ABSTRACT
Southern right whales (Eubalaena australis) were widespread in New Zealand waters before commercial whaling in the nineteenth century caused drastic declines in their abundance and distribution. Following the cessation of whaling, the population has been recovering and is now slowly recolonising its former range. Estimates of population demographics, including reproductive output, are essential for predicting the trajectory of this population. We gathered photo-identification data on female southern right whales during annual field trips to the Auckland Islands, the principal calving area in New Zealand waters. Forty-five calving intervals were observed between 2006 and 2013 (mean interval = 3.31 years, 95% CI = 3.06–3.57). Incorporating the effects of possible missed calving events produced a plausible range of mean calving intervals from 3.17 to 3.31 years. Our results suggest that the calving interval of New Zealand southern right whales is similar to that found in populations elsewhere.
Introduction
Southern right whales (SRW; Eubalaena australis) were once found throughout New Zealand’s (NZ) coastal waters (Gaskin Citation1964). By the end of the nineteenth century, whaling had dramatically reduced the NZ range of SRWs and decreased numbers to less than 5% of the estimated original population size (Jackson et al. Citation2016). Following the cessation of whaling at the start of the twentieth century, the population now appears to be increasing (Carroll et al. Citation2013) and recolonising parts of its former range (Carroll et al. Citation2014). The main calving area for the population is now in the subantarctic Auckland Islands, where sex specific abundance and population growth has recently been estimated at 1162 (95% CI 921–1467) females increasing at 5% p.a. (95% CI 2–13%), and 1007 (95% CI 794–1276) males increasing at 7% p.a. (95% CI 5–9%) (Carroll et al. Citation2013).
Population-specific estimates of demographic parameters, such as survival and reproductive rates, are critical for developing population models and can help focus conservation efforts (Rowntree et al. Citation2010). Population-specific estimates are particularly important for SRWs because they exhibit strong site fidelity to calving areas (Cooke et al. Citation2003; Carroll et al. Citation2011; Brandão et al. Citation2012) and have spatially isolated populations (Kenney Citation2009). Although previous studies have attempted to estimate apparent survival rates for SRWs in NZ (Carroll et al. Citation2013, Citation2016), to date there have been no estimates of reproductive output.
The reproductive cycle of right whales consists of gestation, a nursing phase and a resting phase, each lasting approximately 1 year (Best Citation1994). The typical calving interval for SRWs is therefore 3 years (Best Citation1994; Kenney Citation2009). Because SRWs calve in coastal waters, and individual females can be reliably recognised over many years (Payne et al. Citation1983), the calving interval can be estimated by the observed time between successive calving events using uniquely identifiable natural markings (Burnell Citation2001; Cooke et al. Citation2003; Brandão et al. Citation2010a). In this way, estimates of mean calving intervals have been generated for SRWs, ranging from 3.16 years (95% CI 3.13–3.19) for the South African population (Brandão et al. Citation2010a, Citation2010b) to 3.64 years (95% CI 2.88–3.38) for the western Australian population (Burnell Citation2001), although note that analysis methods differed. Current environmental and human pressures may contribute to the observed regional differences in calving interval. In this study, we used an 8-year photo-identification (photo-ID) dataset of SRWs calving in the subantarctic Auckland Islands to produce the first estimate of observed calving interval for SRWs in NZ ().
