?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Movement patterns of the Southern Rock Lobster Jasus edwardsii in Victoria, Australia were investigated from 8,533 tag-recapture events across a 20-year period (1992–2012). In total, 83% of lobsters were recaptured within 1 km of their tagging site and 93% within 5 km. While largely resident, elevated movements were observed within specific regions, with the overall direction of movement being from inshore to offshore areas. Movement was not impacted by lobster sex, size, or reproductive stage. The fishery for Southern Rock Lobster within Victoria is currently managed into two zones with separate total allowable commercial catches in each area. Given the high levels of site fidelity, our findings suggest that management of the resource at this spatial scale is appropriate and that consideration of significant movement between zones is not warranted in fishery stock assessment models.
Introduction
Southern Rock Lobster Jasus edwardsii (Hutton 1875) is distributed throughout New Zealand and across southern Australia, from Coffs Harbour in New South Wales to Geraldton in Western Australia, including Tasmania and the Tasman Sea (Edmunds Citation1995). The species inhabits limestone and granite reef habitats in depths ranging from 1 to 200 m. Within Australia, the majority of the population is found in South Australia, Tasmania, and Victoria where it supports an important fishery with an annual landed catch of ∼3,000 tonnes, worth approximately $240 million per annum (Econsearch Citation2017). Fishing is undertaken using baited steel framed pots that are usually set and hauled within a 24-hour period.
In Victoria, the commercial fishery is divided into two separately managed zones; the Western Zone (WZ) and the Eastern Zone (EZ), which are each sub-divided into three fishing regions for spatial reporting purposes. Fishing is undertaken all-year round with catch reported daily in mandatory logbooks. Given its recreational and commercial fishing significance, understanding the movement patterns of Southern Rock Lobster within Victoria is particularly important from a resource management perspective. For example, spatial movement is routinely incorporated into population modelling for stock assessment purposes (Walters et al. Citation1993; McGarvey et al. Citation2010).
Movement patterns of lobsters have been broadly categorised under ‘homing’, ‘nomadism’, and ‘migration’ (Herrnkind Citation1980). Homing is characterised by short and frequently random movements, while migration is considered to be the large-scale movement of an individual or a population over distances >5 km. Nomadism differs from migration in that it typically lacks group directedness, periodicity, and temporal confinement. Within spiny lobsters, long-distance and directional migrations are common in the Green Rock Lobster Sagmariasus verreauxii (Booth Citation1997), Caribbean spiny lobster Panulirus argus (Herrnkind and McLean Citation1971), Australian spiny lobster P. cygnus (Phillips Citation1983), Tropical Rock Lobster P. ornatus (Moore and MacFarlane Citation1984; Bell et al. Citation1987), and the Southern spiny lobster Palinurus gilchristi (Groeneveld and Branch Citation2002). However, other species tend to exhibit limited movement. Numerous large-scale tagging studies have detailed movement patterns of J. edwardsii within various fisheries in both Australia and New Zealand (McKoy Citation1983; Gardner et al. Citation2003; Linnane et al. Citation2005). In almost all cases, the species appeared highly resident, as defined by the tendency of individuals to remain in a defined area over an extended period. However, while large-scale movement was not observed, unique spatial migrations by sub-groups of individuals were evident. In many cases, these movements were over considerable distances (>10 km) and highly directional in nature. For example, McKoy (Citation1983) reported migration of immature Southern Rock Lobsters around southern New Zealand while Linnane et al. (Citation2005) observed movement from marginal lobster habitat to adjacent offshore reefs within the Coorong region of South Australia.
Southern Rock Lobsters have been tagged within the Victorian fishery since the early 1990s. Despite this, localised movement patterns remain largely unknown. This study presents the first insight into spatially-explicit lobster movement in the Victorian fishery based on 20 years of tag-recapture to the end of the 2012 fishing season. Specifically, the study investigated movement between zones, fishing regions, and depth, as well as movement by size, sex, and female reproductive condition.
Methods
Tag-recapture programme
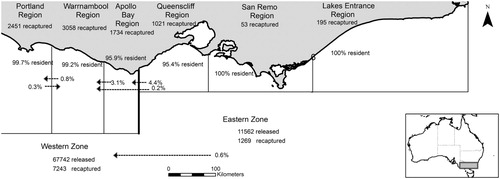
Since August 1992, 79,304 lobsters have been tagged within the Victorian fishery (67,742 WZ and 11,562 EZ; ). All lobsters were caught using standard baited rock lobster traps as required by the commercial lobster fishing industry of Victoria (Anon. Citation2017). Lobsters were tagged using Hallprint T-anchor tags. The tags were 55 mm long with a 30 mm shaft length and 10 mm T-bar length. Each was identified with a unique code and were inserted ventrally into the anterior oblique muscle between the first and second abdominal sterna. Lobsters were released as close to the area of capture as possible and the position recorded using the vessels’ global positioning system (GPS).
