ABSTRACT
Non-indigenous zooplankton species pose a biosecurity threat to New Zealand’s freshwater native taxa. Nine species are known to have established in New Zealand lakes to date. The spread of some zooplankton taxa is linked to the translocation of farmed fish, principally grass carp (Ctenopharyngodon idella), and recreational vessel movements. The aims of this study were to assess the effectiveness of a range of chemical and physical treatments for transport water and associated equipment to kill freshwater cladoceran, copepod, and rotifer zooplankton species, and their risk to non-target fish. Sodium chloride was the most effective and applicable chemical treatment tested at length in the cladoceran and, combined with physical treatment via mechanical filtration of water or hot water immersion of equipment (to also manage the risk of diapausing eggs), represents an effective option for the control of non-indigenous zooplankton, with limited impact on stenohaline fish.
Introduction
New Zealand’s freshwater ecosystems have ecological, economic, and cultural importance. A large proportion of New Zealand’s endemic taxa is at risk or is threatened, by non-indigenous species (NIS) (Clout & Lowe Citation2000; Jay et al. Citation2003). In accordance with the Biosecurity Act 1993 (Parliamentary Counsel Office Citation1993), strict pre-border and border controls are implemented to minimise the risk of NIS entering the country. Yet, it is inevitable that some incursions will occur (Goldson et al. Citation2015). Post-border response tools to eradicate newly established NIS or slow their spread are a vital component of effective biosecurity management plans (Wotton & Hewitt Citation2004).
The most widely publicised recent post-border response to a freshwater NIS incursion in New Zealand was that of the North American diatom Didymosphenia geminata (didymo). Didymo was first recorded in New Zealand in 2004 and has subsequently spread throughout the South Island with major ecological consequences (Kilroy & Unwin Citation2011). This incursion triggered a campaign to manage risks associated with didymo-contaminated equipment associated with commercial and recreational activities (Ministry for Primary Industries Citation2008). While didymo has been placed firmly in the regulatory, media, and public spotlight, other freshwater NIS incursions have been left unaddressed.
At least nine non-indigenous zooplankton species have been introduced to New Zealand lakes (Duggan & Collier Forthcoming). Although the impacts of non-native zooplankton are often inconspicuous, invasions have been implicated in the declines of native zooplankton populations, with flow-on effects impacting other levels of lake food chains (e.g. algae and fish) (Ricciardi Citation2015) . For example, Balvert et al. (Citation2008) observed a large decline in the densities of algae and smaller zooplankton following the establishment of the North American cladoceran Daphnia galeata in Lake Puketirini (Waikato). The North American calanoid copepod Skistodiaptomus pallidus altered zooplankton species composition in Lake Kereta (Rodney District), to the detriment of the native copepod Calamoecia lucasi (Duggan et al. Citation2014). The non-indigenous zooplankton species already established in New Zealand are, as far as we are currently aware, relatively benign from an ecological or economic perspective. However, zooplankton species have had major effects on food webs in other countries, such as the spiny waterflea (Bythotrephes longimanus) (Yan et al. Citation2001) and fishhook waterflea (Cercopagis pengoi) in North America (Laxson et al. Citation2003) by decreasing the abundances of aquatic communities (Gallardo et al. Citation2015).
The management of non-indigenous zooplankton species requires identification of vectors and pathways of spread, and the development of management protocols to minimise their spread and impacts. The establishment of North American Skistodiaptomus pallidus in Lake Kereta coincided with the release of domestically cultured grass carp, Ctenopharyngodon idella (Cyprinidae: Leuciscinae) for weed control (Duggan et al. Citation2014). Subsequent surveys of grass carp aquaculture ponds have found S. pallidus, along with other non-indigenous zooplankton, strengthening the link between grass carp translocations and zooplankton spread (Duggan & Pullan Citation2017). Further, significant differences in zooplankton composition have been observed between Auckland ponds where grass carp have and have not been released, which could be attributed in part to the establishment of hitchhiking zooplankton species originating from aquaculture ponds, including of non-native species (e.g. the North American Daphnia pulex and Skistodiaptomus pallidus; Branford & Duggan Citation2017). The aims of this study were to identify chemicals and assess their efficacy to control three zooplankton species, the copepod S. pallidus (non-native), the rotifer Asplanchna brightwelli (native), and the cladoceran Daphnia pulex (non-native) in transport waters and contaminated equipment and potential risk to non-target freshwater fish, including grass carp.
Materials and methods
Animal collection
Zooplankton species were sourced from ponds in the Auckland region of New Zealand that have been subject to grass carp releases. The species S. pallidus and A. brightwelli were sourced from a pond at Tahuna Torea, Glen Innes (WSG84 −36.872232, 174.882151), and D. pulex was sourced from Sancta Maria Triangle pond at Flat Bush (WSG84 −36.956610, 174.906413). Zooplankton were collected using a 70-µm mesh net hauled horizontally through the water from the shore. The contents of multiple hauls were placed with pond water into 10-L containers for transportation to the laboratory. Zooplankton were maintained in 30-L aquaria with coarse bubblers and fed with a mix of freshwater green microalgae and diatoms until used.
Grass carp (<1 year) were obtained from a fish-rearing facility (Maisey Road Hydroponics Limited, Richmond). Carp (32 individuals 164 ± 7 mm and 62.4 ± 7.9 g) were transported in a 200-L tank and held in the laboratory in a 500-L tank of dechlorinated tap water with continuous aeration for a week prior to testing. Examination of the fish on arrival revealed the presence of gill parasites. The details of the parasite treatment are summarised in the supplementary materials.
