ABSTRACT
Planktonic dinoflagellate records for New Zealand are substantial due to intense monitoring programmes that have taken place on behalf of New Zealand’s biotoxin regulators and the shellfish industry since 1993. At that time a Karenia bloom caused human illnesses and shellfish harvesting ceased until monitoring was instigated. Phytoplankton records are based on morphological identification using light microscopy but are backed up by government funded research programmes which implement techniques such as electron microscopy, DNA sequencing, molecular detection assays and high throughput sequencing of environmental DNA. This checklist will support management of aquaculture industries, recreational shellfish harvests and environmental health initiatives. Some genera are considered benthic or epiphytic, but have lengthy planktonic life stages. Forty-five genera in the class Dinophyceae Fritsch are reported: Akashiwo, Alexandrium, Amphidinium, Amylax, Azadinium, Biecheleria, Bysmatrum, Cachonina, Cochlodinium (synonym: Margalefidinium), Coolia, Dicroerisma, Dinophysis, Diploneis, Diplopsalis, Fragilidium, Glenodinium, Gonyaulax, Gymnodinium, Gyrodinium, Heterocapsa, Karenia, Karlodinium, Lepidodinium, Lingulodinium, Margalefidinium, Ostreopsis, Oxyphysis, Pelagodinium, Pentapharsodinium, Phalacroma, Podolampas, Polarella, Polykrikos, Prorocentrum, Protodinium, Protoodinium, Protoperidinium, Pseliodinium, Scrippsiella, Takayama, Togula, Torodinium, Tripos, Vulcanodinium, Wolosynska. Other genera belonging to the Infraphylum Dinoflagellata also occur in New Zealand waters. They are not in the list but include Noctiluca, Pronoctiluca and Spatulodinium (Class: Noctilucophyceae) and Oxyrrhis (Class: Oxyrrhidophyceae).
Introduction
The planktonic dinoflagellates of New Zealand () are a relatively well studied group compared to the benthic and epiphytic dinoflagellates (Rhodes and Smith Citation2018). The on-going research and resultant knowledge of these micro-algae in New Zealand’s coastal waters is largely due to the economic importance of the approximately NZ$1.8 billion per annum seafood industry, which includes finfish and shellfish aquaculture (Ministry for Primary Industries Citation2018), and also the importance of the recreational harvest.
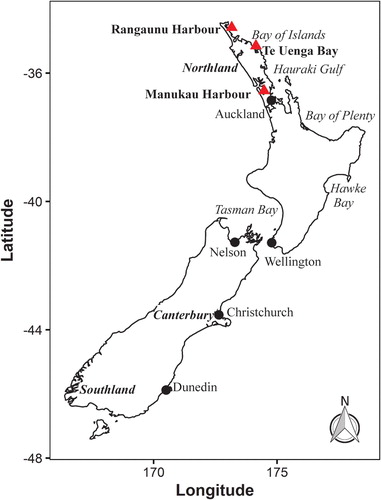
Figure 1. Map of New Zealand highlighting sites where planktonic dinoflagellates referred to in this study were collected.
With warming average sea surface temperatures (SST; Ministry for the Environment Citation2016), there is the risk of toxic sub-tropical introductions to the known microflora. For example, Cochlodinium polykrikoides Margalef (synonym: Margalefidinium polykrikoides (Margalef) F.Gómez, Richlen & D.M.Anderson (Gomez et al. Citation2017); Family Gymnodiniaceae) is an ichthyotoxic dinoflagellate with an over-wintering cyst phase, which forms long-lasting blooms in South East Asia (Kim et al. Citation2016). The species has been tentatively recorded in New Zealand (Chang et al. Citation2012; New Zealand’s National Marine Biotoxin monitoring programme records (NZMBMP)), and with the current average annual SST range in New Zealand’s Northland of approximately 13–23°C (https://terra.nasa.gov/data/modis-data) and coastal SSTs rising, C. polykrikoides is a potential risk for fish aquaculture in the future.
Potential shellfish-toxin producing dinoflagellate genera that fall within the class Dinophyceae include Alexandrium Halim (MacKenzie Citation2014), Dinophysis Ehrenberg (MacKenzie Citation2019), Karenia Hansen & Moestrup (Haywood et al. Citation2004; de Salas et al. Citation2005; Smith et al. Citation2007), Protoceratium Bergh (Satake et al. Citation1997) and Gymnodinium Stein (for example, PST producer, G. catenatum Graham; MacKenzie Citation2014) (). Planktonic blooms of all these species occur regularly around New Zealand’s coastline. Many of the causative species have multiple life stages, including temporary or ecdysal cysts, but the longest life stage is planktonic. An exception is Alexandrium pacificum (=catenella) Litaker, which can develop both long-lasting blooms and long-lasting resting cyst (hypnozygote) beds (MacKenzie Citation2014; Rhodes and Smith Citation2018).
Table 1. Toxin producing marine planktonic dinoflagellates recorded in New Zealand’s coastal waters, the toxins they produce and the regulatory biotoxin limits in New Zealand.
El Niño is one phase of a naturally occurring global climate cycle known as the El Niño-Southern Oscillation (ENSO), and resultant climatic events have been linked to harmful algal blooms (HAB) in New Zealand. Colder than usual SSTs occurred during the 1992 El Niño event, as well as high winds with accompanying nutrient upwelling, and this led to the formation of Noctiluca scintillans (Macartney) Kofoid & Swezy and raphidophyte blooms in the Hauraki Gulf during the austral spring and early summer (Rhodes et al. Citation1993). This was followed by a dinoflagellate bloom, dominated by Karenia mikimotoi (Miyake & Kominami ex Oda) Hansen & Moestrup, which led to more than 100 human illnesses and the closure of shellfish harvesting throughout New Zealand (Jasperse Citation1993). The bloom was the trigger for the implementation of phytoplankton and shellfish biotoxin monitoring programmes (Rhodes et al. Citation2013).
