexible generalised additive models (GAMs) to test our hypothesis of sperm viability across fish size and weight distribution. GAMs look for both linear and non-linear relationships and as such are a more powerful tool than multivariate or principal component analysis. GAMs were fitted with a gamma error distribution (GAMs, R-package mgcv, Wood Citation2011), which looks for relationships between sperm viability and somatic parameters of length and weight. In an overarching model, we used the percentage of live sperm cells as a response variable against length and weight. We used model diagnostic plots to assess the underlying assumptions of variance homogeneity (residuals vs. fitted values plot) and normality (quantile–quantile plots).
Results
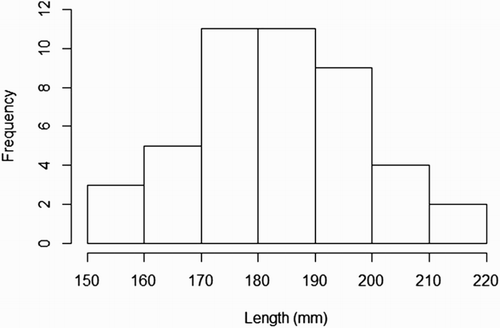
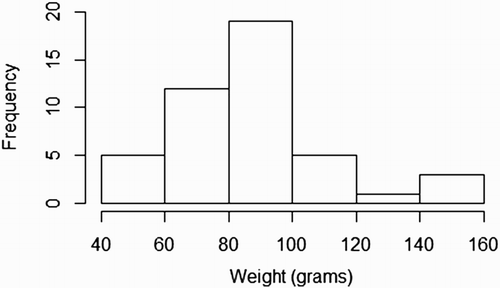
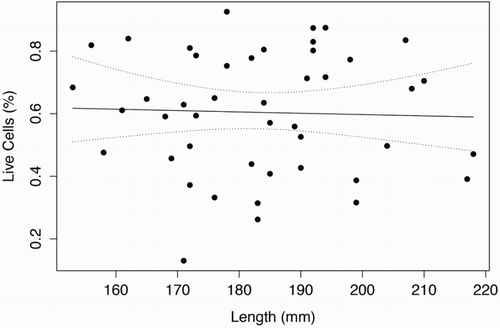
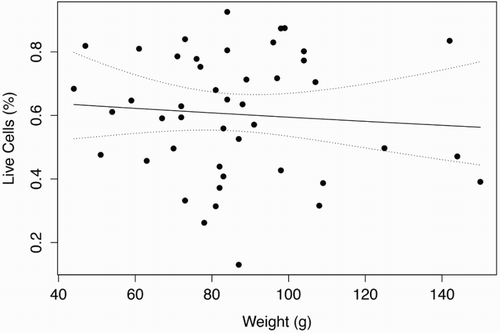
shows a scatter plot from the flow cytometer depicting viable and dead cells, in which a test sample was judged to have 4,621 viable cells and 1,168 dead cells. The graph also shows what is regarded to be cellular debris unstained by either PI or SYBR-14. and show the length (mm) and weight (g) distributions, respectively, of the 45 G. argenteus from which sperm samples were extracted. All samples were of the exact same age, hatched 4 years ago and reared under the same RAS conditions to date. The G. argenteus population at New Zealand Premium Whitebait Limited are grown together and not size-graded. As such, our random sample of 45 fish contained a heterogeneous range of size and weight. The smallest fish was 44 g and 153 mm long, while the heaviest fish was 150 g and 217 mm long and the longest fish was 218 mm weighing 144 g. The average weight was 86 g and the average length was 183 mm. GAM analysis of live sperm cell percentage showed no relationship with length (P = .82) or weight (P = .59) of G. argenteus, indicating that no linear or non-linear relationship was found between sperm concentration and paternal parameters of length and weight. Concentrations of live sperm cells are depicted against length () and weight (), where solid lines represent fits from GAMs with gamma error distribution, while dotted lines indicate 95% confidence intervals.
