ABSTRACT
The Río de la Plata estuary (RdlP) exhibits environmental gradients associated with the freshwater input and oceanic water intrusion. The aim of this study was to assess diatom species distribution in surface sediment samples related to such environmental gradients. The internal section of RdlP was dominated by Aulacoseira spp., Eunotia spp., Staurosirella martyi, Actinocyclus normanii and Thalassiosira baltica, indicatives of low salinity levels and high trophic conditions, associated with the riverine and estuarine regimes. The external section was dominated by Coscinodiscus radiatus, Thalassiosira spp., Paralia sulcata, Cyclotella striata, among other marine taxa, indicatives of high salinity and low trophic conditions, associated with the influence of the Southwestern Atlantic Ocean. Furthermore, the intermediate section presents a mixture of both diatom groups representing mixing conditions. The observed diatom species groups capture fairly well the RdlP environmental variability and can be reliably used for paleoenvironmental studies in this and other similar estuarine systems.
Introduction
Diatoms represent a very common tool in both paleolimnological and paleoceanographical studies. To use these biological indicators as proxies of the environmental conditions, correct identification of taxa and reliable knowledge about the environmental conditions they represent are required (ie auto-ecology; Denys and de Wolf Citation1999; Vos and de Wolf Citation1988, Citation1993, Citation1994; Hassan Citation2010). In estuaries, salinity is a major determinant of diatom distribution, hence, diatom species analyses have become widely used in paleoenvironmental studies in such environments, eg in determining the continental versus marine influence (Denys and de Wolf Citation1999; Hassan Citation2010; Perez et al. Citation2017). Diatom assemblages from surface sediments are widely used as modern analogues of paleoenvironments, as they reliably reflect the environmental conditions at the sampling point and have been shown to integrate the annual seasonal scale variability (Juggins Citation1992; Hassan et al. Citation2008).
Diatoms in the Río de la Plata (RdlP) and adjacent coastal lagoons have been studied for taxonomic description (Frenguelli Citation1941, Citation1945; Müller Melchers Citation1945, Citation1952, Citation1953, Citation1959). In other studies, diatoms were utilised as proxies for paleosalinity changes to document Holocene sea level changes (coastal lagoons) and continental versus marine influence (RdlP) (García-Rodríguez et al. Citation2004a, Citation2004b, Citation2004c, Citation2010; Inda et al. Citation2006; Mourelle et al. Citation2015; Perez et al. Citation2016, Citation2017). However, on only a few occasions (Licursi et al. Citation2006, Citation2010), auto-ecological data (ie abundance and distribution of diatoms in relation to environmental variables) have been presented. This means that there are only few studies on modern environmental data of diatoms, and hence, there is a need for further information on modern diatom distribution with respect to environmental gradients in the RdlP, a basis for more robust paleoenvironmental reconstructions (Hassan Citation2010).
This study aims to contribute information about modern diatom distribution by analysing surface sediment samples from the RdlP, from the riverine through to the marine section. We aim to identify representative diatom species groups associated with the environmental condition (ie salinity and trophic state) at different locations along a transect in the RdlP. Such diatom groups will be used as modern analogues for inferring continental versus marine influence within the RdlP system in future regional paleoenvironmental studies.
Materials and methods
Study area
The RdlP estuary (35′00°; 36′10°S to 55′00°; 58′10°W) covering an area of 36,103 km2 is shared by Uruguay and Argentina (). This funnel-shaped river forms a large estuary, the second largest of South America, characterised by a semidiurnal tide with a low tidal amplitude (<1 m). It provides very important socio-economic and environmental services, and has a highly variable water chemistry because of human impacts (Bisbal Citation1995; Kurucz et al. Citation1998; Nagy et al. Citation2002) and natural variability at seasonal, inter-annual, decadal and centennial scales (López-Laborde et al. Citation2000; Acha et al. Citation2003). The main tributaries are the Paraná and Uruguay River, with an annual average flow of 16,000 and 6000 m3 s−1, respectively, thus the RdlP outflow exhibits an average value of 22,000 m3 s−1 (CARP Citation1989). The RdlP exhibits intra-annual variability regarding with the river discharge and wind patterns. In this sense, the RdlP outflow shows a maximum river discharge during summer (28,000 m3 s−1) and a minimum during winter (17,500 m3 s−1; Depetris and Pasquini Citation2007), associated with the intensification/weakening of the South American summer monsoon system, respectively (SAMS; Zhou and Lau Citation1998). In addition, a northeasterly/southwesterly wind pattern during summer/winter leads to a southward/northward displacement of the low salinity RdlP waters (Guerrero et al. Citation1997; Möller et al. Citation2008; Piola et al. Citation2008). There is also inter-annual and inter-decadal hydrological variability (Garreaud et al. Citation2009), related to the climatic mode of oscillations, ie El Niño–Southern Oscillation (ENSO), and the Pacific Decadal Oscillation (PDO), respectively. Increasing freshwater input into the RdlP is associated with the El Niño events and is enhanced by positive PDO phases, while the opposite is registered during La Niña and negative PDO phases (Depetris and Kempe Citation1990; Ciotti et al. Citation1995; Depetris et al. Citation2003; Depetris and Pasquini Citation2007; Garreaud et al. Citation2009; Barreiro Citation2010). Some studies have identified changes in the salinity of the system and in the trophic state of the sediments related to such hydrological variability of the RdlP (García-Rodríguez et al. Citation2014; Marrero et al. Citation2014; Perez et al. Citation2016, Citation2017; Bergamino et al. Citation2017).
