ABSTRACT
New Zealand estuaries and harbours are subjected to increasing sediment deposition that can smother and bury infaunal communities, yet how coastal species respond to sediment deposition is not well understood. Here, we experimentally examined the effects of native marine sediment deposition on the NZ cockle (Austrovenus stutchburyi). Cockles were found to be highly mobile and capable burrowers, able to resurface within days from beneath 2–25 cm of sediment where no physical disturbance to their natural orientation occurred. Cockles were also resilient to daily (2 cm) reburials. However, following disturbance to their natural orientation, inverted cockles were significantly impeded when buried under 5–10 cm of sediment, with fewer adults resurfacing than sub-adults. Cockle populations are likely to be resilient to native sediment deposition, unless physically disturbed. When disturbed from their natural orientation in the sediment, higher mortality of larger adult-sized cockles would be predicted, with mortality increasing under thicker sediment deposits.
Introduction
Infaunal bivalve beds, including commercially, recreationally and culturally valued species such as cockles, can play an important, and often critical functional role in our coastal and marine ecosystems (Thrush et al. Citation2006). Bivalve beds are known to improve water quality, stabilise and oxygenate sediments, as habitat modifiers and active bio-engineers (Norkko et al. Citation2001; Gibbs et al. Citation2005; Hewitt et al. Citation2006; Norkko et al. Citation2006; Thrush et al. Citation2006). They also provide habitats for other animals (i.e. habitat-formers), both as living individuals (Thomsen et al. Citation2016; Yakovis and Artemieva Citation2017) and as beds (Hewitt et al. Citation2006; Thrush et al. Citation2006). Habitat provision is also facilitated through the accumulation of shell debris that can support diverse benthic assemblages (Hewitt et al. Citation2005; MacDiarmid et al. Citation2013). Bivalve beds may be important indicators of ecosystem change because of these roles (Thrush et al. Citation2004).
New Zealand has witnessed large increases in sedimentation within sheltered coastal areas since humans, particularly Europeans, arrived (Hume and McGlone Citation1986). Sediment deposition in New Zealand’s coastal environments can smother and bury the animals that live there, including those living on and in sediments (Morrison and Browne Citation1999; Anderson et al. Citation2004; Lohrer et al. Citation2016). Bivalve beds often occur in these areas, so they are vulnerable to smothering and burial by natural and anthropogenic sedimentation, events that may be chronic or acute. Natural causes of acute sedimentation include the re-suspension and deposition of marine and terrestrial sediments during storms. Terrigenous sediment inputs to coastal areas due to heavy rainfall and associated landslips have increased in step with anthropogenic changes in land use (Hume and McGlone Citation1986). Acute sedimentation due to navigational dredging and resource extraction (e.g. oil, gas, aggregates and minerals), commonly requiring extraction from one place and deposition elsewhere in the coastal environment, is also becoming increasingly important. Assessments of effects of these acute events require knowledge of the resilience and recovery ability of the organisms affected.
Studies examining sedimentation effects on intertidal soft-sediment infauna have already identified negative relationships between the health of bivalve populations and sedimentation, especially for functional species associated with low/moderate depositional environments (Turk and Risk Citation1981; Norkko et al. Citation2002; Anderson et al. Citation2004; Lohrer et al. Citation2004). For example, deposition of small amounts of terrigenous fine clay sediments (c. 3 cm deposits) on sandflats in New Zealand was shown to cause a 90% decrease in macrofaunal populations after 10 days (Norkko et al. Citation2002). Susceptibility or resilience of organisms to depositional events may be dictated by a combination of species-specific physiological tolerances, behavioural responses (e.g. mobility) and body size and shape (Hinchey et al. Citation2006). Physiological tolerance (e.g. to reduced oxygen or increased sulphide and ammonia concentrations) may be related to preferred burial depth and sediment composition. These studies indicate that a shift in grain-size distribution relative to a species’ native sediment is likely to be an important factor in determining species-specific resurfacing success. Susceptibility or resilience may also reflect the degree of disturbance incurred during the deposition event (Glude Citation1954; Hull et al. Citation1998; Hinchey 2006). The processes of deposition, such as dredge spoils, storms, landslides and sediment-flows, have the potential to disorient bivalves before burying them. Animals that become upended or knocked over during a sedimentation event may incur more intense impact than those left in a natural upright orientation (Glude Citation1954). At present our ability to determine the likely resilience and recovery of bivalve-dominated communities subjected to different forms of sediment deposition is very limited. Given that the magnitude and frequency of sediment deposition, and the degree of physical disturbance, are likely to be important direct and indirect factors that determine the environmental consequences and likely recovery of the affected benthic communities (Thrush et al. Citation2003; Gibbs and Hewitt Citation2004), understanding the relative importance of these factors will be crucial for managing increasing human impacts on the coastal zone.
The endemic New Zealand cockle, or tuangi, (Austrovenus stutchburyi) is a shallow-burrowing suspension-feeding bivalve that is common on intertidal sediment flats (mostly soft mud to coarse sand) in sheltered estuaries and harbours around New Zealand (Morton and Miller Citation1968).
