ABSTRACT
Diet analysis can provide an insight into the structure and function of an ecosystem, and can be used in ecosystem-based frameworks to inform management and conservation decisions. Diet composition of rough skate (Zearaja nausta) from the south east coast of New Zealand, was investigated for the first time. We examined 35 stomachs from three trawls between March and June 2017. Prey importance was assessed by a prey specific index of relative importance (PSIRI). This population of rough skates (n = 32) was found to be specialised feeders, primarily preying on one species, Nectocarcinus antarcticus. This study provides the first insight into rough skate trophic interactions in the surrounding marine community.
Introduction
The rough skate, Zearaja nasuta (Müller & Henle 1841) is the most common endemic skate in New Zealand waters and an increasingly important commercial fish species (Ministry for Primary Industries Citation2017). Managed in New Zealand’s Quota Management System (QMS) with a second skate species, smooth skate, Dipturus innominatus, rough skate reported landings have increased from 325 to 1,553 tonne between 1996–97 and 2015–16 (Ministry for Primary Industries, Citation2017). A recent qualitative risk assessment of New Zealand elasmobranchs suggested rough skates are among the most vulnerable species to overexploitation, due to its high range overlap with commercial fishing grounds, being caught for more than 300 days a year and a lack of information pertaining to ecological interactions (Ford et al. Citation2018). Recently, regional fisheries management organisations have recognised the value of diet studies as one of the main components supporting ecosystem-based fisheries management frameworks (e.g. Juan-Jordá et al. Citation2018). Diet descriptions can also provide insight into the structure and functioning of ecological communities by quantifying interactions between a predator and its prey (Caddy and Sharp Citation1986; Cortés Citation1999). Therefore, understanding feeding patterns and the ecological role played by respective species in ecosystems are imperative to develop smarter management strategies and make smarter conservation decisions (De Ruiter et al. Citation2005). No dietary information is available for rough skates in any location surrounding New Zealand and without information pertaining to trophic interactions and feeding ecology, the ecological role of this species remains unclear.
In this short communication, we describe the diet composition of rough skates from one geographical area over a three-month period using an index of relative importance. Although we are limited by the spatio-temporal scale of this study, the information is relevant in providing the first diet composition description for this species and first insight into rough skate trophic interactions with the surrounding marine community.
Materials and methods
Specimen collection and dissection
Rough skates were collected in waters around Nugget Point, off the eastern coast of New Zealand (approximately 46.4481°S, 169.8147°E) by a commercial bottom trawler on three occasions between March and June 2017. Collections occurred between <1–20 km offshore at a depth of approximately 40 metres.
Stomach analysis
The stomach state was recorded, being either empty or not empty. Any empty stomachs were excluded from the dietary analysis. Stomach contents were rinsed, counted, and weighed to the nearest gram. Contents were examined and prey items were identified to the lowest taxonomic level possible using reference guides (e.g. Naylor et al. Citation2005; Tracey et al. Citation2005; McMillan, Francis, et al. Citation2011; McMillan, Griggs, et al. Citation2011). Whenever fragments of prey items were found, the number of individuals was taken as the smallest possible number of individuals from which the fragments could have originated.
To determine the importance of each prey in the diet, the prey-specific index of relative importance (%PSIRI) (Brown et al. Citation2012) was used according to the equation: %PSIRI = 0.5 x %O x (%PNi + %PWi), where %O is the percent frequency of occurrence (the number of stomachs containing prey i divided by the total number of stomachs, n), and %PNi and %PWi are the prey-specific abundances by number and weight, respectively. Prey-specific abundance was calculated with the equation: %PAi = , where %Aij is the abundance (by number %PNi or weight %PWi) of prey i in stomach sample j and ni is the number of stomachs containing prey i. The %PSIRI is a modification of the index of relative importance (IRI) (Pinkas et al. Citation1971).