Figure 1. Movement dynamics of Jasus edwardsii between zones and sub-regions within Victoria, Australia Solid black line represents border between Western and Eastern zones.
Scientists from the Victorian Fisheries Authority executed the tagging programme in conjunction with volunteer commercial fishers. Recaptures were largely reported by commercial fishers and therefore primarily occurred during the commercial fishing season from 16 November to 14 September for males, and 16 November to 31 May for females. Other avenues of data collection were by fish processors, recreational fishers and, occasionally, by restaurants or individuals who purchased a tagged lobster.
Data recorded for all tagging and recaptures included details on date of capture, location (GPS), sex, reproductive condition, carapace length (CL), and depth. Location was recorded using fishing zone (WZ and EZ) and sub-regions within these zones, i.e. Portland, Warrnambool and Apollo Bay (WZ), and Queenscliff, San Remo and Lakes Entrance (EZ) (). Sex was determined by direct observations of pleopod morphology and the position of the gonophores, and occasionally by the presence or absence of an opposable claw on the fifth leg (only present in females). Female reproductive condition was determined by setal development and categorised into unsetosed (immature), setosed (mature), and berried (egg-bearing). Carapace length (CL) was measured linearly from the point of union of the second antennae to the centre of the posterior margin of the carapace, and recorded to the nearest mm. Lobster size data were categorised in three classes, as per Linnane et al. (Citation2005): small (<100 mm CL), medium (100–130 mm CL), and large (>130 mm CL).
Data analysis
Contingency tables were used to test whether there was a difference in the spatial and temporal distribution of capture and recapture effort. Distance moved was calculated as the straight-line displacement between initial capture and recapture as recorded by latitude and longitude. Following descriptive comparison of movement between regions, we investigated the effect of Region, Depth, Size class, and Reproductive condition on movement >5 km, the arbitrary distance above which Booth (Citation1997) defined as large-scale movement, using a generalised linear model. We determined the most appropriate statistical family and error distribution (Gaussian distribution) by examining the distribution of the response variable and visually inspecting the residuals for the saturated models. The full model was dredged, creating a set of new models containing all possible combinations of the factors. We did not include interactions between variables because of the low number of recaptures once split across more than one response variable. Models were ranked on decreasing model fit, using Akaike’s information criterion corrected for small sample size (AICc) (Burnham and Anderson Citation2002). The bias-corrected relative weight of evidence for each model, given the data and the suite of candidate models considered, was the AICc weight; the smaller the weight, the lower its contribution to parameter estimates (Burnham and Anderson Citation2002).
Results
Recaptures
To June of 2012, 8,512 (11%) of the 79,304 lobsters had been recaptured. Of these, 633 were caught more than once, with the highest number of recaptures being 5. There were no significant differences in the temporal distribution of catches between capture and recapture for either zones ( = 0.67, p = 0.411) or regions (
= 1.6, p = 0.901). The number of lobsters tagged and recaptured was not homogeneously distributed throughout the year (capture
= 6581.7, p < 0.001, recapture
= 6659.9, p < 0.001). Catches primarily occurred between November and February, when 74% and 68% of original captures and recaptures took place, respectively, reflecting the period of highest fishing effort in Victoria (Linnane et al. Citation2016).
Similarly, there were no significant differences in the spatial distribution of capture and recapture for either zones ( = 0.67, p = 0.410) or regions (
= 1.6, p = 0.909). However, the number of lobsters caught lacked spatial uniformity, with 85.3% of recaptures occurring in the WZ (
= 8461.63, p = < 0.001). Spatial differences in catches (capture and recapture) also existed regionally (
= 7509.77, p < 0.001), with 42% of the WZ catches occurring in Warrnambool and 81% of the EZ catches occurring in Queenscliff () reflecting the high contribution of these regions to overall catches within each zone (Linnane et al. Citation2016).