Blooms of the large, bioluminescent species, N. scintillans are regularly recorded in New Zealand’s Hauraki Gulf, particularly in spring. The genus Noctiluca is classified as Superclass: Dinoflagellata, but falls within the Class Noctilucophyceae Fensome, Taylor, Norris et al. (Guiry Citation2018) and is not included in this checklist. In the checklist of New Zealand Myzozoa, in the New Zealand inventory of biodiversity (Chang et al. Citation2012), the classification used includes Infraphylum Dinoflagellata, Superclass Dinokaryota, and the Classes Noctilucea and Peridinea. The species N. scintillans and Pronoctiluca acuta Lohmann are included in the former. The website AlgaeBase (http://www.algaebase.org/; accessed 26 January 2019), which provides the taxonomic classification followed in this publication, uses the classification Infraphylum Dinozoa, Superclass Dinoflagellata, Classes Dinophyceae, Ellobiophyceae, Noctilucophyceae, Oxyrrhidophyceae, Perkinsea and Syndiniophyceae (Guiry Citation2018).
The species Oxyrrhis marina Dujardin has also been reported in New Zealand waters within the Peridinea (Chang et al. Citation2012), but under the AlgaeBase classification falls within the Class Oxyrrhidophceae Cavalier-Smith (Guiry Citation2018) and in this checklist only marine genera in the Class: Dinophyceae Fritsch have been included.
Reports of major HAB events for all micro-algae classes are now accessible through the ‘Harmful algae event meta database’ (HAEDAT: http://haedat.iode.org/; Jaffrezic et al. Citation2019), which enables future risk inferences to be drawn based on global data. For example, a link between cooler SSTs in New Zealand and rainfall in southwestern USA, via a western Pacific Ocean atmosphere pathway, was recently reported (Mamalakis et al. Citation2018).
Planktonic dinoflagellate species from sub-tropical New Zealand (, and )
References to the subtropics infer those geographic and climate zones located approximately between the tropics (latitude 23.5°) and temperate zone (latitudes 35.0° – 66.5°) to the south of the equator. For Northland, New Zealand, the average annual SST range is from approximately 13–23°C (a 10°C range; https://terra.nasa.gov/data/modis-data). Rangitāhua/Kermadec Islands is not included here as samples received and analysed to date have been of epiphytic dinoflagellates (Rhodes and Smith Citation2018). This will be rectified in the future.
Table 2. Planktonic families and genera in the Class Dinophyceae reported in New Zealand. The list includes taxa recorded in the New Zealand Inventory of Biodiversity (Chang et al. Citation2012).
Table 3. List of planktonic dinoflagellate species (Superclass Dinoflagellata; Classes Dinophyceae, Noctilucophyceae and Oxyrrhidophyceae) identified by light microscopy in New Zealand coastal waters. Results are from New Zealand’s National Marine Biotoxin monitoring programme data records (NZNMBMP; 2008–2018) and authors’ records. Note that some species may have only ever been recorded once or are only identified to genus level.
The 1993 Karenia mikimotoi dominated bloom led to further research into this genus and several species have since been reported in both the warmer northern Hauraki Gulf as well as more temperate waters. The actual cause of the human illnesses is still not convincingly established, and may never be, and several other Karenia species formed a minor component of the bloom. Karenia mikimotoi continues to be a bloom former and has been responsible for fish deaths, most events attributable to anoxia due to the bloom conditions rather than due to biotoxins. A very closely related species, K. concordia Chang & Ryan, has also impacted on marine life (Chang et al. Citation2008). The genus Karlodinium Larsen also has a wide distribution, K. veneficum (Ballantine) Larsen (heterotypic synonym: K. micrum (Leadbeater & Dodge) Larsen) being reported in both the Hauraki Gulf and the Marlborough Sounds (de Salas et al. Citation2005). The identification of Karenia and Karlodinium spp. has been simplified by use of molecular assays (quantitative polymerase chain reaction (qPCR); Smith et al. Citation2007).
Other planktonic dinoflagellates isolated from Northland include Amphidinium trulla Murray, Rhodes & Flø Jørgensen (Murray et al. Citation2004), A. thermaeum Dolapsakis & Economou-Amilli and Akashiwo sanguinea (Hirasaka) Hansen & Moestrup. Not all dinoflagellates are a potential health risk to seafood consumers, or to fin fish and shellfish, and these three species can be considered benign (NZMBMP). Heterocapsa niei (Loeblich) Morrill & Loeblich, isolated from KeriKeri in Northland, was identified by electron microscope examination of thecal plate structure and scale morphology, and an isolate from the Bream Bay in the Hauraki Gulf was identified as H. circularisquama Horiguchi, based on plate structure alone (L. MacKenzie, pers. comm.). The latter species may be lethal to molluscs.
The palytoxin-like compound producer, Ostreopsis cf siamensis Schmidt, is also considered an epiphytic species but has regularly been reported in the water column (collected by bottle and hose samplers at the surface or at approximately 3–5 m) in Northland and throughout the Hauraki Gulf following late austral summer benthic/epiphytic blooms (NZMBMP; Shears and Ross Citation2009; Rhodes Citation2011; Rhodes and Smith Citation2018).
