ABSTRACT
Spinal abnormalities are common in farmed Chinook Salmon in New Zealand. We report spinal abnormalities in adult Chinook salmon which were predominantly hatchery reared and released as smolts and which we term free-living. We compare these to rates seen in farmed New Zealand salmon. 101 free-living adult salmon were radiographically assessed for spinal curvatures (lordosis, kyphosis, scoliosis; LKS) and other vertebral abnormalities. Severity of abnormality was assessed on a three-point scale. Abnormal vertebral bodies were detected in 88.1% of free-living salmon. Spinal curvatures were the most common abnormality type with 83.2% of fish showing this abnormality but only one free-living Chinook had LKS of severity greater than 1. Farmed Chinook salmon are reported to have LKS rates of 29% with 18% of LKS abnormalities of severity greater than 1. These results suggest that free-living Chinook salmon frequently develop spinal abnormalities, but these abnormalities are less severe than those observed in farmed salmon.
Introduction
Farmed Chinook salmon in New Zealand exhibit a range of vertebral abnormalities including spinal curvatures (lordosis, kyphosis, scoliosis; LKS) and fusion abnormalities (Perrott et al. Citation2018). The prevalence of vertebral abnormalities in farmed Chinook salmon in New Zealand varies widely between 4% and 70% as it does in farmed Atlantic salmon (Fjelldal, Hansen, Breck, et al. Citation2012) and can be a significant problem for fish production. LKS can comprise a significant proportion of all abnormality types at harvest (Perrott et al. Citation2018) and are much more common in farmed Chinook than reported in harvest-sized farmed Atlantic salmon (Witten et al. Citation2009; Fjelldal et al. Citation2016). Chinook salmon with LKS have unilateral perivertebral fibrosis (Munday et al. Citation2016). This perivertebral fibrosis is present in some farmed Chinook which have no radiographic evidence of LKS and may arise in response to inflammation. The cause(s) of inflammation leading to fibrosis and then to radiologic spinal curvature (LKS) in farmed Chinook is not known.
Abnormalities of vertebrae in wild salmon have received much less attention than those of farmed salmon. Wild Pacific salmon (Oncorhynchus spp.) have been described in the early literature on salmonid morphology. Seymour (Citation1959) reported that vertebral body fusions (ankyloses) were the most common deformity in hatchery-reared juvenile Chinook salmon (Oncorhynchus tshawytscha) spawned from wild parents taken from Washington and California rivers. Less commonly, hyperdense vertebrae and centra with spiral suturing were observed. This latter deformity was also described by Gill and Fisk (Citation1966) who studied abnormalities in wild adult Pacific salmon in North America and identified a range of abnormalities. In addition to fusions, Gill and Fisk (Citation1966) described reduced intervertebral spaces and compressed vertebrae along with ‘distorted’ vertebrae which appear to be vertically shifted vertebrae. These descriptions of abnormalities fit within the classification system of vertebral abnormalities in Atlantic salmon later proposed by Witten et al. (Citation2009). Gill and Fisk (Citation1966) reported that 2.8% of adult Sockeye, Oncorhynchus nerka (Walbaum); 3.3% of Pink, Oncorhynchus gorbushca (Walbaum) and 2.9% of Chum, Oncorhynchus keta (Walbaum) had at least one deformed vertebra with samples from some catchments exhibiting the maximum deformity prevalence of 8%–10% depending on species. Interestingly, neither Seymour (Citation1959) nor Gill and Fisk (Citation1966) reported spinal curvatures (LKS) in any of the Pacific salmon species that they studied. Seymour (Citation1959) observed that the rates of fusions were strongly influenced by the hatchery-rearing temperature of the fish with the lowest prevalences at rearing temperatures between 10°C and 12°C.
Abnormalities of vertebrae in wild Atlantic salmon Salmo salar (L.) have received less attention than in farmed Atlantic salmon (Fjelldal, Hansen, Breck, et al. Citation2012) with only three published studies assessing spinal abnormalities of wild Atlantic salmon. Fjelldal et al. (Citation2006) found no abnormalities in 12 wild smolt or post-smolt of Atlantic salmon collected from seawater. In contrast, Fraser et al. (Citation2014) examined 18 adult Atlantic salmon from the Arne River, Norway and reported 35% had at least one deformed vertebra. Abnormal vertebrae showed pronounced biconcave shape (75%), compression (11%) and reduced intervertebral space (11%). Sambraus et al. (Citation2014) reported abnormalities in 43% (28/65) of spawning wild Atlantic salmon (1.1–5.8 kg) collected between 2010 and 2012 from the Figgjo River, Norway. Vertebral fusions, the major deformity phenotype in Figgjo Atlantic salmon, varied in prevalence between 5% and 29% in different years of the study. One-sided compressions (Type 5 ‘K’ shaped; Witten et al. Citation2009) affected the second vertebra in 20% of fish. 18.5% of wild Atlantic salmon had fusion-related abnormalities (Sambraus et al. Citation2014). As with the Pacific salmon studies noted above, neither Fjelldal et al. (Citation2006), Fraser et al. (Citation2014) nor Sambraus et al. (Citation2014) reported spinal curvatures (LKS) in wild Atlantic salmon.