Materials and methods
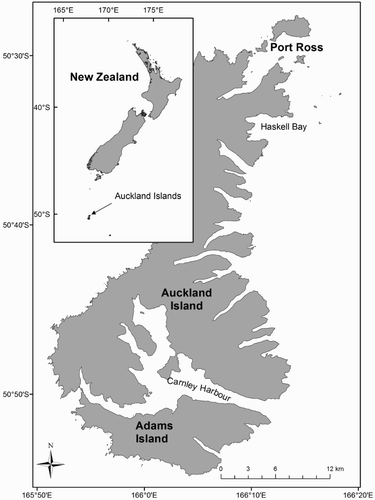
Photographic identification (photo-ID) methods (Evans and Hammond Citation2004) have been used extensively to estimate demographic parameters of right whale populations (Cooke et al. Citation2003, Brandão et al. Citation2012). In this study, photo-ID data were gathered in and around Port Ross, Auckland Islands (), annually from 2006 to 2013 during field seasons of approximately 3 weeks in July and August. This time of year coincides with the peak abundance of SRWs in Port Ross (Patenaude Citation2002), which is thought to be the only significant calving area in NZ waters (Rayment et al. Citation2012; Carroll et al. Citation2014; Torres et al. Citation2017). Photo-ID surveys were conducted from small (<6 m) outboard-powered vessels. When a whale or group of whales was sighted, the vessel was slowed to idle speed and the whales were approached to obtain photo-ID images. Whales were considered part of the same group if they were within one and a half body lengths of each other, and a calf was defined as a whale less than half the length of an accompanying adult (Patenaude Citation2002; Carroll Citation2011). A whale in consistent, close association with a calf was assumed to be its mother. Young SRW calves typically remain submerged for less than 1.5 minutes (Thomas and Taber Citation1984), which makes it easy to determine if a calf is present in each group. Images obtained from sightings were included in the photo-ID analysis if they were in sharp focus and clearly showed the pattern of callosities on the whale’s head or other permanent distinguishing marks, such as dorsal blazes or ‘grey-morph’ colouration (Payne et al. Citation1983; Schaeff et al. Citation1999).
Photo-ID matching was facilitated by classifying each individual according to 17 distinguishing characteristics (eg nature of lip callosity, the number of rostral islands; Payne et al. Citation1990). These data were stored in the custom-written database ‘BigFish’ (Pirzl et al. Citation2006), which could be queried each time a new image was compared to the existing catalogue. To avoid possible duplication of individuals in the database, only high-quality left-side images were used. Calving intervals for each mature female with at least two different calving events were extracted from the database. Calving events that were not observed would lead to an overestimate of mean calving interval. Calving events may have been missed for one of three reasons:
a female had a calf in the Auckland Islands before or during the annual 3-week study period, but it was not recorded, either because it died or was not observed;
a female had a calf in the Auckland Islands after the annual 3-week study period;
a female had a calf at a different location.
The potential sampling error associated with missed calving events was investigated by modifying the observed calving data. The 6-year calving intervals in the database could, in fact, have resulted from two 3-year calving intervals, with one of the calves not observed. Likewise, observed 5-year intervals could have been a 3-year interval and a 2-year interval and observed 4-year intervals could have been two 2-year intervals (Cooke et al. Citation2015). Therefore, we made two modifications of the recorded data to investigate the effect of the possible overestimation of calving interval. Firstly, the observed 4-year intervals were reduced to 2-year intervals, the 5-year intervals into a 2-year and a 3-year interval, and all the 6-year calving intervals into 3-year intervals to provide an estimate of the minimum possible mean calving interval. A second, less extreme correction, consisted of only modifying the observed 6-year intervals to two 3-year intervals.
Estimates of mean calving interval in other populations of SRWs have been generated using widely varying sample sizes which affect the precision of the estimates. For example, standard errors range from 0.17 (n = 117; Burnell Citation2001) to 0.015 (n = 1968; Brandão et al. Citation2010a). To investigate the potential effect of sample size and detection error on our estimate of precision, we performed two bootstrapping exercises. Firstly, a sample of n = 10 calving intervals was randomly selected with replacement from the entire calving interval dataset and the mean of that subsample calculated. This process was then repeated a further 999 times to produce a distribution of 1000 means. The median of this distribution was extracted as an estimate of the mean calving interval and the 25th and 975th quantiles were used as estimates of the 95% confidence limits. The process was repeated using n = 100, 500, 1000 and 2000 to represent a wide range of potential sampling effort. Secondly, to incorporate detection error we randomly resampled the observed sighting histories to generate 2000 calving intervals, and randomly deleted 5% of the sightings each year. The mean calving interval was then estimated from these data. We repeated this process 999 times and extracted the median and 25th and 975th quantiles as above.
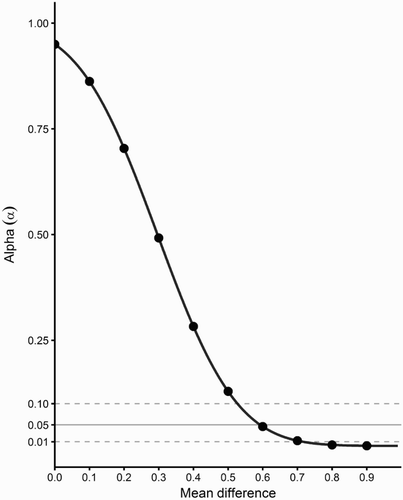
Finally, we conducted a power analysis to investigate the increase in mean calving interval which could be detected for a given alpha level, assuming that another 45 intervals were observed (sample size of 45) and the standard deviations of the samples were similar to our dataset.