Sampling of surface sediments in Northland, New Zealand, has been carried out on numerous occasions and the presence of Prorocentrum lima (Ehrenberg) Stein and Vulcanodinium rugosum Nézan & Chomérat has been noted (Rhodes and Smith Citation2018). The former species has, however, also been reported in phytoplankton monitoring reports (NZMBMP) and the diarrhetic shellfish toxins it produces are of concern for shellfish safety. The species P. balticum Schütt, P. compressum (Bailey) Abé ex Dodge, P. gracile Schütt, P. micans Ehrenberg and P. triestinum Schiller have all been reported as planktonic species in New Zealand waters (Rhodes and Smith Citation2018). The name P. compressum is currently regarded as a synonym of Tryblionella compressa (Bailey) Poulin.
Vulcanodinium rugosum is most commonly found in its non-motile form in Rangaunu Harbour, where it forms dense mats on the sediments (Rhodes and Smith Citation2018), but cells do become motile and cause pinnatoxin contamination of farmed oysters (Rhodes et al. Citation2011). Pinnatoxin F has a high oral toxicity which raises concerns regarding potential adverse effects in shellfish consumers, although no toxic effects in humans have been recorded with pinnatoxins or with any other compound of the cyclic imine group to date (Munday et al. Citation2012a, Citation2012b). As well as pinnatoxins, V. rugosum strains produce portimine, a polycyclic ether toxin containing a cyclic imine moiety which is a potent and selective inducer of apoptosis (McNabb et al. Citation2011; Selwood et al. Citation2013).
Planktonic dinoflagellate species from temperate New Zealand (, and )
The annual average range in SSTs for the temperate region is approximately 9–20°C. In Canterbury the range is 8–12°C (https://terra.nasa.gov/data/modis-data). There are several species, however, that have been found in both the sub-tropical northern and the cooler southern waters of New Zealand’s mainland. For example, Ostreopsis cf siamensis has been reported in low cell concentrations in plankton samples from as far south as temperate Wellington coastal waters (NZNMBMP records; Rhodes Citation2011).
The bloom forming Alexandrium pacificum is now a regular HAB species in the main shellfish production region of New Zealand, the Marlborough Sounds (MacKenzie Citation2014). Seafood export earnings for New Zealand were $1.8 billion for the year ending June 2018 and are expected to increase in 2019. Prices for GreenshellTM mussels were lifted temporarily in 2018 due to the disruption of production in Pelorus Sound during the A. pacificum bloom (Ministry for Primary Industries Citation2018). Alexandrium minutum Halim also occurs in the Marlborough Sounds (MacKenzie and Berkett Citation1997) and both A. pacificum and A. minutum have also bloomed and caused illnesses in the Bay of Plenty (eastern North Island). The spirolide producer, A. ostenfeldii (Paulsen) Balech & Tangen, is also regularly detected through the phytoplankton monitoring programmes (MacKenzie et al. Citation1996a). Other species reported in the monitoring data include A. camurascutulum MacKenzie & Todd (MacKenzie and Todd Citation2002), A. gaarderae L.Nguyen-Ngoc & J.Larsen (previously classified as A. concavum (Gaarder) Balech), A. fraterculus (Balech) Balech, A. margalefii Balech and A. pseudogonyaulax (Biecheler) Horiguchi ex Yuki & Fukuyo. (MacKenzie Citation2014; Ruvindy et al. Citation2018).
Species from the diarrhetic shellfish poisoning genus Dinophysis are common in New Zealand’s coastal waters. The species D. acuminata Claparède & Lachmann, D. acuta Ehrenberg and D. tripos Gourret have all been reported (NZMBMP) and major Dinophysis blooms have been recorded. Phalacroma rotandatum (Claparède & Lachmann) Kofoid & Michener (previously D. rotundata Claparède & Lachmann) is also regularly recorded. In 1996, GreenshellTM mussels were experimentally hung out on long lines in the Marlborough Sounds during a bloom of D. acuta and Protoceratium reticulatum (Claparède & Lachmann) Bütschli. On that occasion the highest toxin concentrations in the mussels were from yessotoxin derivatives produced by P. reticulatum, although okadaic acid from D. acuta was detected in wild blue mussels (Mytilus galloprovincialis) (MacKenzie et al. Citation1998). Blooms of D. acuminata occur annually in the major GreenshellTM mussel producing region, the Marlborough Sounds. Despite the regular blooms, toxin concentrations in mussels have rarely exceeded regulatory limits (MacKenzie Citation2019).
Another yessotoxin producing species, with affinities to the gonyaulacoid species P. reticulatum and Lingulodinium polyedra (Stein) Dodge, is Gonyaulax spinifera (Claparède & Lachmann) Diesing (Rhodes et al. Citation2006). This species bloomed in the Marlborough Sounds, in both the autumn and spring of 2004; G. cf. elegans Rampi was also reported at that site. The related G. hyalina Ostenfeld & Schmidt was isolated from Tasman Bay and was determined as the cause of a massive mucilage event there (MacKenzie et al. Citation2002). Bioluminescence is exhibited by some dinoflagellate species including L. polyedra and G. spinifera. There have been many anecdotal reports from the Hauraki Gulf to the Marlborough Sounds. Some species of Alexandrium, Protoceratium and Tripos (formerly Ceratium) may also express bioluminescence.
The chain-forming G. catenatum, discussed under sub-tropical species, has been detected in its motile form in phytoplankton samples collected from the outer Marlborough Sounds in New Zealand’s South Island and the benthic/epiphytic P. lima is also found in sea water samples from the sub-tropics to the cooler temperate regions and is observed in the phytoplankton samples (NZMBMP; L. Rhodes unpublished data).