This species supports an important recreational, traditional and commercial fishery (Morrison et al. Citation2009), and when in high densities (up to 3000 individuals m−2 have been recorded: Stephenson and Chanley Citation1979), is a functionally important species able to influence water clarity, nutrient cycling and the productivity of microphytobenthos on sediment flats. Abundances of cockles correlate positively with seagrass cover, organic matter and the efflux of ammonium from sediments (Lohrer et al. Citation2012, Citation2016), and beds of cockles may act as the basal habitat-former in multi-level habitat cascades in New Zealand estuaries (de Juan and Hewitt Citation2011; Thomsen et al. Citation2016). Individual cockles bury just below the sediment surface to enable their very short siphons to reach the surface to feed (Stephenson Citation1981; Hewitt et al. Citation1996; Marsden and Bressington Citation2009). Vertical and horizontal movement of cockles through sediments occurs by burrowing and crawling using their muscular foot; horizontal distances moved range from a few cm’s to 1.5 m per tide, but individuals are rarely found deeper than 5–10 cm below the sediment surface (Larcombe Citation1971; Stephenson Citation1981; Mouritsen Citation2004), so cockles may be particularly sensitive to burial by sediment deposition.
In this study, we experimentally investigated the effects of native marine sediment deposition on the New Zealand cockle to test the hypothesis that ability to resurface is proportional to burial depth. We also examined differences in response to two forms of sediment deposition (the amount of sediment deposited [0, 2, 5 and 10 cm], the frequency of deposition [one single event or 2 cm daily for 5 days]), relative to cockle size (subadult versus adult) and cockle orientation (natural, upright orientation versus disturbed, inverted orientation). In their natural habitat within sheltered estuaries, cockles are subjected to sediment deposition in relatively small amounts and at infrequent intervals. Consequently, before the start of the burial experiments we expected that 10 cm of deposition would be an appropriate maximum depth that cockles could burrow to resurface, and that this amount would prevent some individuals from reaching the surface or cause mortality. However, cockles were found to be very capable at burrowing back to the surface. Therefore, we added a ‘deep burial’ experiment by depositing 25 cm of sediment (in a single event) to estimate their maximum resurfacing potential.
Materials and methods
Experimental design and set-up
We conducted experiments to examine the ability of sub-adult (<15 mm max width, Mean = 11.5 mm ± 0.32 SEM, range 5–15 mm) and adult (≥20 mm, Mean = 22.4 mm ± 0.19 SEM, range 20–27 mm) cockles to resurface from beneath increasing depths of sediment (2, 5, 10 and 25 cm) and from a pulsed burial event (2 cm added daily for 5 days) (). Individuals were positioned in either a natural (upright) orientation or a disturbed (inverted) orientation to simulate a dis-orientation during a deposition event (only the natural orientation was used in the 25-cm burial: ).
Table 1. Summary of deposition experiments.
Austrovenus stutchburyi individuals and their native surficial sediments were collected from a cockle bed in Delaware Bay (41°10′S, 173°26′E), 25 km north east of Nelson, at the northern end of the South Island of New Zealand. Sediments were wet sieved to remove particles larger than 1 cm. Two size classes of cockles – sub-adult and adult (150 each) – were collected under NIWA’s Ministry for Primary Industries special permit (No. 597).
Sediment grain size
To determine the native sediment grain sizes of the Delaware Bay cockle bed, and to ensure that sediments used in the depositional experiments were not significantly different from these, replicate 50-ml sediment samples were taken from Delaware Bay at the time of collection, and from treatment and control buckets at the end of each experiment (frozen for storage). Grain-size distributions (the proportion of mud, sand and gravel) was determined by oven drying each sample at 100°C overnight and washing each subsample through stacked sieves with meshes of 2 mm and 63 μm sieves, to provide the proportion of gravel (>2 mm), sand (63 μm–2 mm), and mud (<63 μm). The fraction retained on each sieve was dried and reweighed. The dry weights of each fraction were then expressed as percentages of the total dry weight.
Experimental deposition on upright and inverted cockles
To examine their ability to resurface after being buried, cockles were placed in experimental aquaria in an upright orientation (Experiment 1) or in an inverted (‘disturbed’) orientation (Experiment 2a–b). For these experiments, a 5-cm deep basal layer of sediment was placed in each of 30 experimental buckets (20.3 cm high × 18 cm wide). The sediment was left to settle and the water to clear for 24 h. Water temperature for all experiments was measured and maintained at 18–20°C. Five sub-adult or adult cockles were carefully pushed into the sediment of each bucket to a depth of half their shell. Upright cockles were placed into the sediment with their siphons facing up, as they would occur in their natural environment, while inverted cockles were placed into the sediment with their hinge pointing upwards, simulating cockles that had been disturbed and up-ended (). All cockles (per size group) were haphazardly positioned within the buckets, interspaced equally between each other and the bucket edges.
Figure 1. Experimental design for sediment deposition, where cockles were placed in either a natural (upright) or disturbed (inverted) orientation. Each bucket had a 5-cm basal sediment layer prior to 5 cockles (in 2 size classes) being placed in either an upright (experiment 1) or inverted (experiment 2a-b) orientation within the buckets. Control buckets received no sediment deposits; Treatments 1–3 varied in the amount of sediment deposited (2, 5 and 10 cm, respectively), while Treatment 4 had 2 cm deposited daily for 5 days (10 cm total).