A variety of diverse factors is known to cause vertebral abnormalities in both wild and cultured fish species which have been recently reviewed (Fjelldal, Hansen, Breck, et al. Citation2012; Boglione, Gavaia, et al. Citation2013; Boglione, Gisbert, et al. Citation2013). For salmonids, important risk factors include temperature, timing of smoltification, vaccination and dietary phosphorus. While low phosphorus diets predispose Atlantic salmon to deformity (Fjelldal, Hansen, and Albrektsen Citation2012), no studies have shown differences in vertebral minerals between deformed and normal Atlantic salmon (Witten et al. Citation2005) or rainbow trout (Deschamps et al. Citation2008) when fed adequate dietary phosphorus. Maturation of salmonids is associated with demineralisation from bones including vertebrae and scales (Kacem et al. Citation2000; Kacem and Meunier Citation2003). While the earlier studies described some changes in the radiographic appearance of transverse (frontal) sections of caudal vertebrae with age in both Salmo and Oncorhynchus (Desse Citation1976), Kacem et al. (Citation1998) noted the absence of any differences in external vertebral morphology with maturation but observed increased vertebral trabeculation, with loss of intertrabecular bone (Kacem et al. Citation1998).
Toxins such as antifouling agents and insecticides acting on neural and neuromuscular junctions can generate spinal curvature deformities (Silverstone and Hammell Citation2002; Mochida et al. Citation2009) rather than fusion-related abnormalities. Infectious agents (Sanders et al. Citation2012) may also be risk factors in spinal curvatures. It is apparent that vertebral abnormalities in both wild and farmed fish are likely to have a complex aetiology with multiple causative factors including the effects of husbandry on farms and genetic selection of fish for aquaculture.
Both free-living and farmed Chinook salmon are derived from stocks successfully introduced into the Waitaki River, New Zealand from the Sacramento River in California between 1901 and 1907 (Haworth Citation2010). No further imports have been made to New Zealand since 1907. Kinnison et al. (Citation2002) have shown that New Zealand free-living salmon have lower levels of heterozygosity and allelic richness compared with North American stocks, consistent with an overall founder effect upon introduction of the species to New Zealand. Farmed Chinook salmon in New Zealand were originally from these introduced stocks but since the 1980s have been derived from captive brood stock. Farmed fish are either raised to harvest at around 3.5 kg in freshwater farms or more commonly transferred from freshwater hatcheries as smolts to sea cages and raised to harvest at around 4 kg.
In this study, we sought to determine the prevalence of vertebral abnormalities in free-living adult Chinook salmon from two catchments and to compare them with data reported from farmed Chinook salmon. When free-living Chinook salmon return to breed they are of comparable sizes to immature harvested New Zealand Chinook salmon from farms. This is the first time free-living New Zealand Chinook salmon have been evaluated for the presence of vertebral abnormalities or for mineral content of bones as a possible factor in vertebral abnormality development. Comparing the type and prevalence of abnormalities in free-living and farmed salmon with similar early life history in hatcheries could provide valuable information regarding possible causes of spinal abnormalities in farmed salmon.
Materials and methods
Samples of free-living and farmed adult Chinook salmon
Samples of adult female Chinook were obtained from fish traps maintained by Fish and Game New Zealand on the Waimakariri (43.43°S 172.15°E) and Rangitata (43.81°S 171.26°E) Rivers on the East coast of the South Island, New Zealand as these fish returned to their natal streams to spawn. These fish were primarily captured for gonad harvest.
Seventy-seven frozen eviscerated carcasses of ripe and stripped adult females which returned to the Waimakariri River to spawn in May 2014 were obtained. These fish averaged 2.6 kg (eviscerated ± 0.50 SE) and had an average of 663 mm fork length. Waimakariri fish were spawned from wild-caught parents derived from the Rangitata or Waimakariri Rivers, raised to smolts of around 50 g at Montrose Hatchery on the upper Rakaia River before being released into the Waimakriri River.
Twenty-four whole fresh adult female Chinook salmon, average weight of 3.45 kg (±0.15 SE), were obtained in May 2013 as they returned to spawn in the Rangitata River. The origin(s) of fish sampled from the Rangitata river is less clear. Fish and Game estimate that between 50% and 60% of adult fish in Rangitata River spawning runs are derived from wild Rangitata parents whose progeny were hatched by the McKinnon’s Creek Salmon Hatchery on the lower Rangitata River and released as smolts. These fish are similar to those in the Waimakariri sample. The remainder of fish in the Rangitata River sample, however, were from wild parents spawned in the river and we cannot tell which is which.
Examination of scales from 10 of the fish from the Waimakariri River revealed either one or two winter seawater growth checks in roughly equal proportions making them either two- or three-year returning fish (Shearer Citation1984).
