ABSTRACT
The Northland region of New Zealand includes numerous high-value, macrophyte-dominated dune lakes. Recent water policy reforms offer limited guidance on managing for aquatic macrophytes. In addition, dune lake histories are poorly known as regular monitoring dates to 2005 AD. Here, ca. 4000 years of lake functional behaviour is reconstructed from sedimentary archives in two Northland dune lakes (Humuhumu and Rotokawau). Results demonstrated that macrophyte dominance is sensitive to catchment erosion and hydrological drawdown. Degradation of macrophyte communities occurred in the nineteenth and twentieth centuries, earlier at Lake Humuhumu than Lake Rotokawau (post-1880 AD and post-1930 AD, respectively). In both lakes, increased erosional influx reduced macrophyte productivity, before later increases to wider trophic state (post-1970 AD). Lake-level decline is linked to increased nutrient loading at Lake Rotokawau but less so, Lake Humuhumu which is more strongly groundwater-fed. In Northland dune lakes, water-level reduction and erosional influx from land use have driven macrophyte degradation.
Introduction
Dune lakes are biodiverse ecosystems of high cultural and recreational value in New Zealand (Wells and Champion Citation2015). Although dune lakes are distributed widely throughout the country, the Northland region (north of 35.6° S; ) contains a disproportionate number of dune lakes, including many of outstanding trophic status (Kelly et al. Citation2016). Here, approximately 367 dune lakes support flora and fauna unique to New Zealand, and on occasion, endemic to Northland (Champion and de Winton Citation2012). Notably, many Northland dune lakes host diverse submerged plant communities often dominated by Characeae (Nitella and Chara spp.) and several threatened species (Trithuria inconspicua, Utricularia australis) (Champion and de Winton Citation2012). Like their Northern Hemisphere counterparts, macrophyte dominance is integral to resilient dune lake ecosystems in Northland (Kelly et al. Citation2016). In addition to providing valuable habitat for indigenous fish like the endemic dwarf inanga (Galaxias maculatus), native macrophytes stabilise lake beds and restrict sedimentary nutrient cycling, thereby limiting the negative impacts of algal biomass proliferations on ecological, recreational and cultural lake values (de Winton and Schwarz Citation2004).
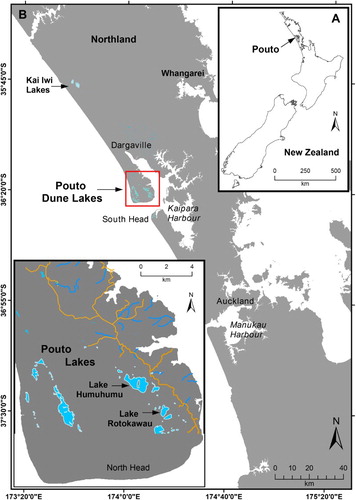
Figure 1. Location map showing Poutō lakes on North Kaipara Barrier and the study lakes: Rotokawau and Humuhumu.
Despite comparatively recent human arrival to New Zealand, widespread forest clearance by fire from ca. 1250 AD, resulting in a rapid transformation of much of the New Zealand landscape (McWethy et al. Citation2010; Perry et al. Citation2012), especially following European arrival (ca. 1800 AD) (McGlone and Wilmshurst Citation1999; McWethy et al. Citation2010). Approximately 51.4% of the country has been cleared of native vegetation, albeit much of this historic; from 1996 to 2001 AD less than 0.1% of indigenous vegetation cover was cleared (Walker et al. Citation2006). These land cover changes have adversely impacted lake ecosystems. In particular, variation in lake trophic state across New Zealand has been linked to the proportion of catchment cleared of indigenous vegetation (Sorrell et al. Citation2006; Hughes et al. Citation2016). Verburg (Citation2012) found 23% of New Zealand lakes were of ‘unacceptable’ trophic state with annual median algal biomass exceeding a threshold indicating an algal-dominated state (12 mg/m3). A disproportionate number of such ‘unacceptable’ lakes are located in pastoral, lowland catchments (Verburg Citation2012), supporting earlier findings by Verburg et al. (Citation2010) that pastoral land-use is associated with ecological deterioration.
In 2010, water quality was rated the single most important environmental issue facing New Zealand (Hughey et al. Citation2010). Accordingly, in 2011 the Government instigated water policy reform recognising that the Resource Management Act had effectively addressed anthropogenic point-source pollutants, but was less effective at managing diffuse contaminant effects on aquatic ecosystems (Rouse and Norton Citation2017). In 2014, the National Policy Statement for Freshwater Management (NPS-FM) was introduced to maintain or improve water quality within quantitative limits for key ‘attributes’ for ecosystem and recreational health (MfE Citation2014). Underpinning the NPS-FM is the National Objectives Framework (NOF), a framework for policy makers to utilise scientific consensus on aquatic functions, to set quantitative limits for their ecological state throughout New Zealand. The NOF is broadly equivalent to the water quality criteria determined by the US Environmental Protection Agency for the Clean Water Act, albeit at a national rather than ecoregion scale (McDowell et al. Citation2015). The NPS-FM requires that all New Zealand lakes must be managed for ecosystem health, to limits on at least five attributes: total nitrogen (TN), ammoniacal nitrogen (NH4N), nitrate nitrogen (NO3N), total phosphorus (TP) and phytoplankton biomass (Chl-a) (MfE Citation2014). Three tiers of nutrient stress have been set to protect lakes from an algal-dominated state, with a requirement that current water quality across a region cannot be degraded and must meet a minimum standard or national bottom-line (MfE Citation2014). In addition, the NPS-FM also directs resource managers to provide for the habitat needs of flora and fauna including, therefore, the management of aquatic macrophyte communities.
Northland Regional Council are responsible for implementing the NPS-FM on dune lakes across Northland, including gathering scientific evidence to underpin policy and management actions. A regional State of Environment (SoE) monitoring programme supports this, with regular monitoring of 25 dune lakes since 2005 AD. In addition to NOF attributes, annual surveys of Lake Submerged Plant Index (LakeSPI; Clayton and Edwards Citation2006) are conducted to record the ratio of native-to-invasive macrophytes and their colonisable depth ranges (de Winton et al. Citation2012). Investigation of Northland monitoring records by Kelly et al. (Citation2016) revealed moderate-to-strong relationships between NOF attributes and LakeSPI variables were limited to nutrient availability and colonisable depth (p < .05; R2 = 0.39–0.83). Poor statistical relationships were found between TN and TP, and overall LakeSPI score suggesting non-linear responses to nutrient availability are likely from macrophyte influences on physicochemistry (and/or hysteresis). Similarly, in Europe few simple relationships between nutrient availability and submerged macrophytes have been encountered (Sayer et al. Citation2010a). In contrast, strong responses of phytoplankton productivity to nutrient availability, principally TP, have been reported across Northland dune lakes (Gibbs et al. Citation2014). Whilst NOF guidance on nutrient availability is useful for managing phytoplankton in Northland dune lakes, it appears of more limited value for managing aquatic macrophyte communities. Furthermore, macrophyte community dynamics typically operate over decadal-scales of changing seasonal recruitment (Madgwick et al. Citation2011; Sayer et al. Citation2010b), limiting the potential for 12-years of regular monitoring to inform which dune lake processes to target for macrophyte health.