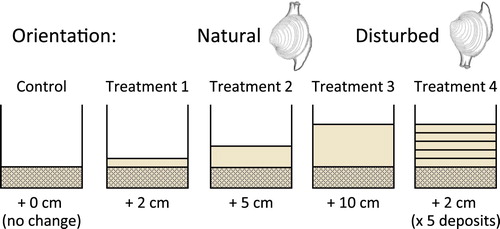
Deposition experiments: For Experiments 1 and 2b, four sediment-deposition treatments and a control were randomly assigned to buckets within aquaria using a fully orthogonal random design (within experiment), with three replicate buckets assigned for each treatment/control per size class. Two forms of sediment deposition (‘one-off-amount’ and ‘repeated-deposition’) were applied, whereby sediment was experimentally deposited in a single event in 2, 5 and 10 cm depths (Treatments 1–3, respectively), or applied by adding 2 cm of sediment each day for five days (Treatment 4, ). Sediments were systematically added in a water slurry to allow even settlement. The control treatment received no sediment. This set of experiments involved 150 cockles in total across five treatments, two-size classes and three replicate buckets per size class × treatment/control combination.
The treatments were monitored once daily for seven days. Each bucket was visually inspected each day and the number of cockles seen at the sediment surface (shells and/or siphons and siphon holes present) were recorded. At the end of seven days, all cockles at the surface were removed and measured with calipers across the maximum width (to the nearest mm). Each bucket was then drained, and any cockles not at the surface were exhumed by systematically removing layers of sediment, 1 cm at a time, following the method of Kranz (Citation1974). The width of each individual was measured, and the depth at which they were found (measured in cm relative to the surface mark) was recorded.
No deposition: To determine how quickly inverted cockles were able to right themselves without any sediment deposition, we ran a short Experiment (2a) that mirrored the design above for inverted orientation (Experiment 2b), but no sediment was deposited. This required 150 cockles in total, two-size classes with fifteen replicate buckets per size class. The orientation of each cockle was recorded every hour for 6 h and the time taken to reorientate to an upright position noted. At the end of 6 h, each cockle was removed, and its final orientation recorded.
Deep-burial (25 cm) experiment
In Experiment 3, six tall plastic buckets (40.6 cm high × 30.5 cm wide) were placed in each of six glass aquaria. Each of these tall buckets was given a 5-cm deep basal layer of sediment and left to settle for 24 h. After this time, 10 sub-adult or adult cockles were carefully pushed into the sediment of each bucket in an upright position to a depth of half their shell height. This gave a design of 60 cockles in total, two-size classes with three replicate buckets. Placement of cockles within the buckets used the same method as the previous experiments. Sediment was then deposited into each bucket to a deposition-depth of 25 cm and left to settle. The ‘deep-burial’ Experiment was then monitored once daily for seven days and harvested as described above.
Data analysis
Multivariate analysis of variance using PROC GLM in SAS was used to determine if sediments (percentage of mud, sand and gravel) varied by ‘experiment’ (treatment-type and collection site), with Pillai’s Trace used as the test statistic. To determine if adults and sub-adults differed in their rate to resurface from beneath 2, 5, and 10 cm of sediment in upright (experiment 1), inverted (experiment 2b) orientations, and deep-burial (experiment 3), repeated-measures Analysis of Variance (ANOVA) with Tukey post-hoc tests were run using the Mixed Procedure in SAS. Resurfacing rates were calculated as percentage of total cockles resurfaced at the end of each experiment. We used fixed factor design with time (days since burial) as the repeated measure, where ‘size-class’ and ‘size-class*time’ were used as the significant test. To determine if there was any correlation (or monotonic trend) between vertical burrowing distance (i.e. distance between final and starting depth, measured in mm) and cockle size, we ran Spearman’s rank correlation analyses using the PROC CORR procedure in SAS, for each treatment. The mean percentage (± SEM) were then calculated and graphed for each size-class and treatment.
Results
Sediment grain size
Native sediments from the cockle bed in Delaware Bay, Nelson were dominated by sand (>90%), with small quantities of mud (4–8%) and gravel (<1%). Although coarsely sieving sediments to remove large animals and shell debris did remove some gravels, these differences were negligible (<0.02%), and resulted in no significant difference in the grain size composition between the native sediments from Delaware Bay and those used in the experiments.
Effects of orientation and depth of burial (Experiments 1, 2a and 2b)
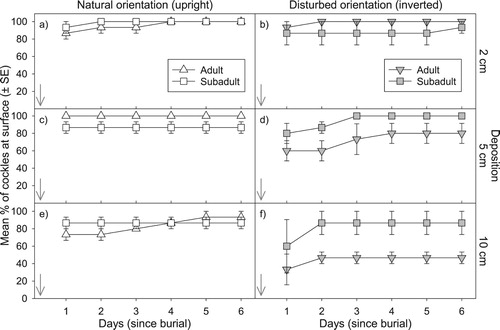
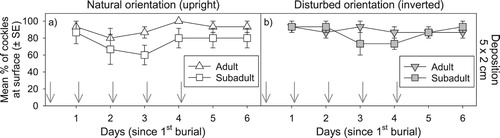
Cockles placed in a natural upright orientation and buried by 2, 5 or 10 cm of sediment (Experiment 1), resurfaced quickly and were observed feeding at the surface within a week (a–c). When buried under 2 cm of sediment, naturally orientated cockles quickly resurfaced with >90% of cockles present at the surface within 2 days and all cockles resurfacing after 1 week (a). When buried under 5–10 cm of sediment, most naturally orientated cockles in both size classes quickly resurfaced, with >70–80% of cockles present at the surface within 2 days, and >80–90% after 1 week (b,c). Although the total number of cockles recorded at the surface over time differed significantly between adults and sub-adults in some treatments (F(1,5) = 24.0, P < 0.0001) (e.g. b), there was no consistent trend in adult or sub-adult cockles rate to resurface (a–c), and no significantly correlation between cockle size and the vertical distance burrowed.
