ABSTRACT
Lake Manapouri in the South Island of New Zealand has a significant hydroelectric generation facility, which potentially has a negative impact on the successful emigration of longfin eels (Anguilla dieffenbachii) from the upper Waiau catchment. Consequently, a trap-and-transfer programme has been implemented to allow silver (migratory) eels access to the sea. The present study analysed data on the timing of captures and numbers of silver eels over four seasons, including the influence of possible migration cues. Silver eels were captured over an extended season of seven months (November–May), with peak numbers during December and January. Flow at the lake outflow was found to have a weak relationship with the number of eels caught, as did lake level, but moon phase and rainfall had no significant effect. These results highlight the variability in silver longfin eel behaviour both in terms of timing and response to potential environmental cues.
Introduction
New Zealand longfin eels (Anguilla dieffenbachii) are large, long-lived endemic fish. They are found throughout the country, often occurring in alpine waterways, and streams far inland, and moving much further inland than New Zealand's other common eel species, the shortfin eel (Anguilla australis), (Jellyman Citation1995, Citation1998; Jellyman and Chisnall Citation1999; Hoyle and Jellyman Citation2002). Like other anguillids, they are catadromous, making them vulnerable to barriers, such as dams, constructed across waterways as they move between inland habitat and the sea (Beentjes et al. Citation2005; Jansen et al. Citation2007; Righton and Walker Citation2013; Chen et al. Citation2014; Jacoby et al. Citation2015).
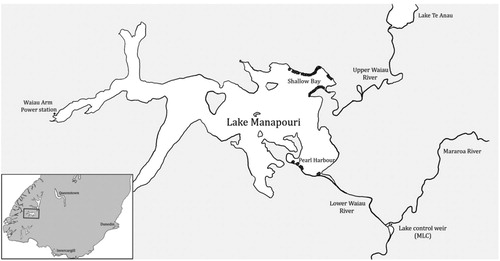
Lake Manapouri, a large natural lake (153 km2) on the edge of Fiordland National Park, is a significant source of hydroelectric generation in New Zealand. Two large tailrace tunnels in the West Arm of the lake discharge into Deep Cove, Doubtful Sound, providing 177 m of head for electricity generation (Meridian Energy factsheet). The water level in the lake is controlled by a weir (Manapouri Lake Control structure, MLC) 11 km below the lake outlet (). MLC can divert water from the Mararoa River into Lake Manapouri, resulting in an upstream flow of water back into the lake. Such reversed flows are permissible so long as minimum flow requirements to the Waiau River are met (12–16 m3 s−1), but must cease when Mararoa River flows exceed 40 m3 s−1 to prevent turbid water entering lake Manapouri.
Figure 1. Locations of nets within Lake Manapouri over the four eel fishing seasons (there is often overlap as nets can be set in the same or similar location for multiple seasons). The two primary net locations – Shallow Bay and Pearl Harbour are marked, as are the locations of the power station and the lake control structure (MLC).
Silver eels attempting to emigrate from the lake can encounter outflows at the power station or the natural outlet. Unfortunately, entry into the power station penstocks results in 100% mortality (Beentjes et al. Citation2005), while outflows at the natural outlet can be interrupted by periods of inflow from the diversion of the Mararoa River. Accordingly, to provide successful egress for silver eels, the power company (Meridian Energy Ltd.) are required to operate a trap-and-transfer and monitoring programme. For this, silver eels are caught in the lake and transferred downstream of MLC, thus allowing them to continue their downstream migration.
This long-term trap-and-transfer and monitoring programme provides an opportunity to investigate the activity of silver longfin eels. Previous work on this population of longfins has found that silver eels can move extensively within the lake before emigration; they may also approach the lake outlet several times before exiting the lake (Jellyman and Unwin Citation2017). Unfortunately, because of high boat traffic, it was not possible to set nets immediately within the outlet area, meaning that the catch records cannot be interpreted as indicating actual migration behaviour, but rather as silver eel activity. Large catches are assumed to indicate increased activity, which could be a response to environmental cues and the beginning of an attempt to emigrate from the lake.