Five normal adult female salmon farmed in freshwater at Waiau, Canterbury (42.65°S 173.04°E) and five with mild spinal curvature were provided by New Zealand King Salmon (Nelson, New Zealand) for vertebral mineral analyses.
Radiographic assessment
Waimakariri River salmon were thawed and then radiographed using a Phillips Bucky Diagnostics Ceiling System and Canon CXDI-50G sensor at 80 cm, 40 kV, 16 mA, 2.2 ms.
Rangitata River salmon were radiographed whole and without prior freezing using methods described in Perrott et al. (Citation2018). In brief, we used an Atomscope HF80/15 (Mikasa, Tokyo) and a Sound Technologies Tru-DR panel (DLC Australia Pty Ltd, Melbourne). All radiographs were assessed for the presence and severity of four deformity types: LKS are dorsal, ventral and lateral bends, respectively, of the normal straight vertebral column (Witten et al. Citation2009), fusions were identified by the absence of a radiolucent intervertebral space between adjacent vertebrae, compressions of vertebral bodies are characterised by shorter than normal longitudinal dimensions while reduction of the intervertebral space was identified as a smaller than normal width of the radiolucent space between adjacent bodies, and vertical shifts are misalignment of adjacent vertebrae in the dorso-ventral direction compared with their neighbours. LKS, fusions, compressions and vertical shifts (see Witten et al. Citation2009) in each of four regions along the body (Region 1 V1–V8, Region 2 V9–V31, Region 3 V32–V50, Region 4 V51–V63+) were assigned a severity on a three-point scale based on either the angle of the curvature or the number of vertebrae involved. Abnormalities were classified as mild (severity 1: 0°–20°, 1 or 2 vertebrae), moderate (severity 2: 20°–40°, 2–5 vertebrae) or severe (severity 3: >40°, >5 vertebrae). Two researchers independently scored all images and discrepancies were resolved by reassessing those fish by the two observers together.
Mineral analysis
Vertebrae 30 and 31 from eight Waimakariri fish (average 2.7 kg, eviscerated) were dissected, cleaned and defatted by the methods described by Deschamps et al. (Citation2008). Vertebrae 30 and 31 from 10 Chinook salmon which had been farmed in freshwater to similar sizes (average 3.5 kg, whole fish) but which were not reproductively active were prepared in an identical fashion. Half of these farmed fish (five) showed mild LKS. Vertebrae 30 and 31 were chosen based on their high mineral content and high loss of minerals with maturation compared with vertebrae from other regions (Kacem et al. Citation2000). Mineral content (ash) was determined by first slow air drying for 16 h at 105°C, then samples were muffle furnaced overnight. The ashed samples were digested in 6 M hydrochloric acid for 20 min at 200°C (Official Methods of Analysis of AOAC INTERNATIONAL (2016) 20th Ed., AOAC INTERNATIONAL, Gaithersburg, MD, USA, Official Method 986.08D). For phosphorus, the above digest was analysed colorimetrically with an arsenomolybdate method. For Calcium, the digest was analysed colorimetrically using o-cresol phenolphthalein complexone (Roche kit, Cobas FARA II, Roche, Basel, Switzerland).
Statistical analyses
To compare the overall severity of vertebral abnormality, scores for each abnormality in each region were summed to give a vertebral abnormality score for each fish. The possible range of scores was 0–48. The significance of differences between means, assuming unequal variances, was assessed using t-Tests in Microsoft Excel 2010 analysis tools.
Results
At least one abnormal vertebra of any kind (LKS, fusion, compression, vertical shift) was seen in 89 of 101 (88.1%) adult free-living Chinook salmon included in the study (). However, most abnormalities were of low severity with eight fish having at least one moderate abnormality and three fish identified with severe abnormalities.
Table 1. Prevalence of abnormality phenotypes by region in 101 free-living New Zealand Chinook salmon.
Vertebral abnormality score, calculated as the sum of scores for each abnormality in each region, of fish from the Waimakariri River was 1.97 (±0.17 SE) which was significantly less than the average vertebral abnormality score calculated for Rangitata fish (3.41 ± 0.42 SE, P = .003, d.f. = 31). The range of scores recorded was 0–10 with a median vertebral abnormality score of 2 for Waimakariri salmon and 3 for Rangitata salmon. The average number of vertebral abnormalities in each fish was 2.0 ± 0.2(SE) for fish from the Waimakariri river which was significantly fewer than the average number of abnormalities observed in fish from the Rangitata river (2.9 ± 0.3 SE, P = .004, d.f. = 42).
The most common abnormality observed in the fish was LKS ( and B,D,D’). However, the majority of LKS lesions were of low severity score and only one fish had a moderate LKS in the 101 wild-caught fish and no fish had LKS that was graded as severe.