Distance and direction of movement
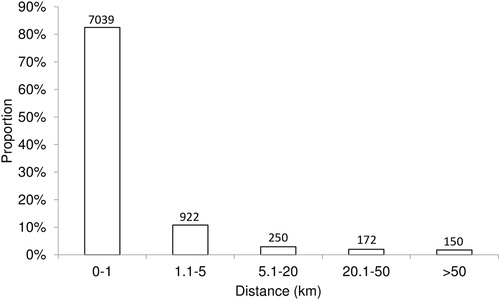
The majority (83%) of tagged J. edwardsii moved <1 km from their original capture location (). Of those remaining, 11% moved 1–5 km, 3% moved 5–20 km, and 4% moved distances >20 km. Movement rarely occurred between regions or zones, with only 0.6% of the tagged J. edwardsii moving from the EZ to the WZ and no recorded movement from the WZ to the EZ (). The highest movement between regions occurred in Queenscliff (4.6%) followed by Apollo Bay (3.1%). Of the lobsters moving out of Queenscliff, 4.4% moved into Apollo Bay and 0.2% to Warrnambool. A small percentage of lobsters tagged in Warrnambool (0.8%) were recaptured in Portland. Combined, these represented westward movement of 8.5% while only 0.3% of individuals moved eastwards from Portland to Warrnambool. None of the lobsters tagged in the San Remo or Lakes Entrance regions were recaptured in any other region.
Figure 2. Distance travelled (km) by Jasus edwardsii based on each capture-recature location in Victoria.Numbers above bars reflect recaptures.
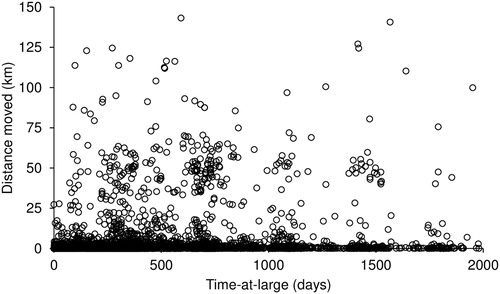
Time-at-large varied extensively from lobsters being recaptured on the same day of release up to 4,461 days (12.2 years) later (mean ± standard error: 461.5 ± 4.5 days) (). The distance between capture and recapture was weakly correlated to time-at-large (R2 = 0.02) with the slope of the regression low (0.004), indicating that time-at-large had minimal effect on the extent of movement.
Effects of region, size, depth, and reproductive condition on movement >5 km
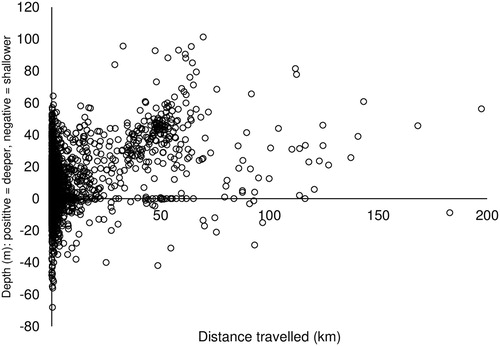
Region and Depth had the greatest effect on lobster movement >5 km, while Reproductive condition and Size classes had little influence on distance travelled (; and ). Lobsters off Warrnambool, Apollo Bay, and Queenscliff moved the most (37 ± 0.7 km) compared to other regions (16 ± 1.6 km) (A). The difference in depths between capture and recapture suggested that J. edwardsii movement was predominantly offshore towards deeper waters, with this pattern increasing with distance travelled (). Movement offshore was generally to a maximum of 50–60 m, with few recaptures observed beyond this depth range. In addition to offshore movement, the amount of movement >5 km increased with depth of original capture from 24 ± 0.7 km at capture depth 0–50 m to 46 ± 0.9 km by lobsters caught >50 m (B; and ). Region and Depth were the variables most often included in the top four models and were both included in the top-ranked model (AICc weight: 0.60; ). Distance travelled >5 km of large lobsters (5.3 ± 0.01 km) was shorter than that of small (33.1 ± 0.42 km) and medium (33.9 ± 0.49 km) lobsters (C; and ). This was, however, based on a small number of large lobster recaptured compared to small- and medium-sized lobsters (105 vs. >3,500). No differences in movement could be detected between small and medium lobsters (). Female reproductive condition and sex had limited effects on the movement of lobsters, with the first model including reproductive condition having an AICc weight of 0.13 (D; ).
Figure 4. (A–D) Movement patterns of Jasus edwardsii in Victoria by region (A), depth (B) size (C) and sex and reproductive condition (D). Median values are indicated by the horizontal bar; length of the box is the inter-quartile range; whiskers represent quartiles; circles are data points; and diamonds are the mean.