This study used data collected on silver longfin eel catch numbers to identify potential triggers for increasing activity and patterns in timing. We hypothesised that there would be a relationship between the number of eels that were caught and the lake level, rainfall and moon phase, as these are all parameters that have been found to influence anguillid eel movement in New Zealand (Burnet Citation1969; Todd Citation1981a; Boubée et al. Citation2001) and elsewhere (Vøllestad et al. Citation1986). Based on the results of such studies, it was predicted that numbers would be higher during high lake levels, high rainfall events and during the new moon. We also predicted that the timing of the peak silver eel catches would be in autumn, similar to that observed in Lake Ellesmere, a South Island lake (Todd Citation1981a; Jellyman et al. Citation1996; Lokman et al. Citation1998) and that the beginning of male migration would precede female migration.
Methods
Study location
Data obtained during the Lake Manapouri eel trap-and-transfer programme were used for this study. This programme involves the capture of longfin eels in unbaited fyke nets positioned around the eastern shores of Lake Manapouri. Nets were set in two areas, Shallow Bay near the entry of the Upper Waiau River, and around Pearl Harbour, the natural outlet (). The possibility to trap eels in the lake tributaries was investigated but decided against as they are generally steep, flashy and difficult to access. However, the Upper Waiau River, which connects Lake Te Anau to Lake Manapouri, flows into Shallow Bay where many nets were set.
Fishing methods
Fishing seasons are typically from early summer through to late autumn, commencing at the first new moon period in late November or early December. Fishing ends when the catch numbers decline to low levels, usually in late April to May. As a result, some seasons started later or finished earlier than others.
Fyke nets were set overnight and emptied the following morning. Nets were made of 12 mm mesh, with an opening height of either 1 or 1.5 m, and wings between 10 and 20 m long and up to 3 m deep, allowing their use with variations in lake level. Nets were unbaited to avoid attraction of yellow eels. Silver eels caught were then transferred to the Waiau River below MLC, where they can migrate to the sea unimpeded. Any yellow eels caught were counted and then returned to the lake. The catch consisted almost entirely of longfin eels, with shortfin eels rarely being caught (<1% of total catch).
The same fisher has been employed for the trap and transfer since 2005, providing a consistency in observations and data collection. Silver eels from each net were individually placed in a net-bag and weighed to the nearest 10 g using electronic fish scales. Sex was determined by using weight as a proxy; any eel over 1 kg was recorded as a female (E. Brunton, pers. comm.). This method was used, as size dimorphism in longfin eels is well recognised, and there are no reliable non-invasive alternatives to identify sex in eels (Burnet Citation1952; Oliveira Citation1999; McCleave and Jellyman Citation2004; Davey and Jellyman Citation2005). Eels were identified as being silver eels based on external physical characteristics such as black dorsal colouration, tapered head shape, prominent pectoral fins and increased eye size (Jellyman and Unwin Citation2017). These factors are well-recognised traits and identified by the same fisherman across all seasons ensuring a consistent comparison (Boubée et al. Citation2008).
Due to high catch numbers at the beginning of the 2010–2011 season, the data collection changed after the first 25 days of fishing, i.e. individual measurements were not taken and only the total daily catch of males and females was recorded. This meant data for the majority of that season could not be used for the spatial analysis, as there were no records of which net eels were caught in. There is also a gap in catch data over the Christmas and New Year period, as nets were removed from the lake to avoid conflict with the increased level of recreational boating activity during this time.