Figure 1. Examples of deformities found in free-living female Chinook salmon captured during spawning migrations up the Rangitata (A,B) and Waimakriri Rivers (C,D,E), in Canterbury, New Zealand. Types refer to the classification system of Witten et al. (Citation2009). A, A salmon with multiple deformities. Compressions of four vertebrae, numbers 26–29, and reduction of intervertebral spaces. Some loss of internal radiologic structure (left inset, Types 3,4). Vertical shifts (Type 17) of vertebrae numbers 39–43 (right inset). B, Kyphosis (Type 15) in the ural region without radiological changes in vertebral morphology. More caudal vertebrae normally show reduced length towards the tail fin. C and C’, Radiograph (C) of part of postcranial and abdominal regions showing dorsal vertical shifts (Type 17) of vertebrae 7–10 and in bone preparations (C’), showing uneven spacing between vertebrae. D and D’, Radiograph (D) and bone preparations (D’) showing lordosis (Type 14) at the level of vertebrae 10–12 and dorsal compression of vertebra 11. Vertebra 11 was 15.1% shorter dorsally than ventrally. E and E’, Radiograph (E) and bone preparations (E’) of multiple vertical shifts (Type 17) of vertebrae 40–46. Alternating compression dorsally and ventrally (see vertebrae 40,42,44) is more easily identified in articulated bones than in radiographs. Dorsal lengths of vertebrae 40,42,44 were significantly longer by 4.8% than ventral lengths (paired t-test, P = .017, d.f. = 2).
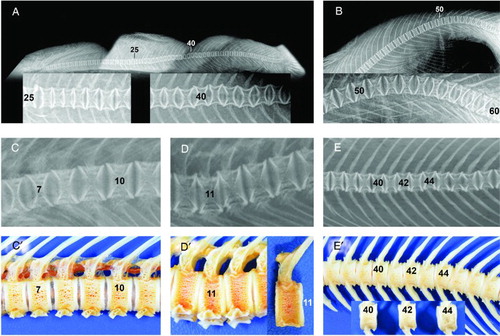
The non-LKS abnormalities observed included 10 fish which had pairs of fused vertebrae and in five of these individuals, the affected vertebrae showed signs of centrum remodelling. One fish had four fused vertebrae in region 3. Nineteen fish had compressions involving 2–5 vertebrae (see and A). Vertical shifts were common (see A, C,C’,E,E’) and all three fish with severe abnormalities were from the Rangitata river each had one severe vertical shift in region 3 (V32–V50).
Over 71% of all abnormalities were found in regions 2 and 3 (). However, this distribution was weighted heavily by a large number of LKS abnormalities and the greater numbers of vertebrae in regions 2 and 3 (). Non-LKS abnormalities were less common in region 4 of free-living Chinook salmon.
Mineral content
Wild sexually mature females had significantly lower minerality (ash as % dry mass) in vertebrae 30 and 31 than farmed freshwater reared salmon (P < .000, d.f. = 13; ). There were no differences in minerality between deformed and normal freshwater farmed salmon (data not shown).
Table 2. Mineral content of vertebrae 30 and 31 from 8 free-living reproductively active and 10 immature freshwater farmed female Chinook salmon.
Discussion
The prevalence of spinal curvature (LKS) in free-living Chinook was 83.2%. This is higher than the 29.4% that has been reported for harvested farmed Chinook (Perrott et al. Citation2018). While it is uncertain why free-living salmon appear to develop LKS more frequently than farmed salmon, the high prevalence of LKS in the free-living fish suggests that the factors that cause LKS in farmed Chinook could also influence LKS development in free-living salmon. While additional research is required, this is consistent with the hypothesis that the high rates of LKS seen in Chinook salmon are not due to factors that have been introduced by farming systems but that this species of fish in New Zealand frequently gets this abnormality.
It is possible that hatchery-rearing may predispose fish to LKS later in life. Between 40% and 50% of fish in the Rangitata samples were from wild parents and were spawned in the river and therefore had no hatchery experience. Only two Rangitata fish had either no or a low LKS score (≤1). If hatchery experience foreshadowed LKS then we might have expected more Rangitata fish to have no LKS or a low score. Hatchery-reared Atlantic salmon typically have abnormality rates between 4% and 9% (Sullivan, Hammond, et al. Citation2007) which comprise predominantly of compressions but some spinal curvatures were seen as they were noted in the discussion as being culled. Post-hatch Chinook show spinal curvatures of a variety of types (De Clercq et al. Citation2018), but these deformity phenotypes are not seen in smolts and these deformed hatchlings presumably die. Perrott et al. (Citation2018) reported 2% LKS in salmon after 129 days at sea (432 g). While we have no data on the abnormalities of these free-living Chinook at release as smolts, spinal curvatures are rare in juvenile Chinook salmon. As all the salmon in New Zealand are originally from the same founder population, this could be a genetic effect. However, studies by commercial companies (unpublished data) suggest that genetics plays only a small role in the development of LKS in farmed Chinook salmon in New Zealand as has been reported for Atlantic salmon (Sullivan, Guy, et al. Citation2007). It should be noted that no male fish were examined in this study. However, as male salmon are not farmed in New Zealand, potential differences in LKS between the sexes are not the cause of the higher rates of LKS seen in free-living compared to farmed Chinook salmon.