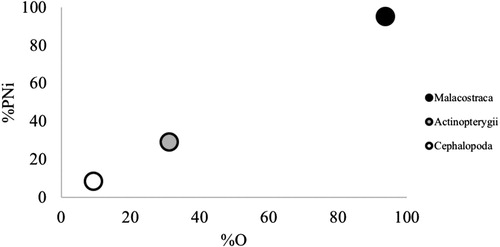
A cumulative prey curve was computed using the software EstimateS version 9.1.0 (Colwell Citation2013) to determine whether our sample size was sufficient to recover all prey items comprising rough skate diet. Diversity settings were set to 100 randomizations and rarefaction curve was extrapolated a further 32 samples. Adequacy of the number of stomachs sampled was assessed using the slope of the linear regression through the last five sub-samples or through five consecutive sub-samples until the slope reached 0.05 (considered adequately close to the asymptote as per Bizzaro et al. Citation2009). The feeding strategy was identified by plotting the prey-specific abundance by number (%PNi) of the three main prey groups (Malacostraca, Actinopterygii and Cephalopoda) against the mean %O (Costello Citation1990; Amundsen et al. Citation1996). In this method, any prey located close to 100% PNi and 100% O represents specialisation.
Results
Sample description
In total, 35 rough skate stomachs were collected, of which three (8.6%) were empty. The number of skates collected per sampling date ranged from 10 to 14 individuals. Twelve specimens were male (TL 56.0 to 85.0 cm) and 23 were female (TL 71.0 to 94.0 cm).
Diet composition %PNi and %PWi
In total, 13 prey species were identified, including five species of Malacostraca, six species of Actinopterygii, and two species of Cephalopoda (). In terms of %PSIRI, rough skates fed primarily on Malacostraca (85.2%), while Actinopterygii (13.0%) and Cephalopoda (1.9%) had a much lower contribution to the diet. Additionally, Malacostraca dominated the diet in terms of %PNi (95.2%), %O (93.8%), and %PWi (86.5%). Among Malacostraca, one species of crab, Nectocarcinus antarcticus contributed the most to the diet: %PNi (87.8%), %O (84.4%), %PWi (79.2%), and %PSIRI (70.5%) (). The second most important prey species to the diet of rough skate was another species of Malacostraca, Pterygosquilla armata, with a %PSIRI of 5.8%. Actinopterygii accounted for considerably less of the overall diet with %PNi, %O, and %PWi of 29.9%, 31.3%, and 51.0%, respectively (). Cephalopoda contributed less to the overall diet of rough skate in terms of all dietary indices (%PNi = 8.3%, %O = 9.4%, %PWi = 31.6%).
Table 1. Diet composition of Z. nasuta off the coast of Nugget Point in terms of frequency of occurrence (%O), per cent prey-specific abundance by number (%PNi), prey-specific abundance by weight (%PWi), and prey-specific index of relative importance (%PSIRI), where N is number of prey and ni is number of stomachs containing specific prey.
Feeding strategy
Overall, rough skates show specialisation on Malacostraca with %PNi of 95.2% and %O of 93.8% (). Few skates preyed on both Cephalopoda and Actinopterygii, respectively, represented by PNi of less than 30% for both groups.
Adequacy of sample size
Our cumulative prey curve failed to reach asymptote at 32 stomachs and 13 prey items. Rarefaction curve estimated that between 14 and 15 prey items would comprise rough skate diet at 55 stomachs samples (when our projected curve would reach a slope of 0.0475).
Discussion
Our study shows that off the shore of Nugget Point and between the months of March and June, New Zealand’s endemic rough skate has a specialised diet, primarily consuming one species of crab, N. antarcticus. A crustacean dominated diet is a trait shared by many other small bodied skates (e.g. Ebert et al. Citation1991; Walmsley-Hart et al. Citation1999; Braccini and Perez Citation2005; San Martin et al. Citation2007; Carrier et al. Citation2012). All other prey items contributed little to the overall diet, possibly a result of opportunistic or incidental prey capture.
The rough skates examined in this study have a continuous feeding strategy. This is based on observations of few empty stomachs and different levels of prey digestion (Pers Obs.). Continuous feeding strategies are evident in many skate species (e.g. Wetherbee et al. Citation1990, Citation2004; Ebert et al. Citation1991; Muto et al. Citation2001; Motta Citation2004; Wilga et al. Citation2012) and is possibly explained by the abundance of readily available prey living on the surface and subsurface layer of the benthos (Wetherbee et al. Citation1990, Citation2004; Jacobsen and Bennett Citation2013).