Data acquisition and sorting
Data from the eel trap-and-transfer programme were provided by NIWA on behalf of Meridian Energy Ltd. These contained the weight, sex, net location and date of capture for every silver longfin eel caught over four fishing seasons between 2008 and 2012 (10,178 longfin eels in total), as well as the coordinates of the nets each season, with the exception for part of the 2010–2011 season previously identified. Meridian Energy provided data on daily lake level, rainfall and water flows associated with the lake. Rainfall data were recorded in West Arm; flow measurements were recorded for the Mararoa River, just upstream of MLC, and for the Lower Waiau River, just below MLC. Flows in the Waiau Arm are not directly measured but were calculated by subtracting the Mararoa flows from the Lower Waiau flows, with a negative value indicating that some Mararoa flows were being diverted, reversing the flow in the Waiau Arm. Moon phase for each day was also included in the dataset. Day of the season was defined relative to the earliest start date (November 28).
Statistics
We examined how the parameters of interest (moon phase, rainfall, lake level, Waiau Arm flows) influenced eel catch numbers over time, and across the two fishing areas, using generalised linear models (GLMs) with Poisson distribution. Data were log transformed to improve normality. For the temporal analysis, total catch and the number of males and females caught were used as response variables in separate GLMs, with season, day of the season, moon phase, Waiau Arm flows and rainfall as the predictors (). To identify any variation between catches at Shallow Bay compared to Pearl Harbour, two GLMs were run, one with number of eels caught at Shallow Bay as the predictor, the other with numbers at Pearl Harbour; both had moon phase, lake flows, rainfall and day of the season being used as predictors (). This spatial dataset was smaller, as only 25 out of the 99 days fished over the 2010–2011 season included location data. Only one- and two-way interactions were of interest as these are the most likely to show features of biological relevance.
Table 1. Formula and AIC values for each of the GLMs used in the analysis.
In addition to the GLMs, a correlation matrix was generated for both the spatial and temporal datasets. Interactions that were significant and considered biologically relevant were investigated further to identify the probability (p) and correlation coefficient (r). When a significant interaction was observed, the parameter of interest was checked for significant correlations with other parameters to ensure that co-linearity was not the cause of the interaction. Trends in average body weight over the length of the season were analysed by Pearson correlation test. All tests were considered significant if p ≤ .05.
Results
Silver eel numbers and timing
The total number of eels caught each season varied significantly (p < .001), with the highest number of eels being caught during the 2010–2011 season. Variation between seasons may be affected by varying catch effort, start times and duration of each fishing season (); however, the 2010–2011 season is clearly different. Total catch varied with a day of the season (p = .017), with the catch higher at the start of each season and declining towards the end ().
Figure 2. Number of Lake Manapouri longfin eels caught compared to the day of the fishing season, day 0 is the 27th of November. Four fishing seasons are combined. n = 336 days. A , number of males caught (p = .002). B, number of females caught (p = .39). The break in the data (day 25–45) is over the Christmas–New Year period.
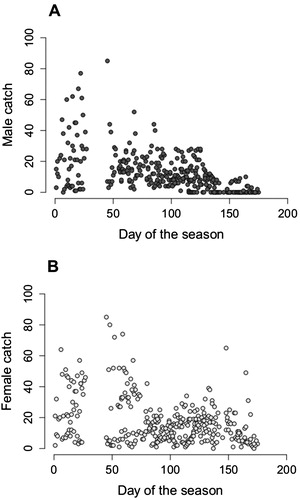
Table 2. Dates fished, total eel catch and other catch statistics for each of the four Lake Manapouri longfin eel trap-and-transfer seasons.
Male and female catches exhibited different temporal patterns. The female catch rate was relatively consistent throughout the fishing season, whereas male catch declined towards the end of the season, with a day of the season relating significantly to male catch (p = .002), but not female catch (p = .39). During late April and May, it was common for no males to be caught, but the capture of large numbers of females continued ().