Several species in the genus Karenia have been discussed in sub-tropical waters (Section 2). In Hawke Bay, K. papilionaceae Haywood & Steidinger, was first reported as a Gymnodinium species (Haywood et al. Citation2004), but the genus name was later revised to Karenia (Daugbjerg et al. Citation2000). In 1994, gymnodimine was found in high concentrations in Bluff oysters (Tiostrea chilensis) and widespread clam (tuatua; Paphies subtriangulata) mortalities occurred on Southland beaches. K. selliformis Haywood, Steidinger & MacKenzie was found to be the causative organism (MacKenzie et al. Citation1996b). The bloom moved as far north as the Marlborough Sounds.
A major bloom of K. brevisulcata Chang occurred in 1998 (Chang Citation1999). The bloom caused a yellow-brown discolouration of the water and all marine life in Wellington Harbour was devastated. The toxins characterised included ten lipid-soluble K. brevisulcata toxins (KBTs) and six water-soluble brevisulcatic acids (BSXs) (Holland et al. Citation2012).
The species Karenia umbella Salas, Bolch & Hallegraeff (de Salas et al. Citation2005) was first isolated from Nelson harbour and a bloom in Akaroa Harbour, Canterbury was recently responsible for salmon mortalities (C. Moisan unpublished data; species confirmation by qPCR assay). The species is now considered synonymous with K. longicanalis Yang, Hodgkiss & G.Hansen (Luo et al. Citation2018). It is unclear whether the deaths were due to the production of an associated biotoxin or to oxygen depletion. The related Gymnodinium aureolum (Hulbert) Gert Hansen has also been isolated from throughout New Zealand, including the Hauraki Gulf. Two species from the related genus Takayama were reported from the Marlborough Sounds and Nelson’s Tasman Bay: T. helix de Salas, Bolch, Botes & Hallegraeff and T. tasmanica deSalas, Bolch & Hallegraeff (de Salas et al. Citation2005). As mentioned in Section 2, Karlodinium veneficum was reported for the Marlborough Sounds (de Salas et al. Citation2005).
Gymnodinium catenatum was first observed in the Manukau Harbour, Auckland, in 2000 (MacKenzie and Beauchamp Citation2001; Taylor and MacKenzie Citation2001). At that time paralytic shellfish toxins reached >4000 µg saxitoxin equivalents per 100 g in GreenshellTM mussels (Perna canaliculus) (MacKenzie and Beauchamp Citation2001). Since then blooms have expanded from the north west coast, around the northern tip of New Zealand, and down the north east coast and so while this species is considered temperate it can withstand warmer temperatures. It has also been reported in the Cook Strait on occasion. A look-alike species, G. impudicum (Fraga & Bravo) Gert Hansen & Moestrup, is also found in those waters and to differentiate the harmless G. impudicum from the toxic G. catenatum both fluorescent in situ hybridisation and sandwich hybridisation assays were developed for use by phytoplankton monitoring technicians (Rhodes et al. Citation2007). Since then a qPCR assay has also been developed which can rapidly determine the species present (Smith et al. Citation2014). The related G. cf. microreticulatum Bolch, Negri & Hallegraeff has been isolated from the Hauraki Gulf and G. simplexsimplex (Lohmann) Kofoid & Swezy from the Bay of Plenty.
The genus Amphidinium (family Gymnodiniaceae) is common in the sub-tropical north, but A. carterae Hulburt and A. operculatum Claparède & Lachmann are also regularly found in temperate New Zealand waters (NZMBMP). There is high intraspecific diversity within Amphidinium and, in recent years, the genus was re-investigated and many species reclassified. Of those removed from Amphidinium there are still species which are deemed ‘sensu lato taxa’, with their classification remaining uncertain (Murray et al. Citation2004; Hoppenrath et al. Citation2014). Amphidinols produced by A. carterae, have antifungal and haemolytic properties (Houdai et al. Citation2001; Echigoya et al. Citation2005) and this species has also been associated with fish kills in southeast Australia. The fish deaths were most likely due to low dissolved oxygen levels in the coastal lagoon, but the presence of Luteophenol A-like compounds produced by A. carterae cannot be ruled out as a contributor to these deaths (Murray et al. Citation2015).
The azaspiracid producing dinoflagellate genus, Azadinium Elbächter & Tillmann (Family Amphidomataceae), has been reported in temperate New Zealand waters (NZMBMP) and motile cells of Azadinium poporum Tillmann & Elbrächter have been isolated from the Marlborough Sounds (Smith et al. Citation2016). The isolates proved non-toxic and the cause of low levels of azaspiracids present in shellfish in New Zealand is still being investigated. Species of the genera Heterocapsa F. Stein and Biecheleria Moestrup, Lindberg & Daugbjerg can be easily mis-identified as Azadinium during light microscope monitoring of phytoplankton samples and qPCR assays are now regularly used to differentiate them. An isolate of H. illdefina (Herman & Sweeney) Morrill & Loeblich was identified by electron microscopy from the Bay of Plenty (L. MacKenzie, unpublished data) and was associated with skin irritations of swimmers. This species swims in a jerky manner similar to the swimming motion of Azadinium species, which further confuses light microscopy identification. An isolate of H. triquetra (Ehrenberg) Stein has identified from the waters off Canterbury and is maintained in the Cawthron Institute Culture Collection of Microalgae (CICCM). There is currently discussion about the validity of the classification of H. triquetra as the type species of the genus, there being a proposal to rename it as a species of Kryptoperidinium Er.Lindem (Gottschling et al. Citation2019).