Figure 2. Cockle resurfacing success relative to single 0, 2, 5 and 10 cm deposition events, with 3 replicates per treatment and control. The time of deposition is depicted by an arrow for each graph. Mean percentage (±SE) of adult versus sub-adult sized cockles that burrowed to the surface in days since burial. a-c, Cockles placed in a natural upright position at the time of deposition; d-e, Cockles placed in a simulated disturbed (inverted) orientation.
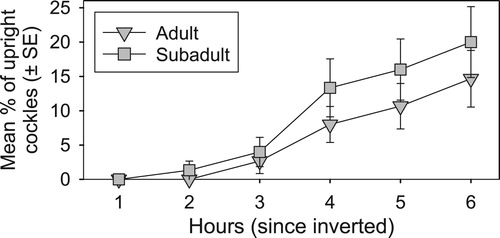
Cockles placed in a disturbed (inverted) orientation, but with no sediment added (Experiment 2a), were slow to re-orientate to an upright position, with <25% of cockles re-orientating within the first 6 h (). There was no significant difference between the rate of inverted adult and sub-adult cockles to right themselves, partly due to high variability within-size class ().
Figure 3. Cockle righting success after being inverted into the sediment (no sediment added). Mean percentage (±SE) of adult versus sub-adult cockles found upright every hour for six hours following simulated disturbance.
Cockles placed in a disturbed inverted orientation with sediment deposited on top of them (Experiment 2b) were much slower to resurface than those placed in an upright position (). Resurfacing rate varied as a function of both cockle size and the amount of sediment deposited (d–f). When buried under 2 cm of sediment, most inverted cockles (>75%) were able to right themselves and resurface within 2 days, with almost all cockles observed feeding at the surface within a week (d). In contrast, when buried under 5 and 10 cm of sediment, many inverted cockles failed to re-surface, with significantly fewer adults resurfaced than sub-adults (F(1,5) = 15.38, P < 0.001, F(1,5) = 21.81, P < 0.0001, 5 and 10 cm treatments respectively) (e–f). The number of sub-adults that resurfaced from an inverted position decreased as more sediment was added, but most sub-adults had re-orientated themselves and re-surfaced in less than 2–3 days (>70–80%). Almost all sub-adult cockles were feeding at the surface after 3 days (e). In contrast, significantly fewer inverted adults resurfaced over the same time frame (F and P values as above, f). Inverted adult cockles fared worst when buried under 10 cm of sediment, with only 40% of adult cockles (> 20 mm) able to right themselves and resurface over the course of a week. Resurfacing success was significantly correlated with cockle shell size, but only for inverted cockles buried beneath 10 cm of sediment (Spearman Correlation Coefficient −0.543, P = 0.0019). Most inverted cockles that hadn’t resurfaced in this treatment were adults ≥ 22 cm (62% of all adults), with 75% of these still in their original inverted position.
Repeated reburial (Experiments 1 and 2b)
Cockles quickly resurfaced after being buried under the first 2 cm of sediment (>70% of all cockles had resurfaced 24 h after the first burial). However, reburial under 2 cm of sediment daily for 5 days impeded some cockles, with fewer cockles resurfacing on days 2 through 5. In the naturally orientated reburial treatment (Experiment 1), fewer sub-adults re-surfaced on these days than adults (F(1,5) = 6.53, P < 0.05) (), although this was not significant across the entire week. The reburial of inverted cockles (Experiment 2b) did not differ in their resurfacing rates compared to naturally-orientated cockles. Although a reduction in re-surfacing rates was observed on days 3 and 4 for sub-adult cockles, the size comparison was not significant due to within-treatment variability (). There was also no significant correlation between cockle size and the vertical distance burrowed for natural or inverted orientation. A comparison between the reburial (5 × 2 cm) and the single 10 cm deposition treatments (both having 10 cm total deposition) found no significant difference in the resurfacing rates of upright and inverted subadults or upright adults (e and a). However, while inverted adults were able to consistently resurface following continual daily reburial and disturbance, their resurfacing rates were significantly impeded under a single 10 cm deposition (f and b).
Figure 4. Cockle resurfacing success after repeated depositional events (burial under 2 cm of sediment daily for 5 days). The timing of repeated deposition events are depicted by arrows. Mean percentage (±SE) of adult versus sub-adult cockles found at the surface each day after being placed initially in either a natural upright (left-hand plot) or disturbed inverted (right-hand plot) orientation.