Here, we use a multi-proxy paleolimnological approach to reconstruct changes in lake environment and macrophyte-phytoplankton dynamics at two dune lakes on the Poutō peninsula, Northland (). Our aim is to identify dune lake macrophyte responses to catchment or in-lake processes impacted by land-use change, particularly the historic conversion of two thirds of the Poutō Peninsula to pasture and exotic forestry. Insodoing, we are able to demonstrate how paleolimnology can inform limit-setting in New Zealand lakes under the NPS-FM.
Setting
Northland dune lakes are mainly located in three clusters: the Poutō Peninsula, Kai-Iwi Lakes and the Aupouri Peninsula (). The Poutō Peninsula contains ∼50 dune lakes spread along the northern arm of the Kaipara Harbour on the southwest coast of Northland (). Most were formed by dune activity during the late Quaternary, resulting in Pleistocene and Holocene coastal sand and alluvial deposits of high erosion potential, overlying Miocene marine sediments (Edbrooke Citation2001). Lake origins vary from consolidation of dunes into impermeable bases through to drowned valley-lakes, produced by active dune migration. Hydraulic connections also vary between perched and groundwater-supplemented (‘window’) lakes, depending on whether the basin is underlain by an iron pan (Champion and de Winton Citation2012). Recent hydrological modelling of seven Poutō dune lakes identified an east–west groundwater divide along the length of the Poutō Peninsula, with lake inflows dominated by direct rainfall (37–59%), runoff (12–60%) and groundwater (0–40%) (Savoldelli Citation2014).
Northland dune lake typology was explored by Hughes et al. (Citation2016). Differences in depth explained significant (p < .001) variance in TN, TP, Chl-a and Secchi depth (SD). Deeper dune lakes have lower nutrient concentrations, greater clarity and lower algal biomass than shallow lakes. The threshold between deep and shallow lakes, associated with differing in-lake processes altering trophic state, were most marked at a maximum depth threshold of 10 m (Hughes et al. Citation2016).
Lake Rotokawau (12 m-depth, 0.26 km2 catchment area) is a deep dune lake in a mixed catchment of pine forest (46%), pasture (25%), with remnant native bush (18%) and minor fringing wetland () (Land Cover Database; LCDB, v.4). There are no permanent inlet or outlet streams and only minor spring-fed inputs (7% of inflow and 45% of outflow is via groundwater) (Savoldelli Citation2014). Submerged macrophytes are dominated by charophytes (Chara spp.) that grow to 11.1 m water depth. Considerable variation in maximum colonisable depth has been observed during occasional macrophyte surveys since 1984 AD and prior to water quality improvement during annual surveys from 2005 AD (Wells and Champion Citation2013). The lake is currently mesotrophic with median TN, TP, Chl-a and SD of 0.349 g/m3, 0.008 g/m3, 2.35 mg/m3 and 6.0 m, respectively (2009–2014; Hughes et al. Citation2016) ().
Table 1. Summary characteristics of Lake Humuhumu and Lake Rotokawau.
Table 2. Radiometric age markers in the Humuhumu and Rotokawau lake sediment records.
Lake Humuhumu (16 m-depth, 1.39 km2 catchment area) is a deep, window lake containing two basins in a drowned valley. The lake receives considerable spring-fed input (40% of inflow and 59% of outflow is via groundwater) (Savoldelli Citation2014). The catchment is predominantly plantation pine forest (42%) and pasture (27%) with areas of remnant bush (16%) (). Recent lake surveys indicate high ecological condition and submerged macrophytes growing to a depth of 9.1 m. Little change in macrophyte colonisable depth has been noted since 1984 (Wells and Champion Citation2015). The lake is also mesotrophic with median TN, TP, Chl-a and SD of 0.311 g/m3, 0.011 g/m3, 6.25 mg/m3 and 3.7 m, respectively (2009–2014; Hughes et al. Citation2016) ().
Land-use across the entire Poutō peninsula (3468 km2) is broadly similar to that in both study catchments (38% pasture; 34% plantation pine forest; 14% native forest/scrub; 8% active dunes and 5% open water [LCDB, v.4]). Anthropogenic impacts began with Māori arrival ca. 1250 AD and subsequent fire clearance for horticulture (McGlone and Wilmshurst Citation1999; McWethy et al. Citation2010). Harvesting of timber for export accelerated land clearance following European settlement in the Kaipara Harbour in 1820 AD and the opening of a navigable harbour route in 1840 AD (Byrne Citation2002). The Kaipara became the centre of the national kauri timber and gum industry between 1870 and 1920 AD, further enhancing deforestation (Smale et al. Citation2009). In 1931 AD, a road to the nearest major settlement of Dargaville was opened, permitting conversion to pasture of the previously remote Poutō Peninsula (Smale et al. Citation2009). After 1968 AD, exotic pine (Pinus radiata) was introduced to the peninsula, following earlier planting of active dunes on the western peninsula with nitrogen-fixing herbs to develop a topsoil on highly permeable sands with little to no organic content (Farnsworth et al. Citation1976; Thode Citation1983) (see ).
Materials and methods
Coring and chronology
A UWITEC piston coring system was used to collect two overlapping sediment cores from the deepest basin of Lakes Rotokawau and Humuhumu (April 2015). Cores varied in length from 18 to 78 cm and captured the sediment-water interface. Each core was split laterally and half archived at 4°C. The working half was described for stratigraphy before being subsampled for radiometric dating and then subsampled at 1 cm-intervals for a range of proxies for lake and catchment processes ().
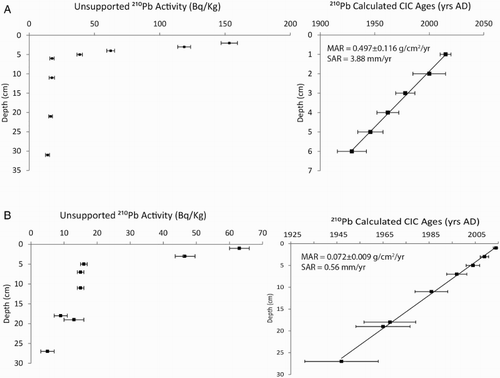
Sediment core chronologies and sediment accumulation rates (SAR) were established from simple linear regression for a mix of 5–8 210Pb and 3 14C bulk-sediment samples in each core series. In total, 11 age markers were collected from Lake Rotokawau and 9 from Lake Humuhumu. 210Pb ages were determined by alpha spectrometry (Attahan et al. Citation2015) at the Australian Nuclear Science and Technology Organisation (ANSTO), Sydney. Sedimentation rate was calculated from the slope of the least squares fit for 210Pb excess, plotted versus depth, using the Constant Initial Concentration (CIC) method (Appleby Citation2001; Augustinus et al. Citation2006). The CIC model requires that the 210Pb activity reduces monotonically with increasing depth. The slope (plotted logarithmically) versus depth is a measure of the sedimentation rate (SAR) and sediment mass accumulation rate (g/cm2/yr) when normalised by sediment bulk density. Radiocarbon ages of bulk plant detritus subsamples were determined by accelerator mass spectrometry (AMS) at DirectAMS (Seattle, USA) using an acid-alkali-acid washing sequence prior to production of a graphite target. All 14C ages were calibrated in OxCal v.4.2 (Ramsey and Lee Citation2013) using the SHCal13 calibration dataset (Hogg et al. Citation2013), and presented with two sigma errors
Erosional indicators
Sedimentological and geochemical indicators (proxies) of erosion included: magnetic susceptibility (MS), organic-matter content, grain-size distribution and conservative element down core profiles (e.g. Ti, Fe), which were analysed at varying resolution, ranging from 1 cm subsamples to 1 mm elemental measurements during core scans by ITRAX micro-X-Ray Fluorescence (μ-XRF). MS was measured at 1 cm-intervals using an MS2e1 Bartington sensor (Nowaczyk Citation2001). Down core patterns in MS reflect catchment erosion and influx of iron-bearing minerals as well as post-depositional changes in pore-water Eh and lake bottom water REDOX state (Nowaczyk Citation2001). Sediment organic-matter and moisture-content were determined for 1 cm-spaced subsamples using the loss-on-ignition (LOI) method and equations in Heiri et al. (Citation2001). Organic matter and water content vary inversely with erosion rate and positively with biological production (autochthonous and allochthonous), allowing changes in SAR to be determined as erosional and/or biological in origin (Stephens et al. Citation2012a). Grain-size analyses were also conducted on 1 cm-subsamples following the method of Parris et al. (Citation2010) to estimate distributions from 0.02 to 2000 μm with a Malvern Master-Size 2000 laser particle size analyser (precision ± 1%). Larger grain sizes can accompany reductions in lake level and/or enhanced erosional influx, and vice-versa under rising lake level and/or reduced catchment disturbance (Stephens et al. Citation2012a).