The species Scrippsiella acuminata (Ehrenberg) Kretschmann, Elbrächter, Zinssmeister, Soehner, Kirsch, Kusber & Gottschling (previously reported in New Zealand as S. trochoidea (F.Stein) A.R.Loeblich) is known throughout the Hauraki Gulf.
The heterotrophic dinoflagellate family Thoracosphaeraceae includes Pfiesteria piscicida Steidinger & J.M.Burkholder and Pseudopfiesteria shumwayae (Glasgow & J.M.Burkholder) Litaker, Steidinger, P.Mason, Shields & P.Tester (Litaker et al. Citation2005) (synonym: Pfiesteria shumwayae Glasgow & Burkholder) and has only been reported in New Zealand from sediment samples (Rhodes et al. Citation2002, Citation2006; Rhodes and Smith Citation2018) and so are not included in the planktonic list. Species from the genera may, however, have planktonic stages.
The Southern Oceans ()
The Ross Dependency is the part of Antarctica claimed by New Zealand. Research into the microalgae associated with sea ice and the underlying water column in the Ross Sea, Antarctica, has shown a predominance of pennate diatoms (Ryan et al. Citation2006). The late summer dominance of the sea ice dinoflagellate Polarella glacialis Montresor, Procaccini & Stoecker (family Suessiaceae) was noted at Scott Base, Antarctica (McMinn et al. Citation2017) and a dinoflagellate closely related to P. glacialis (determined by DNA sequencing) was reported in sea ice by Torstensson et al. (Citation2015). Balech (Citation1976) and Scott and Marchant (Citation2005) described armoured dinoflagellate species from Antarctic waters, but little research has been carried out on the marine planktonic dinoflagellates of New Zealand’s sector of the Southern Oceans.
The presence of the genera Karlodinium and Takayama, both in the family Kareniaceae, has been recorded for the Australian sector (de Salas et al. Citation2008) and it can reasonably be inferred that those genera will also exist in the New Zealand sector, but no reports have been published at this time. Six new species (Kareniaceae) have been described from the Australian Southern Ocean: K. antarcticum de Salas, K. ballantinum de Salas, K. conicum de Salas, K. corrugatum de Salas, K. decipiens de Salas et Laza-Martinez and T. tuberculata de Salas. Both genera have been reported in New Zealand’s temperate waters although the species differ (refer Section 2). Similarly, from transects along the Antarctic coast, McMinn and Scott (Citation2005) published a comprehensive report of marine dinoflagellate species in the genera Alexandrium, Amphidinium, Blepharocysta, Dinophysis, Diplopsalis, Gonyaulax. Gymnodinium, Gyrodinium, Heterocapsa, Katodinium, Mesoporos, Oxytoxum, Phalacroma, Podolampas, Polykrikos, Preperidinium, Prorocentrum, Protoperidinium, Scrippsiella, Torodinium and Tripos (formerly Ceratium). An earlier description of dinoflagellates published in Argentina (Balech Citation1976) also names many of the same genera, in particular, Amphidinium, Dinophysis, Diplopeltopsis (synonym of Preperidinium), Gymnodinium, Gyrodinium, Heterocapsa, Prorocentrum and Protoperidinium. Balech (Citation1976) notes that some species move between sub-Antarctic and Antarctic waters, and it is therefore probable that they will also be found in the New Zealand sector.
Conclusion
New Zealand has a rich, well researched, planktonic microflora, known mainly due to the risk from toxic microalgae to the economically valuable seafood industry. More research is needed into the planktonic dinoflagellates of Rangitāhua/Kermadec Islands, to the north-east of mainland New Zealand, and of the New Zealand sector of the Southern Ocean (). Many isolates of many of the species mentioned are maintained in the CICCM (http://cultures.cawthron.org.nz/). The list will be updated as the currently active dinoflagellate research continues.
Acknowledgements
Thanks for technical help to Janet Adamson for technical support and Krystyna Ponikla and Sarah Challenger for maintaining the unique Cawthron Institute Culture Collection of Microalgae. Thanks also to Laura Biessy and Kati Doehring for providing the maps of New Zealand and Antarctica respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Balech E. 1976. Clave ilustrada de dinoflagelados Antárticos. Buenos Aires: Instituto Antárctico Argentino, Dirección Nacional del Antártico. 99 p.
- Calado AJ, Huisman JM. 2010. Commentary: Gómez F, Moreira D, López-Garcia P (2010). Neoceratium gen. nov., a new genus for all marine species currently assigned to Ceratium (Dinophyceae). Protist. 161:517–519. Protist 161: 517–519. doi: 10.1016/j.protis.2010.04.003
- Chang FH. 1999. Gymnodinium brevisulcatum sp. nov. (Gymnodiniales, Dinophyceae), a new species isolated from the 1998 summer toxic bloom in Wellington Harbour, New Zealand. Phycologia. 38:377–384. doi: 10.2216/i0031-8884-38-5-377.1
- Chang FH, Charleston T, McKenna PB, Clowes CD, Wilson GJ, Broady PA. 2012. Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans. In: Gordon DP, editor. New Zealand inventory of biodiversity. Vol 3, Chap 13. Christchurch, New Zealand: Canterbury University Press; p. 175–216.