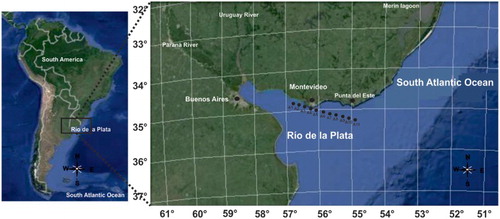
Figure 1. Map showing location of the study area. Black dots in the Río de la Plata indicate the position of the sampling stations A1 through A11.
The location of turbidity and salinity fronts depends on the wind direction/intensity, rainfall in the RdlP watershed and the associated interaction between the freshwater input and the marine intrusions from the Southwestern Atlantic Ocean (SWAO, Möller et al. Citation2008; Piola et al. Citation2008; Acha et al. Citation2008). Thus the system is characterised by two salinity fronts: the bottom salinity front, located in the innermost part of the bottom salt wedge, and the surface salinity front, indicating the transition between the turbid river and the less turbid marine surface waters (Framiñan and Brown Citation1996).
The area of investigation of this study consists of a transect parallel to the Uruguayan coast (34°85′51″S, 56°88′64.3″W to 35°25′37″S, 54°90′56″W) (; ). The 11-station transect was undertaken on board the oceanographic vessel Aldebarán during May 2009. The 11 stations ranged between 6 and 26 metres depth, from the innermost (landward) to the outermost station (seaward, ). During sampling, conductivity, salinity, temperature and depth were measured using a CTD SBE-19, and surface sediment samples (from the uppermost cm) were taken with a Smith–McIntyre bottom grab for measuring organic matter, chlorophyll a and diatom valve identification and counting.
Table 1. Geographical coordinates (latitude and longitude), depth (Z), and environmental variables and measurements (OM = organic matter, Chl a = chlorophyll a, bottom and superficial salinity) of all 11 stations from the Río de la Plata (A1–A11).
Laboratory analyses
Organic matter and chlorophyll a
Organic matter (OM) and chlorophyll a (Chl a) concentration in surface sediments are commonly used as proxies for productivity changes within the estuarine systems, as their concentration change with a shift in trophic state of aquatic systems (Rabalais et al. Citation2007; García-Rodríguez et al. Citation2014).
Aliquots of ca. 1 g wet surface sediment (uppermost cm of the grab) were taken for OM and for Chl a determinations. OM measurements were performed using the loss on ignition technique at 550°C according to Byers et al. (Citation1978). For Chl a determinations, the samples were extracted in 90% acetone in the dark at 4°C for 24 h, and centrifuged at 3000 rpm and absorbance was measured using a UV/VIS Beckman DU-650 at 750 and 665 nm (Strickland et al. Citation1972).
Diatom analyses
Diatoms samples were first treated with Na2P2O7 to defloculate the sediment and eliminate clay particles. Then the samples were treated with 35% HCl to remove inorganic carbonate material. Finally, the samples were boiled with 30% H2O2 for 2 hours to eliminate OM (Metzeltin and García-Rodríguez Citation2003). Between each treatment samples were rinsed at least four times with distilled water. Permanent slides were mounted using Entellan® mounting medium (Refractive Index: 1.54). A minimum of 400 valves was counted on each slide with a light microscope at 1250× magnification with oil immersion. Diatom species were identified according to Frenguelli (Citation1941, Citation1945), Müller-Melchers (Citation1945, Citation1953, Citation1959), Hasle and Syversten (Citation1996), Witkowski et al. (Citation2000), Metzeltin and García-Rodriguez (Citation2003), Metzeltin et al. (Citation2005), Sar et al. (Citation2010). Furthermore, the species were separated into groups according to their ecological salinity preference, ie in groups indicating freshwater (F), marine (M) and brackish (b) conditions; and their habitat type, ie in groups indicating benthic (B), planktonic (P), Tychoplanktonic (T) according to Pankow (Citation1970), de Wolf (Citation1982), Vos and de Wolf (Citation1988, Citation1993, Citation1994), Juggins (Citation1992), Van Dam et al. (Citation1994), Hasle and Syversten (Citation1996), Gómez and Bauer (Citation2000), Al-Kandari et al. (Citation2009) and Guiry and Guiry (Citation2017). Relative abundances of individual species were calculated by dividing the number of valves from each species by the total number of valves counted on each slide/station.
Data analyses
For analyses of the surface sediment diatom species, we used the most significant taxa (ie 2% in at least three stations, Karst and Smol Citation2000). Diatom Association Zones (DAZ) were determined using stratigraphically constrained cluster analyses (CONISS) using the software Tilia v. 2.0.38. To identify the most representative species of each DAZ and its association with environmental variables, a Canonical Correspondence Analysis (CCA) was performed using the CANOCO (ver. 4.5) program.
The CCA included the most significant diatom taxa and the associated environmental physical variables (ie salinity and temperature), proxies of productivity, ie P (phosphorus), TN (total nitrogen), Corg (organic carbon), OM (organic matter) and chlorophyll a (Chl a). Furthermore, we used Ti/Ca ratio as proxy for the terrigenous versus marine input and C/N ratio as proxy for the composition of the OM. P, C/N, TN, Corg and Ti/Ca data were extracted from Burone et al. (Citation2013).
Results
Environmental variables
We registered an increasing trend in the values of salinity from the innermost (landward) to the outermost (seaward) stations (A1–A11), with bottom salinity ranging between 4.9 and 30.6 and superficial salinity ranging from 4.2 and 28.6 (). Furthermore, OM ranged between 1.02% and 9.47% (A11 and A6, respectively) and the highest values were recorded from A1 to A8 (6.81–9.47%) and the minimum values were found in A9–A11 with a decreasing trend (2.17–1.02%). Regarding the Chl a, it ranged between 0.98 and 2.63 µg g−1 and there is no specific trend in the values, with the maximum value found in A8 and the minimum in A6 ().