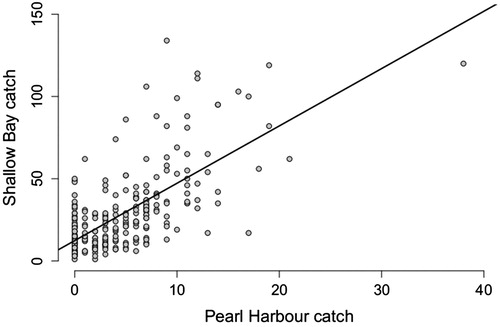
The number of eels caught at Pearl Harbour and Shallow Bay were closely correlated (p < .001, correlation coefficient = 0.56), hence high catches were observed at the same time in both areas (). Catches at both locations were significantly related to the day of the season (Pearl Harbour p < .001, Shallow Bay p = .012).
Potential migration cues
Weak significant relationships were identified between the total number of all eels caught and both lake level (p = .0028, r = −0.17) and flows (p = .0099, r = 0.13). However, these are unlikely to be independent influences, as lake level and Waiau flows are closely correlated (p < .001, r = 0.57). There was a weak relationship at the outlet site between catch and daily rainfall, with the catch being inversely related to rainfall (p = .015, r = −0.15), but this trend was not observed with the Shallow Bay catches.
There were no detectable relationships between eel catch and either moon phase () or rainfall (). When catches from the two areas were analysed separately, there were no significant relationships between the catch at Shallow Bay and any of the environmental parameters examined, but there was a weak significant relationship between catch at Pearl Harbour and rainfall (p = .015, r = −0.15).
Figure 4. Number of longfin eels caught in Lake Manapouri compared to moon phase, starting and ending at the full moon, p = .37, n = 336 days.
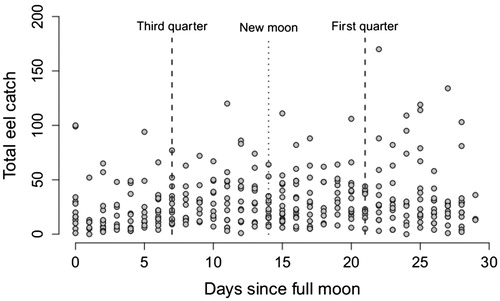
Table 3. Deviance and significance levels (p) for variables examined for their influence on total catch of silver eels.
Eel size
Male size ranged from 0.26 to 1.0 kg with an average of 0.73 kg. Female size ranged from 1.0 to 11.82 kg, with an average of 2.37 kg. A significant inverse relationship between day and average size was found for females, but not for males (females p < .0001, r = −0.516, males p = .426).
Discussion
Silver eel numbers and timing of capture
There was a significant difference in the timing of movement between male and female eels, with the number of males captured declining before the number of females showed any indication of a decline. This pattern is similar to that observed elsewhere in New Zealand (Todd Citation1981a; Boubée et al. Citation2001). In Lake Ellesmere, male longfins begin their seaward migration approximately a month earlier than females; they also finish migrating before females (Todd Citation1981a). However, it is important to note that there is extensive exploration prior to emigration from Lake Manapouri (Jellyman and Unwin Citation2017); therefore, this study is an indicator of activity and movement of silver eels, rather than direct migration.
The number of silver eels captured in Lake Manapouri peaked over summer and had a capture season ranging from November to May, with a high possibility of the season beginning even earlier. This temporal pattern was also observed in an acoustic tagging study of Lake Manapouri silver eels (Jellyman and Unwin Citation2017). Lake Manapouri eels have a longer migration season than has typically been observed elsewhere. In Lake Ellesmere and two North Island systems, silver eels were recorded from March to May (Todd Citation1981a), although a migration period of February to May has been observed more frequently (Burnet Citation1969; McDowall Citation1995). This suggests that the eels in Lake Manapouri have a greater flexibility in the timing of their migration. Assuming that the eel activity recorded in the present study is indicative of likely emigration season, then these results support the finding of Jellyman and Unwin (Citation2017) that longfin silver eels display considerable variability in the commencement of their seaward migration. Such ‘risk spreading’ has also been noted for the European eels (Righton et al. Citation2016) where not all silver eels reach their spawning ground in the year of departure.