Discussion
This study set out to apply an easily repeatable flow cytometry protocol for the analysis of sperm viability in G. argenteus. To test the effectiveness of this methodology against another closely related species, we also applied the same protocol to assess sperm viability in Inanga G. maculatus (unpublished data), which also produced accurate and repeatable results consistent with findings in this study. Examples of recent flow cytometry studies include assessing its usefulness and accuracy compared to traditional microscopy methods in rainbow trout Oncorhynchus mykiss (Nynca et al. Citation2016), assessing the impact of storage time on semen viability in O. mykiss (Trigo et al. Citation2015), or the effects of heavy metal impact on sperm viability in zebra fish Danio rario (Acosta et al. Citation2016; Torres and Tiersch Citation2016; Yang et al. Citation2016). As with this study, the objective of these cited studies was also to develop repeatable flow cytometry protocol and procedures in order to test sperm cellular parameters. Yang et al. (Citation2016) and Nynca et al. (Citation2016) showed the benefits of flow cytometry by comparing it to traditional methods of hemocytometery, computer-aided fluorescence microscopy and micro-spectrophotometry. For example, Nynca et al. (Citation2016) showed that flow cytometry produced significantly higher sperm concentration values compared to traditional sperm viability analyses in O. mykiss, and proved to be an accurate and rapid assessment tool for sperm concentration and viability. Torres and Tiersch (Citation2016) compared flow cytometry to the use of amine-reactive dyes in order to investigate the effect of storage time and cryopreservation agents, showing flow cytometry to be a more effective option for such analysis. Acosta et al. (Citation2016) successfully used flow cytometry to assess the impact of cadmium toxicity on external fertilisation, in addition to examining sperm plasma membrane viability and fluidity, motility, mitochondrial function and ROS.
Flow cytometry will increasingly replace and ultimately make obsolete laborious and traditional microscopy methods for sperm analysis. Given its ability to specifically target cytoplasmic organelles, the opportunities for gamete research in fisheries science will undoubtedly expand, especially given the advent and increasing prevalence of mobile flow cytometers (eg Nynca et al. Citation2016).
The size and weight distribution of our sample fall within the normal distribution range of wild G. argenteus (see Jellyman et al. Citation2013) and are thus a fair representation of size and weight. However, due to the fact that our samples were hatched and reared exactly 4 years ago, it was not possible to acquire a wide range of age groups. This may be a shortcoming as it would have been interesting to have tested sperm viability across various age classes. The fact that sperm viability showed no trend across size or weight in fish that were reared in an aquaculture setting is interesting. However, the high variability in sperm viability values of fish in the same size or weight class, as was shown here, is more interesting and does suggest that gamete quality parameters may be more a function of biological pathways or species-specific genetic disposition, and less so a function of diet or environmental conditions. For example, in analysing gamete quality of captive snapper Chrysophrys auratus, Martin (Citation2009) found high variability in lipid content, even though the specimens were all reared in the same RAS system and fed the same diet.
This study has successfully used flow cytometry to produce repeatable experimental results in assessing sperm viability of G. argenteus. Having also applied the same protocol to G. maculatus (unpublished data), we are confident that the procedures set out in this study can be applied to other galaxiid species.