Diatoms
A total of 78 diatom taxa representing 47 genera were identified in the surface sediment samples from the RdlP transect. Of the taxa, 52 were identified to species level using an optic microscope. The significant taxa belong to 17 genera (ie Aulacoseira, Staurosirella, Eunotia, Actinocyclus, Thalassiosira, Coscinodiscopsis, Coscinodiscus, Cyclotella, Paralia, Thalassionema, Chaetoceros, Trigonium, Diploneis, Fallacia, Psammodictyon, Fragilariopsis, Actinoptychus). In addition, we identified Chrysophyte cysts and Dictyocha fibula ().
Figure 2. Relative abundance of diatom species. Individual species are grouped according to salinity preferences on left side of diagram and into salinity-habitat group at right side as follows: the freshwater planktonic (FP) and benthic (FB), brackish planktonic (bP) and benthic (bB), and marine planktonic (MP), benthic (MB) and tychoplanktonic (MT). Furthermore, clustering groups and observed superficial and bottom salinity are shown to the right of the plot.
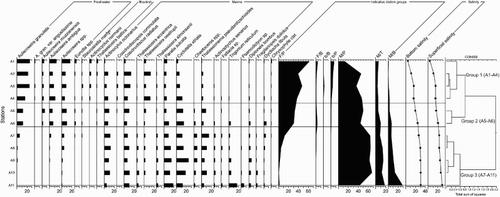
Diatom species with freshwater preference dominate the landward sample sites (A1–A4) and those with marine preference dominate the seaward sites (A7–A11) as indicated by the relative abundances of diatom salinity preference groups in . Furthermore, stations A5 and A6 showed intermediate abundances of species with freshwater and marine preference in relation with the landward and the marine sites (). These diatom abundances displayed a similar trend to that of salinity (). Planktonic taxa dominated in all samples throughout the RdlP, while the benthic taxa showed low abundances but an increasing trend towards the marine sites was recorded, especially in A11 (20%).
The cluster analysis () identified three DAZ: A1 through A4, A5–A6, and A7 through A11 (group 1, 2 and 3, respectively). The inner RdlP stations (group 1), A1 through A4, exhibited clear dominance of the freshwater genera Aulacoseira, Eunotia and Staurosirella, and also brackish taxa, ie Actinocyclus normanii and Thalassiosira baltica were observed. On the other hand, stations A7 through A11 (group 3) were dominated by marine genus Thalassiosira, and the species Coscinodiscus radiatus, Coscinodiscopsis commutata, Cyclotella striata, Diploneis bombus, Triceratium reticulum, Actinoptychus senarius, Fragilariopsis doliolus and Thalassionema pseudonitzschioides. Furthermore, intermediate stations A5 and A6 (group 2) were co-dominated by freshwater, brackish and marine species ().
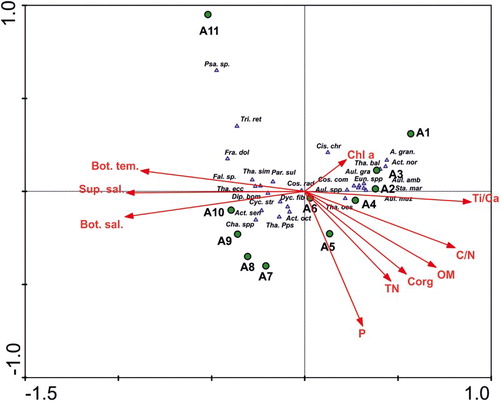
The axes 1 and 2 of the CCA ordination diagram shown in presented 60.9% and 75.5% of the accumulated variance, respectively. Salinity and temperature explained much of the variation in the diatom data as shown by their strong correlation with axis 1 in the CCA ordination diagram (). Axis 2 explains a fair amount of variation between the marine samples (A7–A11) and it appears to be strongly correlated with the productivity proxies, ie P, Corg and TN. Stations A1 through A4 were positively associated with Ti/Ca ratios, Chl a, and negative with both salinity and temperature (ie they are low salinity, low temperature stations). The most representative species of these sites Aulacoseira granulata, A. granulata var. angustissima, A. muzzanensis, A. ambigua, Staurosirella martyi, Eunotia spp., Actinocyclus normanii, Thalassiosira baltica and Coscinodiscopsis commutata relate well to cluster group 1. Station A7 through A11 showed a positive relationship with salinity and temperature and negative with the rest of the variables. The most representative species were those related to cluster group 3, ie Coscinodiscus radiatus, T. oestrupii, Actinocyclus octonarius, Thalassiosira eccentrica, T. simonsenii, Paralia sulcata, Cyclotella striata, Actinoptychus senarius, Diploneis bombus, Triceratium reticulum, Fragilariopsis doliolus and Thalassionema pseudonitzschioides. Finally, stations A5 and A6 (cluster group 2) occur in the middle of the salinity gradient and were positively associated with the productivity proxies P, Corg, OM and TN, especially A5, but no diatom species were clearly associated to these stations ().
Figure 3. CCA tripolt ordination diagram showing position of species (triangles), sites (circles) and environmental variable (arrows). The axes 1 and 2 presented 60.9% and 75.5% of the accumulated variance, respectively. P, C/N, TN (total nitrogen), Corg (organic carbon) and Ti/Al data were extracted from Burone et al. (Citation2013).