There are a number of factors believed to influence sex determination, including, eel density, habitat, latitude and growth rates (Davey and Jellyman Citation2005). In New Zealand rivers, females often dominate inland sites, with some locations having no males, despite high male proportions in the lower reaches (Burnet Citation1969; Todd Citation1981a). However, this is not universal, with other studies finding no geographical differences between male and female numbers (Beentjes et al. Citation2006). In our study, females made up 58% of the catch, but overall male numbers were also high.
Migration cues
As discussed earlier, external features were used to differentiate silver (migratory) eels from feeding (yellow, non-migratory) eels; this can be somewhat misleading, especially early in season when diagnostic features like eye size, head shape and colouration are less pronounced than later in season. Although identification was done by an experienced operator, there is always the possibility that some eels may have been misidentified, as silvering is a progressive process and not all eels exhibit the full range of characteristics at the same time (Svedaung et al. Citation1996; Durif et al. Citation2005; Bultel et al. Citation2014). This issue was discussed in a separate study (Jellyman and Unwin Citation2017); these authors concluded that significant differences in eye size discriminated between ‘silver’ and ‘yellow’ eels from the lake. This is further supported by the fact that over 180 tagged ‘silver’ eels selected on the basis of external features over a range of five months (December to April) all emigrated, indicating that their identification as silver eels was correct (Jellyman and Unwin Citation2017).
The relationship between flows and lake level is a complex one, as outflows are required to comply with seasonally varying minimum flows, while lake level variation is permitted only over a limited range. Whether this manipulated regime impacts on eel behaviour is uncertain, but it is possible that an artificially maintained and near-constant outflow for extended periods could reduce the behavioural responses of silver eels.
There was a weak inverse relationship between rainfall and the number of eels caught around Pearl Harbour. Rainfall, and the associated increase in flow, is widely considered as having a positive association with silver eel migration (e.g. Burnet Citation1969; Todd Citation1981a; Boubée et al. Citation2001; Durif et al. Citation2003). The slight negative association between rainfall and catch in the present study is at odds with the findings of a positive correlation between flow and emigration in both the present study and that of Jellyman and Unwin (Citation2017 ). Reasons for this anomaly are uncertain but may reflect the relative flow contributions to Lake Manapouri as 65% of inflows come from the Upper Waiau River draining Lake Te Anau (Meridian Energy, pers. comm.), meaning that local rainfall could be of less importance than expected.
There were no significant relationships relating the numbers of silver longfin eels with moon phase or rainfall, both factors that have been shown to influence silver eel movement in A. dieffenbachii and other anguillid species (Lowe Citation1952; Todd Citation1981a; Vøllestad et al. Citation1986; Boubée et al. Citation2001). There is evidence that silver eel numbers tend to cycle with the moon phase, with a peak that is frequently, but not always associated with the new moon (Burnet Citation1969; Todd Citation1981a). While catch rates in the present study were a measure of activity rather than emigration per se, a study of the actual migration of tagged eels from Lake Manapouri also found no influence of moon phase (Jellyman and Unwin Citation2017). However, this latter study also highlighted the potentially confusing cues that silver eels might experience within the lake, with the strongest outflows associated with the operation of the power station, and the likelihood of silver eels arriving at the natural outlet experiencing inflows rather than outflows.
Management implications
Trap-and-transfer programmes are not a common practice, despite their recognition as a means of silver eel escapement; only a few countries utilise this as a management tool to mitigate impacts of hydroelectric facilities. New Zealand and Ireland both have several trap-and-transfer programmes providing safe passage past potentially lethal generation facilities, thus increasing the successful emigration rate of silver eels (MacNamara and McCarthy Citation2014; McCarthy et al. Citation2014; Boubée et al. Citation2008). It is estimated that the Lake Manapouri programme moves 55% of females attempting to emigrate (Jellyman and Unwin Citation2018), ensuring the survival of fish that might otherwise be killed by passage through the turbines.