Results
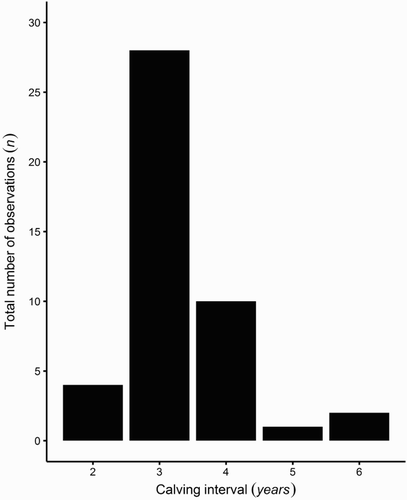
Between 2006 and 2013, 45 calving intervals were observed at Port Ross, ranging from 2 to 6 years (; Appendix 1). The most frequently observed calving interval was 3 years (n = 28), followed by 4 years (n = 10). The mean calving interval for the full, unmodified data set was 3.31 years (95% CI 3.06–3.57).
Figure 2. Distribution of calving intervals observed after 8 years (2006–2013) of data collection in Port Ross, Auckland Islands, New Zealand. Total sample size (n = 45).
The data set included 13 females for which the observed calving interval exceeded 3 years, some of which may have been over-estimated. Two 6-year intervals were observed, along with one 5-year interval and ten 4-year intervals. The shortest possible mean calving interval, estimated after modifying all intervals that exceeded 3 years, was 2.57 (95% CI 2.44–2.70). When only the two 6-year intervals were modified, the resulting mean was 3.17 years (95% CI 2.94–3.27).
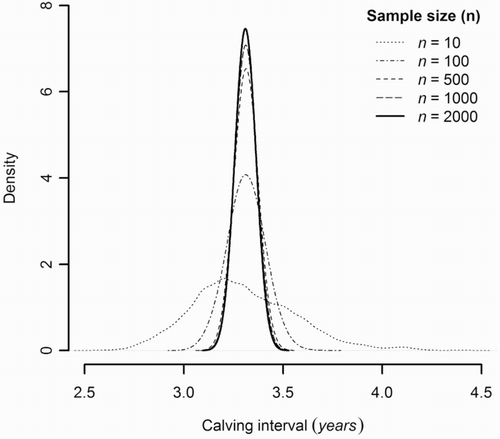
The unmodified data were resampled to explore the effect of sample size and detection error. The medians of the bootstrapped distributions did not differ appreciably across the range of sampling intensities () and were very similar to the estimate of calving interval from the unmodified dataset. As expected, the estimates became progressively more precise as sampling intensity increased (n = 10, 95% CI 2.90–3.90, CV = 7.88%; n = 2000, 95% CI 3.27–3.35, CV = 0.58%; ). Incorporating the 5% annual detection error into the sample of 2000 calving intervals increased the mean interval slightly from 3.31 to 3.32 years, with a consequent increase in CV to 0.63%.
Figure 3. Density distributions of the 1000 resampled mean calving intervals for the range of sample sizes tested (n = 10, 100, 500, 1000, 2000).
The power analysis estimated the magnitude of the difference between mean values which could be detected with a sample size of 45 calving intervals (). To observe a difference with an alpha of 0.05 or less, the mean difference would have to be greater than 0.6 (i.e. the mean calving interval would have to increase from 3.31 to 3.90 years).
Discussion
Accurate and precise estimates of demographic rates are essential for understanding the status of populations (Beissinger and Westphal Citation1998). Estimating reproductive output is particularly important for charting the trajectory of recovering populations. This analysis has produced the first estimate of calving interval for NZ SRWs of 3.31 years (95% CI 3.06–3.57 years). This estimate is very similar to one derived from SRWs at Head of the Bight in Australia (mean = 3.33 years; Burnell Citation2001) but slightly shorter than the Australia-wide estimate (mean = 3.63 years; Burnell Citation2001).