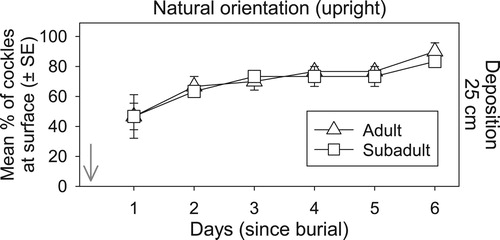
Deep burial (Experiment 3)
Naturally orientated cockles were able to ascend quickly through 25 cm of native sediment. Within 2 days >50% of all cockles had reached the surface, with >70% of cockles resurfacing after 1 week (). There was no significant difference in resurfacing rate or success between adult and sub-adult cockles (), and no significant correlation between vertical burrowing distance and cockle size. Individuals that had not resurfaced after 6 days varied in size from 11 to 20 mm (5 subadult and 2 adult cockles). Most of these individuals were found at depths between 10 and 18 cm, showing they had made some vertical progress, while three cockles (two 20 mm adults and one 12 mm sub-adult) had not moved from their original position and one 20 mm individual was dead. While those cockles that had not moved had perished or would likely perish, it is unclear whether the cockles still buried between 10 and 18 cm depths could have resurfaced given more time.
Discussion
Large sediment-deposition events can smother sediment habitats, burying the animals that live there (Anderson et al. Citation2004; Lohrer et al. Citation2016). Austrovenus stutchburyi, along with other recreationally and culturally important shellfish species (e.g. Perna canaliculus (green lipped mussels), Paphies australis, Paphies ventricosa (toheroa) and Paphies subtriangulata (tuatua)) are known to be vulnerable to sedimentation and deposition (Anderson et al. Citation2004; Cummings and Thrush Citation2004; Norkko et al. Citation2006; Morrison et al. Citation2009; Österling et al. Citation2010).
Previous studies have generally considered responses to deposition of non-native sediments. Large deposition events are most likely to occur due to storms or anthropogenic deposition activities (e.g. following dredging), when effects of deposition may be exacerbated by disturbance to the orientation of bivalves in the sediment. In the present study, both subadult (mean 11.5 mm ± 0.32 SEM) and adult (mean 22.4 mm ± 0.19 SEM) cockles were found to be rapid and effective burrowers, able to resurface within days (often hours) from under 2, 5, 10 and even 25 cm of native sandy sediment where no physical disturbance to their natural (in situ) orientation had occurred. Rapid and effective burrowing has also been found in a range of other bivalve species. For example, the North American hard-shell clam (Mercenaria mercenaria), which is similar in shape to the NZ cockle, but grows significantly larger to ≤120 mm in length, and the very small-sized (<10 mm) Atlantic nut clam (Nucula proxima) can burrow up through at least 16 and 24 cm of experimentally deposited sediment (respectively) within 1–3 days (Maurer et al. Citation1978). A few M. mercenaria individuals also resurfaced from beneath 50 cm of native sediment within 4–18 days, although most individuals did not vertically migrate more than 20 cm. In a study of the New Zealand pipi, Paphies australis, Hull et al. (Citation1998) found that individuals rapidly resurfaced following native sand deposits of at least 10 cm, with some individuals able to burrow up through 40 cm of sand.
Extreme smothering of bivalve beds by sedimentation events has been documented for a wide range of New Zealand species (McKnight Citation1969; Stephenson Citation1981; Morrison et al. Citation2009). Many estuaries that are now dominated by clays and silt have layers of dead cockle shell >1 m below the surface, highlighting how vulnerable coastal populations can be to sedimentation events (Morrison et al. Citation2009; Marsden and Adkins Citation2010). For example, Reeve (1854, cited in Stephenson Citation1981) described the death of extensive mature cockle beds in South Canterbury following inundation by a thick (50–60 cm) layer of distinctive mud from the Avon and Heathcote Rivers in the early 1850s. Stephenson (Citation1981) noted ‘beds of shells in a natural orientation’, providing further evidence of this event. These events raise the question of why, if cockles are so good at resurfacing, are there death assemblages under deep sediment flows? The ability of soft-sediment bivalves’ to burrow and resurface after sediment deposition, is likely to be impacted by several factors. These include sediment depth, animal size, the orientation of the cockle in the sediment after deposition, the rate of deposition, the nature of the deposited sediment (e.g. grain size), and other environmental, often synergistic, changes that accompany deposition (e.g. higher organic content and decreased salinity from freshwater incursions of land-derived depositional events) (e.g. Stephenson Citation1981; Hull et al. Citation1998; Lohrer et al. Citation2004; Marsden Citation2004; Thrush et al. Citation2004).
Most soft-sediment infauna live in the upper 5 cm, partly because deeper sediments are more resistant to burrowing and other movements due to reduced sediment pore space and the increasing weight of the overlying sediments (Hines and Comtois Citation1985). Chang and Levings (Citation1978) found that the broader shell of the large-sized heart cockle Clinocardium nuttallii impeded their ability to resurface when burrowing under increased amounts of sediment (100%, 50% and 0% survival under ≤5, 10 and 20 cm of sediment, respectively). Depth of burial would, therefore, be expected to influence the ability of shellfish to resurface. In the present study, naturally-orientated cockles showed little reduction in their ability to resurface with increasing depth of sediment, even from under 25 cm of sediment. Although Austrovenus and Clinocardium have a characteristically similar deeply-rounded shape, the Clinocardium used in Chang and Levings’ experiments were more than twice the size (range 50–70 mm) of the Austrovenus individuals used in our study (range 5–27 mm). In the 25 cm deposition experiment, of those individuals that had not resurfaced, larger-sized adults had made more progress than most small-sized cockles (although no significant monotonic size-trend was present), suggesting that sediment weight was not a deterrent to these adult sizes. A. stutchburyi adults commonly grow to 40–50 mm, but can grow up to 65 mm in length (Cook Citation2010), but these larger sizes were not present at the Delaware Bay collection site, so we were unable to test the resurfacing rates of these larger-sized cockles.