The numbers of eels caught early in the season indicates that the initial increase in activity is being missed. The activity of silver eels is positively associated with water temperature (e.g. Boubée et al. Citation2001; Durif and Elie Citation2008; Jellyman and Unwin Citation2017), and temperature may provide a physiological ‘window’ within which other factors can operate (Euston et al. Citation1998). Water temperatures <10–12°C are usually considered inhibitory for silver eel activity (Sloane Citation1984; Boubée et al. Citation2001; Reckordt et al. Citation2014; Jellyman and Unwin Citation2017), but once temperatures exceed this, fishing for silver eels could commence in Lake Manapouri. In addition, the female eels caught at the start of the season tend to be larger; with an average weight of 2.7 kg in December compared to 2.1 kg in May. A decline in fecundity of 33% was calculated between females in December compared to May by utilising the fecundity-weight equation described by Todd (Citation1981b). As a result, an earlier start time for the trap-and-transfer programme could allow an increased number of eels, that are potentially more fecund, to be transferred over the season; it would also enable more information to be gathered on the characteristics of silver eel activity, including any differences between the sexes, early in the migration season.
The lack of correlation between catch (numbers) and environmental parameters means that it would be difficult to determine times when large catches are more likely, unlike other areas where moon phase or rainfall can guide capture attempts. In Lake Manapouri, there is also the potential that the unnatural lake level fluctuations and flow variability due to the hydropower facility may have altered the sensitivity of eels to these migration cues, further complicating the ability to predict their movement.
The recent inclusion of longfin eels in the Department of Conservation list of 150 priority threatened species (Department of Conservation Citation2017) indicates some concern about the overall well-being of this species. Results from the present study highlight the variability in silver eel activity, meaning it is difficult to generalise about such behaviour. However, this variability must be recognised and factored into management proposals.
Conclusions
This study has provided strong additional evidence that longfin eels in Lake Manapouri have an extended out-migration period of over six to seven months. These findings further support the theory that migration timing in longfin eels has significant flexibility, rather than a fixed autumn-only out-migration period. It also highlights how migration cues are not universal and may differ between populations. Both of these factors mean that any conservation efforts need to be specified for particular locations rather than generalising on the species as a whole.
Acknowledgements
The authors express their sincere thanks to E. Brunton (Trap-and-transfer fisherman) for sharing his knowledge on the programme, and anecdotes about the eels, and to E. Stead (on behalf of Meridian Energy) for providing Lake Manapouri environmental data. The advice from Dr T. Ingram (University of Otago) about statistical analysis is also greatly appreciated. The authors thank Meridian Energy for providing permission to use the data collected during their eel management programme.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beentjes M, Boubée J, Jellyman D, Graynoth E. 2005. Non-fishing mortality of freshwater eels (Anguilla spp.). New Zealand Fisheries Assessment Report No. 2005/34. Wellington: Ministry of Fisheries, 38p.
- Beentjes MP, Jellyman DJ, Kim SW. 2006. Changing population structure of eels (Anguilla dieffenbachii and A. australis) from southern New Zealand. Ecology of Freshwater Fish. 15:428–440. doi: 10.1111/j.1600-0633.2006.00165.x
- Boubée J, Jellyman D, Sinclair C. 2008. Eel protection measures within the Manapouri hydro-electric power scheme, South Island, New Zealand. Hydrobiologia. 609:71–82. doi: 10.1007/s10750-008-9400-6
- Boubée JA, Mitchell CP, Chisnall BL, West DW, Bowman EJ, Haro A. 2001. Factors regulating the downstream migration of mature eels (Anguilla spp.) at Aniwhenua Dam, Bay of Plenty, New Zealand. New Zealand Journal of Marine and Freshwater Research. 35:121–134. doi: 10.1080/00288330.2001.9516982
- Bultel E, Lasne E, Acou A, Guillaudeau J, Bertier C, Feunteun E. 2014. Migration behaviour of silver eels (Anguilla Anguilla) in a large estuary of Western Europe inferred from acoustic telemetry. Estuarine, Coastal and Shelf Science. 137:23–31. doi: 10.1016/j.ecss.2013.11.023
- Burnet A. 1952. Studies on the ecology of the New Zealand long-finned eel, Anguilla dieffenbachii Gray. Australian Journal of Marine and Freshwater Research. 3:32–63. doi: 10.1071/MF9520032
- Burnet A. 1969. Migrating eels in a Canterbury river, New Zealand. New Zealand Journal of Marine and Freshwater Research. 3:230–244. doi: 10.1080/00288330.1969.9515292
- Chen J-Z, Huang S-L, Han Y-S. 2014. Impact of long-term habitat loss on the Japanese eel Anguilla japonica. Estuarine, Coastal and Shelf Science. 151:361–369. doi: 10.1016/j.ecss.2014.06.004
- Davey A, Jellyman D. 2005. Sex determination in freshwater eels and management options for manipulation of sex. Reviews in Fish Biology and Fisheries. 15:37–52. doi: 10.1007/s11160-005-7431-x
- Department of Conservation. 2017. New Zealand threatened species strategy – draft for consultation. Wellington: Department of Conservation; 50p.