Most other published estimates of calving interval in right whales account for the possibility of missed calving events. For example, Cooke et al. (Citation2003) and Brandão et al. (Citation2010a) applied a model which imposes a maximum interval of 5 years. By applying a similar constraint to our data, we estimated a mean calving interval of 3.17 years (95% CI 2.94–3.27). Since the estimate of mean calving interval from the unmodified dataset is undoubtedly biased upward, we suggest that this lower estimate is more realistic. Indeed, the fact one of the whales in our dataset with an observed 6-year interval was recorded on the calving ground in the interim but without a calf, suggests that we missed a calving event. Furthermore, our estimate of 3.17 years is very similar to the one derived from the South African population (mean = 3.16 years), which has the most comprehensive dataset on SRW reproduction (35 annual surveys, 1968 individual calving events; Brandão et al. Citation2010a). Both estimates are shorter than that derived from Peninsula Valdes in Argentina (mean = 3.35 years, 29 annual surveys, 1298 individual calving events; Cooke et al. Citation2003), suggesting there could be real differences in reproductive rates among populations of SRWs. It is known that reproductive output of large whales is affected by food availability, whaling and other human impacts (Baker and Clapham Citation2004), and these influences vary among populations. For example, reproductive success of SRWs has been linked to climate-driven variations in abundance of krill, their primary food resource (Leaper et al. Citation2006, Seyboth et al. Citation2016). Cooke et al. (Citation2003) suggest that calving intervals of 4 or more years may result when females remain in the resting stage for at least 2 years in response to low food availability during the summer months.
Obtaining precise estimates of demographic rates is important for comparisons among populations. As expected, our resampling analysis showed that increased sample sizes resulted in estimates of mean calving interval with greater precision. The largest simulated sample size (n = 2000) resulted in a similar estimate of precision (CV = 0.58%) to the South African dataset (CV = 0.48%, n = 1968; Brandão et al. Citation2010a). Introducing additional detection error resulted in a very slight increase in the mean calving interval and a small decrease in precision. Note that the original data which were resampled were likely to already contain some detection error.
Overall these results suggest that the observed differences in precision of the real datasets could also be a product of the differing population sizes and durations of the respective studies. If the longitudinal study of SRWs in NZ continues, estimates of calving interval with greater precision can be expected. We estimate that we would obtain a similar sample size to the South African dataset if annual surveys at the Auckland Islands were continued until 2033.
Our estimates of calving interval provide some insight into the likely status of SRWs in NZ waters. With a calving interval of 3.16 years, the South African population is growing at 7% per annum (Brandão et al. Citation2010b). Assuming that other demographic parameters are similar between the populations we can, therefore, expect that the abundance of SRWs in NZ is also increasing. The NZ population live in a relatively unmodified habitat, and since commercial whaling ceased, are not thought to experience high levels of direct human impact. In comparison to the other populations of SRW, the South African population is possibly the least impacted by anthropogenic effects, although ship strike and entanglement are known to occur (Best et al. Citation2011; Meÿer et al. Citation2011). In a more extreme contrast, North Atlantic right whales (E. glacialis) live off one of the most urbanised stretches of coastline in the world, where they suffer lethal interactions with shipping and fishing gear (Kraus and Rolland Citation2007). In the 1990s, at a time when this population was declining at 2% p.a., mean calving interval was estimated to be more than 5 years (Fujiwara and Caswell Citation2001).
The range of calving intervals presented in this paper can be used in future modelling of the demographics of SRWs to explore the effect of parameter uncertainty on estimates of population growth rate. We suggest that there should be a continued focus on annual photo-ID in the Auckland Islands to allow for the application of more sophisticated reproductive models (Payne et al. Citation1990). Estimates of calving interval will become more accurate and precise with additional years of research. The development of age estimation techniques (Brandão et al. Citation2012) will also allow for estimates of age at first reproduction, and age-specific survival, the other parameters required for accurate estimates of reproductive output.
