ABSTRACT
Knowledge of stock structure is key for the effective management of any fish species. Amphidromous fish, which live and spawn in freshwater but spend a pelagic larval period at sea, have typically been assumed to disperse widely during their larval phase, resulting in populations being sourced from a single unstructured larval pool. We used otolith microchemical analysis to examine the stock structure of bluegill bully (Gobiomorphus hubbsi), a declining amphidromous eleotrid endemic to New Zealand, along the west coast of South Island, New Zealand. Some drainages – even those in close proximity (c. 20 km) – were readily distinguishable based on otolith trace element concentrations, while little structure was evident between other geographically disparate locations. These results indicate that, at least in some cases, locally retained larvae, rather than a single unstructured larval pool, dominates recruitment. Management of bluegill bully and other amphidromous species must therefore consider the possibility of regionally distinct populations.
Introduction
Amphidromy is a type of diadromous migration common in many aquatic species, involving an early larval migration out of the adult freshwater habitat into the marine environment, followed by a return migration to freshwater as juveniles (McDowall Citation2007). Such migrations are important, as dispersal during the marine phase is the primary means of gene flow between otherwise isolated adult populations (Waters and Wallis Citation2001), and the wide dispersal of marine larvae is thought to promote recolonisation and the resilience of amphidromous populations (McDowall Citation2010a). However, the migratory movements undertaken by amphidromous larvae in their marine nursery habitat are poorly understood, as fish larvae in general are small and hard to detect, making tracking in the marine environment difficult (Nickols et al. Citation2015). The extent of dispersal between populations of amphidromous fish is thus largely unknown, a lack of knowledge which is recognised as having led to poor management in the past (Miles et al. Citation2013). As such, a more complete understanding of larval marine migratory behaviour is necessary for making informed management and conservation decisions for amphidromous species, many of which are threatened or declining (e.g. Thuesen et al. Citation2011; Walter et al. Citation2012; Goodman et al. Citation2014).
Panmixia is suggested to be the default condition for amphidromous fish and invertebrates (Crandall et al. Citation2010; Russ et al. Citation2010; Dennenmoser et al. Citation2010; Schmidt et al. Citation2011). However, evidence for panmixia in amphidromous stocks is based primarily on genetic markers, which generally measure gene flow and rates of exchange between populations at multi-generational time scales (Lord et al. Citation2012). Rates of exchange as small as a single individual per generation can suppress population genetic structure (Slarkin Citation1985). This means that for genetic population structure to arise in amphidromous taxa, there must be almost no transmission of larvae between populations at a life stage when returning larvae can number in the millions for a single river (Bell Citation1999). Studies using genetic tools may thus underestimate the prevalence of local retention of larvae, and are uninformative in determining the primary larval sources involved in sustaining populations (Hicks et al. Citation2017). Additional insights into larval migratory movements during the marine phase can be gained using analytical tools that generate signals within the lifespan of a single individual, such as the trace element analysis of otoliths (Elsdon et al. Citation2008).
Otolith trace element analysis has been used for stock identification and assessment for over two decades (Campana Citation1999; Sturrock et al. Citation2012). Progressive refinement of sampling techniques has led to the extensive use of laser ablation as a precise and reliable means of sampling trace elements in biogenic carbonates such as otoliths (Russo et al. Citation2002; Jochum et al. Citation2012). The elemental composition of the otolith reflects individual life history changes and movements, especially between fresh and salt water (Walther and Limburg Citation2012). The exact mechanisms behind changes in otolith microchemistry based on life history are unclear, but are likely to reflect physical changes in the ambient environment (Hicks et al. Citation2010) as well as physiological responses to environmental and developmental change (Sturrock et al. Citation2015). Whatever the mechanism, trace element analysis is a robust tool for assaying stock structure (Elsdon and Gillanders Citation2004).