While the prevalence of LKS was high in free-living fish, the severity of LKS observed was much lower than in farmed fish. The prevalence of moderate and severe LKS (severity 2 and 3) was 1% in free-living spawning Chinook compared with 18% in farmed Chinook (Perrott et al. Citation2018). While farmed Chinook were of similar size to the free-living fish, farmed fish were immature. The cause of the lower severity in free-living salmon is unknown. However, it could be because free-living fish are exposed to the factors that cause LKS for less time than farmed salmon. Alternatively, it is possible that free-living fish with severe LKS were not identified simply because they did not survive or cannot migrate up the rivers. Presumably due to reduced food competition and predation, fish on farms, unlike their free-living counterparts, can survive with moderate and even severe LKS. This is supported by studies on Atlantic salmon that suggest that mild spinal abnormalities that affect fewer than six vertebrae are unlikely to significantly affect the growth of this fish (Hansen et al. Citation2010; Sambraus et al. Citation2014). It is interesting to note that Sambraus et al. (Citation2014) similarly reported that vertebral abnormalities in wild Atlantic salmon were less severe than those seen in farmed Atlantic salmon.
A previous histological study of farmed Chinook salmon revealed that LKS was associated with unilateral perivertebral fibrosis (Munday et al. Citation2016). Histology was not possible in the current study and it is unknown whether or not free-living Chinook salmon have similar histological lesions associated with LKS.
As only mild LKS was observed in the present study, it was considered possible that the observed curvatures could be artefacts caused by radiographing salmon that had been dead for some days or stripped, frozen and then defrosted. However, as Rangitata salmon, which were radiographed whole and fresh, had significantly higher LKS scores than the Waimakariri fish that were stripped prior to freezing and thawing, this suggests that handling was unlikely to cause the observed LKS in the present study.
The prevalence of moderate or severe (severity 2 or 3) abnormalities of any phenotype (LKS, fusion, compression and vertical shift) was significantly less in the Waimakariri river sample (2 fish, 2.6%) than in the Rangitata river sample (9 fish, 37.8%). Why there are differences among catchments and samples is not known, but it is noted that samples were taken in different years for each catchment. Deformity prevalence varies between years of sampling of wild Atlantic salmon (Sambraus et al. Citation2014) and between geographical sampling locations of free-living Pacific salmon from North America (Seymour Citation1959; Gill and Fisk Citation1966).
The frequency of abnormalities varied along the vertebral column, to some extent reflecting the numbers of vertebrae in each region. The posterior truncal and anterior caudal regions, R2 and R3, contained 40.5% and 31.2% of all abnormalities, respectively, in free-living Chinook salmon (). All moderate and severe vertical shift abnormalities were found in region 3, a common location for abnormalities in harvest-sized farmed salmon (Fjelldal et al. Citation2009; Munday et al. Citation2016). Sambraus et al. (Citation2014) found that most abnormalities in wild Atlantic salmon were in the postcranial (R1) or posterior truncal (R2) regions (33% and 36%, respectively) and this contrasts with farmed Atlantic salmon at harvest which show a higher proportion of deformed vertebrae in the anterior caudal and ural regions (Fjelldal et al. Citation2009).
In addition to the prevalence of LKS observed in the free-living Chinook, many fish also had other abnormality phenotypes (see ). Fused vertebrae were the most commonly described abnormality in early studies (types 7 and 8 of Witten et al. Citation2009), at least in part because they are easy to diagnose from radiographs (Gill and Fisk Citation1966). We found one Rangitata salmon with a severe fusion in region 3. We found that 10 Waimakariri salmon (13%) had fusions across regions R1, R2, R3 compared to the prevalence of 18.5% reported for wild Atlantic salmon (Sambraus et al. Citation2014).
Completely fused vertebrae showed remodelling of the centrum in Atlantic salmon and are regarded as stable (Witten et al. Citation2005, Citation2009) with little capability to further progress by fusing with adjacent vertebrae so enlarging to form a fusion centre (Fusion type 8, Witten et al. Citation2009). In this study, evidence for remodelling of fused centra was seen in 5/10 two-verterba fusions found in free-living Chinook salmon. All complete fusions in wild Atlantic salmon were regarded as stable with evidence of remodelling (Sambraus et al. Citation2014).
Dietary phosphorous is essential for optimal bone health and since deposition and resorption are in dynamic equilibrium, factors which affect the availability of minerals such as Ca and P will impact on bone health. Salmon migrating to spawn in freshwater do not feed and derive minerals from scales and bones including vertebrae (Kacem et al. Citation2000, Citation2013; Kacem and Meunier Citation2003; Deschamps et al. Citation2008). The differences seen here between spawning free-living and immature farmed Chinook salmon from freshwater are similar to those seen in migrating and immature female Atlantic salmon (Kacem et al. Citation2000; Kacem and Meunier Citation2003). Current evidence suggests that the mineral content of vertebrae does not influence the morphology of the vertebrae as demineralisation occurs by an halastic process without the loss of the organic bone matrix (Kacem and Meunier Citation2003). We detected no association between mineral content of the vertebra and spinal deformity in Chinook salmon farmed in fresh water. It remains to be determined how lower mineral content may have played a role in the high prevalence of spinal curvature in free-living and spawning Chinook salmon.