Acknowledgements
We would like to thank Paul Decker from New Zealand Premium Whitebait Limited for access to their facility and also supplying us with specimens for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acosta IB, Varela AS, De Silva EF, Cardoso TF, Caldas JS, Jardim RD, Corcini CD. 2016. Effects of exposure to cadmium in sperm cells of zebrafish, Danio rerio. Toxicology Reports. 3:696–700. doi: 10.1016/j.toxrep.2016.08.002
- Baker CF, Smith JP. 2007. Habitat use by banded kokopu (Galaxias fasciatus) and giant kokopu (G. argenteus) co-occurring in streams draining the Hakarimata Range, New Zealand. New Zealand Journal of Marine and Freshwater Research. 41(1):25–33. doi: 10.1080/00288330709509893
- Bobe J, Labbé C. 2010. Egg and sperm quality in fish. General and Comparative Endocrinology. 165(3):535–548. doi: 10.1016/j.ygcen.2009.02.011
- Bonnett ML, Lambert PW. 2002. Diet of giant kokopu, Galaxias argenteus. New Zealand Journal of Marine and Freshwater Research. 36(2):361–369. doi: 10.1080/00288330.2002.9517093
- Bonnett ML, Sykes JRE. 2002. Habitat preferences of giant kokopu, Galaxias argenteus. New Zealand Journal of Marine and Freshwater Research. 36(1):13–24. doi: 10.1080/00288330.2002.9517067
- Brito LFC, Althouse GC, Aurich C, Chenoweth PJ, Eilts BE, Love CC, Luvoni GC, Mitchell JR, Peter AT, Pugh DG, Waberski D. 2016. Andrology laboratory review: evaluation of sperm concentration. Theriogenology. 85(9):1507–1527.
- Cabrita E, Martínez-Páramo S, Gavaia P, Riesco M, Valcarce D, Sarasquete C, Herraez P, Robles V. 2014. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture. 432:389–401.
- Cabrita E, Robles V, Hares P. 2009. Sperm quality assessment. In: Cabrita E, Robles V, Hares P, editors. Methods in reproductive aquaculture marine and freshwater species. Boca Raton (FL): CRC Press; p. 94–135.
- Cadwallader PL. 1976. Breeding biology of a non-diadromous galaxiid, Galaxias vulgaris stokell, in a New Zealand river. Journal of Fish Biology. 8:157–177. doi: 10.1111/j.1095-8649.1976.tb03929.x
- Ciereszko A, Dabrowski K. 1993. Estimation of sperm concentration of rainbow trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture. 109(3–4):367–373. doi: 10.1016/0044-8486(93)90175-X
- Ciereszko A, Dietrich M, Nynca J. 2016. Fish semen proteomics – new opportunities in fish reproductive research. Aquaculture. DOI:10.1016/j.aquaculture.2016.03.005.
- Fauvel C, Suquet M, Cosson J. 2010. Evaluation of fish sperm quality. Journal of Applied Ichthyology. 26(5):636–643. doi: 10.1111/j.1439-0426.2010.01529.x
- Franklin PA, Smith J, Baker CF, Bartels B, Reeve K. 2015. First observations on the timing and location of giant kōkopu (Galaxias argenteus) spawning. New Zealand Journal of Marine and Freshwater Research. 49(3):419–426. doi: 10.1080/00288330.2015.1045004
- Goodman JM, Dunn NR, Ravenscroft PJ, Allibone RM, Boubee JAT, David BO, Rolfe JR. 2014. Conservation status of New Zealand freshwater fish, 2013 (New Zealand Threat Classification Series 7). [accessed 2017 October 1]. Department of Conservation website: http://doc.org.nz/documents/science-and-technical/nztcs7entire.pdf.