- Chang FH, Uddstrom MJ, Pinkerton MH, Richardson KM. 2008. Characterising the 2002 toxic Karenia concordia (Dinophyceae) outbreak and its development using satellite imagery on the north-eastern coast of New Zealand. Harmful Algae. 7:532–544. doi: 10.1016/j.hal.2007.11.004
- de Salas MF, Laza-Martinez A, Hallegraeff GM. 2008. Novel Unarmored Dinoflagellates from the Toxigenic family Kareniaceae (gymnodiniales): five new species of Karlodinium and one new Takayama from the Australian sector of the Southern Ocean. Journal of Phycology. 44:241–257. doi: 10.1111/j.1529-8817.2007.00458.x
- de Salas MF, Rhodes LL, Mackenzie LA, Adamson JE, Ponikla K. 2005. The gymnodinoid genera Karenia and Takayama (Dinophyceae) in New Zealand coastal waters. New Zealand Journal of Marine and Freshwater Research. 39:135–139. doi: 10.1080/00288330.2005.9517296
- Daugbjerg N, Hansen G, Larsen J, Moestrup O. 2000. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia. 39:302–317. doi: 10.2216/i0031-8884-39-4-302.1
- Echigoya R, Rhodes L, Oshima Y, Satake M. 2005. The structures of five new antifungal and haemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae. 4:383–389. doi: 10.1016/j.hal.2004.07.004
- Gómez F. 2010. A genus name for the marine species of Ceratium: Reply to commentary by A. Calado and J.M. Huisman on Neoceratium gen. nov., a new genus for all marine species currently assigned to Ceratium (Dinophyceae). Protist. 161:35–54. Protist 161: 520–522. doi: 10.1016/j.protis.2009.06.004
- Gómez F. 2013. Reinstatement of the dinoflagellate genus Tripos to replace Neoceratium, marine species of Ceratium (Dinophyceae, Alveolata). CICIMAR Oceánides. 28:1–22.
- Gómez F, Moreira D, López-Garcia P. 2010. Neoceratium gen. nov., a new genus for all marine species currently assigned to Ceratium (Dinophyceae). Protist. 161:35–54. doi: 10.1016/j.protis.2009.06.004
- Gomez F, Richlen ML, Anderson DM. 2017. Molecular characterisation and morphology of Cochlodinium strangulatum, the type species of Cochlodinium, and Margalefidinium gen. nov. for C. polykrikoides and allied species (Gymnodiniales, Dinophyceae). Harmful Algae. 63:32–44. doi: 10.1016/j.hal.2017.01.008
- Gottschling M, Tillmann U, Elbrächter M, Kusber W-H, Hoppenrath M. 2019. Glenodinium triquetrum Ehrenb. is a species not of Heterocapsa F.Stein but of Kryptoperidinium Er.Lindem. (Krytoperidiniaceae, Peridiniales). Phytotaxa. 391:155–158. doi: 10.11646/phytotaxa.391.2.11
- Guiry MD. 2018. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. [accessed 8 February 2019]. http://www.algaebase.org.
- Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, MacKenzie L. 2004. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. Journal of Phycology. 40:165–179. doi: 10.1111/j.0022-3646.2004.02-149.x
- Holland PT, Shi F, Satake M, Hamamoto Y, Ito E, Beuzenberg V, McNabb P, Munday R, Briggs L, Truman P, et al. 2012. Novel toxins produced by the dinoflagellate Karenia brevisulcata. Harmful Algae. 13:47–57. doi: 10.1016/j.hal.2011.10.002
- Hoppenrath M, Murray SA, Chomèrat N, Horiguchi T. 2014. Marine benthic dinoflagellates – unveiling their worldwide biodiversity. Stuttgart, Germany: Schweizerbart. 276 pp.
- Horiguchi T. 1995. Heterocaspa circularisquama sp. nov. (Peridinales, Dinophyceae): A new marine dinoflagellate causing mass mortality of bivalves in Japan. Phycological Research. 43:129–136. doi: 10.1111/j.1440-1835.1995.tb00016.x
- Houdai T, Matsuoka S, Murata M, Satake M, Ota S, Oshima Y, Rhodes LL. 2001. Acetate labeling patterns of dinoflagellate polyketides, amphidinols 2, 3 and 4. Tetrahedron. 57:5551–5555. doi: 10.1016/S0040-4020(01)00481-1
- Jaffrezic E, Rhodes L, Mackenzie L, Schweibold L, Hallegraeff GM. 2019. Overview of New Zealand HAB species and HAEDAT Events, with a comparison to Australian events.: Proceedings of the 17th International Conference on Harmful Algae (submitted).
- Jasperse JA., editor. 1993. Marine toxins and New Zealand shellfish. Proceedings of a workshop on research issues, 10–11 June 1993. The Royal Society of New Zealand, Miscellaneous Series. 24:1–68.