Heavy and light (Al-U) elemental profiles were determined for erosional (Ti, K) and lake redox state (Fe/Mn) by μ-XRF spectrometry using an ITRAX X-ray density/micro-XRF elemental core-scanner at ANSTO, Sydney. The micro-XRF core scanner was used with both the Mo and Cr anodes to optimise the detection of the lithophile and lighter elements (Croudace et al. Citation2006). Ti is commonly used to indicate eroded catchment input to lakes as it is conservative (i.e. undergoes little diagenetic change by variable REDOX) and strictly terrigenous in provenance (Arnaud et al. Citation2012).
Hydrological proxy indicators
Indicators of changing lake hydrology in the sediment cores (i.e. depth, water column mixing) included Fe/Mn ratios and Total Sulphur (TS). These were analysed at 1 mm-increments for ITRAX-derived elemental profiles of Fe, Mn (Mo anode) and S (Cr anode), and 1 cm subsamples for TS using a LECO CNS elemental analyser at the University of Auckland following the methods of Stephens et al. (Citation2012a). TS analyses were corrected for drift by introducing two blanks and two EDTA standards after every tenth sample; limit of detection in ±0.02 wt%. Note that the Itrax µ-XRF scans give relative element counts whilst the TS measurement is quantitative and acts as a check on the reliability of the trends in the former. Mn is more soluble than Fe under reducing conditions, meaning that lower Fe/Mn ratios correspond to greater benthic oxygenation through weaker stratification, or stronger water column mixing (Brown et al. Citation2007; Davies et al. Citation2015). TS content varies with productivity (input of organic-S) and benthic hypoxia (diagenetic precipitation of iron pyrite from dissolved-S under conditions of greater oxygenation in pore-water, or at the sediment-water interface) (Talbot Citation2001).
Productivity proxy indicators
Biomass productivity indicators included loss-on-ignition (LOI%), total organic carbon (TOC), total nitrogen (TN), stable carbon and nitrogen isotopic composition (δ13C, δ15N), and diatom assemblage concentrations. TOC and TN content were analysed at 1 cm-increments on a LECO TruSpec C and N determinator using the methods outlined in Stephens et al. (Citation2012a) at the University of Auckland (limit of detection < ± 0.01%TOC and ± 0.25%TN). Isotope geochemistry was determined for bulk sediment samples at 1–2 cm-increments to 30 cm depth and then at 4 cm-increments to basal samples in each core, using standard methods on a Europa Scientific 20/20 continuous-flow isotope-ratio mass spectrometer (University of Waikato Stable Isotope Unit (limit of detection ± 0.5‰ for δ13C and ± 1.0‰ for δ15N).
Changes in past biological productivity, nutrient cycling and sediment diagenesis can be revealed by changes in the availability of total organic-C and N (TOC, TN) (Talbot Citation2001). Greater TOC and TN is generally indicative of conditions favouring greater aquatic primary productivity, but can undergo competing changes through mineralisation of sediment organic matter if well-mixed and/or oxygenated (e.g. Meyers and Teranes Citation2001). Similarly, greater TOC and/or TN can occur in lake sedimentary records through increased terrestrial organic-matter inputs. Hence, the TOC/TN ratio is useful for determining whether increased sedimentary nutrient or organic content was caused by allochthonous (terrestrial; TOC/TN > 20) or autochthonous production (aquatic; TOC/TN < 20) (Meyers and Teranes Citation2001). Autochthonous production can be discriminated into mixed but predominantly plant macrophyte (TOC/TN ratios 10–20) or algal sources (TOC/TN < 10) (Talbot Citation2001).
The historical availability and competition for nutrients by aquatic primary producers can be reconstructed from δ13C and δ15N (Talbot Citation2001). Algae and macrophytes will discriminate against heavier C and N isotopes during nutrient uptake (Talbot Citation2001). Hence, isotopic enrichment can record periods of nutrient scarcity (i.e. increased biological nutrient demand or reduced external/internal nutrient supply), independent of changes to macrophyte-phytoplankton balance (e.g. Stephens et al. Citation2012a). The opposite is indicated by depletion in δ13C or δ15N, although changes can also be driven by pH, temperature and kinetic effects (Talbot Citation2001). Reliable interpretation of sedimentary δ13C or δ15N, therefore, requires a multi-proxy approach that examines changes to each of production, diagenesis and sediment provenance (Birks and Birks Citation2006).
Diatoms are an excellent additional proxy for assessing lake processes including: pH (Battarbee et al. Citation2001), nutrient availability (Saunders et al. Citation2008) and depth or mixing (Stephens et al. Citation2012b). Diatom frustules were prepared and mounted onto slides before counting at least 300 per sample slide at 1000× magnification following the techniques of Battarbee et al. (Citation2001). Diatoms were identified to species level where possible using the keys listed in Stephens et al. (Citation2012b). Unidentified diatoms were categorised into morphotypes. Diatoms were enumerated using microspheres as per Stephens et al. (Citation2012b). Only taxa with >2% occurrence are reported here. Diatom abundance was plotted versus depth and sediment age using C2 (Juggins Citation2017).
Results
Stratigraphy and chronology
Cores RK3 and H1, from Lakes Rotokawau and Humuhumu respectively, captured the sediment-water interface. Cores RK2 and H2 were longer with basal depths of 100 and 87 cm respectively. Sediments in both series of cores varied from silt to fine sand, including fine laminae at basal depths. Due to loss of the upper 6 cm of sediment in core RK2, a 6 cm offset was introduced to align the sequence with RK3 (supported by sediment magnetic susceptibility [MS]). Notable stratigraphic changes included textural and visual change above 18 cm-depth in the Rotokawau series (lightening, coarsening) and several sandy horizons in the Humuhumu series between 42–37 cm and 6–2 cm-depth.