Chemical and physical treatments
Nine chemicals were selected based on their known efficacy against related pest species, potential low risks to food safety (for fish destined for human consumption), receiving environments and safety of the operators conducting the treatments (Table s1): spinosad, sodium percarbonate (Sigma Aldrich), chloramine-T, potassium chloride, potassium permanganate (BDH), hydrogen peroxide 30% (Pronalys, ThermoFisher), Tsunami® (Ecolab), sodium hypochlorite (commercial bleach), non-iodised sodium chloride (grocery source).
The physical treatment options tested were based on marine ballast water treatment approaches, which also aim to render microscopic life-stages inert (Tsolaki & Diamadopoulos Citation2010). They included UV-C sterilisation (Sutherland et al. Citation2001; Waite et al. Citation2003), mechanical filtration (Parsons & Harkins Citation2002), and heated water (Mountfort et al. Citation1999; Quilez-Badia et al. Citation2008). Equipment was treated with five of the most effective chemicals toward zooplankton (Tsunami®, potassium permanganate, hydrogen peroxide, sodium hypochlorite, and sodium chloride). Air drying of equipment was also tested as a simple and universally used control option.
Zooplankton toxicity tests
Range-finding tests were conducted on all species but definitive tests were conducted only on the most resistant one as the treatment levels would also be effective against other species (Results in Table s2). Mortality of the target zooplankton species within the time of exposure (1–3 h) was the endpoint in both range-finding and full-range tests. The highest concentrations tested were 1 g/L (the highest non-toxic concentration based on the GESAMP rating scheme for acute aquatic toxicity in water (GESAMP Citation2013)) for chloramine-T, KCl, KMnO4, NaOCl, and sodium percarbonate (higher concentrations of these would be too toxic to fish), and 320 mg/L for spinosad, its limit of solubility. Spinosad was first suspended in methanol and added to tap water to reach a maximum concentration of methanol at 0.5%. For the liquid products tested (hydrogen peroxide and Tsunami®), the highest concentration was a 10% dilution of the proprietary product. Next, a dilution series of 1:10 was applied to all chemicals up to five concentration levels.
Concentrations for the chemicals tested are reported as nominal as they were for range finding only. Sodium chloride concentrations were measured as salinity with an HACH HQ40d multi-parameter (conductivity probe CDC401).
A further experiment was carried out after the fish toxicity test (see section below) to better define the maximum time with no effect and the lethal time (LT) affecting 50% and 99.9% (LT50 and LT99.9) of the tested zooplankton with the selected chemical. Survival (n = 5), with 40–60 animals per replicate, was monitored every 30 s for 10 min.
Range-finding acute tests were carried out in six well plates with 10 mL of test solution with 5–10 individuals per well (n = 5) with 1 control. Full-range tests were carried out in 96-well plates with a 350 µL of test solution with 1–4 individual per well. Ten concentrations were tested (n = 16) plus control (n = 32)
Treatment of transport water
Treatments were applied to dechlorinated tap water inoculated with 5–20 fully grown individuals of each of the three test zooplankton species. Mixed cultures of D. pulex, A. brightwelli, and S. pallidus used in pilot tests demonstrated that mixing the three species had no discernible effects on survival or retention within the maximum 24-h testing periods used in this study (data not shown). Three replicates were performed in all cases. Default treatment conditions were 18 ± 1°C, 7.0 ± 0.1 pH, and ambient light. The viability of zooplankton was assessed in six-well plates by counting live and dead individuals. Individuals were considered dead if they did not respond to gentle prodding. In the case of UV-C exposure, a secondary count was made after 24 h to account for the possibility of delayed mortality (expected based on the mode of action of UV-C).
Chemical treatment concentrations were based on the mean and upper limits of the 20 min LC99 values, when available, or the next reliable values from the toxicity assessment experiments: 0.8 and 1.2% for Tsunami®; 10 and 11 mg/L for sodium hypochlorite; 9.1 and 13.0 g/L for hydrogen peroxide; 75 and 138 mg/L for potassium permanganate; and 26 and 35 g/L for sodium chloride, corresponding to three-quarters and full-strength seawater. Approximately, 20 test animals were exposed to the chemical treatments in 1 L of dechlorinated tap water. Blank controls contained freshwater and zooplankton only. After 20-min exposure, zooplankton were collected on a 5-µm sieve, rinsed five times with freshwater, and washed into six-well plates with 10 mL freshwater to assess viability.
For UV-C exposure, 2 L volumes of dechlorinated tap water containing the three zooplankton species were passed into 9, 18, or 36 W UV filters (Pond One ClearTech). The UV filters were switched on, with exposure times of 5, 10, 30 s and 1, 2, 5, and 10 min. Procedural controls were passed through the UV filter when it was not turned on. Blank controls were not passed through the UV filter. Post treatment, zooplankton were collected on a 5-µm sieve and washed into six-well plates with 10 mL of freshwater for viability assessment.
For mechanical filtration, volumes (10 L) of dechlorinated tap water containing the three zooplankton species were passed through a Filter Pure cartridge filter fitted with pleated polyester cartridges. Pore sizes trialled were 1, 5, 10, and 50 µm. Procedural controls were passed through the filter with no cartridge installed. Blank controls were not passed through the filter. Post treatment, zooplankton were collected on a 5-µm sieve and washed into 6-well plates with 10 mL freshwater for viability assessment.
Treatment of equipment
Out of eleven equipment types tested, only wool (4 mm yarn) and felt (7 mm wool-rayon blend) were found to retain sufficient numbers of zooplankton for meaningful experimental analysis of treatment efficacy (details in Supplementary Material). Equipment samples (10 cm2) were inoculated within a bath containing 50–75 individuals/mL of each of the three zooplankton species in a mixed culture (n = 3). The inoculated samples were submerged in baths (1 L) containing the test chemicals at concentrations used to treat water for 20 min. Samples were removed from the bath and allowed to drain for 5 s, before being placed in six-well culture plates with 10 mL dechlorinated tap water to count the number of live zooplankton adhered to the sample and released into the water. As NaCl was the most effective treatment and equipment is likely to be treated without fish being present, concentrations over 99% lethal values were used: 3.5, 35, 70, and 175 g/L. The inoculated samples were submerged in baths containing the NaCl solutions for 30 s and 1, 5, or 10 min (n = 3).