Acknowledgements
The field data were collected with funding from the Foundation for Research, Science and Technology (FRST), the New Zealand Whale and Dolphin Trust, Otago Museum and the University of Otago Marine Science Department. We thank the Captain and crew of Evohe and Polaris II; Bill Dickson, Phil Heseltine, Steve Little, Evan Kenton, Sophie Fern and Tim Lever, for support to and from the Auckland Islands. From 2010 to 2013, research was conducted under Marine Mammal Research Permit Per/NO/2010/05 led by Dr Will Rayment and Professor Steve Dawson (University of Otago). Additional thanks to Simon Childerhouse who made data from between 2006 and 2009 available for this research (conducted under Marine Mammal Research Permit SO-25791-MAR and Animal Ethics Approval (University of Auckland): AEC/02/2005/R334), and to Glen Dunshea for assistance with the photo-ID data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Baker CS, Clapham PJ. 2004. Modelling the past and future of whales and whaling. Trends in Ecology and Evolution. 19:365–371. doi: 10.1016/j.tree.2004.05.005
- Beissinger SR, Westphal MI. 1998. On the use of demographic models of population viability in endangered species management. The Journal of Wildlife Management. 62:821–841. doi: 10.2307/3802534
- Best PB. 1994. Seasonality of reproduction and the length of gestation in southern right whales Eubalaena australis. Journal of Zoology. 232:175–189. doi: 10.1111/j.1469-7998.1994.tb01567.x
- Best PB, Meÿer MA, Kotze D, Hofmeyr G, Thornton M. 2011. Mortalities of right whales in South African waters and associated factors (excluding entanglement), 1999–2010. Report of the International Whaling Commission SC/S11/RW. 14:1-14.
- Brandão A, Best PB, Butterworth DS. 2010a. Estimates of demographic parameters for southern right whales off South Africa from survey data from 1979 to 2006. Report of the International Whaling Commission SC/62/BRG30; 1–17.
- Brandão A, Best PB, Butterworth DS. 2010b. A note on possible changes in some demographic parameters for southern right whales off South Africa. Report of the International Whaling Commission SC/62/BRG31; 1–9.
- Brandão A, Butterworth DS, Müller A, Best PB. 2012. Application of a photo-identification based assessment model to southern right whales in South African waters. Report of the International Whaling Commission SC/64/BRG2; 1–15.
- Burnell SR. 2001. Aspects of the reproductive biology, movements and site fidelity of right whales off Australia. Journal of Cetacean Research and Management Special Issue. 2:89–102.
- Carroll EL. 2011. Return of the right whale: assessment of abundance, population structure and gene flow in the New Zealand southern right whale [Unpublished PhD thesis]. Auckland, New Zealand: Auckland University. p. 271.
- Carroll EL, Childerhouse SJ, Fewster RM, Patenaude NJ, Steel D, Dunshea G, Boren L, Baker CS. 2013. Accounting for female reproductive cycles in a superpopulation capture–recapture framework. Ecological Society of America. Ecological Applications. 23:1677–1690. doi: 10.1890/12-1657.1
- Carroll EL, Fewster RM, Childerhouse SJ, Patenaude NJ, Boren L. 2016. First direct evidence for natal wintering ground fidelity and estimate of juvenile survival in the New Zealand southern right whale Eubalaena australis. PLoS One. 87:1–17.
- Carroll EL, Patenaude NJ, Alexander AM, Steel D, Harcourt R, Childerhouse S, Smith S, Bannister JL, Constantine R, Scott Baker C. 2011. Population structure and individual movement of southern right whales around New Zealand and Australia. Marine Ecology Progress Series. 432:257–268. doi: 10.3354/meps09145
- Carroll EL, Rayment WJ, Alexander AM, Baker CS, Patenaude NJ, Steel D, Constantine R, Cole R, Boren LJ, Childerhouse S. 2014. Reestablishment of former wintering grounds by New Zealand southern right whales. Marine Mammal Science. 30:206–220. doi: 10.1111/mms.12031
- Cooke JG, Rowntree VJ, Payne RS. 2003. Analysis of inter-annual variation in reproductive success of South Atlantic right whales (Eubalaena australis) from photo-identifications of calving females observed off Península Valdés, Argentina, during 1971–2000. Report of the International Whaling Commission SC/55/O23; 1–16.
- Cooke J, Rowntree VJ, Sironi M. 2015. Southwest Atlantic right whales: interim updated population assessment from photo-id collected at Peninsula Valdés, Argentina. Report of the International Whaling Commission SC/66a/BRG; 1–9.