Calibrated radiocarbon ages (±2σ) of 4094 ± 68 and 4029 ± 59 years Before Present (cal. yr BP; BP refers to 1950 AD) were determined for core bases from Rotokawau (90 cm-depth) and Humuhumu cores (83 cm-depth), respectively. Pre-human SAR for both lakes was equivalent at 0.22 mm/yr in Rotokawau between 4094 and 1400 cal. yr BP and 0.18 mm/yr in Humuhumu between 3733 and 1145 cal. yr BP.
Excess 210Pb activity was lower (<63 Bq/Kg) in core RK2 than in H2 (<160 Bq/kg), reflecting generally low 210Pb in New Zealand lake sediments (Graham et al. Citation2004). In both lakes, excess 210Pb decay versus depth plots demonstrated linear sedimentation, validating the assumptions of the CIC decay model (). Post-European (twentieth Century) SAR markedly increased relative to pre-human rates at both lakes but varied through time, ranging from 0.56 mm/yr in Humuhumu (2015–1929 AD) to 3.88 mm/yr in Rotokawau (2015–1947). A change in stratigraphy was noted in core RK2 at 30 cm-depth between the oldest 210Pb marker of 1947 AD (27 cm) and youngest 14C age marker of 550 AD (30 cm-depth). Given that both 210Pb and 14C down core trends in SAR are highly linear, it is likely the intervening sediment record at Lake Rotokawau was not captured by coring and represents a loss of sediment.
Dune lake ecosystem health histories
Lake Rotokawau
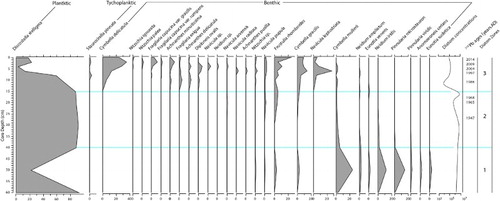
In Lake Rotokawau, limited change was observed in the erosional proxies prior to 1968 AD (18 cm-depth) (). After 1968 AD, Ti and K increased markedly, mean grain size increased from <40 μm (silt) to 160 μm (fine sand), MS increased and both LOI and TOC decreased (). Erosional proxies remained elevated until the present day, including during influxes of greater silt and organic matter content at ca. 1990 and 2010 AD. These organic-rich horizons were not associated with reduced erosional influx or changes in grain size (). While lake-level decline could explain increased SAR and mean grain size from 1968 AD by reducing the distance from shore and concentrating sediment loads onto a smaller lake bed area, Fe/Mn ratios declined only after 1982 AD becoming consistently lower (more oxygenated) from 1990. These results suggest that increased erosion occurred decades before changes to depth and/or mixing.
Figure 4. Stratigraphic plot of sedimentology and geochemistry for Lake Rotokawau cores RK2 and RK3.

Increased catchment erosion and algal dominance were inferred between ca. 1977 and 1997 AD, from TOC/TN ratios <5 (). Reduced TOC and TN without a change in SAR over this period, indicated reduced organic matter production and/or greater remineralisation also occurred (). Enrichment of δ13C between ca. 1968 and 1997 AD also corroborates changes in TOC/TN by indicating that increased algal biomass drove greater seasonal demand for dissolved inorganic carbon. Changes to δ15N were unusual, becoming increasingly enriched prior to and depleted after heightened erosion (ca. 1955–1968 AD), well before changes to macrophyte-phytoplankton dynamics. The δ15N enrichment was accompanied by limited change to other proxies. The absence of variation in Fe/Mn at this time suggested little change in benthic REDOX or denitrification. Later δ15N depletion is aligned to reduced TOC/TN reflecting greater algal productivity post-1977 AD (i.e. greater access to atmospheric-N and/or lesser N-limitation of phytoplankton during production).
Shifts in diatom assemblages occurred only well after erosion increased. A marked decline in the planktic/euplanktic (open-water) taxon, Discostella stelligera, occurred between ca. 1977 and 1990 AD (from > 85% dominance to 20% today) (). Replacement by a more diverse diatom community of meroplanktic and benthic taxa associated with shallower, turbulent water after 1977 AD indicated that greater mixing and/or lake-level decline likely contributed to reduced coeval macrophyte dominance. Oligotrophic benthic taxa including Frustulia rhomboides and Cymbella spp. have become more common since 1968 AD and account for 60% of present-day diatoms. This indicates nutrient-driven changes to diatom communities have been relatively modest compared to the changes driven by variations in depth.
Lake Humuhumu
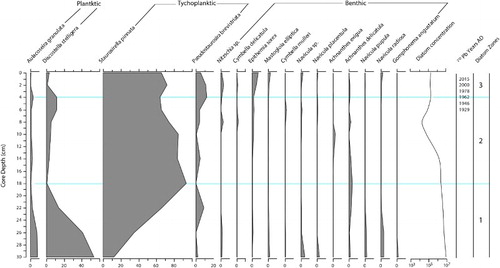
In Lake Humuhumu, pre-human erosional changes are more distinct than in Lake Rotokawau. These changes include an early erosive phase from 2200 to 1145 cal. yr BP (55–36 cm-depth; ). However, the most marked changes occurred after ca. 1880 AD (7 cm-depth). From 1880 to 2000 AD, sand content rose (50–95%), LOI fell (15–7%) and mean grain size increased (56–158 μm). Corresponding increases in Ti, K and MS suggest that increased catchment erosion was the cause of greater SAR from 1880 to 2000 AD. Erosional indicators decreased later at Lake Humuhumu (post-2000 AD) but, like Lake Rotokawau, decreased lake level is unlikely to have driven increases in the erosional indicators since Fe/Mn ratios only reduced consistently from the 1960's.
Increased catchment erosion after ca.1880 AD corresponded to reduced macrophyte dominance inferred from TOC/TN ratios reducing to below 15 (). The trend in TOC/TN ratios continued after 2000 AD with ratios remaining below 10 ().
After ca. 1978 AD, a shift to greater aquatic productivity and nutrient availability was recorded by doubled TOC, trebled TN content, and enriched δ13C and δ15N (as also occurred at Lake Rotokawau) (). However, enriched δ13C and δ15N at Lake Humuhumu were not accompanied by altered Fe/Mn, suggesting lake level and/or mixing were not important drivers of the stable isotope signatures. Increased nutrient availability (and algal productivity) was inferred for both lakes from the 1960/1970s onwards, suggesting increased external nutrient loading might have occurred thereafter across the Poutō Peninsula.
Diatom concentrations were generally lower and underwent limited change prior to ca. 1880 AD in Lake Humuhumu (). A shift in dominance from D. stelligera to the meroplanktic Staurosira pinnata from ca.1145 cal. yr BP cannot simply be explained through a change in mixing or depth because of limited change in Fe/Mn, although it should be noted D. stelligera is otherwise sensitive to changes in mixing depth (Saros et al. Citation2016). Instead, the continued dominance of S. pinnata and low levels of (<20%) benthic taxa today are evidence for less marked change in lake depth than at Lake Rotokawau. However, growth in the epipsamic taxon Pseudostaurosira brevistriata between ca. 1880 and 2000 AD supports sediment and geochemical evidence above for greater coeval catchment erosion and reduced macrophyte dominance. Increased taxonomic diversity, principally of diatoms with broad nutrient tolerance (e.g. S. pinnata, P. brevistriata, Epithemia sorex), at this time also suggests greater variation in nutrient availability over the last century.