Disturbance during sediment deposition events may significantly alter the resurfacing success and survival of some species (Hull et al. Citation1998; Hinchey et al. Citation2006). Glude (Citation1954) suggested that for a given size of individual and depth of burial, deposition-events would be more damaging to animals that have been laid on their side or inverted than to those remaining upright. Disturbed cockles in our study were very slow (80% of individuals took > 6 h) to re-orientate to their natural orientation following physical disturbance (simulated by placing cockles in an inverted position in the sediment). Although able to resurface from an inverted position under 2 cm of deposited sediment, inverted cockles were significantly impeded in their ability to resurface when buried to greater depths (5 and 10 cm). Hull et al. (Citation1998) also found that disturbed pipis (placed on their sides) took longer to resurface than naturally-oriented (upright) ones, while soft-shell clams (Mya arenaria) buried in upright and horizontal positions were more likely to survive than those placed in an inverted position (Glude Citation1954). In our experiments, cockle size, only in combination with disturbance, was an important factor impeding a cockles’ resurfacing ability. Large inverted cockles that failed to resurface had not moved from their original inverted position, suggesting that ‘righting-ability’ relative to cockle size and overlying sediment weight are likely to be the factors impeding resurfacing success of larger-sized A. stutchburyi, rather than the ability to burrow up through the overlying sediment per se.
Ongoing or repeated burials may also have important consequences. Hull et al. (Citation1998) suggested that pipis were more susceptible to continual reburial (under 5, 10 or 15 cm of sediment daily, for 4 days) than a single equivalent deposition event (20, 40 and 60 cm of total sediment, respectively). Pipi size was important in resurfacing success, with medium-sized individuals (40–50 mm) faring much better than smaller or larger ones. In the present study, cockles regardless of size or orientation repeatedly re-surfaced following daily reburial (i.e. 2 cm added daily for 5 days), indicating that cockles were resilient to at least low levels of reburial. However, when continual reburial and a single equivalent deposition (totalling 10 cm) were compared, size and orientation were both important factors in predicting resurfacing success. This was only significant for inverted adult cockles, which in contrast to pipis, were 40% more susceptible to a single deposition than continual reburial (10 cm of total sediment). Equivalent treatments of continual burial were not examined in this study due to a lack of aquarium capacity.
Changes in the composition and grain size distributions of natural and anthropogenically deposited sediments are also known to alter the burrowing ability and survival of infaunal species (Anderson et al. Citation2004). Species including the hard-shelled clam, the San Francisco gem clam (Gemma gemma), the deep-burrowing soft-shell clam (M. arenaria) the Atlantic nut clam (N. proxima), pipis, and the New Zealand cockle (this study) were all found to be capable at resurfacing from under thick deposits of native sediments, yet are known to have high mortality under thin deposits of foreign sediments (Shulenberger Citation1970; Maurer et al. Citation1978; Norkko et al. Citation2002; Lohrer et al. Citation2004). New Zealand cockles are most abundant in sandy sediments, while markedly lower abundances occur in muddy sediments (e.g. Anderson 2004), and decrease with increasing proportions of silt/clay, with no live individuals were found in experimental treatments with >60% silt/clay Norkko et al. (Citation2001). Sediment depositions comprising high proportions of foreign sediments (i.e. silt/clay) are therefore likely to be highly detrimental to the resurfacing success and survival of cockles. When combined with freshwater this effect can be dramatic. A. stutchburyi were negatively impacted by even thin layers of clay deposits, when sediment was deposited as a slurry of freshwater (simulating the effects of terrestrial runoff) (Norkko et al. Citation2002; Lohrer et al. Citation2004), with individual cockles unable to resurface from under 3, 6 and 9 cm of clay/silt (Norkko et al. Citation2002), indicating that terrestrial run-off deposition events carried by freshwater incursions are extremely detrimental to cockles.
Conclusions
Our study suggests that natural or anthropogenic events that deposit sediments with similar grain sizes to those already present would have limited direct impacts on local populations of A. stutchburyi. However, if individuals were also physically disturbed and their orientation in the sediment changed, some mortality of larger-sized (>20 mm) cockles would be predicted. Higher mortality would also be expected with thicker deposits of sediments. Where deposited sediment differs in texture from the native sediment, or are comprised of terrestrial muds, or where freshwater incursion also occurs, changes in community structure and loss of critical species, such as cockles, may occur. More frequent storms, associated with long-term climate change, are also likely to increase the risk of burial with non-native sediments accompanied by physical disturbance.
Acknowledgements
Thanks to Marlborough District Council (especially Steve Urlich) for their support of this project; Louis Olsen, Mike Page, Jon Stead and Anna Bradley (NIWA) for their assistance with aquaria setup and saltwater collections; James Mackman (University of Birmingham) for his help with collecting sediments and cockles; Stephen Brown and Megan Carter (NIWA) for their advice with grain size analyses; Sean Handley, Ken Grange, Alison MacDiarmid and Judy Hewitt (NIWA) for their comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson MJ, Ford RB, Feary DA, Honeywill C. 2004. Quantitative measures of sedimentation in an estuarine system and its relationship with intertidal soft-sediment infauna. Marine Ecology Progress Series. 272:33–48. doi: 10.3354/meps272033
- Chang BD, Levings CD. 1978. Effects of burial on the heart cockle Clinocardium nuttallii and the Dungeness crab Cancer magister. Estuarine and Coastal Marine Science. 7(4):409–412. doi: 10.1016/0302-3524(78)90093-2
- Cook S. 2010. New Zealand coastal marine invertebrates. Christchurch (NZ): Canterbury University Press; 640 p.