In this study, we used depth-profiling LA-ICP-MS to sample otolith trace elements (Warburton et al. Citation2017) in an effort to determine the stock structure of Gobiomorphus hubbsi (Stokell, 1959), an amphidromous eleotrid fish endemic to New Zealand. There is a conservation imperative to document stock structure in amphidromous fishes in New Zealand, as these species are under increasing threat from human activities (Goodman et al. Citation2014). Efforts to conserve amphidromous species in New Zealand and abroad need to come from an informed perspective, as the effectiveness of conservation actions may differ based on larval marine stock structure (Hicks et al. Citation2017). Management actions will proceed differently if species recruit from a single unstructured marine population vs. separate cohorts sourced from a specific region or watershed. In species with unstructured larval marine populations [e.g. Australian Grayling (Schmidt et al. Citation2011)], resources can be focused across the species’ range. In species with regional or even watershed level structuring [e.g. Southern Smelt (Hughes et al. Citation2014)], resources must be focused on individual rivers. Ultimately, effective conservation cannot take place without some a-priori knowledge of larval marine stock structure.
Materials and methods
Study species
G. hubbsi (Stokell, 1959) is a small, benthic fish endemic to the rivers of New Zealand. Part of the eleotrid amphidromous radiation into New Zealand, G. hubbsi is one of the three species in the monophyletic group typically classified as having an obligate diadromous life history (Stevens and Hicks Citation2009), although one of these, the closely related redfin bully (G. huttoni), has recently been demonstrated to be capable of non-diadromous recruitment (Hicks et al. Citation2017). The species is locally common across both the North and South Islands of New Zealand, but absent from surrounding islands as well as large sections of the coastal stream catchments of New Zealand (NIWA Citation2013). Spawning is typical of most eleotrids, with males guarding nests of adhesive eggs under rocks, often courting multiple females (Jarvis et al. Citation2017). Males will guard the eggs until they hatch, at which point parental care ceases. Once hatched, larvae drift downstream and into the marine environment (Jarvis and Closs Citation2015). Nothing is known about their larval life history once they enter the marine environment, including the timing of and age at larval return, or stock structure of the species across New Zealand. Although most native freshwater fishes in New Zealand are considered to be under pressure, populations of G. hubbsi and two other riffle specialists are considered to be in marked decline (Goodman et al. Citation2014).
Field sampling
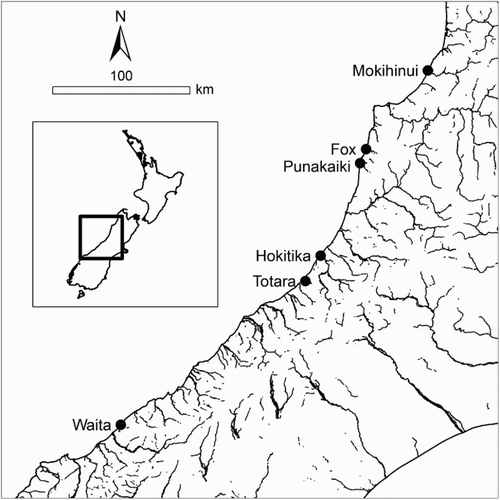
To examine population structure, we sampled six rivers on the West Coast of the South Island of New Zealand (). G. hubbsi were collected using a Kainga 300 backpack electrofisher (National Institute of Water & Atmospheric Research Ltd). Specimens were collected from the lower reaches of rivers, but upstream of tidal influence. Collections took place in February and March of 2012, in rivers with estuaries that were separated by 20 km or less of coastline, and the greatest distance between rivers was approximately 350 km (). A total of 10 individuals from the same young-of-year cohort (total length (TL) <45 mm) from each river was euthanised and retained for analysis. Individuals were euthanised using an overdose of Aqui-S fish anaesthetic (Aqui-S New Zealand Ltd.) and then transferred into 95% ethanol for preservation prior to being transported to the laboratory for dissection. All sampling was conducted under a University of Otago Animal Ethics sampling permit, as well as a DOC-sensitive species sampling permit.
Otolith dissection and preparation
Sagittal otoliths were selected for analysis because they are large and could be reliably extracted, even in very small individuals (TL ∼ 25 mm). Sagittal otoliths were dissected from specimens and mounted whole, sulcus down, on acid-washed glass slides in two-part epoxy (Araldite™; Huntsman Advanced Materials, Switzerland). Otoliths were mounted randomly to control for potential instrument drift bias during analysis. Slides were then sonicated for 3 min in nano-pure water and allowed to air-dry overnight in a laminar flow hood.