The efficacy of air drying and hot water was also assessed. Equipment samples were left in empty six-well plates with the lids removed for 5, 10, 30 min and 1, 5, 12, 24, 48, and 72 h (n = 3). The ambient conditions were 19 ± 1°C, 100% relative humidity, and 12:12 h light:dark. Exposure to hot water spanned 30°C, 35°C, 40°C, and 50°C for 30 s and 1, 5, 10, and 20 min (n = 3). For hot water treatments, equipment samples were exposed to water at temperatures of 30°C, 35°C, 40°C, and 50°C for 30 s and 1, 5, 10, and 20 min (n = 3). Samples inoculated with zooplankton were placed in water at target temperatures (±2°C). The water baths were housed in 5 L polystyrene bins and the temperatures monitored continuously using digital thermometers. Following drying and hot water treatments, 10 mL of dechlorinated tap water was added to the appropriate wells and live and dead zooplankton counted. Blank controls were placed immediately into dechlorinated tap water.
Fish toxicity test
The exposure followed the OECD Guideline (OECD Citation1992) with modifications. Fish (n = 20) were individually exposed in continuously aerated 10-L aquaria filled to a single concentration of NaCl at 26 g/L, which is the highest concentration that grass carp can tolerate in dechlorinated tap water. Sodium chloride concentrations (salinity) and oxygen saturation were measured with a HACH HQ40d multi-parameter (salinity probe CDC401 and oxygen probe LDO101). Control fish (n = 5) were housed in individual aquaria with dechlorinated and aerated tap water. Fish were continuously observed until the first signs of morbidity. If erratic respiratory patterns and movements, or irregular reflexes and loss of equilibrium were observed (Kilambi & Zdinak Citation1980), time of exposure was recorded and fish were immediately removed and transferred into an aerated and recirculating freshwater tank to recover for 24 h.
Statistical analysis
The numbers of live and dead animals in each test chamber were recorded. The log-logistic model applied to determine the lethal concentrations (LC) only allowed the determination of the response levels higher than the LC0 and lower than the LC100 (Ritz & Streibig Citation2007). The same approach was used for effective time (ET). Survival endpoints were calculated using bootstrap resampling with associated 95% confidence intervals (CIs). Normality of the weight and length distributions of the fish was tested using a Shapiro–Wilk test. Statistical analyses were conducted with R (R Core Team Citation2016) with the dose–response studies package (Ritz & Streibig Citation2007), along with Statistica 13 for hypothesis testing (Dell Inc., Tulsa, USA).
For the transport water and equipment, mortality of each species of zooplankton was calculated relative to blank controls using the following formula:Treatments were only considered to be effective if they resulted in 100% mortality of all three zooplankton species.
Results
Zooplankton toxicity testing
Based on the results from the range-finding test (Table S2), Tsunami®, hydrogen peroxide, potassium permanganate, sodium hypochlorite, and sodium chloride were selected for definitive toxicity evaluations. The Daphnia was selected to determine the toxicity parameters (lethal doses) in a full-range definitive test because it was the hardiest species in the range-finding test (Table S2). All the chemicals tested achieved full control of the most tolerant species, D. pulex, at all the exposure times and the toxicity results are summarised in .
Table 1. Lethal concentrations LC10, LC50, and LC99 (n = 16) with 95% CIs for the chemicals tested on Daphnia pulex at different exposure times.
Treatment of transport water
All chemical treatments were 100% effective against D. pulex and S. pallidus in transport water; no live individuals were recovered from any of the sodium hypochlorite, Tsunami®, H2O2, KMnO4, or NaCl treatments. A. brightwelli was consistently the least resistant taxon in all other treatments, and as such NaCl was not tested on this species. Survival in blank controls was 84 ± 2% for D. pulex, 42 ± 7% for A. brightwelli, and 78 ± 11% for S. pallidus.
All mechanical filtration treatments effectively removed all zooplankton from water. The exception was the 50-µm filtration treatment where one S. pallidus individual was recovered from the filtered water. No differences between blank and procedural controls were observed.
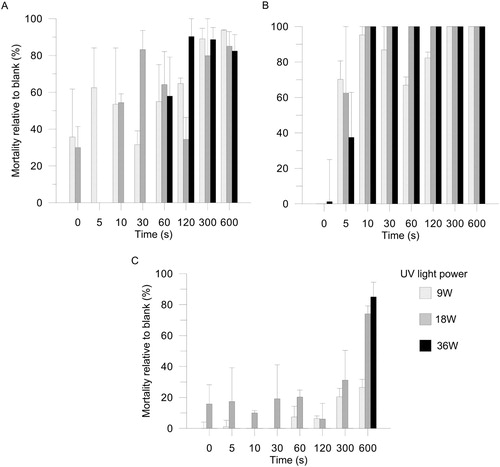
The three test organisms displayed varying sensitivities to UV-C sterilisation. Daphnia pulex were negatively affected by UV-C but none of the treatments resulted in 100% mortality; in the 10 min, 36 W treatment, mortality was 82 ± 9% (A). The rotifer A. brightwelli was the most sensitive, with 18 or 36 W UV-C for 10 s or longer sufficient to kill all individuals (B). The copepod S. pallidus was the most tolerant to UV-C, with 9 W having little or no negative effect and only the longest exposures to 18 and 36 W reducing survival by more than half (C). It should be noted that the number of live Daphnia recovered from the procedural controls was approximately 40% lower than in the blank controls.