Spinal curvature is common in adult and spawning Chinook salmon that have lived most of their lives in the wild (this study) and in adult farmed Chinook (Perrott et al. Citation2018). This contrasts with the low prevalence of LKS in farmed or wild Atlantic salmon (Fjelldal, Hansen, Albrektsen Citation2012; Fraser et al. Citation2014; Sambraus et al. Citation2014). A major difference in life histories between Chinook and Atlantic salmon is that Chinook are semelparous and normally die after spawning while Atlantic salmon can spawn many times (iteroparous). Preparation for migration is preceded by rapid growth. Wild Pacific salmon gain about half of their mature body mass in the last 6 months at sea (Hinch et al. Citation2006) and is described as a ‘pubertal growth spurt’ (Taranger et al. Citation2010). New Zealand Farmed Chinook can triple in weight in the last six months of the production cycle. High-fat reserves in wild fish are a further signal to continue preparation for migration and subsequent gonad growth (Rowe et al. Citation1991). Gonad growth occurs largely after migration up-stream has started and is inhibited in farmed Chinook by continuous lighting (Unwin et al. Citation2005). Thus, adult wild or farmed salmon in early stages of preparation for migration and maturation may look immature (silver) but physiologically (Hinch et al. Citation2006) and hormonally (Unwin et al. Citation2004; Taranger et al. Citation2010) preparation for migration and maturation may have started.
Restricted food intake (cessation of feeding) is a key trigger for changes associated with preparing for migration and subsequent spawning in Pacific salmonids. During migration, salmon do not feed and use muscle protein for energy (Bowerman et al. Citation2017). Chinook with LKS and other abnormalities have lower body weights than normal salmon (Perrott et al. Citation2018) as do Atlantic salmon with spinal abnormalities (not LKS, Hansen et al. Citation2010) and we interpret this as either lower than optimal nutrition or increased metabolism in affected fish. During feeding cessation as occurs during migration free-living or wild salmon, breakdown of muscle proteins that join sarcomeres together, the Z discs, has been observed in white muscle of migrating Pacific salmon and is most likely as a result of calcium-activated proteases (Mommsen Citation2004). We suggest that salmon with diminished nutrition and/or elevated metabolic demands may breakdown muscle for energy and subsequent fibrosis of necrotic muscle fibres (Munday et al. Citation2016) may contribute to the spinal curvature abnormality in adult and especially in maturing Chinook salmon.
Conclusions
Free-living Chinook salmon showed a high prevalence of LKS. While the cause of LKS is currently unknown, these results confirm that this abnormality is not restricted to farmed Chinook salmon. Therefore, it appears that both free-living and farmed salmon are exposed to the factors that cause this abnormality. Bone mineral content was significantly less in spawning free-living Chinook compared with immature Chinook salmon farmed in freshwater to similar sizes. In contrast to farmed salmon, severe LKS was not observed in free-living salmon. While the cause of these differences is unknown, it is plausible that severe LKS reduces survival of fish and prevents them from migrating up rivers to spawn. Free-living salmon also developed other abnormalities such as fusions and compressions and these abnormalities occurred at rates within the ranges reported for wild and farmed Atlantic salmon and for farmed Chinook salmon.
Acknowledgements
We are grateful to the National Institute of Water and Atmospheric Research (NIWA), Fish and Game New Zealand, Salmon Smolts New Zealand Co and New Zealand King Salmon, Nelson New Zealand for assistance in obtaining samples of free-living and farmed salmon. NIWA is also thanked for providing the digital X-ray unit and technical assistance enabling taking radiographs of Rangitata River fish samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Peter S. Davie http://orcid.org/0000-0002-5394-7519
Additional information
Funding
References
- Boglione C, Gavaia P, Koumoundouros G, Gisbert E, Moren M, Fontagné S, Witten PE. 2013. Skeletal anomalies in reared European fish larvae and juveniles. Part 1: normal and anomalous skeletogenic processes. Reviews in Aquaculture. 5(SUPPL.1):S99–S120. doi: 10.1111/raq.12015
- Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagné S, Koumoundouros G. 2013. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Reviews in Aquaculture. 5(SUPPL.1):S121–S167. doi: 10.1111/raq.12016
- Bowerman TE, Pinson-Dumm A, Peery CA, Caudill CC. 2017. Reproductive energy expenditure and changes in body morphology for a population of Chinook salmon Oncorhynchus tshawytscha with a long distance migration. J Fish Biol. 90(5):1960–1979. doi: 10.1111/jfb.13274
- De Clercq A, Perrott MR, Davie PS, Preece MA, Huysseune A, Witten PE. 2018. The external phenotype-skeleton link in post-hatch farmed Chinook salmon (Oncorhynchus tshawytscha). J Fish Dis. 41(3):511–527. doi: 10.1111/jfd.12753
- Deschamps MH, Kacem A, Ventura R, Courty G, Haffray P, Meunier FJ, Sire JY. 2008. Assessment of “discreet” vertebral abnormalities, bone mineralization and bone compactness in farmed rainbow trout. Aquaculture. 279(1–4):11–17. doi: 10.1016/j.aquaculture.2008.03.036
- Desse G. 1976. Les vertèbres des Salmonidés Actes 2e Congrès Européen des Ichtyologistes Paris 8 au 15 septembre 1976 Revue des Travaux de l’Institut des Pêches Maritimes. 40:557.