Otolith trace element analysis
When assaying for trace elements using larval or post-larval individuals, such as in this study, otolith specimens are small (generally >300 µm in diameter). Accordingly, depth-profiling LA-ICP-MS was used to estimate elemental concentrations, a technique which reduces handling requirements, and is particularly effective as it does not require that individual otolith specimens be cut and polished prior to analysis (Warburton et al. Citation2017).
Eight isotopes of elements commonly found in otoliths, plus 43Ca were sampled during LA-ICP-MS analysis, including 23Na, 24Mg, 39K, 55Mn, 64Zn, 85Rb, 88Sr and 138Ba. During LA-ICP-MS all elements were consistently measured at values above the calculated limits of detection (3× SD of background values). Mn values were recorded because elevated Mn concentrations are commonly associated with the primordium of otoliths (Ruttenberg et al. Citation2005). These elevated Mn levels were used to navigate ontogenetic zones within traces. A restricted subset of elements (Rb, Sr, Ba) was used to conduct a conservative stock assessment of G. hubbsi. Sr has been shown to substitute readily for Ca in aragonite matrixes and is therefore considered to be a stable non-labile marker of environmental conditions in fish otoliths (Doubleday et al. Citation2014). Ba has similar properties in crystalline carbonic matrixes and is speculated to substitute for Ca in a fashion similar to Sr (Thomas et al. Citation2017). Rb was included in the restricted element list because it is believed to be informative about environmental conditions (Thomas et al. Citation2017), and acts as a natural tag for identifying fish stocks (Thresher Citation1999) in spite of the fact that it does not substitute for Ca and may be less stable within the crystal matrix of the otolith in vivo (Thomas et al. Citation2017).
To examine stock structure in G. hubbsi we collected depth-profiling ablation transects from the distal surface of the otolith to the proximal surface, through the centroid of the otolith, following the method of Macdonald et al. (Citation2008). This method has been shown to be a reliable means of sampling otolith microchemistry in whole otolith applications (Warburton et al. Citation2017). We used an Agilent 7500cs ICP-MS coupled to a Resonetics RESOlution M-50 laser ablation system located in the Centre for Trace Element Analysis at the University of Otago, Dunedin, New Zealand. The ablation system was powered by a Coherent 193 nm ArF excimer laser. Otolith mounts were placed in the sampling cell and visually located via a 400× magnification video imaging system. Each sample was then ablated in a vertical transect from the distal surface to the proximal surface of the unpolished whole otolith using a 75 µm circular spot size. The laser was operated with a 5 Hz repetition rate and an average on sample fluence of 2.2 ± 0.1 J/cm2. Ablation occurred in an atmosphere of pure helium to minimise re-condensation of ablated materials and potential elemental fractionation (Eggins et al. Citation1998). Software-controlled gas flows of helium and nitrogen and ICP-MS-controlled argon carrier gas were tuned to maximise signal intensities on 138Ba and 88Sr while minimising oxides and mass fractionation by monitoring ablation of the NIST SRM 612 certified reference calibration glass (National Institute of Standards and Technology, USA). Each ablation was carried out for ∼200 s to ensure that transects completely traversed the core. To reduce the lag time associated with washout, no signal smoothing manifold was employed (Woodhead et al. Citation2008).
Signal baselines were established by collecting 30 s of background prior to each ablation transect. NIST SRM 612 was employed as the calibration standard, and was ablated for 40 s after every 10 samples. United States Geological Survey MACS-3 powdered calcium carbonate standard was ablated at the beginning and end of every laser session for ∼200 s to model potential downhole effects associated with depth profiling (Woodhead et al. Citation2008). We had previously determined rates of ablation for the selected spot size and fluence, and used this to estimate ablation pit depth by laser on time. Fractionation of lithophilic elements is negligible when ablation pit aspect ratios are greater than 2:1 depth:width but worsens at aspect ratios greater than 2:1 (Woodhead et al. Citation2004). We therefore restricted the analysis to signals generated at aspect ratios less than 2:1.