A small sample size and large variation in food categories can alter the dietary importance of each prey items (Hyslop Citation1980). The minimum number of stomachs required for a precise description of a predator’s diet should be determined. For rough skates, 55 stomachs are required to reach a predicted stable prey diversity asymptote suggesting our sample size does not capture the true diversity of rough skate diet. Our study does, however, allow the first insight into the diet composition of rough skates off the coast of Nugget point between autumn and winter months for 2017. Additional sampling in other years or other months would be necessary to make broader generalisations and to reach a sufficient sample size, although we would expect only one or two additional prey items to be recorded by increased sampling.
Given that our samples were obtained from a commercial fisherman, it was not possible to investigate ontogenetic shifts in feeding habits of rough skates, as our sample showed a significant size bias towards larger adult specimens. In many skate species, as they grow, their diet tends to shift towards larger and heavier prey items (e.g. Wetherbee et al. Citation2004; Treloar et al. Citation2007; Lucifora et al. Citation2009; Carrier et al. Citation2012; Forman and Dunn Citation2012; Kadri et al. Citation2013). Typically, skates feed on crustaceans and move to a more teleost-based diet as they grow (e.g. Treloar et al. Citation2007; Carrier et al. Citation2012; Forman and Dunn Citation2012). We would expect rough skate to undergo ontogenetic shift in diet as they grow and mature, but this will need to be investigated further.
The dietary information presented in this study provides a snapshot of the diet composition of rough skates between the autumn and winter months of a given year. It is very likely that rough skates will exhibit seasonal variation in feeding ecology, such that reflects environmental changes that influence the distribution and abundance of prey items. Feeding patterns will differ at other times of the year as found in other skate species (Motta Citation2004). However, the benthic invertebrate fauna and its seasonal patterns off the southeast coast of New Zealand are poorly studied with only brief reports on the communities from these areas (e.g. Probert et al. Citation1979). N. antarcticus is an endemic species that is common and abundant at depths of 20–60 m (Main Citation1974), but whose relative and seasonal abundances have yet to be assessed.
Rough skates are an increasingly important resource within New Zealand’s commercial fisheries (Ministry for Primary Industries Citation2017). According to the most recent fisheries report, it is not yet known if the current catch levels or total allowable catch are sustainable (Ministry for Primary Industries Citation2017). This information lacks as a result of under researched ecological parameters pertaining to population productivity and life history characteristics. Dietary studies are an important step in supporting ecosystem-based fisheries management (Brodeur et al. Citation2017; Livingston et al. Citation2017; Rohan and Buckley Citation2017). Understanding basic dietary composition and trophic interactions of a predator and its prey within a localised environment may help prevent the collapse of, or reduce the risks of, local extirpation of these species while keeping fishing levels at a sustainable level.
In summary, the rough skate population located off Nugget Point, around the east coast of the South Island of New Zealand, during the autumn and winter months is a specialised and continuous feeder that primarily feeds on crustaceans, and in particular on a single species of crab, N. antarcticus. These results suggest that rough skates might play a unique functional role in New Zealand’s benthic food web due to its strong predation interaction with N. antarcticus. Despite rough skate’s potential food web importance and vulnerability to overexploitation (Ford et al. Citation2018), its life history characteristics and overall ecological role is still poorly known within this environment, however we now have basic information pertaining to diet composition and trophic interactions of this species.
Acknowledgements
The authors are grateful to Gavin Heineman for collecting the skate specimens, to Fatima Jorge for guidance with the analyses and to Olwyn Friesen, Brandon Ruhele and Bronwen Presswell for help with the skate dissections. We also thank members of the Evolutionary and Ecological Parasitology Research Group for their comments on the early daft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Jerusha Bennett http://orcid.org/0000-0003-1037-894X
Haseeb S. Randhawa http://orcid.org/0000-0001-6262-970X
Additional information
Funding
References
- Amundsen P-A, Gabler H-M, Staldvic FJ. 1996. A new approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. J Fish Biol. 48(4):607–614.