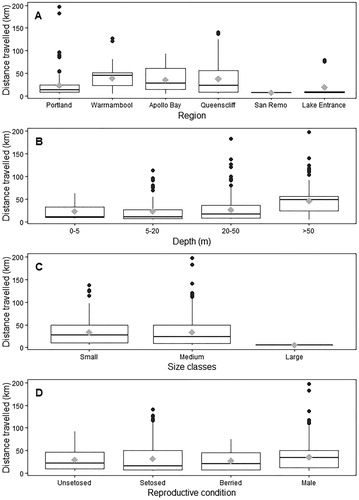
Table 1. Results from the dredge function assessing the effects of variables on Jasus edwardsii over 5 km.
Table 2. Estimated coefficients (β) and their standard errors (SE) for each variable and factor, t-values of factors included in the top-ranked model (indicated for each variable).
Discussion
Consistent with previous findings, there was no evidence to suggest that J. edwardsii within Victoria exhibited large-scale migration behaviour. In New Zealand, Street (Citation1969, Citation1971) first reported movement patterns in tagged J. edwardsii released in Otago, Foveaux Strait, and Fiordland regions between 1957 and 1970. Almost all lobsters were recaptured within 16 km of their release points. McKoy (Citation1983) similarly reported that 87% of tagged recaptures were within 5 km of release in Stewart Island, New Zealand. In Australia, a study of 39,000 tag-recapture events in sites around Tasmania between 1973 and 2001 showed that more than 90% of animals moved <5 km (Gardner et al. Citation2003) while in South Australia between 1993 and 2003, 68% of tagged lobsters were recaptured within 1 km of their release site and 85% within 5 km (Linnane et al. Citation2005). The results from Victoria are consistent with these studies where 83% of lobsters were recaptured within 1 km of release, confirming that the species is largely resident within these spatial scales and is likely to exhibit high levels of site fidelity.
Despite the high levels of residency, all studies across the range of J. edwardsii identified regions where elevated levels of movement were prevalent. In many cases, this was directional, and thereby migrational, in nature. For example, in South Australia, Linnane et al. (Citation2005) reported directional movement >100 km from within a lobster sanctuary to adjacent lobster grounds. Similarly, in Tasmania, Pearn (Citation1994) analysed movement from a cohort of tagged animals in Tasmania and concluded that immature females and similar-sized males appeared to migrate distances >8 km to deep-water sites in the far northwest of the State.
In Victoria, higher levels of directional movements (>5 km) also occurred for a small percentage of lobsters within Warrnambool, Apollo Bay, and Queenscliff. In these regions, J. edwardsii move from inshore to offshore grounds. While no differences were observed in movement patterns between sex or reproductive conditions within Victoria, previous studies have suggested that the migratory behaviour of J. edwardsii, especially to deeper grounds, may be linked to reproductive, moulting, and feeding cycles. For example, within New Zealand, aggregations of male lobsters are known to peak offshore at times of elevated feeding rates in July (after mating), whereas ovigerous female numbers are highest in August before larval release in September–October (MacDiarmid Citation1989; Kelly et al. Citation1999; Kelly Citation2001). Similarly, in South Australia and New Zealand, movement to offshore grounds was highest in small (<100 mm CL) sexually immature females (Annala and Bycroft Citation1993; Kendrick and Bentley Citation2003; Linnane et al. Citation2005). Movement to offshore grounds prior to sexual maturity and subsequent egg production may have advantages from a reproductive perspective. Booth (Citation1997) suggested that movement by females to offshore sites might facilitate larval survival by allowing for dispersal away from reef-dwelling planktivores, while Factor (Citation1995) speculated that females remain offshore to brood their eggs over the colder winter months due to the more constant cool temperatures that are optimal for egg development. While movement was predominantly offshore, some individuals did move to inshore grounds. MacDiarmid (Citation1991) noted that higher densities of females in shallow waters (<10 m depth) in New Zealand coincided with periods of moulting, although the benefits of this behaviour were not clear, particularly given that predatory fish are generally more abundant in these depths.
Accounting for movement patterns within rock lobster fisheries is important in terms of resource management, especially where TACCs have been implemented. Based on similar findings to those in Victoria where some inshore-offshore movement was observed, McGarvey et al. (Citation2010) integrated recapture-conditioned movement estimation into a spatial stock assessment model in South Australia. Overall, the study showed that, combined with mortality and recruitment estimates, the integration of movement can substantially improve biomass estimates in spatially-resolved stock assessments.