LA-ICP-MS results for the marine larval phase were identified by simultaneous low 138Ba/43Ca and elevated 88Sr/43Ca readings. The marine larval phase was classified as the period between the clear Sr/Ba inversion indicating a return to fresh water, and elevated Mn concentrations indicating the otolith core (see Hicks et al. Citation2017). Data were processed using IOLITE version 2.50 (Paton et al. Citation2011). The internal standard was 43Ca, and elements were expressed as MWElement/43Ca molar ratios. NIST SRM 612 was used as the calibration standard and NIST SRM 610 and MACS-3 were used as reference standards. All data analysis took place in the R statistical computing environment (R Core Team Citation2014). Data were log10-transformed to meet assumptions of normality prior to conducting a one-way MANOVA (manova function in the base package) and Linear Discriminant Function analysis (DFA) (lda function in the MASS package (Venables and Ripley Citation2002)) to test for patterns in trace element concentrations between populations.
Results
Examination of Sr/Ca and Ba/Ca values across depth-profiling transects indicated that all G. hubbsi included in the analysis were diadromous, and had experienced a period of marine residence. This was evident through a readily observable Sr/Ca and Ba/Ca inversion at the onset of the traces, which is typically associated with transitions between marine and freshwater environments (Crook et al. Citation2006; Elsdon et al. Citation2008; Feutry et al. Citation2011). Additionally, Sr/Ca values during the marine phase were consistent in the 2 mmol/mol range, which was similar to values collected for other diadromous New Zealand Gobiomorphus fishes (Hicks et al. Citation2017).
Traces displayed elevated Mn readings in the putative core region in concentrations similar to those observed by Ruttenberg et al. (Citation2005). We used these elevated Mn values as a marker to identify the natal core. In conjunction with the Sr/Ca and Ba/Ca inversion, this allowed us to identify the larval marine portions of the trace. Results of the MANOVA indicated differences in multi-elemental signatures for the larval marine period between drainages were significant for all samples (F(5,15) = 12.33, p < .0005, Wilk’s Λ= 0.103). The order of contribution to the first discriminant function by element was as follows, from most to least important: Rb, Ba, Sr. The first and second linear discriminants accounted for 93% and 5% of the variance in the dataset, respectively. Mean concentration and standard deviation of Rb, Ba and Sr were as follows; Rb: 0.45 ± 0.16 µg/g, Ba: 0.83 ± 0.22 µg/g, Sr: 5191 ± 756 µg/g.
DFA classification success differed between rivers, with some rivers exhibiting high classification success (Correct/Incorrect): Fox; 7/3, Hokitika; 10/0, Mokihinui; 7/3, while others approached random chance (Totara, Punakaiki) (). Classification success when examining all rivers using Rb, Sr and Ba was 63% (38/60 Correct/Incorrect), relative to only 16% expected under random chance. Rivers that were in close proximity (Fox and Punakaiki, 11 km coastal distance; Totara and Hokitika, 21 km coastal distance) exhibited little or no overlap in DFA classification success.
Table 1. DFA classification success of G. hubbsi for west coast rivers based on larval otolith microchemistry.
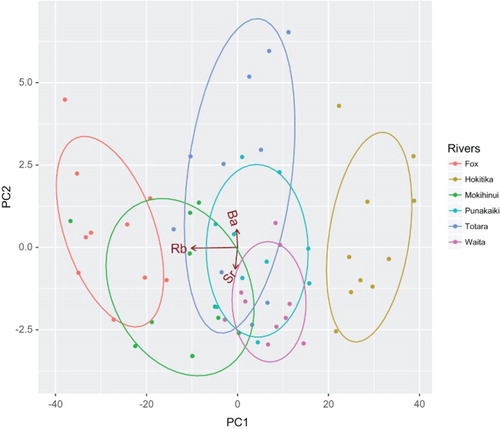
A principal components plot reflected similar patterns to the classification analysis (). Hokitika was readily distinguished from all other rivers, including the Totara River (21 km coastal marine distance). The Fox and Punakaiki rivers were also readily distinguishable from each other, with no overlap in the plot. DFA classification success was 100% when the analysis was restricted to single river pairs (Totara/Hokitika and Fox/Punakaiki).