Discussion
The salinity gradient observed in this study (; ) is the characteristic of the RdlP system, and previous studies have reported distinguished domains (ie riverine, estuarine and marine) (Guerrero et al. Citation1997; Nagy et al. Citation2002; Calliari et al. Citation2009; Martínez and Ortega Citation2015). The OM and Chl a values recorded in the superficial sediments () are in accordance with other studies carried out in the RdlP (Burone et al. Citation2013; García-Rodríguez et al. Citation2014). The maximum OM concentration values recorded in the present study from the innermost station A1 to A8 could be associated with the high seston load related to the terrestrial supply in the RdlP waters (Calliari et al. Citation2009; Burone et al. Citation2013), and the maximum OM recorded in A6 could be a consequence of the flocculation process related to the position of the maximum turbidity front (, Framiñan and Brown Citation1996; Burone et al. Citation2013). Burone et al. (Citation2013) assessed the sediment footprint of riverine versus marine influence along the salinity gradient between the RdlP estuary and the adjacent SWAO shelf, and observed a transition from a tidal river to estuarine and marine zones based on foraminiferous, geochemical and sedimentological analyses. They observed increases in sand and clay content at the transition between tidal river and the estuarine zone associated with the maximum turbidity zone, where increased organic matter content and productivity was observed. In addition, Bergamino et al. (Citation2017) showed that the isotopic signals of surface sediments of the RdlP determined that the upper reaches (ie riverine domain) were influenced by riverine particulate matter, ie δ13C range: −24‰ to −26‰ relative to Pee Dee Belemnite (PDB). The lower reaches represented a depositional environment of marine algae, ie δ13C range: −21‰ to −23‰ relative to PDB.
The high diatom species number and the co-occurrence of both freshwater and marine diatom species () is probably associated with the intrinsic high productivity and the RdlP estuarine hydrological dynamics (Acha et al. Citation2008; Möller et al. Citation2008; Piola et al. Citation2008). Through this study, we determined that diatom distribution was associated with the hydrological features of the RdlP, related to the continental input of freshwater from the rivers and the intrusion of marine waters. Thus we recorded a strong correlation among salinity, sites and diatom species, ie the landward sample sites were associated with low salinity and high abundance of freshwater diatom species while seaward stations were related to high salinity and marine diatoms ().
The diatom composition/distribution from surface sediments, the environmental variables and the proxies of productivity, origin of OM and terrigenous versus marine input, registered in this study, allowed us to divide the study area into three sections (). The inner RdlP section related to group 1 was influenced by continental input of freshwater, which exhibits low salinity and temperature values, high trophic state as recorded by the productivity proxies (OM, P, TN, Corg and Chl a), and continental input (Ti/Ca and C/N), all determining a dominance of freshwater diatom species. The marine section (group 3), which is characterised by low productivity and low supply of terrestrial OM, and therefore low trophic state, and high salinity and temperature values, was related to marine diatom species. We also identified a transitional section, related to group 2, characterised by mixing conditions. Such a transitional zone is related to the position of the turbidity front (Burone et al. Citation2013), and it is characterised by increases in the productivity proxies and a co-dominance of both freshwater and marine species. Burone et al. (Citation2013) also divided the study area into such sections and determined for the inner section (up to the station 7) a high influence of terrigenous material, ie finer sediments, high particulate OM, Chl a, Corg, TN, P, C/N, Fe/Ca, Ti/Ca and more negative δ13C, while the opposite was registered for the marine section highly influenced by the SWAO waters.
Each of the identified sections, ie inner, transitional and marine, are characterised by specific trophic conditions and salinity values, with distinct diatom associations. The inner section was dominated by planktonic freshwater species (group 1), ie Aulacoseira granulata, A. granulata var. angustisima, A. ambigua, A. muzzanensis and the benthic Eunotia spp. (Coste and Prygiel Citation2000), but some planktonic brackish species Actinocyclus normanii and Thalassiosira baltica, were also dominant (Hasle and Syvertsen Citation1996). Such a mixture of species is the characteristic of estuarine environments and has been previously recorded by other authors for the riverine and estuarine sections of the RdlP (Frenguelli Citation1941; Müller Melchers Citation1953; Gómez and Bauer Citation2000; Ferrari and Pérez Citation2002; Licursi et al. Citation2006, Hassan Citation2010). Furthermore, the most significant species of the inner RdlP (Aulacoseira spp., Actinocyclus normanii and Staurosirella martyi) are all indicators of fairly eutrophic conditions (, Vos and de Wolf Citation1993; Van Dam et al. Citation1994; Coste and Prigiel Citation2000).
Table 2. Significant (ie 2% in at least three stations, Karst and Smol Citation2000) diatom species recorded from the RdlP superficial sediment transect (A1–A11), their ecological preferences and the RdlP environmental condition.
Within diatom group 1, the most abundant species were Aulacoseira spp. (). These planktonic species show an adaptive advantage, as they can form long filamentous chains and have a morphology that allows them to have a greater surface light absorption and contain accessory pigments that increase their spectrum of absorption (Gómez and Bauer Citation2000). Consequently, they are adapted to live in systems with a high load of suspended particulate material (Wang et al. Citation2008), such as the case of the RdlP (Licursi et al. Citation2006). These species (ie Aulacoseira granulata, A. granulata var. angustisima, A. ambigua and A. muzzanensis) were recorded previously for the RdlP coastal waters (Gómez and Bauer Citation2000; Licursi et al. Citation2006; Hassan Citation2010). Furthermore, they are also indicators of eutrophic conditions (Van Dam et al. Citation1994; Bicudo et al. Citation2016), hence, this group is indicative of high trophic levels in the RdlP. Thus the stations on the inner RdlP (related to group 1) are all influenced by nutrient-rich and suspended material coming from the Paraná and Uruguay Rivers (Nagy et al. Citation2002; Depetris and Pasquini Citation2007; Calliari et al. Citation2009).