- Evans PGH, Hammond PS 2004. Monitoring cetaceans in European waters. Mammal Review 34:131–156. doi: 10.1046/j.0305-1838.2003.00027.x
- Fujiwara M, Caswell H. 2001. Demography of the endangered North Atlantic right whale. Nature. 414:537–541. doi: 10.1038/35107054
- Gaskin DE. 1964. Return of the southern right whale (Eubalaena australis) to New Zealand waters 1963. Tuatara. 12:2–5.
- Jackson JA, Carroll EL, Smith TD, Zerbini AN, Patenaude NJ, Baker CS. 2016. An integrated approach to historical population assessment of the great whales : case of the New Zealand southern right whale. Royal Society Open Science. 3(3): 150669. doi: 10.1098/rsos.150669
- Kenney RD. 2009. Right whales Eubalaena glacialis, E. japonica, and E. australis. In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopaedia of marine mammals. London: Academic Press. p. 962–972.
- Kraus SD, Rolland RM. 2007. The urban whale: North Atlantic right whales at the crossroads. Cambridge, MA: Harvard University Press. p. 1-576.
- Leaper R, Cooke J, Trathan P, Reid K, Rowntree V, Payne R. 2006. Global climate drives southern right whale (Eubalaena australis) population dynamics. Biological Letters. 2:289–292. doi: 10.1098/rsbl.2005.0431
- Meÿer MA, Best PB, Anderson-Reade MD, Cliff G, Dudley SFJ, Kirkman SP. 2011. Trends and interventions in large whale entanglement along the South African coast. African Journal of Marine Science. 33:429-439. doi: 10.2989/1814232X.2011.619064
- Patenaude NJ. 2002. Demographic and genetic status of southern right whales at the Auckland Islands New Zealand [Unpublished PhD thesis]. Auckland, New Zealand: Auckland University. p. 223.
- Payne R, Brazier O, Dorsey EM, Perkins JS, Rowntree VJ, Titus A. 1983. External features in southern right whales (Eubalaena australis) and their use in identifying individuals. US Marine Mammal Commission. pp. 371–445.
- Payne RS, Rowntree VJ, Perkins JS. 1990. Population size, trends and reproductive parameters of Right Whales (Eubalaena australis) off Peninsula Valdes, Argentina. Report of the International Whaling Commission SC/A88/ID1; 271–278.
- Pirzl R, Murdoch G, Lawton K. 2006. Bigfish: computer assisted matching software and data management system for photo-identification. Horsham, Australia: Skadia Pty Ltd.
- Rayment W, Davidson A, Dawson S, Slooten E, Webster T. 2012. Distribution of southern right whales on the Auckland Islands calving grounds. New Zealand Journal of Marine and Freshwater Research. 463:37–41.
- Rowntree VJ, Uhart MM, Sironi M, Chirife A, Sala LL, Pozzi LM, Musmeci L, Mohamed N, Andrejuk J, Sala EJ, Carribero A, Franco M, Seger J, Brownell RL, Rowles T. 2010. High mortalities of southern right whales (Eubalaena australis) on their nursery ground at Peninsula Valdes, Argentina. Report of the International Whaling Commission SC/S11/RW24.
- Schaeff CM, Best PB, Rowntree VJ, Payne R, Jarvis C, Portway VA. 1999. Dorsal skin colour patterns among southern right whales (Eubalaena australis): genetic basis and evolutionary significance. The Journal of Heredity. 90:464–471. doi: 10.1093/jhered/90.4.464
- Seyboth E, Groch KR, Dalla Rosa L, Reid K, Flores PAC, Secchi ER. 2016. Southern right whale (Eubalaena australis) reproductive success is influenced by Krill (Euphausia superba) density and climate. Nature Publishing Group. Scientific Reports. 6:28205. doi: 10.1038/srep28205
- Thomas PO, Taber SM. 1984. Mother–infant interaction and behavioural development in Southern Right Whales, Eubalaena australis. Behaviour. 88:42–60. doi: 10.1163/156853984X00470
- Torres LG, Rayment W, Olavarría C, Thompson DR, Graham B, Baker CS, Patenaude N, Bury SJ, Boren L, Parker G, Carroll EL. 2017. Demography and ecology of southern right whales Eubalaena australis wintering at sub-Antarctic Campbell Island, New Zealand. Polar Biology. 40(1):1–12. doi: 10.1007/s00300-016-1926-x