Discussion
The otolith trace element composition of all G. hubbsi included in this study (specifically Sr/Ca and Ba/Ca ratios) indicated a period of larval marine residence prior to re-entry into freshwater, supporting the previous classification of the species as diadromous (McDowall Citation2010b). Multi-elemental signatures taken from the larval marine phase indicated distinct patterns of larval migratory behaviour that are previously undescribed for this species. The elemental signatures, represented by variation in the concentration of Sr, Ba and Rb in the otoliths of these fish, indicate that fish captured from different rivers were likely to have reared in different marine environments. The results of the DFA indicated unique larval signatures at the drainage scale for some samples. Importantly, the larval marine signatures from drainages that were geographically proximate were readily distinguishable.
G. hubbsi eggs are extremely small, and at 0.7 mm diameter (McCarter Citation1994) they are amongst the smallest eggs of any fish (Winemiller and Rose Citation1993). Small larvae, pelagic or otherwise, have a weak swimming ability and therefore could therefore be expected to be largely subject to external forces such as prevailing currents. However, as fish larvae develop in the marine environment, their swimming competency increases (Kopf et al. Citation2014). It may be that G. hubbsi larvae have the ability to maintain their position within the freshwater plumes of the rivers they will eventually recruit into once they reach a certain point in development, as has been suggested for some ecologically similar amphidromous gobies (Sorensen and Hobson Citation2005). River plume association, and the resulting limited dispersal and/or increased exposure to the unique elemental signature of the river they ultimately recruit into, may be resulting in the drainage level classification success we observed for some rivers. Similar trace-element signatures among fish from a single drainage could be the result of G. hubbsi remaining associated with the rivers they recruit out of, or nearby rivers during the larval marine phase.
G. hubbsi has been observed to have a disjunct distribution, being largely absent from Fiordland, the Catlins on the South Island, and south of Hawke’s Bay on the North Island (McDowall Citation2000). This patchy distribution across the landscape could be promoting or reinforcing the pattern of region-level phylopatry we observed in this study. Alternately, a hypothetical absence of distinct chemical markers in freshwaters of the West Coast could result in reduced differences in multi-elemental concentrations. Even with strict local recruitment, this could lead to overlapping distributions in chemical signatures, even between drainages separated by large distances. A third possibility is that the oceanographic processes in the Tasman Sea may enhance oceanic mixing of G. hubbsi larvae in localised portions of the study area. Oceanic currents on the West Coast of New Zealand’s South Island are complex, with northward and southward flows originating from the Subtropical Convergence Current and Tasman Front mixing inshore of northward prevailing currents forming the West Coast Current (Heath Citation1985). These complex nearshore currents on the West Coast could promote differential larval mixing interacting with the migratory competency of G. hubbsi larvae, leading to the patterns of isolation and mixing that we observed in the current study.
Our work, and other studies using analysis of signals generated within a single generation (otolith microchemistry, stable isotope analysis) show that local to regional recruitment, as indicated by the larval trace element signatures in G. hubbsi observed in this study, can be the dominant mode of some diadromous migrations (Sorensen and Hobson Citation2005; Hughes et al. Citation2014). This stands in contrast to results from studies of amphidromous populations using genetic tools, which generally indicate panmixia (Waters et al. Citation2000; Crandall et al. Citation2010; Schmidt et al. Citation2011; Lord et al. Citation2012). Even for an amphidromous species with an extended pelagic larval duration, local recruitment could be the dominant migratory mode (Sorensen and Hobson Citation2005). Local recruitment in amphidromous species has important implications for conservation management (Hogan et al. Citation2014). Previous work has referred to unstructured larval marine stocks when discussing recolonisation and recovery from disturbance (McDowall Citation1995; Leathwick et al. Citation2009). Our findings indicate that management of bluegill bully must consider the possibility of regionally distinct populations.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Matt G. Jarvis http://orcid.org/0000-0001-8276-1097
Additional information
Funding
References
- Bell KNI. 1999. An overview of goby-fry fisheries. Naga, the ICLARM Quarterly. 22:30–36.