The freshwater diatom assemblages, group 1, were gradually replaced along the transect by marine species (group 3), ie Coscinodiscus radiatus, Thalassiosira eccentrica, T. simonsenii, T. oestrupii, Paralia sulcata, Cyclotella striata, Actinocyclus octonarius, Actinoptychus senarius, Diploneis bombus, Triceratium reticulum, Fragilariopsis doliolus and Thalassionema pseudonitzschioides, which were most previously recorded for the RdlP and adjacent Uruguayan coastal waters (Müller Melchers Citation1959; Ferrando Citation1962; Burone Citation1984; Ferrari and Pérez Citation2002; Metzeltin et al. Citation2005; Calliari et al. Citation2009). Such a diatom assemblage was also observed in the adjacent coast of Brazil and Argentina (Lange and Mostajo Citation1985; Negri et al. Citation1988; Sar et al. Citation2001, Citation2007; Hassan Citation2010). The fact that both Coscinodiscus and Thalassiosira were the most abundant genera can be explained because these planktonic genera are the best adapted to neritic and oceanic waters worldwide (Hasle and Syvertsen Citation1996). Some of the species (Actinoptychus senarius, Paralia sulcata, Cyclotella striata, Diploneis bombus and Coscinodiscus radiatus) were also recorded in sediment cores of SE Uruguayan coastal lagoons during the Holocene transgressive phases, where the systems exhibited marine/brackish conditions because they were permanently connected to the ocean (García-Rodríguez et al. Citation2004a, Citation2004b, Citation2004c). This transgression event was also related to relatively low trophic state levels as low OM and nutrient levels were observed. In this sense, such diatom species were also observed in station A7 through A11 where low values of OM and also Chl a were measured. Furthermore, Thalassiosira oestrupii, T. simonsenii, Triceratium reticulum, Fragilariopsis doliolus and Thalassionema pseudonitzschioides are indicative of warm water regions (). Therefore, this species group is indicating a warm and marine water influence (Hasle and Syvertsen Citation1996; Méndez et al. Citation1998; Sar et al. Citation2001, Citation2007, Citation2010) which is evident as the higher temperature values recorded within this section (), probably associated with the intrusion of the subtropical waters within the continental shelf related to a la Niña event that occurred during 2009 (Martínez and Ortega Citation2007; García-Rodríguez et al. Citation2014).
Group 2 did not show diatom species exclusively associated to this zone (), but it was rather observed a mixture of freshwater, brackish and marine diatom species. Therefore, we inferred intermediate values in relation to the adjacent zones (ie groups 1 and 3). This zone indicates the transition between the continental and the marine influence, and it is associated with the position of the turbidity front as previously registered by Burone et al. (Citation2013). The high turbidity inherent of this zone explains the dominance of planktonic species in group 2 and the highest values of benthic species observed in group 3. The latter is a consequence of the flocculation process of particulate matter in the turbidity zone (A5 and A6), which led to a highest light penetration in the external section (seaward), and allowed the development of benthic species, ie Diploneis bombus, Triceratium reticulum and Fragilariopsis doliolus (Calliari et al. Citation2009).
Final remarks
The diatom species distribution from superficial sediments is related to the continental input of freshwater into the RdlP and the marine intrusion. Three distinct diatom groups were identified along the main environmental gradients within the RdlP (evolved from the interaction between terrigenous continental input and oceanic water intrusion). Group 1 is associated with high productivity proxies and low salinity within the riverine and estuarine sections, and the related diatom species were mostly indicative of a freshwater and eutrophic system. On the other hand, group 3 is related to low productivity proxies and high salinity related to the marine domain and the associated diatom species are marine planktonic and benthic and some are indicative of subtropical waters. Group 2 presents a mixture of freshwater, brackish and marine diatom species. Thus presenting intermediate values respect to the adjacent zones (groups 1 and 3).These diatom groups are reliable modern analogues and can be used as proxies for paleoenvironmental studies of the continental versus the marine influence within the RdlP and adjacent continental shelf.
Table S1. Supplementary material of Perez et al. New Zealand Journal of Marine and Freshwater Research. List of total diatoms species from surface sediments of Rio de la Plata (transect A1-A11, 2009)
Download MS Word (16.3 KB)Table S2. Supplementary material of Perez et al. New Zealand Journal of Marine and Freshwater Research. Total counts (relative abundances-%) of the diatoms species from surface sediments of Rio de la Plata (transect A1-A11, 2009)
Download MS Word (23.5 KB)Acknowledgements
We would like to thank to DINARA (Dirección Nacional de Recursos Acuáticos), PEDECIBA (Programa para el Desarrollo de las Ciencias Básicas), ANII (Agencia Nacional de Investigación e Innovación) and RLB (Red Latinoamericana de Botánica). We would like to express special thanks to the editor and reviewers for their critical comments and suggestions, which improved the manuscript content.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acha EM, Mianzan H, Iribarne O, Gagliardini D, Lasta C, Daleo P. 2003. The role of the Río de la Plata bottom salinity front in accumulating debris. Marine Pollution Bulletin. 46:197–202. doi: 10.1016/S0025-326X(02)00356-9
- Al-Kandari M, Rifaie K, Yamani FY, editors. 2009. Diatoms. En: ‘Marine phytoplankton atlas of Kuwait’s water’. Safat, Kuwait: Kuwait Institute for Scientific Research.
- Barreiro M. 2010. Influence of ENSO and the South Atlantic Ocean on climate predictability over Southeastern South America. Climate Dynamics. 35:1493–1508. doi: 10.1007/s00382-009-0666-9
- Bergamino L, Schuerch M, Tudurıí A, Carretero S, García-Rodríguez F. 2017. Linking patterns of freshwater discharge and sources of organic matter within the Río de la Plata estuary and adjacent marshes. Marine and Freshwater Research. 68:1704. doi: 10.1071/MF16286
- Bicudo DC, Tremarin PI, Almeida PD, Zorzal-Almeida S, Wengrat S, Faustino SB, Costa LF, Bartozek ECR, Rocha ACR, Bicudo CEM, Morales EA. 2016. Ecology and distribution of Aulacoseira species (Bacillariophyta) in tropical reservoirs from Brazil. Diatom Research. 31(3):199–215. doi: 10.1080/0269249X.2016.1227376
- Bisbal GA. 1995. The southeast South American shelf large marine ecosystem: evolution and components. Marine Policy. 19:21–38. doi: 10.1016/0308-597X(95)92570-W
- Burone F. 1984. Estudio taxonómico de las Bacillariophyceae de la Bahía de Montevideo (República Oriental del Uruguay). Montevideo, Uruguay: Tesis de Licenciatura, Facultad de ciencias. p. 230.