Discussion
Lake management requires an understanding of in-lake and catchment change affecting functional processes and ecosystem structure. In New Zealand lakes, degradation has been linked to changing land-use, and the introduction of invasive species altering light regime, hydrology, nutrient availability or trophic structure (Kelly and Hawes Citation2005; Schallenberg et al. Citation2011). Pastoral land-use, in particular, has been widely associated with reduced water quality, largely through eutrophication (Verburg et al. Citation2010). Changes introduced by the NPS-FM in 2014 aimed to prevent further water quality degradation (MfE Citation2014). Specifically these changes involved use of the NOF to set objectives on attributes for lake ecosystem health that should limit nutrient from land-use. These attributes include algal biomass and nutrient concentration.
Whilst NOF attributes do not yet exist specifically for macrophyte communities, the NPS-FM does require that aquatic habitat provides for the needs of resilient native flora and fauna. Aquatic macrophytes perform key functional roles mitigating sediment resuspension, benthic de-oxygenation, nutrient cycling and phytoplankton growth in New Zealand lakes (e.g. de Winton and Schwarz Citation2004). Hence, there could be merit in additional ecosystem health attributes based on macrophyte communities to target with limit-setting beyond simply nutrient or algal concentration (Schallenberg et al. Citation2017). Especially so in the Northland region, where a disproportionate number of New Zealand's dune lakes remain in macrophyte-dominated states (Champion and de Winton Citation2012).
Using paleolimnology to inform dune lake management – the importance of visual clarity & hydrology
The paleolimnology of Lakes Humuhumu and Rotokawau support adopting an erosion-linked attribute to protect macrophyte communities in Northland dune lakes. Enhanced erosion was accompanied in both lakes by increased algal contributions to autochthonous production (ca. 1880 and 1968 AD, respectively). As erosional changes preceded marked change to nutrient proxies by a decade or more, reduced macrophyte dominance likely resulted initially from burial and/or reduced light transmission. Greater phytoplankton production is likely to have reduced light availability, feeding-back positively to further reduce colonisable depth and macrophyte dominance.
Reduced dominance of primary production by aquatic macrophytes at Lake Humuhumu corresponds broadly with native timber harvesting and conversion to pasture in the nineteenth Century after the Kaipara Harbour became navigable (post-1840 AD; Byrne Citation2002). At Lake Rotokawau, enhanced erosional influx to Lake Rotokawau from the late 1960's coincided with the expansion of pine forestry on the Poutō Peninsula in 1968 AD (Thode Citation1983). Erosion-driven degradation of submerged macrophyte communities have been recorded by paleolimnological reconstructions elsewhere, including from the United States (Brush and Hilgartner Citation2000), Australia (Reid et al. Citation2007) and United Kingdom (Salgado et al. Citation2010). Submerged macrophytes indigenous to New Zealand are particularly sensitive to changes in light regime, which restricts colonisable depth and the balance of respiration-to-production (de Winton and Schwarz Citation2004). Even charophytes, which are adapted to low-light availability by utilising carbohydrate stores to offset periodic reductions in clarity, can only do so for periods of days to weeks (Howard-Williams et al. Citation1995). Long-term erosional influx recorded by the paleolimnology of both Poutō lakes are, therefore, likely to have driven declines in macrophyte dominance of autochthonous production. Furthermore, the dissimilar timings of changes in erosional proxies such as the later reduction in erosional influx at Lake Humuhumu (after 1997 AD) cf. Lake Rotokawau (after 1968 AD), suggests that macrophyte histories likely vary across the Poutō Peninsula. Consequently, targeted paleolimnological reconstructions are recommended to inform water quality objectives for particular lakes. Equally, the two paleolimnologies here present evidence for the value of developing water clarity guidance to better manage for lake macrophytes. Wider paleolimnological reconstructions and development of a clarity proxy, coupled to ongoing monitoring of macrophyte and lake water quality is recommended. This would contribute improved understanding of macrophyte relationships to altered light-climate, across lake types and plant assemblages and satisfy the national-basis of attributes in the NOF.
The paleolimnology of Lake Rotokawau also highlights that altered lake hydrology can stress macrophyte communities. For instance, greater water column mixing (benthic oxygenation), coincided with increased nutrient availability and algal productivity after 1977 AD. The lack of equivalent timing in changes to hydrological proxies at Lake Humuhumu, tentatively suggests a region-wide climatic cause (increased westerlies) was unlikely. Without a climatic driver, it is more likely that lake-level decline at Lake Rotokawau increased benthic oxygenation. Any lake shallowing would feedback positively with nutrient from land-use, helping to both concentrate external and recycle internal nutrient loads (e.g. Hughes et al. Citation2016). Consequently, water level decline at Lake Rotokawau appears to be linked to the observed increases in benthic oxygenation, TN availability and algal production noted since 1977 AD. In combination with their external nutrient loading, managing land-use effects on depth appears to be integral to regulating the nutrient availability and protecting macrophyte communities in Northland dune lakes.
Implications for managing land-use effects on Northland dune lake macrophytes
The impacts of sediment on water quality is receiving increased attention in New Zealand. In Northland, geochemical finger-printing has demonstrated that plantation forestry and pastoral losses to water predominate (Gibbs Citation2006; Gibbs et al. Citation2012; Swales et al. Citation2012, Citation2015a, Citation2015b). Topsoil and subsoil disturbance associated with forest harvesting and re-planting is typically restricted to the late and early forest growth cycle (2–3 years) with sediment mobilised at rates nearly fivefold pre-harvest (Basher et al. Citation2011). Over full harvest cycles, pastoral losses are generally estimated at one-and-a-half those of forestry, though with wide variation reflecting slope, soil type, climate and land practice (Quinn and Phillips Citation2016). Analysis by Hughes et al. (Citation2016) demonstrated that exotic forestry extent is a significant predictor of water clarity across Northland lakes, along with information on maximum lake depth, soil phosphorus content, lake elevation and erosional proxies (adj. R2 = 0.87; p < .0001). Although differences in pastoral cover were insignificant at explaining additional variance in clarity or Trophic Level SD, this might record greater importance and variation in land management, rather than the absence of effect. For instance, modelling indicates pastoral yields of sediment to water are nearly twice that of forestry in Northland but both account for approximately 60% and 8% of total sediment loss across Northland, respectively (Daigneault and Morgan Citation2017). Hence, any clarity attribute for Poutō dune lakes will likely affect pastoral and forestry land-uses, with both occupying ∼72% of the 3500 km2 of Poutō Peninsula.
Ongoing clearance of indigenous vegetation is of less pressing concern as both land-uses have declined in extent; pasture by 2.7% and forestry by 6.6% between 1996 and 2012 AD (LCDB, v.1–4). Instead, land-use practices are expected to have a greater future impact on dune lake macrophytes. In particular, for pastoral land users, cropping and livestock access to waterways are likely to be the principal drivers of sediment loss, placing greater emphasis on land and riparian management (Scarsbrook et al. Citation2016). Equally, the harvesting of existing forests will be the greatest driver of sediment loss from plantation forest, whose affected area whilst modest, increased between 1996 and 2012 from 0.3% to 3.9% of the Poutō Peninsula, respectively (LCDB, v.1–4).