- Grote B, Hagen W, Lipinski MR, Verheye HM, Stenevik EK, Ekau W. 2011. Lipids and fatty acids as indicators of egg condition, larval feeding and maternal effects in Cape hakes (Merluccius paradoxus and M. capensis). Marine Biology. 158(5):1005–1017. doi: 10.1007/s00227-011-1626-6
- Hansen EA, David BO, Closs GP. 2004. Diel patterns of feeding and prey selection in giant kokopu (Galaxias argenteus). New Zealand Journal of Marine and Freshwater Research. 38:341–345. doi: 10.1080/00288330.2004.9517242
- Hickford M, Schiel D. 2014. Experimental rehabilitation of degraded spawning habitat of a diadromous fish, Galaxias maculatus (Jenyns, 1842) in rural and urban streams. Restoration Ecology. 22:319–326. doi: 10.1111/rec.12079
- Hossain M, Johannisson A, Wallgren M, Nagy S, Siqueira A, Rodriguez-Martinez H. 2011. Flow cytometry for the assessment of animal sperm integrity and functionality: state of the art. Asian Journal of Andrology. 13(3):406–419. doi: 10.1038/aja.2011.15
- Jellyman P, Booker D, Crow S, Bonnett M, Jellyman D. 2013. Does one size fit all? An evaluation of length-weight relationships for New Zealand’s freshwater fish species. New Zealand Journal of Marine and Freshwater Research. 47(4):450–468. doi: 10.1080/00288330.2013.781510
- Martin JL. 2009. Investigating maternal effects in a batch spawning teleost [dissertation]. Auckland: University of Auckland.
- McDowall RM. 2010. New Zealand freshwater fishes: An historical and ecological biogeography. Dordrecht: Springer.
- Mylonas CC, Papadaki M, Divanach P. 2003. Seasonal changes in sperm production and quality in the red porgy Pagrus pagrus. Aquaculture Research. 34:1161–1170. doi: 10.1046/j.1365-2109.2003.00922.x
- Nynca J, Dietrich GJ, Liszewska E, Judycka S, Karol H, Dobosz S, Krom J, Ciereszko A. 2016. Usefulness of a portable flow cytometer for sperm concentration and viability measurements of rainbow trout spermatozoa. Aquaculture. 451:353–356.
- Petrunkina A, Harrison R. 2010. Systematic misestimation of cell subpopulations by flow cytometry: a mathematical analysis. Theriogenology. 73(7):839–847. doi: 10.1016/j.theriogenology.2009.09.007
- R Development Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: Austria.
- Ritchie H, Marshall DJ. 2013. Fertilisation is not a new beginning: sperm environment affects offspring developmental success. Journal of Experimental Biology. 216:3104–3109.
- Rurangwa E, Kime D, Ollevier F, Nash J. 2004. Review article: the measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 234(1–4):1–28. doi: 10.1016/j.aquaculture.2003.12.006
- Salze G, Tocher DR, Roy WJ, Robertson DA. 2005. Egg quality determinants in cod (Gadus morhua L.): egg performance and lipids in eggs from farmed and wild broodstock. Aquaculture Research. 36(15):1488–1499. doi: 10.1111/j.1365-2109.2005.01367.x
- Torres L, Tiersch TR. 2016. Amine reactive dyes: an alternative to estimate membrane integrity in fish sperm cells. Aquaculture. 46:371–378.
- Trigo P, Merino O, Sánchez R, Risopatrón J, Figueroa E, Valdebenito I. 2015. Effect of short-term semen storage in salmon (Oncorhynchus mykiss) on sperm functional parameters evaluated by flow cytometry. Andrologia. 47(4):407–411. doi: 10.1111/and.12276
- Trippel EJ. 2003. Estimation of male reproductive success in marine fishes. Journal of Northwest Atlantic Fishery Science. 33:81–113. doi: 10.2960/J.v33.a6
- Valdebenito II, Gallegos PC, Effer BR. 2013. Gamete quality in fish: evaluation parameters and determining factors. Zygote. 23:177–197. doi: 10.1017/S0967199413000506
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society (B). 73(1):3–36. doi: 10.1111/j.1467-9868.2010.00749.x
- Yang H, Daly J, Tiersch T. 2016. Determination of sperm concentration using flow cytometry with simultaneous analysis of sperm plasma membrane integrity in zebrafish Danio rerio. Cytometry Part A. 89(4):350–356. doi: 10.1002/cyto.a.22796
- Zilli L, Schiavone R, Vilella S. 2016. Role of protein phosphorylation/ dephosphorylation in fish sperm motility activation: state of the art and perspectives. Aquaculture. DOI:10.1016/j.aquaculture.2016.03.043.