- Bizzarro JJ, Smith WD, Márquez-Farías JF, Tyminski J, Hueter RE. 2009. Temporal variation in the artisanal elasmobranch fishery of Sonora, Mexico. Fish Res. 97:103–117. doi: 10.1016/j.fishres.2009.01.009
- Braccini JM, Perez JE. 2005. Feeding habits of the sandskate Psammobatis extenta (Garman, 1913): sources of variation in dietary composition. Mar Freshwater Res. 56(4):395–403. doi: 10.1071/MF04205
- Brodeur RD, Smith BE, McBride RS, Heintz R, Farley E Jr. 2017. New perspectives on the feeding ecology and trophic dynamics of fishes. Environ Biol Fish. 100(4):293–297. doi: 10.1007/s10641-017-0594-1
- Brown SC, Bizzarro JJ, Cailliet GM, Ebert DA. 2012. Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environ Biol Fish. 95(1):3–20. doi: 10.1007/s10641-011-9959-z
- Caddy JF, Sharp GD. 1986. An ecological framework for marine fishery investigations. Rome: Food and Agriculture Organization of the United Nations (FAO). Fisheries Technical Paper No 283.
- Carrier JC, Musick JA, Heithaus MR, editors. 2012. Biology of sharks and their relatives. Boca Raton: CRC Press.
- Colwell RK. 2013. EstimateS: statistical estimation of species richness from samples (Software and User’s Guide), Version 9.1.0. USA: University of Conneticut.
- Cortés E. 1999. Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci. 56(5):707–717. doi: 10.1006/jmsc.1999.0489
- Costello MJ. 1990. Predator feeding strategy and prey importance: a new graphical analysis. J Fish Biol. 36(2):261–263. doi: 10.1111/j.1095-8649.1990.tb05601.x
- De Ruiter PC, Wolters V, Moore JC, editors. 2005. Dynamic food webs: multispecies assemblages, ecosystem development and environmental change. New York: Academic Press.
- Ebert DA, Cowley PD, Compagno LJV. 1991. A preliminary investigation of the feeding ecology of skates (Batoidea: Rajidae) off the west coast of southern Africa. South African J Mar Sci. 10(1):71–81. doi: 10.2989/02577619109504621
- Ford RB, Francis MP, Holland L, Clark MR, Duffy CAJ, Dunn MR, Jones E, Wells R. 2018. Qualitative (Level 1) Risk Assessment of the impact of commercial fishing on New Zealand chondrichthyans: an update for 2017. Wellington: Ministry for Primary Industries. New Zealand Aquatic Environment and Biodiversity Report No 201.
- Forman JS, Dunn MR. 2012. Diet and scavenging habits of the smooth skate Dipturus innominatus. J Fish Biol. 80(5):1546–1562. doi: 10.1111/j.1095-8649.2012.03255.x
- Hyslop EJ. 1980. Stomach contents analysis – a review of methods and their application. J Fish Biol. 17(4):411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
- Jacobsen IP, Bennett MB. 2013. A comparative analysis of feeding and trophic level ecology in stingrays (Rajiformes; Myliobatoidei) and electric rays (Rajiformes: Torpedinoidei). PLoS One. 8(8):e71348. doi: 10.1371/journal.pone.0071348
- Juan-Jordá MJ, Murua H, Arrizabalaga H, Dulvy NK, Restrepo V. 2018. Report card on ecosystem-based fisheries management in tuna regional fisheries management organizations. Fish Fish. 19(2):321–339. doi: 10.1111/faf.12256
- Kadri H, Saïdi B, Marouani S, Bradai MN, Bouaïn A. 2013. Food habits of the rough ray Raja radula (Chondrichthyes: Rajidae) from the Gulf of Gabès (central Mediterranean Sea). Ital J Zool. 80(1):52–59. doi: 10.1080/11250003.2012.697925
- Livingston PA, Aydin K, Buckley TW, Lang GM, Yang M-S, Miller BS. 2017. Quantifying food web interactions in the North Pacific – a data-based approach. Environ Biol Fish. 100(4):443–470. doi: 10.1007/s10641-017-0587-0
- Lucifora LO, García VB, Menni RC, Escalante AH, Hozbor NM. 2009. Effects of body size, age and maturity stage on diet in a large shark: ecological and applied implications. Ecol Res. 24(1):109–118. doi: 10.1007/s11284-008-0487-z
- Main, WdeL. 1974. Distribution and ecology of Nectocarcinus antarcticus and N. bennetti (Brachyura: Portunidae) in the New Zealand region. New Zeal J Mar Fresh. 8(1):15–38. doi: 10.1080/00288330.1974.9515489
- McMillan PJ, Francis MP, James GD, Paul LJ, Marriott PJ, Mackay E, Wood BA, Griggs LH, Sui H, Wei F. 2011. New Zealand fishes, Volume 1: a field guide to common species caught by bottom and midwater fishing. Wellington: Ministry of Fisheries. New Zealand Aquatic Environment and Biodiversity Report No 68.