Managing the impacts of land-use on catchment hydrology presents a different challenge to sediment loss, given the focus on water consumption rather than discharge to, dune lakes. Water levels reduced and nutrient availability increased at Lake Rotokawau within a decade of plantation pine forestry expanding onto active dunes in the Poutō Peninsula (Thode Citation1983). Plantation pine forestry generates considerably greater water demand by area than either pasture or native scrub (e.g. Fahey et al. Citation2004). For instance, New Zealand field trials have demonstrated that pine afforestation reduced runoff by 30–50% and groundwater recharge by up to 70% relative to pastoral land-use (Smith Citation1987; Fahey Citation1994).
Water yields under pine stands reduce linearly with age but on average over a 30-year pine rotation equate to an annualised loss in water yield of 160–260 mm relative to pasture, or 100 mm compared to indigenous forest (Beets and Oliver Citation2007). Consequently, if increased nutrient supply resulted from pastoral land conversion, a net gain in lake level would be expected. The opposite is evident in the sediment record at Lake Rotokawau after 1977 AD, whereby, the timing and reduction in lake level suggests afforestation was a likely driver of reduced water levels and indirectly, of increased nutrient availability. The lack of equivalent hydrological change in Lake Humuhumu, which receives greater groundwater recharge (40% cf. 7% in Lake Rotokawau; Savoldelli Citation2014) but possesses a similar area of pine plantation (42% cf. 46% in Lake Rotokawau; LCDB, v.4), suggests that plantation forestry has greater impact on the hydrology of perched dune lakes than on window dune lakes. Whilst the paleolimnological evidence from Lake Rotokawau suggests that plantation forestry altered hydrological and nutrient balances to degrade ecosystem health, it could equally hold for intensification of pastoral or horticultural land-use in other lakes (e.g. through greater livestock, dairy-shed or irrigation demand for water [Higham et al. Citation2017]). This finding does not warrant a reduced focus on external nutrient loading under the NPS-FM. Rather, the paleolimnology of Lake Rotokawau suggests land-use driven effects on lake depth will also need managing to achieve nutrient or phytoplankton limits in Northland dune lakes.
Conclusions
New Zealand is undergoing major water policy reform supported by a national objective framework to set conditions for water quality attributes. Due to the short (decadal) duration of lake water quality monitoring records in New Zealand, and NOF guidance being restricted to phytoplankton and nutrient management limit-setting in lakes, management for aquatic macrophyte objectives remains uncertain despite their integral role to lake processes (Schallenberg et al. Citation2011). Paleolimnology can be invaluable to addressing this and other barriers to good lake management, by providing evidence of lake processes linked to macrophyte dynamics and catchment land-use (e.g. Bennion and Battarbee Citation2007; Dalton et al. Citation2009; Saulnier-Talbot Citation2016).
The paleolimnology of Lakes Rotokawau and Humuhumu demonstrated the importance of managing erosional inputs and water level for macrophyte communities. The former has direct impacts on macrophyte productivity whilst the latter was shown to be linked indirectly to nutrient availability and algal biomass in Northland dune lakes. Perched dune lakes in particular, appear more sensitive to land-use water consumption altering nutrient availability.
We recommend the NOF be extended to include a visual water clarity attribute for macrophyte health and that lake managers remain aware of the impacts land-use change and/or intensification can have on nutrient availability and macrophyte health, through altered hydrology.
Acknowledgements
BR undertook the research during his Masters of Science (Earth Science, University of Auckland) under the supervision of PA. TS and PA conceived the study. Brendan Hall and David Wackrow (University of Auckland) undertook the fieldwork with BR. Bruce Griffin (Northland Regional Council) and various land owners are thanked for providing access to the Poutō dune lakes. Jean-Charles Perquin, Ben Tait, Susie Osbaldiston, Lisa Forester, Andrew Macdonald, James Griffin and Duncan Kervell from Northland Regional Council are thanked for their insights to land-use history of the Poutō catchment. Paul Champion (NIWA) is thanked for sharing his excellent knowledge of the Poutō lakes. Aslan Wright-Stow (DairyNZ), Marc Weeber (Deltares) and two anonymous reviewers are gratefully thanked for improving the manuscript no end. Any mistakes that remain are the authors own.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Tom Stephens http://orcid.org/0000-0002-0003-9269
Paul Augustinus http://orcid.org/0000-0002-1391-2151
Patricia Gadd http://orcid.org/0000-0001-6725-1603
Additional information
Funding
References
- Appleby P. 2001. Chronostratigraphic techniques in recent sediments. In: Last W, Smol J, editors. Tracking environmental change using lake sediments. Volume 1: basin analysis, coring, and chronological techniques. Dordrecht: Kluwer; p. 171–203.
- Arnaud F, Revillon S, Debret M, Revel M, Chapron E, Jacob J, Giguet-Covex C, Poulenard J, Magny M. 2012. Lake Bourget regional erosion patterns reconstruction reveals Holocene NW European Alps soil evolution and paleohydrology. Quaternary Science Reviews. 51:81–92. doi: 10.1016/j.quascirev.2012.07.025
- Attahan P, Heijnis H, Dodson J, Grice K, Le Metayer P, Taffs K, Hembrow S, Woltering M, Zawadski A. 2015. Pollen, biomarker and stable isotope evidence of late Quaternary environmental change at Lake McKenzie, southeast Queensland. Journal of Paleolimnology. 53:139–156. doi: 10.1007/s10933-014-9813-3
- Augustinus P, Reid M, Andersson S, Deng U, Horrocks M. 2006. Biological and geochemical record of anthropogenic impacts in recent sediments from Lake Pupuke, Auckland City, New Zealand. Journal of Paleolimnology. 35:789–805. doi: 10.1007/s10933-005-5306-8
- Basher L, Hicks D, Clapp B, Hewitt T. 2011. Sediment yield response to large storm events and forest harvesting, Motueka River, New Zealand. New Zealand Journal of Marine and Freshwater Research. 45:333–356. doi: 10.1080/00288330.2011.570350
- Battarbee R, Jones V, Flower R, Cameron N, Bennion H, Carvalho L, Juggins S. 2001. Diatoms. In: Smol J, Birks H, Last W, editors. Tracking environmental change using lake sediments. Volume 3. Terrestrial, algal, and siliceous indicators. Dordrecht: Kluwer Academic; p. 155–202.
- Beets P, Oliver G. 2007. Water use by managed stands of Pinus radiata, indigenous podocarp/hardwood forest, and improved pasture in the central North Island of New Zealand. New Zealand Journal of Forestry Science. 37:306–323.
- Bennion H, Battarbee R. 2007. The European Union water framework directive: opportunities for paleolimnology. Journal of Paleolimnology. 38:285–295. doi: 10.1007/s10933-007-9108-z
- Birks H, Birks H. 2006. Multi-proxy studies in palaeolimnology. Vegetation History and Archaeobotany. 15:235–251. doi: 10.1007/s00334-006-0066-6
- Brown E, Johnson T, Scholz C, Cohen A, King J. 2007. Abrupt change in tropical African climate linked to the bipolar seesaw over the past 55,000 years. Geophysical Research Letters. 34:L20702. doi: 10.1029/2007GL031240
- Brush G, Hilgartner W. 2000. Paleoecology of submerged macrophytes in the upper Chesapeake Bay. Ecological Monographs. 70:645–667. doi: 10.1890/0012-9615(2000)070[0645:POSMIT]2.0.CO;2
- Byrne B. 2002. The unknown Kaipara. Five aspects of history 1250–1875. Auckland: Jason Books.
- Champion P, de Winton M. 2012. Northland Lakes strategy. NIWA client report HAM2012-121 prepared for Northland Regional Council, Hamilton.