Treatment of equipment
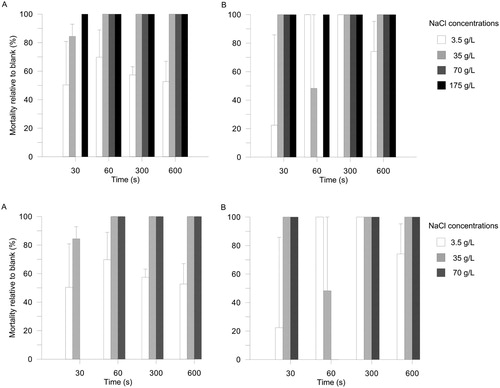
Tsunami®, potassium permanganate (KMnO4) and hydrogen peroxide (H2O2) were 100% effective against D. pulex and S. pallidus adhered to wool or felt. In the case of the lowest bleach concentration, mortality was 75 ± 20% for D. pulex on felt. The 11 g/L bleach treatment resulted in 71 ± 15% mortality relative to the blank for D. pulex on wool. All other bleach-equipment combinations reduced survival to zero. The procedural controls had slightly reduced survival (0%–22% mortality). Exposure to 35 g/L NaCl for ≥5 min rendered all D. pulex adhered to wool inert. For felt, 35 g/L NaCl was 100% effective after only 60 s. Insufficient S. pallidus adhered to wool for analysis, but all individuals that adhered to felt were killed by ≥35 g/L NaCl within 60 s ().
Figure 2. Mortality of D. pulex on A, wool or B, felt treated with sodium chloride solutions for various amounts of time. Values are means ± standard errors of numbers retained per 10 cm2 sample unit (n = 3).
For air drying, D. pulex survived for up to 24 h on wool, rope, and braided net, yet they took 72 h to die on felt. All S. pallidus died within 1 h on braided net, 6 h on wool, 24 h on rope, and 72 h on felt. For hot water, very few live A. brightwellii adhered to wool or felt (0.08 ± 0.04 and 0.2 ± 0.06, respectively). Daphnia pulex survival was reduced by the exposure to hot water, with 40°C for 5 min. or longer killing all D. pulex on both wool and felt. The results for S. pallidus were more variable than for D. pulex, due to the small numbers of S. pallidus that adhered to wool or felt (3.9 0.3 vs. 1.1 ± 0.2 and 6.6 ± 0.5 vs. 2.7 ± 0.2, respectively). Nevertheless, 50°C for 30 s or longer resulted in 100% mortality of all S. pallidus.
Fish tolerance
Length and weight of the grass carp were normally distributed, with means ± standard deviation of 164 ± 7 mm and 62.4 ± 7.9 g, respectively. The fish were successfully treated for parasites found in their gills (details provided in the supplementary material). Oxygen concentration in exposure tank remained at 100% saturation during the experiment. The fish could tolerate exposure to 26 g NaCl/L (26.06 ± 0.35 g/L, n = 20) up to a maximum of 5 min with no visible effects. The ET10, ET20, ET50, and ET90 (with 95% CIs) were 5.9 (4.5–7.4), 8.4 (6.9–9.9), 14.1 (12.7–15.4), and 24.4 (21.8–26.9) min, respectively. When returned to freshwater, 95% of the fish fully recovered after 24 h. At 26 g/L (26.02 ± 0.13 g/L, n = 5), survival of D. pulex steadily decreased within the first 5 min of the exposure. The LT50 and LT99.9 (with 95% CIs) are 2.04 (1.99–2.09) and 3.9 (3.7–4.1) min, respectively. No mortality occurred in the controls (salinity 0.1 g/L).
Discussion
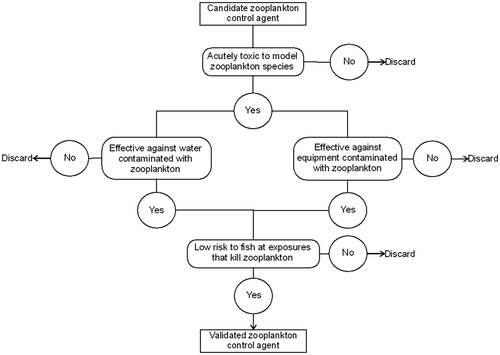
illustrates the approach used to select effective chemical treatments. The zooplankton tolerance to chemical treatments varied among species. The cladoceran D. pulex was consistently the most tolerant to chemical treatments, followed by the copepod S. pallidus and the rotifer A. brightwelli. D. pulex was selected for the definitive toxicity tests as an indicator to ensure total control of the other species. In addition, D. pulex is an established model species in standardised toxicity tests and well suited for the determination of the lethal concentration (OECD Citation1984; ISO Citation1996). All chemicals tested were effective in killing D. pulex in transport water and zooplankton-contaminated equipment.
The physical treatment methods (UV-C and mechanical filtration) evaluated for use on transport water contaminated with zooplankton effectively reduced zooplankton survival or removed individuals. The effectiveness of UV-C treatments varied between species. UV-C with 18 W for 10 s killed all A. brightwelli. None of the treatments totally controlled D. pulex and S. pallidus. The tolerance of S. pallidus to UV-C could be explained by this species being positively phototactic, congregating near the water’s surface (Chapman et al. Citation1985). D. pulex would be expected to be less tolerant given their tendency to migrate downward in the water column during the day (Rhode et al. Citation2001). It is possible that the surviving individuals found some shelter. Regardless, effective treatment requires 100% mortality of the target organism. A single viable copepod was recovered from the 50 µm mechanical filtration treatment, but mechanical filtration at ≤10 µm was 100% effective against the tested species. Mechanical filtration at ≤10 µm is a viable treatment method for contaminated water and is widely used in related fields such as the treatment of ballast water (Parsons & Harkins Citation2002).