- Cummings VJ, Thrush SF. 2004. Behavioural response of juvenile bivalves to terrestrial sediment deposits: implications for post-disturbance recolonisation. Marine Ecology Progress Series. 278:179–191. doi: 10.3354/meps278179
- de Juan S, Hewitt J. 2011. Relative importance of local biotic and environmental factors versus regional factors in driving macrobenthic species richness in intertidal areas. Marine Ecology Progress Series. 423:117–129. doi: 10.3354/meps08935
- Gibbs M, Funnell G, Pickmere S, Norkko A, Hewitt J. 2005. Benthic nutrient fluxes along an estuarine gradient: influence of the pinnid bivalve Atrina zelandica in summer. Marine Ecology Progress Series. 288:151–164. doi: 10.3354/meps288151
- Gibbs M, Hewitt J. 2004. Effects of sedimentation on macrofaunal communities: a synthesis of research studies for ARC. Auckland Regional Council Technical Publication No. 264; 48 p.
- Glude JB. 1954. Survival of soft-shell clams, Mya arenaria, buried at various depths. Maine Department Sea Shore Fisheries Research Bulletin. 22:1–26.
- Hewitt J, Thrush S, Gibbs M, Lohrer D, Norkko A. 2006. Indirect effects of Atrina zelandica on water column nitrogen and oxygen fluxes: the role of benthic macrofauna and microphytes. Journal of Experimental Marine Biology and Ecology. 330:261–273. doi: 10.1016/j.jembe.2005.12.032
- Hewitt JE, Thrush SF, Cummings VJ, Pridmore RD. 1996. Matching patterns with processes: predicting the effect of size and mobility on the spatial distribution of the bivalves Macomona liliana and Austrovenus stutchburyi. Marine Ecology Progress Series. 135:57–67. doi: 10.3354/meps135057
- Hewitt JE, Thrush SF, Halliday J, Duffy C. 2005. The importance of small-scale habitat structure for maintaining beta diversity. Ecology. 86:1619–1626. doi: 10.1890/04-1099
- Hinchey EK, Schaffner LC, Hoar CC, Vogt BW, Batte LP. 2006. Responses of estuarine benthic invertebrates to sediment burial: the importance of mobility and adaptation. Hydrobiologia. 556(1):85–98. doi: 10.1007/s10750-005-1029-0
- Hines AH, Comtois KL. 1985. Vertical distribution of infauna in sediments of a subestuary of central Chesapeake Bay. Estuaries. 8(3):296. doi: 10.2307/1351490
- Hull PJ, Cole RG, Creese RG, Healy TR. 1998. An experimental investigation of the burrowing behaviour of Paphies australis (Bivalvia: Mesodesmatidae). Marine and Freshwater Behaviour and Physiology. 31(3):167–183. doi: 10.1080/10236249809387071
- Hume TM, McGlone MS. 1986. Sedimentation patterns and catchment use change recorded in the sediments of a shallow tidal creek, Lucas Creek, Upper Waitemata Harbour, New Zealand. New Zealand Journal of Marine and Freshwater Research. 20:677–687. doi: 10.1080/00288330.1986.9516188
- Kranz PM. 1974. The anastrophic burial of bivalves and its paleoecological significance. The Journal of Geology. 82(2):237–265. doi: 10.1086/627961
- Larcombe MF. 1971. The ecology population dynamics and energetics of some soft shore molluscs [unpublished PhD thesis]. New Zealand: University of Auckland; 250 p.
- Lohrer AM, Thrush SF, Hewitt JE, Berkenbusch K, Ahrens M, Cummings VJ. 2004. Terrestrially derived sediment: response of marine macrobenthic communities to thin terrigenous deposits. Marine Ecology Progress Series. 273:121–138. doi: 10.3354/meps273121
- Lohrer AM, Townsend M, Hailes SF, Rodil IF, Cartner K, Pratt DR, Hewitt JE. 2016. Influence of New Zealand cockles (Austrovenus stutchburyi) on primary productivity in sandflat-seagrass (Zostera muelleri) ecotones. Estuarine, Coastal and Shelf Science. 181(5):238–248. doi: 10.1016/j.ecss.2016.08.045
- Lohrer AM, Townsend M, Rodil IF, Hewitt JE, Thrush SF. 2012. Detecting shifts in ecosystem functioning: the decoupling of fundamental relationships with increased pollutant stress on sandflats. Marine Pollution Bulletin. 64:2761–2769. doi: 10.1016/j.marpolbul.2012.09.012
- MacDiarmid A, Bowden D, Cummings V, Morrison M, Jones E, Kelly M, Neil H, Nelson W, Rowden A. 2013. Sensitive marine benthic habitats defined. NIWA Client Report WLG2013–18, Prepared for New Zealand Ministry for the Environment. Publication Reference No. CR 147; 72 p. https://www.mfe.govt.nz/publications/marine/sensitive-marine-benthic-habitats-defined.