- Kim D-W, Jo Y-H, Choi J-K, Choi J-G, Bi H. 2016. Physical processes leading to the development of an anomalously large Cochlodinium polykrikoides bloom in the East Sea/Japan Sea. Harmful Algae. 55:250–258. doi: 10.1016/j.hal.2016.03.019
- Kretschmann J, Elbrächter M, Zinssmeister C, Soehner S, Kirsch M, Kusber W-H, Gottschling M. 2015. Taxonomic clarification of the dinophyte Peridinium acuminatum (Ehrenb.), ≡ Scrippsiella acuminata, comb. nov. (Thorasphaeraceae, Peridinales). Phytotaxa. 220:239–256. doi: 10.11646/phytotaxa.220.3.3
- Litaker RW, Steidinger KA, Mason PL, Landsberg JH, Shields JD, Reece KS, Haas LW, Vogelbein WK, Vandersea MW, Kibler SR, Tester PA. 2005. The reclassification of Pfiesteria shumwayae (Dinophyceae): Pseudopfiesteria, gen nov. Journal of Phycology. 41:643–651. doi: 10.1111/j.1529-8817.2005.00075.x
- Luo Z, Wang L, Chan L, Lu S, Gu H. 2018. Karlodinium zhouanum, a new dinoflagellate species from China, and molecular phylogeny of Karenia digitata and Karenia longicanalis (Gymnodiniales, Dinophyceae). Phycologia. 57:401–412. doi: 10.2216/17-106.1
- MacKenzie A. 2014. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. New Zealand Journal of Marine and Freshwater Research. 48:430–465. doi: 10.1080/00288330.2014.911191
- MacKenzie A. 2019. A long-term time series of Dinophysis acuminata blooms and associated shellfish 2 toxin contamination in Port Underwood, Marlborough Sounds, New Zealand. Toxins: 11 (available on-line).
- MacKenzie L, Beauchamp T. 2001. Gymnodinium catenatum in New Zealand: a new problem for public health and the shellfish industry. Cawthron Report No. 633, 10 p.
- MacKenzie L, Berkett N. 1997. Cell morphology and PSP-toxin profiles of Alexandrium minutum in the Marlborough Sounds, New Zealand. New Zealand Journal of Marine and Freshwater Research. 31:403–409. doi: 10.1080/00288330.1997.9516773
- MacKenzie L, Haywood A, Adamson J, Truman P, Till D, Seki T, Satake M, Yasumoto T. 1996b. Gymnodimime contamination of shellfish in New Zealand. In: Yasumoto T, Oshima Y, Fukuyo Y, editors. Harmful and toxic algal blooms. Sendai, Japan: IOC of UNESCO; p. 97–100.
- MacKenzie L, Sims I, Beuzenberg V, Gillespie P. 2002. Mass accumulation of mucilage caused by dinoflagellate polysaccharide exudates in Tasman Bay, New Zealand. Harmful Algae. 1:69–83. doi: 10.1016/S1568-9883(02)00006-9
- MacKenzie L, Todd K. 2002. Alexandrium camurascutulum sp. nov. (Dinophyceae): a new dinoflagellate species from New Zealand. Harmful Algae. 1(3):295–300. doi: 10.1016/S1568-9883(02)00045-8
- MacKenzie L, Truman P, Satake M, Yasumoto T, Adamson J, Mountfort DO, White D. 1998. Dinoflagellate blooms and associated DSP-toxicity in shallfish in New Zealand. In: Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Harmful Algae. Spain: Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; p. 74–77.
- MacKenzie L, White D, Oshima Y, Kapa J. 1996a. The resting cyst and toxicity of Alexandrium ostenfeldii (Diniphyceae) in New Zealand. Phycologia. 35:148–155. doi: 10.2216/i0031-8884-35-2-148.1
- Mamalakis A, Yu J-Y, Randerson JT, AghaKouchak A, Foufoula-Georgiou E. 2018. A new interhemispheric teleconnection increases predictability of winter precipitation in southwestern US. Nature Communications. 9:2332. doi: 10.1038/s41467-018-04722-7
- McMinn A, Müller MN, Martin A, Upgalde SC, Lee S, Castrisios K, Ryan KG. 2017. Effects of CO2 concentration on a late summer surface sea ice community. Marine Biology. 164:87. doi: 10.1007/s00227-017-3102-4
- McMinn A, Scott FJ. 2005. Dinoflagellates. In: Scott FJ, Marchant HJ, editors. Antarctic marine protists. Canberra and Hobart: Australian Biological Resources Study; Australian Antarctic Division; p. 202–250.
- McNabb P, McCoubrey DJ, Rhodes L, Smith K, Selwood A, van Ginkel R, MacKenzie L, Munday R, Holland P. 2011. New perspectives on biotoxin detection in Rangaunu Harbour New Zealand arising from the discovery of pinnatoxins. New Zealand Journal of Marine and Freshwater Research. 13:34–39.
- Ministry for Primary Industries. 2018. Situation and outlook for primary industries. Ministry for Primary Industries, Wellington, New Zealand. Pp 18–19. https://www.mpi.govt.nz/dmsdocument/32260/loggedIn.
- Ministry for the Environment. 2016. Climate change projections for New Zealand. Ministry for the Environment. 127 pp.
- Munday R, Quilliam MA, LeBlanc P, Lewis N, Gallant P, Sperker SA, Ewart S, MacKinnon SL. 2012a. Investigations into the toxicology of spirolides, a group of marine toxins. Toxins. 4:1–14. doi: 10.3390/toxins4010001
- Munday R, Selwood A, Rhodes L. 2012b. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon. 60:995–999. doi: 10.1016/j.toxicon.2012.07.002
- Murray S, Daugbjerg N, Flo Jorgensen M, Rhodes L. 2004. Amphidinium revisited: II. Resolving species boundaries in the Amphidinium operculatum species complex (Dinophyceae), including the descriptions of Amphidinium trulla sp. Nov. and Amphidinium gibbosum comb. nov. Journal of Phycology. 40:366–382. doi: 10.1046/j.1529-8817.2004.03132.x
- Murray SA, Kohli GS, Farrell H, Spiers ZB, Place AR, Dorantes-Aranda JJ, Ruszczyk J. 2015. A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae. 49:19–28. doi: 10.1016/j.hal.2015.08.003
- Ruvindy R, Bolch CJ, MacKenzie L, Smith KF, Murray SA. 2018. QPCR assays for the detection and quantification of multiple paralytic shellfish toxin producing species of Alexandrium. Frontiers of Microbiology. 9:3153. doi:10.3389/fmicb.2018.03153.