- Fjelldal PG, Hansen T, Albrektsen S. 2012. Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long-term bone health. Aquaculture. 334–337:117–123. doi: 10.1016/j.aquaculture.2011.12.043
- Fjelldal PG, Hansen T, Breck O, Ørnsrud R, Lock EJ, Waagbø R, Wargelius A, Witten EP. 2012. Vertebral deformities in farmed Atlantic salmon (Salmo salar L.) – etiology and pathology. J Appl Ichthyol. 28(3):433–440. doi: 10.1111/j.1439-0426.2012.01980.x
- Fjelldal PG, Hansen T, Breck O, Sandvik R, Waagbø R, Berg A, Ørnsrud R. 2009. Supplementation of dietary minerals during the early seawater phase increase vertebral strength and reduce the prevalence of vertebral deformities in fast-growing under-yearling Atlantic salmon (Salmo salar L.) smolt. Aquacult Nutr. 15(4):366–378. doi: 10.1111/j.1365-2095.2008.00601.x
- Fjelldal PG, Hansen TJ, Lock EJ, Wargelius A, Fraser TWK, Sambraus F, El-Mowafi A, Albrektsen S, Waagbø R, Ørnsrud R. 2016. Increased dietary phosphorous prevents vertebral deformities in triploid Atlantic salmon (Salmo salar L.). Aquacult Nutr. 22(1):72–90. doi: 10.1111/anu.12238
- Fjelldal PG, Lock EJ, Grotmol S, Totland GK, Nordgarden U, Flik G, Hansen T. 2006. Impact of smolt production strategy on vertebral growth and mineralisation during smoltification and the early seawater phase in Atlantic salmon (Salmo salar, L.). Aquaculture. 261(2):715–728. doi: 10.1016/j.aquaculture.2006.08.008
- Fraser TWK, Hansen T, Mayer I, Skjæraasen JE, Glover KA, Sambraus F, Fjelldal PG. 2014. The effect of triploidy on vaccine side-effects in Atlantic salmon. Aquaculture. 433:481–490. doi: 10.1016/j.aquaculture.2014.07.009
- Gill CD, Fisk DM. 1966. Vertebral abnormalities in sockeye, pink, and chum salmon. Trans Am Fish Soc. 95(2):177–182. doi: 10.1577/1548-8659(1966)95[177:VAISPA]2.0.CO;2
- Hansen T, Fjelldal PG, Yurtseva A, Berg A. 2010. A possible relation between growth and number of deformed vertebrae in Atlantic salmon (Salmo salar L.). J Appl Ichthyol. 26(2):355–359.
- Haworth J. 2010. Swimming upstream. How salmon farming developed in New Zealand. Christchurch: Wily Publications Ltd.
- Hinch SG, Cooke SJ, Healey MC, Farrell AP. 2006. Chapter 7, behavioural physiology of fish migrations: salmon as a model approach. In: S. Skawrwb, editor. Behaviour and physiology of fish. New York: Academic Press; p. 239–295.
- Kacem A, Baglinière JL, Meunier FJ. 2013. Resorption of scales in Atlantic salmon (Salmo salar) during its anadromous migration: a quantitative study. Cybium. 37(3):199–206.
- Kacem A, Gustafsson S, Meunier FJ. 2000. Demineralization of the vertebral skeleton in Atlantic salmon Salmo salar L. during spawning migration. Comp Biochem Physiol A Mol Integr Physiol. 125(4):479–484. doi: 10.1016/S1095-6433(00)00174-4
- Kacem A, Meunier FJ. 2003. Halastatic demineralization in the vertebrae of Atlantic salmon, during their spawning migration. J Fish Biol. 63(5):1122–1130. doi: 10.1046/j.1095-8649.2003.00229.x
- Kacem A, Meunier FJ, Baglinière JL. 1998. A quantitative study of morphological and histological changes in the skeleton of Salmo salar during its anadromous migration. J Fish Biol. 53(5):1096–1109.