- Campana SE. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series. 188:263–297. doi: 10.3354/meps188263
- Crandall ED, Taffel JR, Barber PH. 2010. High gene flow due to pelagic larval dispersal among South Pacific archipelagos in two amphidromous gastropods (Neritomorpha: Neritidae). Heredity. 104:563–572. doi: 10.1038/hdy.2009.138
- Crook DA, Macdonald JI, O’Connor JP, Barry B. 2006. Use of otolith chemistry to examine patterns of diadromy in the threatened Australian grayling Prototroctes maraena. Journal of Fish Biology. 69:1330–1344. doi: 10.1111/j.1095-8649.2006.01191.x
- Dennenmoser S, Schubart CD, Thiel M. 2010. High genetic variability with no apparent geographic structuring in the mtDNA of the amphidromous river shrimp Cryphiops caementarius (Decapoda: Palaemonidae) in Northern-Central Chile. Journal of Crustacean Biology. 30:762–766. doi: 10.1651/09-3273.1
- Doubleday ZA, Harris HH, Izzo C, Gillanders BM. 2014. Strontium randomly substituting for calcium in fish otolith aragonite. Analytical Chemistry. 86:865–869. doi: 10.1021/ac4034278
- Eggins S, Kinsley LPJ, Shelley JMG. 1998. Deposition and element fractionation processes during atmospheric pressure laser sampling for analysis by ICP-MS. Applied Surface Science. 127–129:278–286. doi: 10.1016/S0169-4332(97)00643-0
- Elsdon TS, Gillanders BM. 2004. Fish otolith chemistry influenced by exposure to multiple environmental variables. Journal of Experimental Marine Biology and Ecology. 313:269–284. doi: 10.1016/j.jembe.2004.08.010
- Elsdon TS, Wells BK, Campana SE, Gillanders BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD. 2008. Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology: An Annual Review. 46:297–330.
- Feutry P, Keith P, Pécheyran C, Claverie F, Robinet T. 2011. Evidence of diadromy in the French Polynesian Kuhlia malo (Teleostei: Percoidei) inferred from otolith microchemistry analysis. Ecology of Freshwater Fish. 20:636–645. doi: 10.1111/j.1600-0633.2011.00514.x
- Goodman JM, Dunn NR, Ravenscroft PJ, Allibone RM, Boubee JAT, David BO, Griffiths M, Ling N, Hitchmough RA, Rolfe JR. 2014. Conservation status of New Zealand freshwater fish, 2013. New Zealand threat classification series 7. Wellington: Department of Conservation. 12 p.
- Heath RA. 1985. A review of the physical oceanography of the seas around New Zealand – 1982. New Zealand Journal of Marine and Freshwater Research. 19:79–124. doi: 10.1080/00288330.1985.9516077
- Hicks AS, Closs GP, Swearer SE. 2010. Otolith microchemistry of two amphidromous galaxiids across an experimental salinity gradient: a multi-element approach for tracking diadromous migrations. Journal of Experimental Marine Biology and Ecology. 394:86–97. doi: 10.1016/j.jembe.2010.07.018
- Hicks AS, Jarvis MG, David BO, Waters JM, Norman MD, Closs GP. 2017. Lake and species specific patterns of non-diadromous recruitment in amphidromous fish: the importance of local recruitment and habitat requirements. Marine and Freshwater Research. 68:2315–2323. doi: 10.1071/MF16387
- Hogan JD, McIntyre PB, Blum MJ, Gilliam JF, Bickford N. 2014. Consequences of alternative dispersal strategies in a putatively amphidromous fish. Ecology. 95:2397–2408. doi: 10.1890/13-0576.1
- Hughes JM, Schmidt DJ, Macdonald JI, Huey JA, Crook DA. 2014. Low interbasin connectivity in a facultatively diadromous fish: evidence from genetics and otolith chemistry. Molecular Ecology. 23:1000–1013. doi: 10.1111/mec.12661
- Jarvis MG, Closs GP. 2015. Larval drift of amphidromous Gobiomorphus spp. in a New Zealand coastal stream: a critical spatial and temporal window for protection. New Zealand Journal of Marine and Freshwater Research. 49:439–447. doi: 10.1080/00288330.2015.1072569
- Jarvis MG, Harland HA, Warburton ML, Closs GP. 2017. The spawning and early life-history of the New Zealand endemic amphidromous Eleotrid, bluegill bully (Gobiomorphus hubbsi). New Zealand Journal of Marine and Freshwater Research. doi:10.1080/00288330.2017.1330760.