- McMillan PJ, Griggs LH, Francis MP, Marriott PJ, Paul LJ, Mackay E, Wood BA, Sui H, Wei F. 2011. New Zealand fishes. Volume 3: a field guide to common species caught by surface fishing. Wellington: Ministry of Fisheries. New Zealand Aquatic Environment and Biodiversity Report No 69.
- Ministry for Primary Industries. 2017. Fisheries assessment plenary May 2017: stock assessments and stock status. Wellington: Ministry for Primary Industries.
- Motta PJ. 2004. Prey capture behavior and feeding mechanics of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; p. 1245–1301.
- Muto EY, Soares LSH, Goitein R. 2001. Food resource utilization of the skates Rioraja agassizii (Müller & Henle, 1841) and Psammobatis extenta (Garman, 1913) on the continental shelf off Ubatuba, South-eastern Brazil. Rev Bras Biol. 61(2):217–238. doi: 10.1590/S0034-71082001000200005
- Naylor JR, Webber WR, Booth JD. 2005. A guide to common offshore crabs in New Zealand waters. Wellington: Ministry of Fisheries. New Zealand Aquatic Environment and Biodiversity Report No 2.
- Pinkas L, Oliphant MS, Iverson ILK. 1971. Food habits of albacore, bluefin tuna and bonito in Californian waters. State of California: The Resources Agency, Department of Fish and Game; Fish Bulletin No 152.
- Probert PK, Batham EJ, Wilson JB. 1979. Epibenthic macrofauna off southeastern New Zealand and mid-shelf bryozoan dominance. New Zeal J Mar Fresh. 13(3):379–392. doi: 10.1080/00288330.1979.9515814
- Rohan SK, Buckley TW. 2017. Spatial and ontogenetic patterns of Pacific cod (Gadus macrocephalus Tilesius) predation on octopus in the eastern Bering Sea. Environ Biol Fish. 100(4):361–373. doi: 10.1007/s10641-016-0561-2
- San Martin MJ, Braccini JM, Tamini LL, Chiaramonte GE, Perez JE. 2007. Temporal and sexual effects in the feeding ecology of the marbled sand skate Psammobatis bergi Marini, 1932. Mar Biol. 151(2):505–513. doi: 10.1007/s00227-006-0499-6
- Tracey DM, Anderson OF, Clark MR, Oliver MD. 2005. A guide to common deepsea invertebrates in New Zealand waters. Wellington: Ministry of Fisheries. New Zealand Aquatic Environment and Biodiversity Report No 1.
- Treloar MA, Laurenson LJB, Stevens JD. 2007. Dietary comparisons of six skate species (Rajidae) in south-eastern Australian waters. Environ Biol Fish. 80:181–196. doi: 10.1007/s10641-007-9233-6
- Walmsley-Hart SA, Sauer WHH, Buxton CD. 1999. The biology of the skates Raja wallacei and R. pullopunctata (Batoidea: Rajidae) of the Agulhas Bank, South Africa. South African J Mar Sci. 21:165–179. doi: 10.2989/025776199784126051
- Wetherbee BM, Cortés E, Bizzaro JJ. 2004. Food consumption and feeding habits. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; p. 225–246.
- Wetherbee BM, Gruber SH, Cortés E. 1990. Diet, feeding habits, digestion, and consumption in sharks, with special reference to the lemon shark Negaprion brevirostris. NOAA Technical Report, NM FS 90(1):29–47.
- Wilga CD, Maia A, Nauwelaerts S, Lauder GV. 2012. Prey handling using whole-body fluid dynamics in batoids. Zoology. 115(1):47–57. doi: 10.1016/j.zool.2011.09.002