For contaminated equipment that poses biosecurity risk, the effects of air drying were highly dependent on equipment type. All zooplankton died within 24 h on wool, rope, and net but survived for up to 72 h on felt. Felt has a high capacity to retain moisture, and it was observed that felt remained moist up until the 48 h sampling time point; at 72 h, felt was dry to the touch. Felt has been shown to be a high-risk equipment type, with felt-soled boots having been banned for use in New Zealand rivers due to the risk of spreading Didymo (Ryan Citation2009; Anderson et al. Citation2014). Heated water was an effective treatment for all the equipment types tested. Exposure to 40°C water for 5 min resulted in 100% mortality of D. pulex, while 50°C for 30 s or longer was effective against copepods. Comparable treatment protocols effective for treating ballast water are in the order of 40–55°C for a few seconds (Rigby et al. Citation2004; Quilez-Badia et al. Citation2008). Tsunami®, hydrogen peroxide, and potassium permanganate were 100% effective against zooplankton adhered to wool or felt.
The chemicals assessed were effective against zooplankton but the treatment doses utilised must not adversely impact the fish within transporters. Tsunami®, sodium hypochlorite, hydrogen peroxide, and potassium permanganate effectively killed zooplankton in water but the required concentrations exceed the tolerance of commonly farmed freshwater fish. For example, approximately 4 g/L hydrogen peroxide is required to effectively kill D. pulex, but the maximum recommended concentration for use in aquaculture is only 0.1 g/L for 60 min (US Fish and Wildlife Service Citation2015). Potassium permanganate must be dosed in excess of 0.075 g/L against zooplankton, a concentration approximately 10 times higher than the known lethal dose for eels (Madsen et al. Citation2000) and grass carp (Wen et al. Citation2007). These chemical treatments could still be applied to decontaminate equipment or bodies of water not containing fish.
Doses of 26 or 33 (full-strength seawater) g/L NaCl may be suitable for treating bodies of water containing farmed fish with low risk to the receiving environment depending on local dilution conditions. Eel, trout, and salmon are diadromous euryhaline fish and can physiologically adapt to transfer from freshwater to seawater (Olivereau & Olivereau Citation1977; Little et al. Citation2012). Small rainbow trout can survive direct transfer into full-strength seawater for up to 10 h and for longer periods at a concentration of around 22 g/L of NaCl (Vosylienė et al. Citation2006), while shortfin eels and Chinook salmon can survive longer period ( > 35 days) (Kearney et al. Citation2008).
The NaCl tolerance of grass carp, the aquaculture species most associated with zooplankton translocations, is less known. The grass carp is a stenohaline cyprinid and has a lower tolerance to salinity. This study showed no visible adverse effect up to 5 min at 26 g/L where loss of balance and gasping are observed. Grass carp can survive moderate salinity (13–15 g/L) for 11–23 h when slowly acclimated (Kilambi & Zdinak Citation1980), but their tolerance to shock-dosing with higher salinities has not been previously determined. In experiments where grass carp were slowly acclimated to salinity (3–9 g/L), the fish could not tolerate concentrations greater than 13–15 g/L during a 48 h exposure (Kilambi & Zdinak Citation1980). Salinity tolerances differ among fry, fingerling, and yearling stages of stenohaline freshwater fish. The 24, 48, and 96 h LD50 of NaCl at 15.7, 15.1, 15.1 g/L were found for grass carp (C. idella) fingerlings (100–120 mm) previously acclimated to 8 g/L of NaCl for 4 days. With shorter exposure time, Cross (Citation1970) found a 5-h survival at 50% full-strength seawater and loss of equilibrium was observed after 2.5 h at 20.2 g/L (Maceina & Shireman Citation1979). Nevertheless, it should not be assumed that the chemical treatment options alone will fully mitigate the risk of zooplankton translocation via transport water.
Zooplankton can be transferred between water bodies via their diapausing eggs. Asplanchna brightwelli and calanoid copepod have eggs > 100 µm diameter. Daphnia eggs are housed inside an ephippium (resting egg pouch), which for D. pulex is >500 × 1000 µm (Gilbert & Wurdak Citation1978; Dowell Citation1997; Gonzalez et al. Citation2008). While large numbers of these eggs may be found in sediments (due to their negative buoyancy), a small number could, if consumed, survive passage through the gut of fishes, or be found suspended in the transport water during the collection of the fish and through fish movement. For example, diapausing eggs of the spiny waterflea Bythotrephes longimanus and various Daphnia species (including D. pulex and D. galeata) remain viable following passage through fish guts (Mellors Citation1975; Jarnagin et al. Citation2000). Diapausing eggs of freshwater zooplankton are known to be resistant to chemical and physical treatments, including exposure to seawater, various chemical treatments and drying (Pati & Belmonte Citation2003; Gray et al. Citation2006; Radzikowski Citation2013).
None of the fish considered in this report are zooplanktivorous at the sizes transported, but some zooplankton could be accidentally consumed and survive as diapausing eggs. The passage of material through the gut of a grass carp is less than 8 h (Hickling Citation1966). However, the numbers transported in this manner would likely be small compared with those of active individuals carried in transport waters. The successful establishment of most zooplankton species typically requires initial high numbers of propagules (Taylor & Duggan Citation2012). As such, removal of active stages will minimise the likelihood of establishment by this vector. If present in the transport water, eggs are likely to be in low numbers and can be removed by filtration. Studies that aimed to separate diapausing rotifer eggs (which are the smallest eggs) from sediments used a mesh size ranging from 30 µm (Garcia-Roger et al. Citation2006) to 45 µm mesh (Bailey et al. Citation2003). Another study used 10 µm but the smallest axial dimension of rotifer eggs was greater than 50 µm (Maia-Barbosa et al. Citation2003).