- Clayton J, Edwards T. 2006. LakeSPI – A method for monitoring ecological condition in New Zealand lakes. NIWA Technical Report Version 2. Hamilton: National Institute of Water & Atmospheric Research Ltd.
- Croudace I, Rindby A, Rothwell R. 2006. ITRAX: description and evaluation of a new multifunction X-ray core scanner. In: Rothwell R, editor. New techniques in sediment core analysis. Vol. 267. London: Geological Society London Special Publications; p. 51–63.
- Daigneault A, Morgan F. 2017. Economic analysis of alternative soil conservation scenarios for the Northland region. Daigneault Consulting Group client report prepared for Northland Regional Council.
- Dalton C, Taylor D, Jennings E. 2009. The role of palaeolimnology in implementing the water framework directive in Ireland. Biology and Environment: Proceedings of the Royal Irish Academy. 109:161–174.
- Davies S, Lamb H, Roberts S. 2015. Micro-XRF core scanning in paleolimnology: recent developments. In: Croudace W, Rothwell R, editors. Micro-XRF studies of sediment cores developments in Paleo-environmental research 17. Dordrecht: Springer Science; p. 189–226.
- de Winton M, Clayton J, Edwards T. 2012. Incorporating invasive weeds into a plant indicate method (LakeSPI) to assess lake ecological condition. Hydrobiologia. 691:47–58. doi: 10.1007/s10750-012-1009-0
- de Winton M, Schwarz A. 2004. Littoral communities: algae and macrophytes. In: Harding J, Mosely P, Pearson C, Sorrell B, editors. Freshwater of New Zealand. Christchurch: The New Zealand Hydrological Society; p. 24.1–24.14.
- Edbrooke S. 2001. Geology of the Auckland area. In: Institute of geological and nuclear sciences 1:250,000 geological map 3. Lower Hutt: Institute of Geological and Nuclear Sciences Limited.
- Fahey B. 1994. The effect of plantation forestry on water yield in New Zealand. New Zealand Forestry. 39:18–23.
- Fahey B, Duncan M, Quinn J. 2004. Impacts of forestry. In: Harding J, Mosely P, Pearson C, Sorrell B, editors. Freshwaters of New Zealand. NZ Hydrological Society and NZ Limnological Society. Christchurch: The New Zealand Hydrological Society; p. 33.1–33.16.
- Farnsworth, M., Male, A., and Russell, I. 1976. Forestry and farming – a practical example. Proceedings of the New Zealand Grassland Association, 37: 32–37.
- Gibbs M. 2006. Sediment source mapping in Mahurangi Harbour. NIWA Client Report for ARC: HAM2006-087.
- Gibbs M, Albert A, Croker G. 2014. Nutrient limitation in Northland lakes. NIWA Client Report No. HAM2014-098 prepared for Northland Regional Council, Hamilton.
- Gibbs M, Olsen G, Swales A, Shaoneng H. 2012. Kaipara Harbour Sediment Tracing: Sediment dispersion across the harbour. NIWA Client Report for Integrated Kaipara Harbour Management Group: HAM2011-091.
- Graham I, Ditchburn R, Barry B. 2004. 210Pb-137Cs dating of glacial lake sediments. New Zealand Science Review. 61:45–47.
- Heiri O, Lotter A, Lemcke G. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology. 25:101–110. doi: 10.1023/A:1008119611481
- Higham C, Horne D, Singh R, Kuhn-Sherlock B, Scarsbrook M. 2017. Water use on nonirrigated pasture-based dairy farms: combining detailed monitoring and modeling to set benchmarks. Journal of Dairy Science. 100:828–840. doi: 10.3168/jds.2016-11822
- Hogg A, Hua Q, Blackwell P, Niu M, Buck C, Guiderson T, Heaton T, Palmer J, Reimer P, Reimer R, et al. 2013. SHCal13 Southern hemisphere calibration, 0–50,000 years cal B.P. Radiocarbon. 55:1889–1903. doi: 10.2458/azu_js_rc.55.16783
- Howard-Williams C, Schwarz A, Vincent W. 1995. Deep-water aquatic plant communities in an oligotrophic lake: physiological responses to variable light. Freshwater Biology. 44:311–326.
- Hughes B, Stephens T, Snelder T, Kelly D. 2016. Lake FMU’s for Northland. Recommendations for policy development. Lyttelton: Land and Water People Client Report for Northland Regional Council.
- Hughey K, Kerr G, Cullen R. 2010. Public perceptions of New Zealand’s environment: 2010. Christchurch: EOS Ecology.
- Juggins S. 2017. C2 Version 1.7. Software for ecological and palaeoecological data analysis and visualization. Newcastle upon Tyne: Newcastle University.
- Kelly D, Hawes I. 2005. Effects of invasive macrophytes on littoral-zone productivity and food web dynamics in a New Zealand high-country lake. Journal of the North American Benthological Society. 24:300–320. doi: 10.1899/03-097.1
- Kelly D, Peacock L, Jiang W. 2016. Nutrient management for Northland’s dune lakes. Cawthron Institute client report No. 2796 for Northland Regional Council, Nelson.
- Land Cover Database. Version 4. Available from Land Information New Zealand, visited on 40/03/2017. https://lris.scinfo.org.nz/document/416-lcdb-v40-cover-class-change-summary-2008-2012/.
- Madgwick G, Emson D, Sayer C, Willby N, Rose N, Jackson M, Kelly A. 2011. Centennial-scale changes to the aquatic vegetation structure of a shallow eutrophic lake and implications for restoration. Freshwater Biology. 56:2620–2636. doi: 10.1111/j.1365-2427.2011.02652.x
- McDowell R, Cox N, Daughney C, Wheeler D, Moreau M. 2015. A national assessment of the potential linkage between soil and surface and groundwater concentrations of phosphorus. Journal of the American Water Resources Association. 51:992–1002. doi: 10.1111/1752-1688.12337
- McGlone M, Wilmshurst J. 1999. Dating initial Maori environmental impact in New Zealand. Quaternary International. 59:5–16. doi: 10.1016/S1040-6182(98)00067-6
- McWethy D, Whitlock C, Wilmshurst J, McGlone M, Fromont M, Li X, Dieffenbacher-Krall A, Hobbs W, Fritz S, Cook E, et al. 2010. Rapid landscape transformation in South Island New Zealand following initial Polynesian settlement. Proceedings of the National Academy of Sciences of the United States of America. 107:21343–21348. doi: 10.1073/pnas.1011801107
- Meyers P, Teranes J. 2001. Sediment organic matter. In: Last W, Smol J, editors. Tracking environmental change using lake sediments. Volume 2. Physical and geochemical methods. Dordrecht: Kluwer Academic; p. 239–269.
- MfE. 2014. National policy statement for freshwater management 2014 issued by notice in gazette on 4 July 2014. Wellington: Ministry for Environment.
- Nowaczyk N. 2001. Logging of magnetic susceptibility. In: Last W, Smol J, editors. Tracking environmental change using lake sediments volume 1: basin analysis, coring and chronological techniques. Dordrecht: Kluwer Academic; p. 155–170.