- Burone L, Ortega L, Franco-Fraguas P, Mahiques M, García-Rodríguez F, Venturini N, Marin Y, Brugnoli E, Nagai R, Muniz P, et al. 2013. A multiproxy study between the Río de la Plata and the adjacent South-Western Atlantic inner shelf to assess the sediment footprint of river vs. marine influence. Continental Shelf Research. 55:141–154. doi: 10.1016/j.csr.2013.01.003
- Byers SC, Mills EL, Stewart PL. 1978. A comparison of methods to determining organic carbon in marine sediments, with suggestion for a standard method. Hydrobiologia. 58:43–47. doi: 10.1007/BF00018894
- Calliari D, Brugnoli E, Ferrari G, Vizziano D. 2009. Phytoplankton distribution and production along a wide environmental gradient in the South-West Atlantic off Uruguay. Hydrobiologia. 620:47–61. doi:10.1007/s10750-008-9614-7.
- CARP. 1989. Estudio para la Evaluación de la Contaminación en el Río de la Plata. Comisión Administradora del Río de la Plata, Informe de Avance. 1:1–72.
- Ciotti AM, Odebrecht C, Fillmann G, Möller OO. 1995. Freshwater outflow and subtropical convergence influence on phytoplankton biomass on the southern Brazilian continental shelf. Continental Shelf Research. 15:1737–1756. doi: 10.1016/0278-4343(94)00091-Z
- Coste M, Prygiel J. 2000. Guide méthodologique pour la mise en oeuvre de l’Indice biologique diatomées. Bordeaux: Cemagref. p. 134.
- Denys L, de Wolf H. 1999. Diatoms as indicators of coastal paleoenvironments and relative sea-level change. In: E. F. Stoermer, J Smol, editors. The diatoms: applications for the environmental and earth science. New York: Cambridge University Press; p. 265–277.
- Depetris PJ, Kempe S. 1990. The impact of the E1 Niño 1982 event on the Paraná River, its discharge and carbon transport. Palaeogeography, Palaeoclimatology, Palaeoecology. 89:239–244. doi: 10.1016/0031-0182(90)90064-E
- Depetris PJ, Pasquini AI. 2007. The geochemistry of the Paraná river: an overview. In: M. J. Parma, editor. Limnology of a subtropical wetland. Berlin/Heidelberg: Springer-Verlag; p. 144–174.
- Depetris PJ, Probst J-L, Pasquini AI, Gaiero DM. 2003. The geochemical characteristics of the Paraná River suspended sediment load: an initial assessment. Hydrological Processes. 17:1267–1277. doi: 10.1002/hyp.1283
- De Wolf H. 1982. Method of coding of ecological data from diatoms for cometer utilization. Mededelingen – Rijks Geologische Dienst. 36:95–99.
- Ferrando HJ. 1962. Frecuencia estacional del microplancton costero de Montevideo durante el año 1959. Montevideo: Contribuciones planctológicas I, Servicio Oceanográfico y de Pesca. 32 pp.
- Ferrari G, Pérez MC. 2002. Fitoplancton de la costa platense Atlántica de Uruguay (1993–1994). Iheringia Série Botânica. 57:263–278.
- Framiñan MB, Brown OB. 1996. Study of the Río de la Plata turbidity front, part I: spatial and temporal distribution. Continental Shelf Research. 16:1259–1282. doi: 10.1016/0278-4343(95)00071-2
- Frenguelli J. 1941. XVI contribución al conocimiento de las diatomeas argentinas. Diatomeas del Río de la Plata. Revista del Museo de La Plata (n. s.) 3. Botánica. 15:213–334.
- Frenguelli J. 1945. XIX contribución al conocimiento de las diatomeas argentinas. Diatomeas del Platense. Revista del Museo de La Plata (n. s.) 3. Paleontología. 16:77–221.
- García-Rodríguez F, Metzeltin D, Sprechmann P, Beltrán-Morales LF. 2004a. Upper Pleistocene and Holocene development of Castillos Lagoon in relation to sea level variation, SE Uruguay. Neues Jahrbuch für Geologie und Paläontologie Monatsheft. 641–661.
- García-Rodríguez F, Metzeltin D, Sprechmann P, Trettin R, Stams G, Beltrán-Morales LF. 2004b. Upper Pleistocene and Holocene paleosalinity and trophic state changes in relation to sea level variation in Rocha Lagoon, southern Uruguay. Journal of Paleolimnology. 32:117–135. doi: 10.1023/B:JOPL.0000029427.36286.d9
- García-Rodríguez F, Sprechmann P, Metzeltin D, Scafati L, Melendi DL, Volkheimer W, Mazzeo N, Hiller A, von TümplingJrW, Scasso F. 2004c. Holocene trophic state changes in relation to sea level variation in lake Blanca, SE Uruguay. Journal of Paleolimnology. 31:99–115. doi: 10.1023/B:JOPL.0000013281.31891.8e
- García-Rodríguez F, Stutz S, Inda H, del Puerto L, Bracco R, Panario D. 2010. A multiproxy approach to infer Holocene paleobotanical changes linked to sea-level variation, paleosalinity levels and shallow lake alternative states in Negra Lagoon, SE Uruguay. Hydrobiologia. 646:5–20. doi: 10.1007/s10750-010-0184-0
- García-Rodríguez F, Brugnoli E, Muniz P, Venturini N, Burone L, Hutton M, Rodríguez M, Pita A, Kandratavicius N, Perez L, Verocai J. 2014. Warm-phase ENSO events modulate the continental freshwater input and the trophic state of sediments in a large South American estuary. Marine and Freshwater Research. 65:1–11.