Holding fish in ponds devoid of zooplankton for a period prior to transportation (e.g., >8 h for grass carp) should adequately address the risk of diapausing eggs. The best option for removing diapausing eggs from water would be using a ≤50 µm diameter mesh filter. For the treatment of equipment, drying will not kill diapausing eggs and hot water treatment may be the most effective option. Treatments with water at 60°C for less than 5 s have been found to be 100% effective in killing Daphnia eggs (Raikow et al. Citation2007).
There may be times when some species are producing eggs and others not. The timing and duration of zooplankton reproduction are highly variable within and among different taxonomic groups and strongly influenced by temperature and food availability (Pourriot & Snell Citation1983; DeMott Citation1989; Kleiven et al. Citation1992). Reproduction may be consistent throughout the year as the holding ponds likely have relatively stable conditions and resources.
Conclusions
Of all the chemicals tested, NaCl meets most requirements for an effective control agent, including availability, sensitivity of the target organisms, and low toxicity to fish. It is commonly used to treat equipment, being readily available, cheap, and non-toxic (Matheson et al. Citation2007), with a 35 g/L NaCl solution for 5 min shown in this study to be 100% effective against three model zooplankton species. However, there is little margin for error as carp may be adversely impacted if exposed longer than 5 min to NaCl. Sodium chloride is not listed in the Substance Exposure Limit Register from the NZ Environmental Protection Authority, is readily available as household table salt or agricultural salt, and poses a very low risk to operators or the environment. Soaking the carp in a 26 g NaCl/L bath for a brief period prior to transfer to a pond may be an option for reducing risk and kill D. pulex. When combined with purging the fish for at least 8 h to empty their guts and with water filtration of at least 50 µm, the chance of inadvertent transfer of non-indigenous zooplankton species will be greatly reduced. The assessment used an approach based on standard ecotoxicological tests and the next step is the development and validation of an effective protocol under field conditions.
Supplementary material
Download MS Word (422.2 KB)Acknowledgements
The authors thank Ken Cain (University of Idaho) for advice on the selection of chemicals, Steve Webb (Cawthron) for pathological examination of fish gill tissue and identification of parasites. The fish toxicity experiment was carried out with approval from the Nelson Marlborough Institute of Technology Animal Ethics Committee AEC2016-CAW01. Permits were obtained from the Department of Conservation for approval under Regulation 63(1) of the Freshwater Fisheries Regulations 1983 for inter-island transfer, and from the Ministry for Primary Industries for authorisation pursuant to section 26ZM(2)(b) of the Conservation Act 1987, to move zooplankton from the Auckland ponds (North Island) to Cawthron Institute in Nelson (South Island). The transfer of grass carp to Cawthron’s laboratory was approved under the Department of Conservation permit 48429-OTH.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Louis A. Tremblay http://orcid.org/0000-0002-3586-3995
Olivier Champeau http://orcid.org/0000-0002-1892-678X
Patrick Cahill http://orcid.org/0000-0003-0109-7206
Ian Duggan http://orcid.org/0000-0002-6037-9759
Additional information
Funding
References
- Anderson LG, White PC, Stebbing PD, Stentiford GD, Dunn AM. 2014. Biosecurity and vector behaviour: evaluating the potential threat posed by anglers and canoeists as pathways for the spread of invasive non-native species and pathogens. PLoS one. 9(4):e92788. doi: 10.1371/journal.pone.0092788
- Bailey SA, Duggan IC, van Overdijk CDA, Jenkins PT, MacIsaac HJ. 2003. Viability of invertebrate diapausing eggs collected from residual ballast sediment. Limnology and Oceanography. 48(4):1701–1710. doi: 10.4319/lo.2003.48.4.1701
- Balvert SF, Duggan IC, Hogg ID. 2008. Zooplankton seasonal dynamics in a recently filled mine pit lake: the effect of non-indigenous daphnia establishment. Aquatic Ecology. 43(2):403–413. doi: 10.1007/s10452-008-9165-z
- Branford SN, Duggan IC. 2017. Grass carp (Ctenopharyngodon idella) translocations, including hitchhiker introductions, alter zooplankton communities in receiving ponds. Marine and Freshwater Research. 68(12):2216–2227. doi: 10.1071/MF17051
- Chapman M, Green J, Northcote T. 1985. Seasonal dynamics of Skistodiaptomus pallidus herrick and other zooplankton populations in deer lake, SW British Columbia. Journal of Plankton Research. 7(6):867–876. doi: 10.1093/plankt/7.6.867
- Clout MN, Lowe SJ. 2000. Invasive species and environmental changes in New Zealand. In: Mooney HA, Hobbs RJ, editors. Invasive species in a changing world. Washington (DC): Island Press; p. 369–383.
- Cross DG. 1970. The tolerance of grass carp Ctenopharyngodon idella (Val.) to seawater. Journal of Fish Biology. 2(3):231–233. doi: 10.1111/j.1095-8649.1970.tb03279.x
- DeMott WR. 1989. The role of competition in zooplankton succession. In: U. Sommer, editor. Plankton ecology. succession in plankton communities. Berlin: Springer-Verlag; p. 195–252.
- Dowell KM. 1997. Evidence for diapause in the freshwater copepod Skistodiaptomus pallidus. American Midland Naturalist. 137:362–368. doi: 10.2307/2426855
- Duggan IC, Collier KC. Forthcoming. Management of non-indigenous lacustrine animals. Lake Restoration Handbook. Springer Books.