- Parris A, Bierman P, Noren A, Prins M, Lini A. 2010. Holocene paleostorms identified by particle size signatures in lake sediments from the northeastern United States. Journal of Paleolimnology. 43:29–49. doi: 10.1007/s10933-009-9311-1
- Perry G, Wilmshurst J, McGlone M, McWethy D, Whitlock C. 2012. Explaining fire-driven landscape transformation during the initial burning period of New Zealand’s prehistory. Global Change Biology. 18:1609–1621. doi: 10.1111/j.1365-2486.2011.02631.x
- Quinn J, Phillips C. 2016. Production forestry. In: Jellyman P, Davie T, Pearson C, Harding J, editors. Advances in New Zealand freshwater science. NZ Freshwater Sciences Society and NZ Hydrological Society. Bookprinting Press; p. 469–482.
- Ramsey C, Lee S. 2013. Recent and planned developments of the program OxCal. Radiocarbon. 55:720–730. doi: 10.1017/S0033822200057878
- Reid M, Sayer C, Kershaw A, Heijnis H. 2007. Palaeolimnological evidence for submerged plant loss in a floodplain lake associated with accelerated catchment soil erosion (Murray River, Australia). Journal of Paleolimnology. 38:191–208. doi: 10.1007/s10933-006-9067-9
- Rouse H, Norton N. 2017. Challenge for freshwater science in policy development: reflections from the science-policy interface in New Zealand. New Zealand Journal of Marine and Freshwater Research. 51:7–20. doi: 10.1080/00288330.2016.1264431
- Salgado J, Sayer C, Carvalho L, Davidson T, Gunn I. 2010. Assessing aquatic macrophyte community change through the integration of palaeolimnological and historical data at Loch Leven, Scotland. Journal of Paleolimnology. 43:191–204. doi: 10.1007/s10933-009-9389-5
- Saros J, Northington R, Anderson D, Anderson N. 2016. A whole-lake experiment confirms a small centric diatom species as an indicator of changing lake thermal structure. Limnology and Oceanography Letters. 1:27–35. doi: 10.1002/lol2.10024
- Saulnier-Talbot E. 2016. Paleolimnology as a tool to achieve environmental sustainability in the Anthropocene: An overview. Geosciences. 6:26. DOI:10.3390/geosciences6020026.
- Saunders K, Hodgson D, Harrison J, McMinn A. 2008. Palaeoecological tools for improving the management of coastal ecosystems: A case study from Lake King (Gippsland Lakes) Australia. Journal of Paleolimnology. 40:33–47. doi: 10.1007/s10933-007-9132-z
- Savoldelli B. 2014. Hydrogeology assessment of Poutō and Kai Iwi Lakes. JACOBS Client report No. AE04657-RP-0001 for Northland Regional Council, Auckland.
- Sayer C, Davidson T, Jones J. 2010a. Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: A eutrophication-driven pathway from plants to plankton. Freshwater Biology. 55:500–513. doi: 10.1111/j.1365-2427.2009.02365.x
- Sayer C, Burgess A, Kari K, Davidson T, Peglar S, Yang H, Rose N. 2010b. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: implications for the stability of macrophyte dominance. Freshwater Biology. 55:565–583. doi: 10.1111/j.1365-2427.2009.02353.x
- Scarsbrook M, McIntosh A, Wilcock B, Matthaei C. 2016. Effects of agriculture on water quality. In: Jellyman P, Davie T, Pearson C, Harding J, editors. Advances in New Zealand freshwater science. NZ Freshwater Sciences Society and NZ Hydrological Society. Bookprinting Press; p. 483–504.
- Schallenberg M, Hamilton D, Hicks A, Robertson H, Scarsbrook M, Robertson B, Wilson K, Whaanga D, Jones H, Hamill K. 2017. Multiple lines of evidence determine robust nutrient load limits required to safeguard a threatened lake/lagoon system. New Zealand Journal of Marine and Freshwater Research. 51:78–95. doi: 10.1080/00288330.2016.1267651
- Schallenberg M, Kelly D, Clapcott J, Death R, MacNeil C, Young R, Sorrell B, Scarsbrook M. 2011. Approaches to assessing ecological integrity of New Zealand freshwaters. Science for Conservation. 307:1–85.
- Smale M, Clarkson B, Clarkson B, Floyd C, Cornes T, Clarkson F, Gilmour D, Snell T, Briggs C. 2009. Natural areas of Kaipara ecological district (Northland conservancy). Reconnaissance survey report for the protected natural areas programme. Wellington: Department of Conservation.
- Smith P. 1987. Variation of water yield from catchments under grass and exotic forest, east Otago. Journal of Hydrology. 26:175–184.
- Sorrell B, Unwin M, Dey K, Hurren H. 2006. Snapshot – lake water quality. NIWA Client Report No. CHC2006-145 for Ministry for the Environment, Wellington.
- Stephens T, Atkin D, Augustinus P, Shane P, Lorrey A, Street-Perrott A, Nilsson A, Snowball I. 2012a. A late glacial Antarctic climate teleconnection and variable Holocene seasonality at Lake Pupuke, Auckland New Zealand. Journal of Paleolimnology. 48:785–800. doi: 10.1007/s10933-012-9644-z
- Stephens T, Atkin D, Cochran U, Augustinus P, Reid M, Lorrey A, Shane P, Street-Perrott A. 2012b. A diatom-inferred record of reduced effective precipitation during the last glacial coldest phase (28.8-18.0 cal. kyr BP) and increasing Holocene seasonality at Lake Pupuke, Auckland, New Zealand. Journal of Paleolimnology. 48:801–817. doi: 10.1007/s10933-012-9645-y
- Swales A, Gibbs M, Hewitt J, Hailes S, Griffiths R, Olsen G, Ovenden R, Wadhwa S. 2012. Sediment sources and accumulation rates in the Bay of Islands and implications for macro-benthic fauna, mangrove and saltmarsh habitats. NIWA Client Report for NRC: Ham2012-048.
- Swales A, Gibbs M, Olsen G, Ovenden R. 2015a. Historical changes in sources of catchment sediment accumulating in Whangerei Harbour. NIWA Client Report for NRC: HAM2015-037.
- Swales A, Gibbs M, Olsen G, Ovenden R, Griffiths R. 2015b. Historical changes in sources of catchment sediment accumulating in Kaipara Harbour. NIWA Client Report for NRC: HAM2015-038.
- Talbot M. 2001. Nitrogen isotopes in palaeolimnology. In: Last W, Smol J, editors. Tracking environmental change using lake sediments. Volume 2. Physical and geochemical methods. Dordrecht: Kluwer Academic; p. 401–439.
- Thode P. 1983. Northland’s forest history and present resources. New Zealand Journal of Forestry. 28:203–224.
- Verburg P. 2012. Classification and objective bands for monitored lakes. NIWA Client Report No. Wellington: HAM2012 for Ministry for the Environment.
- Verburg P, Hamill K, Unwin M, Abell J. 2010. Lake water quality in New Zealand 2010: Status and trends. NIWA Client Report No. HAM2010-107 for Ministry for the Environmen, Wellington.
- Walker S, Price R, Rutledge D, Stephens R, Lee W. 2006. Recent loss of indigenous cover in New Zealand. New Zealand Journal of Ecology. 30:169–177.
- Wells R, Champion P. 2013. Northland Lakes Status Report 2013. NIWA Client Report No. HAM2013-077 for Northland Regional Council, Hamilton.
- Wells R, Champion P. 2015. Northland Lakes Status Report 2014. NIWA Client Report No. HAM2017-015 for Northland Regional Council, Hamilton.