- Garreaud RD, Vuille M, Compagnucci R, Marengo J. 2009. Present-day South American climate. Palaeogeography, Palaeoclimatology, Palaeoecology. 281:180–195. doi: 10.1016/j.palaeo.2007.10.032
- Gómez N, Bauer DE. 2000. Diversidad fitoplanctónica en la franja costera Sur del Río de la Plata. Biología Acuática. 19:7–26.
- Guerrero R, Acha E, Framiñan MB, Lasta C. 1997. Physical oceanography of the Río de la Plata Estuary, Argentina. Continental Shelf Research. 17:727–742. doi: 10.1016/S0278-4343(96)00061-1
- Guiry MD, Guiry GM. 2017. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. [accessed 2017 April 15] http://www.algaebase.org.
- Hasle GR, Syvertsen EE. 1996. Marine diatoms. In: C. R. Tomas, editor. Identifying marine phytoplankton. San Diego, CA: Academic Press; p. 5–383.
- Hassan G. 2010. Paleoecological significance of diatoms in Argentinean estuaries: what do they tell us about the environment? In: J. R. Crane, A. E. Solomon, editor. Estuaries: types, movement patterns and climatical impacts. New York: Nova Science Publishers; p. 71–147.
- Hassan GS, Espinosa MA, Isla FI. 2008. Fidelity of dead diatom assemblages in estuarine sediments: how much environmental information is preserved? Palaios. 23:112–120. doi: 10.2110/palo.2006.p06-122r
- Inda H, García-Rodríguez F, del Puerto L, Acevedo V, Metzeltin D, Castiñeira C, Bracco R, Adams JB. 2006. Relationships between trophic state, paleosalinity and climatic changes during the first Holocene marine transgression in Rocha Lagoon, southern Uruguay. Journal of Paleolimnology. 35:699–713. doi: 10.1007/s10933-005-4841-7
- Juggins S. 1992. Diatoms in the Thames estuary, England: ecology, paleoecology, and salinity transfer function. (J Cramer, Berlin). Bibl Diatomol. 25:1–216.
- Karst TL, Smol JP. 2000. Paleolimnological evidence of limnetic nutrient concentration equilibrium in a shallow, macrophyte-dominated lake. Aquatic Science. 62:20–38. doi: 10.1007/s000270050073
- Kurucz A, Massello A, Méndez S, Cranston R, Wells P. 1998. Calidad ambiental del Río de la Plata. In: PG Wells, GR Daborn, editor. Río de la Plata una revisión ambiental. Halifax: Dalhousie University; p. 71–86.
- Lange CB, Mostajo EL. 1985. Phytoplankton (diatoms and silicoflagellates) from the south western Atlantic Ocean. Botanica Marina. 27:469–476.
- Licursi M, Gómez N, Donadelli J. 2010. Ecological optima and tolerances of coastal benthic diatoms in the freshwater-mixohaline zone of the Río de la Plata estuary. Marine Ecology Progress Series. 418:105–117. doi: 10.3354/meps08865
- Licursi M, Sierra MV, Gómez N. 2006. Diatom assemblages from turbid coastal plain estuary: Río de la Plata (South America). Journal of Marine Systems. 62:35–45. doi: 10.1016/j.jmarsys.2006.03.002
- López-Laborde J, Perdomo A, Gómez-Erache M, editor. 2000. Diagnostico ambiental y socio-demográfico de la zona costera Uruguaya del Río de la Plata. Informe ECOPLATA. p. 180.
- Marcelo Acha E, Mianzan H, Guerrero R, Carreto J, Giberto D, Montoya N, Carignan M. 2008. An overview of physical and ecological processes in the Río de la Plata Estuary. Continental Shelf Research. 28:1579–1588. doi: 10.1016/j.csr.2007.01.031
- Marrero A, Tudurí A, Perez L, Cuña C, Muniz P, Lopes Figueira RC, Mahiques MM, Pittauerová D, Hanebuth T, García-Rodríguez F. 2014. Cambios históricos en el aporte terrígeno de la cuenca del Río de la Plata sobre la plataforma interna uruguaya. Latin American Journal of Sedimentology and Basin Analysis. 21:165–179.
- Martínez A, Ortega L. 2007. Seasonal trends in phytoplankton biomass over the Uruguayan shelf. Continental Shelf Research. 27:1747–1758. doi: 10.1016/j.csr.2007.02.006
- Martínez A, Ortega L. 2015. Delimitation of domains in the external Río de la Plata estuary, involving phytoplanktonic and hydrographic variables. Brazilian Journal of Oceanography. 63(3):217–227. doi: 10.1590/S1679-87592015086106303
- Méndez S, Gómez M, Ferrari G. 1998. Planktonic studies of the Río de la Plata. In: PG Wells, GR Daborn, editor. Río de la Plata una revisión ambiental. Halifax: Dalhousie University; p. 85–112.
- Metzeltin D, García-Rodríguez F. 2003. Las diatomeas uruguayas. Montevideo: DI.R.A.C.-Facultad de Ciencias.
- Metzeltin D, Lange-Bertalot H, García-Rodríguez F. 2005. Diatoms of Uruguay – taxonomy, biogeography, diversity. iconographia diatomologica. Vol. 15. Koenigstein, Germany: Gantner Verlag A R G.