- Durif C, Dufour S, Elie P. 2005. The silvering process of Anguilla Anguilla: a new classification from the yellow resident to the silver migrating stage. Journal of Fish Biology. 66:1025–1043. doi: 10.1111/j.0022-1112.2005.00662.x
- Durif C, Gosset C, Rives J, Travade F, Elie P. 2003. Behavioral study of downstream migrating eels by radio-telemetry at a small hydroelectric power plant. American Fisheries Society Symposium. 33:343–356.
- Durif CMF, Elie P. 2008. Predicting downstream migration of silver eels in a large river catchment based on commercial fishery data. Fisheries Management and Ecology. 15:127–137. doi: 10.1111/j.1365-2400.2008.00593.x
- Euston ET, Royer DD, Simmons CL. 1998. American eels and hydro plants: clues to eel passage. Hydrological Review. 17: 94–103.
- Hoyle S, Jellyman D. 2002. Longfin eels need reserves: modelling the effects of commercial harvest on stocks of New Zealand eels. Marine and Freshwater Research. 53:887–895. doi: 10.1071/MF00020
- Jacoby D, Casselman J, Crook V, DeLucia M-B, Ahn H, Kaifu K, Kurwie T, Sasal P, Silfvergrip AMC, Smith KG, et al. 2015. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Global Ecology and Conservation. 4:321–333. doi: 10.1016/j.gecco.2015.07.009
- Jansen H, Winter H, Bruijs M, Polman H. 2007. Just go with the flow? Route selection and mortality during downstream migration of silver eels in relation to river discharge. ICES Journal of Marine Science: Journal du Conseil. 64:1437–1443. doi: 10.1093/icesjms/fsm132
- Jellyman D, Glova G, Todd P. 1996. Movements of shortfinned eels, Anguilla australis, in Lake Ellesmere, New Zealand: results from mark-recapture studies and sonic tracking. New Zealand Journal of Marine and Freshwater Research. 30:371–381. doi: 10.1080/00288330.1996.9516724
- Jellyman DJ. 1995. Longevity of longfinned eels Anguilla dieffenbachii in a New Zealand high country lake. Ecology of Freshwater Fish. 4:106–112. doi: 10.1111/j.1600-0633.1995.tb00123.x
- Jellyman DJ. 1998. Exploitation of freshwater eels in national parks. Conservation Advisory Science Notes No. 217. Wellington: Department of Conservation.
- Jellyman DJ, Chisnall BL. 1999. Habitat preferences of shortfinned eels (Anguilla australis), in two New Zealand lowland lakes. New Zealand Journal of Marine and Freshwater Research. 33:233–248. doi: 10.1080/00288330.1999.9516873
- Jellyman DJ, Unwin MJ. 2017. Diel and seasonal movements of silver eels, Anguilla dieffenbachii, emigrating from a lake subject to hydro-electric control. Journal of Fish Biology. 91:219–241. doi: 10.1111/jfb.13335
- Jellyman DJ, Unwin MJ. 2018. Fine-scale swimming movement and behaviour of female silver eels, Anguilla dieffenbachii, within a lake affected by hydro-power generation. Fisheries Management and Ecology. 1–13.