- Duggan IC, Neale MW, Robinson KV, Verburg P, Watson NTN. 2014. Skistodiaptomus pallidus (copepoda: diaptomidae) establishment in New Zealand natural lakes, and its effects on zooplankton community composition. Aquatic Invasions. 9:195–202. doi: 10.3391/ai.2014.9.2.08
- Duggan IC, Pullan S. 2017. Do freshwater aquaculture facilities provide an invasion risk for zooplankton hitchhikers. Biological Invasions. 19:307–314. doi: 10.1007/s10530-016-1280-5
- Gallardo B, Clavero M, Sánchez MI, Vilà M. 2015. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology. 22(1):151–163. doi: 10.1111/gcb.13004
- Garcia-Roger EM, Carmona MJ, Serra M. 2006. Hatching and viability of rotifer diapausing eggs collected from pond sediments. Freshwater Biology. 51(7):1351–1358. doi: 10.1111/j.1365-2427.2006.01583.x
- GESAMP. 2013. Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP). Revised GESAMP hazard evaluation procedure for chemical substances carried by ships. 2nd ed. 2nd Edition GESAMP Reports and Studies No. 64; 106 p.
- Gilbert JJ, Wurdak ES. 1978. Species-specific morphology of resting eggs in the rotifer Asplanchna. Transactions of the American Mathematical Society. 97:330–339. doi: 10.2307/3225986
- Goldson S, Bourdot G, Brockerhoff E, Byrom A, Clout M, McGlone M, Nelson W, Popay A, Suckling D, Templeton M. 2015. New Zealand pest management: current and future challenges. Journal of the Royal Society of New Zealand. 45(1):31–58. doi: 10.1080/03036758.2014.1000343
- Gonzalez EJ, Matsumura-Tundisi T, Tundisi JG. 2008. Size and dry weight of main zooplankton species in bariri reservoir (SP, Brazil). Brazilian Journal of Biology. 68(1):69–75. doi: 10.1590/S1519-69842008000100010
- Gray DK, Duggan IC, MacIsaac HJ. 2006. Can sodium hypochlorite reduce the risk of species introductions from diapausing invertebrate eggs in non-ballasted ships. Marine Pollution Bulletin. 52:689–695. doi: 10.1016/j.marpolbul.2005.11.001
- Hickling CF. 1966. On the feeding process in the white Amur, ctenopharyngodon idella. Journal of Zoology. 148(4):408–419. doi: 10.1111/j.1469-7998.1966.tb02960.x
- ISO. 1996. 6341 – Water quality – Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) – Acute toxicity test.
- Jarnagin ST, Swan BK, Kerfoot WC. 2000. Fish as vectors in the dispersal of Bythotrephes cederstroemi: diapausing eggs survive passage through the gut. Freshwater Biology. 43(4):579–589. doi: 10.1046/j.1365-2427.2000.t01-1-00547.x
- Jay M, Morad M, Bell A. 2003. Biosecurity, a policy dilemma for New Zealand. Land Use Policy. 20(2):121–129. doi: 10.1016/S0264-8377(03)00008-5
- Kearney M, Jeffs A, Lee P. 2008. Effects of salinity and temperature on the growth and survival of New Zealand shortfin, Anguilla australis, and longfin, A. dieffenbachii, glass eels. Aquaculture Research. 39(16):1769–1777. doi: 10.1111/j.1365-2109.2008.02053.x
- Kilambi RV, Zdinak A. 1980. The effects of acclimation on the salinity tolerance of grass carp, Ctenopharyngodon idella (Cuv. and Val.). Journal of Fish Biology. 16(2):171–175. doi: 10.1111/j.1095-8649.1980.tb03696.x
- Kilroy C, Unwin M. 2011. The arrival and spread of the bloom-forming, freshwater diatom, Didymosphenia geminata, in New Zealand. Aquatic Invasions. 6(3):249–262. doi: 10.3391/ai.2011.6.3.02
- Kleiven T, Larsson P, Hobæk A, Kleiven OT, Larsson P, Hobaek A. 1992. Sexual reproduction in Daphnia magna requires three stimuli. Oikos. 65:197–206. doi: 10.2307/3545010
- Laxson CL, McPhedran KN, Makarewicz JC, Telesh IV, MacIsaac HJ. 2003. Effects of the non-indigenous cladoceran Cercopagis pengoi on the lower food web of lake Ontario. Freshwater Biology. 48(12):2094–2106. doi: 10.1046/j.1365-2427.2003.01154.x
- Little EE, Calfee RD, Linder G. 2012. Toxicity of copper to early-life stage kootenai river white sturgeon, Columbia river white sturgeon, and rainbow trout. Archives of Environmental Contamination and Toxicology. 63(3):400–408. doi: 10.1007/s00244-012-9782-3
- Maceina MJ, Shireman JV. 1979. Grass carp: effects of salinity on survival, weight loss, and muscle tissue water content. The Progressive Fish-Culturist. 41(2):69–73. doi: 10.1577/1548-8659(1979)41[69:GCOSOS]2.0.CO;2
- Madsen HCK, Buchmann K, Mellergaard S. 2000. Treatment of trichodiniasis in eel (Anguilla anguilla) reared in recirculation systems in Denmark: alternatives to formaldehyde. Aquaculture. 186(3–4):221–231. doi: 10.1016/S0044-8486(99)00379-8
- Maia-Barbosa PM, Eskinazi-Sant'Anna EM, Valadares CF, Pessoa GCD. 2003. The resting eggs of zooplankton from a tropical, eutrophic reservoir (Pampulha reservoir, south-east Brazil). Lakes and Reservoirs: Research and Management. 8(3–4):269–275. doi: 10.1111/j.1440-1770.2003.00229.x
- Matheson FE, Dugdale A, Wells R, Taumoepeau A, Smith J. 2007. Efficacy of saltwater solutions to kill introduced freshwater species and sterilise freshwater fishing nets. DOC Research & Development Series 261. 0478141408. 24 p.
- Mellors WK. 1975. Selective predation of ephippal Daphnia and the resistance of ephippal eggs to digestion. Ecology. 56(4):974–980. doi: 10.2307/1936308
- Ministry for Primary Industries. 2008. Check clean dry. [accessed 2016 Feb 26]. http://www.biosecurity.govt.nz/biosec/camp-acts/check-clean-dry.