- Möller JrOO, Piola AR, Freitas AC, Campos E. 2008. The effects of river discharge and seasonal winds on the shelf off southeastern South America. Continental Shelf Research. 28:1607–1624. doi: 10.1016/j.csr.2008.03.012
- Mourelle D, Prieto A, Pérez L, García-Rodríguez F, Borel M. 2015. Mid and late Holocene multiproxy analysis of environmental changes linked to sea-level fluctuation and climate variability of the Río de la Plata estuary. Palaeogeography, Palaeoclimatology, Palaeoecology. 421:75–88. doi:10.1016/j.palaeo.2015.01.006.
- Müller Melchers FE. 1945. Diatomeas procedentes de algunas muestras de turba del Uruguay. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo. 1:1–25.
- Müller Melchers FE. 1952. Biddulphia chilensis Grev. as indicator of ocean currents. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo. 2:1–25.
- Müller Melchers FE. 1953. New and little known diatoms from Uruguay and the South Atlantic coast. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo. 3:1–25.
- Müller Melchers FE. 1959. Plankton diatoms of the southern Atlantic Argentina and Uruguay coast. Comunicaciones Botánicas del Museo de Historia Natural de Montevideo. 3:1–45.
- Nagy GJ, Gómez-Erache M, López CH, Perdomo AC. 2002. Distribution patterns of nutrients and symptoms of eutrophication in the Río de la Plata river estuary system. Hydrobiologia. 475/476:125–139. doi: 10.1023/A:1020300906000
- Negri RM, Benavides HR, Carreto JI. 1988. Algunas características del fortalecimiento del fitoplancton en el frente del Río de la Plata: Las asociaciones fitoplanctónicas. Publicación de la Comisión Técnica del Frente Marítimo. 4:151–161.
- Pankow H. 1970. Die Kieselalgenf lora mecklenburgischer Salzst ellen. The diatom Flora of some salt waters in the inland of Mecklenburg. Internationale Revue der Gesamten Hydrobiologie und Hydrographie. 55(6):815–843. doi: 10.1002/iroh.19700550602
- Perez L, García-Rodríguez F, Hanebuth TJJ. 2016. Variability in terrigenous sediment supply offshore of the Río de la Plata (Uruguay) recording the continental climatic history over the past 1200 years. Climate of the Past. 12:623–634. doi: 10.5194/cp-12-623-2016
- Perez L, García-Rodríguez F, Hanebuth TJJ. 2017. Paleosalinity changes in the Río de la Plata estuary and on the adjacent Uruguayan continental shelf over the past 1200 cal yr BP: an approach using diatoms as proxy. In: K Weckström, P Saunders, G Skilbeck, editor. Applications of paleoenvironmental techniques in estuarine studies, developments in paleoenvironmental research. Dordrecht: Springer; p. 529–550.
- Piola AR, Möller OO, Guerrero RA, Campos EJD. 2008. Variability of the subtropical shelf front off eastern South America: winter 2003 and summer 2004. Continental Shelf Research. 28:1639–1648. doi: 10.1016/j.csr.2008.03.013
- Rabalais NN, Turner RE, Gupta BK, Platon E, Parsons ML. 2007. Sediments tell the history of eutrophication and hypoxia in the northern Gulf of Mexico. Ecological Applications. 17(5):S129–S143. doi: 10.1890/06-0644.1
- Sar EA, Sunesen I, Castaños C. 2001. Marine diatoms from Buenos Aires waters (República Argentina). I Thalassiosiraceae. Nova Hedwigia. 73:199–228.
- Sar EA, Sunesen I, Fernandez PV. 2007. Marine diatoms from Buenos Aires waters (República Argentina). II. Thalassionemataceae and Rhaphoneidaceae. Revista Chilena de Historia Natural. 80:63–79. doi: 10.4067/S0716-078X2007000100006
- Sar EA, Sunesen I, Lavigne AS. 2010. Cymatotheca, Tryblioptychus, Skeletonema and Cyclotella (Thalassiosirales) from Argentinian coastal waters. Description of Cyclotella Cubiculata sp. nov. Vie Milieu. 60:135–156.
- Strickland JDH, Parsons JR. 1972. A practical handbook of seawater analysis. bulletin 167, 2nd ed. Ottawa: Fisheries Research Board of Canada.
- Van Dam H, Mertens A, Sinkeldam J. 1994. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology. 28: 117–133. doi:10.1007/bf02334251.
- Vos PC, de Wolf H. 1988. Methodological aspects of paleo-ecological diatom research in coastal areas of the Netherlands. Geologic en Mijnbouw. 67:31–40.
- Vos PC, de Wolf H. 1993. Diatoms as a tool for reconstructing sedimentary environments in coastal wetland; methodological aspects. Hidrobiologia. 269/270:285–296. doi: 10.1007/BF00028027
- Vos PC, de Wolf H. 1994. Paleoenvironmental research on diatoms in early and middle Holocene deposits in central North Holland (The Netherlands). Netherlands Journal of Aquatic Ecology. 28(1):97–115. doi: 10.1007/BF02334250
- Wang L, Lu H, Liu J, Gu Z, Mingram J, Chu G, Li J, Rioual P, Negendank JFW, Han JT. 2008. Diatom-based inference of variations in the strength of Asian winter monsoon winds between 17,500 and 6000 calendar years BP. Journal of Geophysical Research. 113: 171. doi:10.1029/2008JD010145 doi: 10.1029/2008JE003126
- Witkowski A, Lange-Bertalot H, Metzeltin D. 2000. Diatom flora of marine coasts 1. iconographia diatomologica 7. Koenigstein: Gantner Verlag A R G.
- Zhou J, Lau K-M. 1998. Does a monsoon climate exist over South America? Journal of Climate. 11:1020–1040. doi: 10.1175/1520-0442(1998)011<1020:DAMCEO>2.0.CO;2