- Lokman P, Vermeulen G, Lambert J, Young G. 1998. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish Physiology and Biochemistry. 19:325–338. doi: 10.1023/A:1007719414295
- Lowe RH. 1952. The influence of light and other factors on the seaward migration of the silver eel (Anguilla Anguilla L. The Journal of Animal Ecology. 21:275–309. doi: 10.2307/1963
- MacNamara R, McCarthy TK. 2014. Silver eel (Anguilla Anguilla) population dynamics and production in the River Shannon, Ireland. Ecology of Freshwater Fish. 23:181–192. doi: 10.1111/eff.12028
- McCarthy TK, Nowak D, Grennan J, Bateman A, Conneely B, MacNamara R. 2014. Spawner escapement of European eel (Anguilla Anguilla) from the River Erne, Ireland. Ecology of Freshwater Fish. 23:21–32. doi: 10.1111/eff.12091
- McCleave J, Jellyman D. 2004. Male dominance in the New Zealand longfin eel population of a New Zealand river: probable causes and implications for management. North American Journal of Fisheries Management. 24:490–505. doi: 10.1577/M03-045.1
- McDowall RM. 1995. Seasonal pulses in migrations of New Zealand diadromous fish and the potential impacts of river mouth closure. New Zealand Journal of Marine and Freshwater Research. 29:517–526. doi: 10.1080/00288330.1995.9516684
- Oliveira K. 1999. Life history characteristics and strategies of the American eel, Anguilla rostrata. Canadian Journal of Fisheries and Aquatic Sciences. 56:795–802. doi: 10.1139/f99-001
- Reckordt M, Ubl C, Wagner C, Frankowski J, Dorow M. 2014. Downstream migration dynamics of female and male silver eels (Anguilla Anguilla L.) in the regulated German lowland Warnow River. Ecology of Freshwater Fish. 23:7–20. doi: 10.1111/eff.12080
- Righton D, Walker A. 2013. Anguillids: conserving a global fishery. Journal of Fish Biology. 83:754–765.
- Righton D, Weaterberg H, Feunteun E, Økland F, Gargan P, Amilhat E, Metcalfe J, Lobon-Cervia J, Sjöberg N, Simon J, et al. 2016. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Science Advances. 2:1–14. doi: 10.1126/sciadv.1501694
- Sloane RD. 1984. Preliminary observations of migrating adult freshwater eels (Anguilla australis australis Richardson) in tasmania. Australian Journal of Marine and Freshwater Research. 35:471–476. doi: 10.1071/MF9840471
- Svedaung H, Neuman E, Wickström H. 1996. Maturation patterns in female European eel: age and size at the silver eel stage. Journal of Fish Biology. 48:342–351. doi: 10.1111/j.1095-8649.1996.tb01432.x
- Todd P. 1981a. Timing and periodicity of migrating New Zealand freshwater eels (Anguilla spp. New Zealand Journal of Marine and Freshwater Research. 15:225–235. doi: 10.1080/00288330.1981.9515915
- Todd P. 1981b. Morphometric changes, gonad histology, and fecundity estimates in migrating New Zealand freshwater eels (Anguilla spp. New Zealand Journal of Marine and Freshwater Research. 15:155–170. doi: 10.1080/00288330.1981.9515908
- Vøllestad L, Jonsson B, Hvidsten N, Naesje T, Haraldstad O, Ruud-Hansen J. 1986. Environmental factors regulating the seaward migration of European silver eels (Anguilla Anguilla). Canadian Journal of Fisheries and Aquatic Science. 43:1909–1916. doi: 10.1139/f86-236