- Jochum KP, Scholz D, Stoll B, Weis U, Wilson SA, Yang Q, Schwalb A, Börner N, Jacob DE, Andreae MO. 2012. Accurate trace element analysis of speleothems and biogenic calcium carbonates by LA-ICP-MS. Chemical Geology. 318–319:31–44. doi: 10.1016/j.chemgeo.2012.05.009
- Kopf SM, Humphries P, Watts RJ. 2014. Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species. Journal of Fish Biology. 84:1820–1841. doi: 10.1111/jfb.12399
- Leathwick JR, Elith J, Rowe D, Julian K. 2009. Robust planning for restoring diadromous fish species in New Zealand's lowland rivers and streams. New Zealand Journal of Marine and Freshwater Research. 43:659–671. doi: 10.1080/00288330909510032
- Lord C, Lorion J, Dettai A, Watanabe S, Tsukamoto K, Cruaud C, Keith P. 2012. From endemism to widespread distribution: phylogeography of three amphidromous Sicyopterus species (Teleostei: Gobioidei: Sicydiinae). Marine Ecology Progress Series. 455:269–285. doi: 10.3354/meps09617
- Macdonald JI, Shelley JMG, Crook DA. 2008. A method for improving the estimation of natal chemical signatures in otoliths. Transactions of the American Fisheries Society. 137:1674–1682. doi: 10.1577/T07-249.1
- McCarter N. 1994. A key to some larval fish from New Zealand fresh water. Hamilton: NIWA Ecosystems. NIWA Ecosystem Publication 10.
- McDowall RM. 1995. Seasonal pulses in migrations of New Zealand diadromous fish and the potential impacts of river mouth closure. New Zealand Journal of Marine and Freshwater Research. 29:517–526. doi: 10.1080/00288330.1995.9516684
- McDowall RM. 2000. Biogeography of the New Zealand torrentfish, Cheimarrichthys fosteri (Teleostei: Pinguipedidae): a distribution driven mostly by ecology and behaviour. Environmental Biology of Fishes. 58:119–131. doi: 10.1023/A:1007666014842
- McDowall RM. 2007. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish and Fisheries. 8:1–13. doi: 10.1111/j.1467-2979.2007.00232.x
- McDowall RM. 2010a. Why be amphidromous: expatrial dispersal and the place of source and sink population dynamics? Reviews in Fish Biology and Fisheries. 20:87–100. doi: 10.1007/s11160-009-9125-2
- McDowall RM. 2010b. New Zealand freshwater fishes: an historical and ecological biogeography. New York: Springer.
- Miles NG, Walsh CT, Butler G, Ueda H, West RJ. 2013. Australian diadromous fishes – challenges and solutions for understanding migrations in the 21st century. Marine and Freshwater Research. 65:1–13.
- Nickols KJ, White JW, Largier JL, Gaylord B. 2015. Marine population connectivity: reconciling large-scale dispersal and high self-retention. The American Naturalist. 185:196–211. doi: 10.1086/679503
- NIWA. 2013. New Zealand freshwater fish database. [accessed 2013 Jun 14]. http://nzffdms.niwa.co.nz.
- Paton C, Hellstrom J, Paul B, Woodhead J, Hergt J. 2011. Iolite: freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry. 26:2508–2518. doi: 10.1039/c1ja10172b
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Russ A, Santos SR, Muir C. 2010. Genetic population structure of an anchialine shrimp, Metabetaeus iohena (Crustacea: Alpheidae), in the Hawaiian Islands. Revista de biología tropical. 58:159–170.