- Kinnison MT, Bentzen P, Unwin MJ, Quinn TP. 2002. Reconstructing recent divergence: evaluating nonequilibrium population structure in New Zealand Chinook salmon. Mol Ecol. 11(4):739–754. doi: 10.1046/j.1365-294X.2002.01477.x
- Mochida K, Ito K, Harino H, Tanaka H, Onduka T, Kakuno A, Fujii K. 2009. Inhibition of acetylcholinesterase by metabolites of copper pyrithione (CuPT) and its possible involvement in vertebral deformity of a CuPT-exposed marine teleostean fish. Comp Biochem Physiol C Toxicol Pharm. 149(4):624–630. doi: 10.1016/j.cbpc.2009.01.003
- Mommsen TP. 2004. Salmon spawning migration and muscle protein metabolism: the August Krogh principle at work. Comp Biochem Physiol Part B Biochem Mol Biol. 139(3):383–400. doi: 10.1016/j.cbpc.2004.09.018
- Munday JS, Perrott MR, Symonds JE, Walker SP, Lovett B, Preece MA, Davie PS. 2016. Unilateral perivertebral fibrosis associated with lordosis, kyphosis and scoliosis (LKS) in farmed Chinook salmon in New Zealand. Dis Aquat Org. 121(3):211–221. doi: 10.3354/dao03056
- Perrott MR, Symonds JE, Walker SP, Hely FS, Wybourne B, Preece MA, Davie PS. 2018. Spinal curvatures and onset of vertebral deformities in farmed Chinook salmon, Oncorhynchus tshawytscha (Walbaum, 1792) in New Zealand. J Appl Ichthyol.
- Rowe DK, Thorpe JE, Shanks AM. 1991. Role of Fat stores in the maturation of Male Atlantic Salmon (Salmo salar) parr. Can J Fish Aquat Sci. 48(3):405–413. doi: 10.1139/f91-052
- Sambraus F, Glover KA, Hansen T, Fraser TWK, Solberg MF, Fjelldal PG. 2014. Vertebra deformities in wild Atlantic salmon caught in the Figgjo River, Southwest Norway. J Appl Ichthyol. 30(4):777–782. doi: 10.1111/jai.12517
- Sanders JL, Watral V, Kent ML. 2012. Microsporidiosis in zebrafish research facilities. ILAR Journal. 53(2):106–113. doi: 10.1093/ilar.53.2.106
- Seymour A. 1959. Effects of temperature upon the formation of vertebrae and fin rays in young chinook salmon. Trans Am Fish Soc. 88(1):58–69. doi: 10.1577/1548-8659(1959)88[58:EOTUTF]2.0.CO;2
- Shearer WM. 1984. The Report on the Salmon Scale Reading Workshop held in Aberdeen, Scotland, 23–28 April 1984. International Council for the Exploration of the Sea Anadromous and Catdromous Fish Committee ICES_M25.
- Silverstone AM, Hammell L. 2002. Spinal deformities in farmed Atlantic salmon. The Canadian Veterinary Journal. 43(10):782.
- Sullivan M, Guy DR, Roberts RJ, Manchester NJ. 2007. The aetiology of spinal deformity in Atlantic salmon, Salmo salar L.: Influence of genetic factors on the frequency and severity in freshwater stages. J Fish Dis. 30(12):753–758. doi: 10.1111/j.1365-2761.2007.00888.x
- Sullivan M, Hammond G, Roberts RJ, Manchester NJ. 2007. Spinal deformation in commercially cultured Atlantic salmon, Salmo salar L.: a clinical and radiological study. J Fish Dis. 30(12):745–752. doi: 10.1111/j.1365-2761.2007.00889.x
- Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien F-A, Dufour S, Karlsen Ø, Norberg B, et al. 2010. Control of puberty in farmed fish. Gen Comp Endocrinol. 165(3):483–515. doi: 10.1016/j.ygcen.2009.05.004
- Unwin MJ, Poortenaar CW, Rowe DK, Boustead NC, Porter MJR. 2004. Seasonal profiles in growth, energy reserves, gonad development, and plasma steroids in age 1+ cultured Chinook salmon (Oncorhynchus tshawytscha) females. N Z J Mar Freshwat Res. 38(1):29–41. doi: 10.1080/00288330.2004.9517215
- Unwin MJ, Rowe DK, Poortenaar CW, Boustead NC. 2005. Suppression of maturation in 2-year-old Chinook salmon (Oncorhynchus tshawytscha) reared under continuous photoperiod. Aquaculture. 246(1):239–250. doi: 10.1016/j.aquaculture.2005.01.022
- Witten PE, Gil-Martens L, Hall BK, Huysseune A, Obach A. 2005. Compressed vertebrae in Atlantic salmon Salmo salar: evidence for metaplastic chondrogenesis as a skeletogenic response late in ontogeny. Dis Aquat Org. 64(3):237–246. English. doi: 10.3354/dao064237
- Witten PE, Gil-Martens L, Huysseune A, Takle H, Hjelde K. 2009. Towards a classification and an understanding of developmental relationships of vertebral body malformations in Atlantic salmon (Salmo salar L.). Aquaculture. 295(1–2):6–14. English. doi: 10.1016/j.aquaculture.2009.06.037