- Marsden ID. 2004. Effects of reduced salinity and seston availability on growth of the New Zealand little - neck clam Austrovenus stutchburyi. Marine Ecology Progress Series. 266:157–171. doi: 10.3354/meps266157
- Marsden ID, Adkins SC. 2010. Current status of cockle bed restoration in New Zealand. Aquaculture International. 18(1):83–97. doi: 10.1007/s10499-009-9270-6
- Marsden ID, Bressington MJ. 2009. Effects of macroalgal mats and hypoxia on burrowing depth of the New Zealand cockle (Austrovenus stutchburyi). Estuarine, Coastal and Shelf Science. 81(3):438–444. doi: 10.1016/j.ecss.2008.11.022
- Maurer DL, Keck RT, Tinsman JC, Leathem WA, Wethe CA, Huntzinger M, Lord C, Church TM. 1978. Vertical migration of benthos in simulated dredged material overburdens Vol. I: Marine benthos. Technical report D–78–35. Published by the U.S. Army Engineer Waterways Experiment Station Vicksburg, Mississippi; 115 p.
- McKnight DG. 1969. A recent, possibly catastrophic burial in a marine molluscan community. New Zealand Journal of Marine and Freshwater Research. 3:177–179. doi: 10.1080/00288330.1969.9515286
- Morrison MA, Browne GN. 1999. Intertidal shellfish population surveys in the Auckland region 1998–1999 and associated yield estimates. New Zealand Fisheries Assessment Research Document 99/43; 21 p.
- Morrison MA, Lowe ML, Parsons DM, Usmar NR, Macleod IM. 2009. A review of land-based effects on coastal fisheries and supporting biodiversity in New Zealand. New Zealand Aquatic Environment and Biodiversity Report No. 39; 100 p.
- Morton J, Miller M. 1968. The New Zealand sea shore. Collins Auckland; 653 p.
- Mouritsen KN. 2004. Intertidal facilitation and indirect effects: causes and consequences of crawling in the New Zealand cockle. Marine Ecology Progress Series. 271:207–220. doi: 10.3354/meps271207
- Norkko A, Talman S, Ellis J, Nicholls P, Thrush S. 2001. Macrofaunal sensitivity to fine sediments in the Whitford embayment. NIWA client report ARC01266/2; 38 p. http://www.knowledgeauckland.org.nz/assets/publications/TP158-Macrofaunal-sensitivity-to-fine-sediments-Whitford-Embayment-2002.pdf.
- Norkko A, Thrush SF, Hewitt JE, Cummings VJ, Norkko J, Ellis JI, Funnell GA, Schultz D, MacDonald I. 2002. Smothering of estuarine sandflats by terrigenous clay: the role of wind-wave disturbance and bioturbation in site-dependent macrofaunal recovery. Marine Ecology Progress Series. 234:23–42. doi: 10.3354/meps234023
- Norkko J, Hewitt JE, Thrush SF. 2006. Effects of increased sedimentation on the physiology of two estuarine soft-sediment bivalves Austrovenus stutchburyi and Paphies australis. Journal of Experimental Marine Biology and Ecology. 333:12–26. doi: 10.1016/j.jembe.2005.11.015
- Österling ME, Arvidsson BL, Greenberg LA. 2010. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: influence of turbidity and sedimentation on the mussel and its host. Journal of Applied Ecology. 47:759–768. doi: 10.1111/j.1365-2664.2010.01827.x
- Shulenberger E. 1970. Responses of Gemma gemma to a catastrophic burial. The Veliger. 13:163–170.
- Stephenson RL. 1981. Aspects of the energetics of the cockle Chione (Austrovenus) stutchburyi in the Avon-Heathcote Estuary Christchurch New Zealand [unpublished PhD thesis]. Christchurch (New Zealand): University of Canterbury; 165 p.
- Stephenson RL, Chanley PE. 1979. Larval development of the cockle Chione stutchburyi (Bivalvia: Veneridae) reared in the laboratory. New Zealand Journal of Zoology. 6:553–559. doi: 10.1080/03014223.1979.10428397
- Thomsen MS, Hildebrand T, South PM, Foster T, Siciliano A, Oldach E, Schiel DR. 2016. A sixth-level habitat cascade increases biodiversity in an intertidal estuary. Ecology and Evolution. 6:8291–8303. doi: 10.1002/ece3.2499
- Thrush SF, Hewitt JE, Cummings V, Ellis JI, Hatton C, Lohrer A, Norkko A. 2004. Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment. 2:299–306. doi: 10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2
- Thrush SF, Hewitt JE, Gibbs M, Lundquist C, Norkko A. 2006. Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems. 9:1029–1040. doi: 10.1007/s10021-005-0068-8
- Thrush SF, Hewitt JE, Norkko A, Cummings VJ, Funnell GA. 2003. Macrobenthic recovery processes following catastrophic sedimentation on estuarine sandflats. Ecological Applications. 13:1433–1455. doi: 10.1890/02-5198
- Turk TR, Risk MJ. 1981. Effect of sedimentation on infaunal invertebrate populations of Cobequid Bay, Bay of Fundy. Canadian Journal of Fisheries and Aquatic Sciences. 38:642–648. doi: 10.1139/f81-086
- Yakovis E, Artemieva A. 2017. Cockles, barnacles and ascidians compose a subtidal facilitation cascade with multiple hierarchical levels of foundation species. Scientific Reports. 7;237 p.