- Rhodes L. 2011. World-wide occurrence of the toxic dinoflagellate genus Ostreopsis Schmidt. Toxicon. 57:400–407. doi: 10.1016/j.toxicon.2010.05.010
- Rhodes L, Smith K. 2018. A checklist of the benthic and epiphytic marine dinoflagellates of New Zealand, including Rangitāhua/Kermadec Islands. New Zealand. New Zealand Journal of Marine and Freshwater Research (available on-line).
- Rhodes LL, Haywood AJ, Ballantine WJ, MacKenzie AL. 1993. Algal blooms and climate anomalies in north-east New Zealand, August-December 1992. New Zealand Journal of Marine and Freshwater Research. 27:419–430. doi: 10.1080/00288330.1993.9516583
- Rhodes LL, Syhre M. 1995. Okadaic acid production by New Zealand Prorocentrum lima isolate. New Zealand Journal of Marine and Freshwater Research. 29:367–370. doi: 10.1080/00288330.1995.9516671
- Rhodes LL, Burkholder JM, Glasgow HB, Rublee PA, Allen C, Adamson JE. 2002. Pfiesteria shumwayae (Pfiesteriaceae) in New Zealand. New Zealand Journal of Marine and Freshwater Research. 36:621–630. doi: 10.1080/00288330.2002.9517117
- Rhodes LL, Adamson JE, Rublee P, Schaeffer E. 2006. Geographic distribution of Pfiesteria piscicida and P. shumwayae (Pfiesteriaceae) in Tasman Bay and Canterbury, New Zealand (2002–2003). New Zealand Journal of Marine and Freshwater Research. 40:211–220. doi: 10.1080/00288330.2006.9517414
- Rhodes L, McNabb P, de Salas M, Briggs L, Beuzenberg V, Gladstone M. 2006. Yessotoxin production by Gonyaulax spinifera. Harmful Algae. 5:148–155. doi: 10.1016/j.hal.2005.06.008
- Rhodes L, Smith K, de Salas M. 2007. DNA probes, targeting large sub-unit rRNA, for the rapid identification of the paralytic shellfish poison producing dinoflagellate, Gymnodinium catenatum. New Zealand Journal of Marine and Freshwater Research. 41:385–390. doi: 10.1080/00288330709509928
- Rhodes L, Smith K, Selwood A, McNabb P, Munday R, Suda S, Molenaar S, Hallegraeff G. 2011. Dinoflagellate Vulcanodinium rugosum Nézan et Chomérat newly identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia. 50:624–628. doi: 10.2216/11-19.1
- Rhodes L, Smith KF, Moisan C. 2013. Shifts and stasis in marine HAB monitoring in New Zealand. Environmental Science and Pollution Research. 20:6872–6877. doi: 10.1007/s11356-012-0898-9
- Ryan KG, Hegseth EN, Martin A, Davy SK, O’Toole R, Ralph PJ, McMinn A, Thorn CJ. 2006. Comparison of the microalgal community within fast ice at two sites along the Ross Sea coast, Antarctica. Antarctic Science. 18:583–594. doi: 10.1017/S0954102006000629
- Satake M, Mackenzie L, Yasumoto T. 1997. Identification of Protoceratium reticulatum as the biogenetic origin of Yessotoxin. Natural Toxins. 5:164–167. doi: 10.1002/19970504NT7
- Scott FJ, Marchant HJ. 2005. Antarctic marine protists. Australian Biological Resources Study and Antarctic Division, Canberra. 563 pp.
- Selwood AI, Wilkins AL, Munday R, Shi F, Rhodes LL, Holland PT. 2013. Portimine: a bioactive metabolite from the benthic dinoflagellate Vulcanodinium rugosum. Tetrahedron Letters. 54:4705–4707. doi: 10.1016/j.tetlet.2013.06.098
- Shears NT, Ross PM. 2009. Blooms of benthic dinoflagellates of the genus Ostreopsis; an increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae. 8:916–925. doi: 10.1016/j.hal.2009.05.003
- Smith K, Rhodes L, Selwood A, Marfell M, Zeewoldt C. 2007. Massive Karenia mikimotoi bloom in Northland, New Zealand: use of traditional and molecular techniques for rapid identification of HAB species. Harmful Algae News (IOC Newsletter). 34:1–3.
- Smith KF, Rhodes L, Harwood DT, Adamson J, Moisan C, Munday R, Tillmann U. 2016. Detection of Azadinium poporum in New Zealand: the use of molecular tools to assist with species isolations. Journal of Applied Phycology. 28:1125–1132. doi: 10.1007/s10811-015-0667-5
- Smith K, de Salas M, Adamson J, Rhodes L. 2014. Rapid and accurate identification by real-time PCR of biotoxin-producing dinoflagellates from the family Gymnodiniaceae. Marine Drugs. 12:1361–1376. doi: 10.3390/md12031361
- Taylor MD, MacKenzie LA. 2001. Delimitation survey of the toxic dinoflagellate Gymnodinium catenatum in New Zealand. Cawthron report No. 661, 32 pp.
- Torstensson A, Dinasquet J, Chierici M, Fransson A, Riemann L, Wulff A. 2015. Physicochemical control of bacterial and protist community composition and diversity in Antarctic sea ice. Environmental Microbiology. 17:3869–3881. doi: 10.1111/1462-2920.12865