- Mountfort D, Hay C, Taylor M, Buchanan S, Gibbs W. 1999. Heat treatment of ships’ ballast water: development and application of a model based on laboratory studies. Journal of Marine Environmental Engineering. 5(3):193–206.
- OECD. 1984. OECD Guidelines for the Testing of Chemicals, Section 2 – Effects on Biotic Systems – Test No. 202: Daphnia sp., acute immobilisation test and reproduction test. Paris. p. 16.
- OECD. 1992. OECD Guidelines for the Testing of Chemicals, Section 2 – Effects on Biotic Systems – Test No. 203: Fish, acute toxicity test. p. 10.
- Olivereau M, Olivereau J. 1977. Effect of transfer to sea water and back to fresh water on the histological structure of the eel kidney. Journal of Comparative Physiology. 115(2):223–239. doi: 10.1007/BF00692533
- Parliamentary Counsel Office. 1993. New Zealand Biosecurity Act 1993. p. 300. http://www.legislation.govt.nz/act/public/1993/0095/latest/DLM314623.html.
- Parsons MG, Harkins RW. 2002. Full-scale particle removal performance of three types of mechanical separation devices for the primary treatment of ballast water. Marine Technology. 39(4):211–222.
- Pati AC, Belmonte G. 2003. Disinfection efficacy on cyst viability of Artemia franciscana (crustacea), Hexarthra fennica (rotifera) and Fabrea salina (ciliophora). Marine Biology. 142:895–904. doi: 10.1007/s00227-003-1026-7
- Pourriot R, Snell TW. 1983. Resting eggs in rotifers. In: Pejler B, Starkweather R, Nogrady T, editors. Biology of rotifers: proceedings of the third international rotifer symposium held at Uppsala, Sweden, August 30 – September 4, 1982. Dordrecht: Springer; p. 213–224.
- Quilez-Badia G, McCollin T, Josefsen KD, Vourdachas A, Gill ME, Mesbahi E, Frid CL. 2008. On board short-time high temperature heat treatment of ballast water: a field trial under operational conditions. Marine Pollution Bulletin. 56(1):127–135. doi: 10.1016/j.marpolbul.2007.09.036
- Radzikowski J. 2013. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. Journal of Plankton Research. 35(4):707–723. doi: 10.1093/plankt/fbt032
- Raikow DF, Reid DF, Blatchley ERI, Jacobs G, Landrum PF. 2007. Effects of proposed physical ballast tank treatments on aquatic invertebrate resting eggs. Environmental Toxicology. 26:717–726. doi: 10.1897/06-403R.1
- R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
- Rhode SC, Pawlowski M, Tollrian R. 2001. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature. 412(6842):69–72. doi: 10.1038/35083567
- Ricciardi A. 2015. Chapter 5 – Ecology of Invasive Alien Invertebrates A2 – James H. Thorp, D. Christopher Rogers. Thorp and Covich’s Freshwater Invertebrates. 4th ed. Boston (MA): Academic Press. p. 83–91.
- Rigby G, Hallegraeff G, Taylor A. 2004. Ballast water heating offers a superior treatment option. Journal of Marine Environmental Engineering. 7(3):217–230.
- Ritz C, Streibig JC. 2007. Bioassay analysis using R. Journal of Statistical Software. 12(5):18.
- Ryan FS. 2009. Banning felt soles in Vermont: a call for state legislative response to the spread of invasive didymo. University of Denver Water Law Review. 13:83.
- Sutherland T, Levings C, Elliott C, Hesse W. 2001. Effect of a ballast water treatment system on survivorship of natural populations of marine plankton. Marine Ecology Progress Series. 210:139–148. doi: 10.3354/meps210139
- Taylor CM, Duggan IC. 2012. Can biotic resistance be utilized to reduce establishment rates of non-indigenous species in constructed waters? Biological Invasions. 14(2):307–322. doi: 10.1007/s10530-011-0063-2
- Tsolaki E, Diamadopoulos E. 2010. Technologies for ballast water treatment: a review. Journal of Chemical Technology and Biotechnology. 85(1):19–32. doi: 10.1002/jctb.2276
- US Fish and Wildlife Service. 2015. Quick desk reference guide to: Approved drugs for use in aquaculture. 2nd ed. https://www.fws.gov/fisheries/aadap/PDF/2nd-Edition-FINAL.pdf.
- Vosylienė MZ, Baltrėnas P, Kazlauskienė A. 2006. Toxicity of road maintenance salts to rainbow trout Oncorhynchus mykiss. Ekologija. 2:15–20.
- Waite T, Kazumi J, Lane P, Farmer L, Smith S, Smith S, Hitchcock G, Capo T. 2003. Removal of natural populations of marine plankton by a large-scale ballast water treatment system. Marine Ecology Progress Series. 258:51–63. doi: 10.3354/meps258051
- Wen R-S, Zheng Q-M, Fang Z-Q, Pan G-H. 2007. Acute toxicity of dipterex and KMnO4 to juvenile grass carp Ctenopharyngodon idellus [J]. Fisheries Science. 7:008.
- Wotton DM, Hewitt CL. 2004. Marine biosecurity post-border management: developing incursion response systems for New Zealand. New Zealand Journal of Marine and Freshwater Research. 38(3):553–559. doi: 10.1080/00288330.2004.9517260
- Yan ND, Blukacz A, Sprules WG, Kindy PK, Hackett D, Girard RE, Clark BJ. 2001. Changes in zooplankton and the phenology of the spiny water flea, Bythotrephes, following its invasion of Harp lake, Ontario, Canada. Canadian Journal of Fisheries and Aquatic Sciences. 58(12):2341–2350. doi: 10.1139/f01-171