- Russo RE, Mao X, Liu H, Gonzalez J, Mao SS. 2002. Laser ablation in analytical chemistry-a review. Talanta. 57:425–451. doi: 10.1016/S0039-9140(02)00053-X
- Ruttenberg BI, Hamilton SL, Hickford MJH, Paradis GL, Sheehy MS, Standish JD, Ben-Tzvi O, Warner RR. 2005. Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Marine Ecology Progress Series. 297:273–281. doi: 10.3354/meps297273
- Schmidt DJ, Crook DA, O’Connor JP, Hughes JM. 2011. Genetic analysis of threatened Australian grayling Prototroctes maraena suggests recruitment to coastal rivers from an unstructured marine larval source population. Journal of Fish Biology. 78:98–111. doi: 10.1111/j.1095-8649.2010.02844.x
- Slarkin M. 1985. Gene flow in natural populations. Annual Review of Ecology and Systematics. 16:393–430. doi: 10.1146/annurev.es.16.110185.002141
- Sorensen PW, Hobson KA. 2005. Stable isotope analysis of amphidromous Hawaiian gobies suggests their larvae spend a substantial period of time in freshwater river plumes. Environmental Biology of Fishes. 74:31–42. doi: 10.1007/s10641-005-3212-6
- Stevens MI, Hicks BJ. 2009. Mitochondrial DNA reveals monophyly of New Zealand’s Gobiomorphus (Teleostei: Eleotridae) amongst a morphological complex. Evolutionary Ecology Research. 11:109–123.
- Sturrock AM, Hunter E, Milton JA EIMF, Johnson RC, Waring CP, Trueman CN, Leder E. 2015. Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution. 6:806–816. doi: 10.1111/2041-210X.12381
- Sturrock AM, Trueman CN, Darnaude AM, Hunter E. 2012. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology. 81:766–795. doi: 10.1111/j.1095-8649.2012.03372.x
- Thomas ORB, Ganio K, Roberts BR, Swearer SE. 2017. Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry. Metallomics. 9:239–249. doi: 10.1039/C6MT00189K
- Thresher RE. 1999. Elemental composition of otoliths as a stock delineator in fishes. Fisheries Research. 43:165–204. doi: 10.1016/S0165-7836(99)00072-7
- Thuesen PA, Ebner BC, Larson HL, Keith P, Silcock RM, Prince J, Russell DJ. 2011. Amphidromy links a newly documented fish community of continental Australian streams to oceanic islands of the West Pacific. PLoS One. 6:e26685. doi:10.1371/JOURNAL.PONE.0026685.
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York (NY): Springer.
- Walter RP, Hogan JD, Blum MK, Gagne RB, Hain EF, Gilliam JF, McIntyre PB. 2012. Climate change and conservation of endemic amphidromous fishes in Hawaiian streams. Endangered Species Research. 16:261–272. doi: 10.3354/esr00404
- Walther BD, Limburg KE. 2012. The use of otolith chemistry to characterize diadromous migrations. Journal of Fish Biology. 81:796–825. doi: 10.1111/j.1095-8649.2012.03371.x
- Warburton M, Reid M, Stirling CH, Closs GP. 2017. Validation of depth-profiling LA-ICP-MS in otolith applications. Canadian Journal of Fisheries and Aquatic Sciences. 74:572–581. doi: 10.1139/cjfas-2016-0063
- Waters JM, Dijkstra LH, Wallis GP. 2000. Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal? Molecular Ecology. 9:1815–1821. doi: 10.1046/j.1365-294x.2000.01082.x
- Waters JM, Wallis GP. 2001. Cladogenesis and loss of the marine life-history phase in freshwater galaxiid fishes (Osmeriformes: Galaxiidae). Evolution. 55:587–597. doi: 10.1554/0014-3820(2001)055[0587:CALOTM]2.0.CO;2
- Winemiller KO, Rose KA. 1993. Why do most fish produce so many tiny offspring? The American Naturalist. 142:585–603. doi: 10.1086/285559
- Woodhead J, Eggins S, Hergt J, Shelley M, Kemp R. 2004. Zircon Hf-isotope analysis with an excimer laser, depth profiling, ablation of complex geometries, and concomitant age estimation. Chemical Geology. 209:121–135. doi: 10.1016/j.chemgeo.2004.04.026
- Woodhead J, Hellstrom J, Paton C, Hergt J, Greig A, Maas R. 2008. A guide to depth profiling and imaging applications of LA-ICP-MS. In: Sylvester P, editor. Laser ablation ICP-MS in the earth sciences: current practices and outstanding issues. Quebec: Mineralogical Association of Canada; p. 135–145.