It is important to highlight that inferring spatial movement patterns based on fishery-dependent trap data has numerous challenges. This is particularly important in Southern Rock Lobster fisheries where fishers spatially and seasonally target lobsters of a particular size and colour to meet oversea market demands (Chandrapavan et al. Citation2009; Linnane and Crosthwaite Citation2009). Specifically, inshore areas (<60 m depth) are highly targeted, leading to sampling bias linked to heterogeneous sampling effort. In addition, the majority of the catch in Victoria tends to taken from November through to February (Linnane et al. Citation2016) thereby limiting any temporal analyses relating to seasonal movement patterns. From a size perspective, commercial lobster pots are selective with individuals <70 mm CL and >140 mm CL rarely observed within the Victorian fishery (Linnane et al. Citation2016). As a result, conclusions on size specific movement patterns should be treated with caution as certain size classes may be under-represented due to gear selectivity or actual availability in the catch. Catchability can also vary by season and sex (Ziegler et al. Citation2003), with catchability of both males and females highest in the early Austral summer and lowest in winter. In addition, lifecycle stages such as moulting, mating and egg extrusion in females can reduce trap entry and therefore potential recapture rates (Ziegler et al. Citation2004).
In summary, the results of this study largely reflect those from other jurisdictions across the range of J. edwardsii in south-eastern Australia. The species appears largely resident and with the exception of specific areas, does not exhibit long-distance movement behaviour. Finally, these findings have implications from a stock assessmentperspective. Currently, the WZ and EZ are managed as distinct spatial units with separate TACCs for each zone (Anon. Citation2017). Annual stock assessments are based on zone-specific biomass estimates that do not account for movement between jurisdictions (Anon Citation2018). The low movement rates between regions and zones indicate that this approach is appropriate and justified.
Acknowledgements
The authors wish to thank all the Victorian commercial and recreational fishers who provided lobster tag returns. We acknowledge the comments from two anonymous referees during the publication process. This research did not receive any specific funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Annala JH, Bycroft BL. 1993. Movements of rock lobsters (Jasus edwardsii) tagged in Fiordland, New Zealand. New Zealand Journal of Marine and Freshwater Research. 27:183–190. doi: 10.1080/00288330.1993.9516556
- Anon. 2017. Victorian rock lobster fishery management plan 2017. Victorian Fisheries Authority (VFA). ISBN 978-1-925733-6.
- Anon. 2018. Victorian rock lobster fishery stock assessment report – 2016/17 Season. Victorian Fisheries Authority (VFA). ISBN 978-1-925733-58-7.
- Bell R, Channells P, MacFarlane J, Moore R, Phillips B. 1987. Movements and breeding of the ornate rock lobster, Panulirus ornatus, in Torres Strait and on the north-east coast of Queensland. Marine and Freshwater Research. 38:197–210. doi: 10.1071/MF9870197
- Booth JD. 1997. Long distance movements in Jasus spp. and their role in larval recruitment. Bulletin of Marine Science. 61:1111–1128.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, USA: Springer-Verlag.
- Chandrapavan A, Gardner C, Linnane A, Hobday D. 2009. Colour variation in the southern rock lobster Jasus edwardsii and its economic impact on the commercial industry. New Zealand Journal of Marine and Freshwater Research. 43:537–545. doi: 10.1080/00288330909510020
- Econsearch. 2017. Economic indicators for the South Australian Southern Zone Rock Lobster Fishery, 2015/16. A report to PIRSA Fisheries and Aquaculture. Adelaide, 72 pp.
- Edmunds M. 1995. The ecology of the juvenile southern rock lobster, Jasus edwardsii (Hutton 1875) (Palinuridae) [PhD thesis]. The University of Tasmania.
- Factor JF. 1995. Biology of the lobster Homarus americanus. San Diego, California: Academic press Inc; pp. 1–528.
- Gardner C, Frusher S, Haddon M, Buxton C. 2003. Movements of the southern rock lobster Jasus edwardsii in Tasmania, Australia. Bulletin of Marine Science. 73:653–671.
- Groeneveld JC, Branch GM. 2002. Long-distance migration of South African deep-water rock lobster Palinurus gilchristi. Marine Ecology Progress Series. 232:225–238. doi: 10.3354/meps232225
- Herrnkind WF. 1980. Spiny lobsters: patterns of movement. In: Cobb J.S., Phillips B.F, editor. The biology and management of lobsters: physiology and behaviour. New York: Academic Press; p. 349–401.
- Herrnkind WF, McLean R. 1971. Field studies of homing, mass emigration, and orientation in the spiny lobster, Panulirus argus. Annals of the New York Academy of Sciences. 188:359–376. doi: 10.1111/j.1749-6632.1971.tb13109.x
- Kelly S. 2001. Temporal variation in the movement of the spiny lobster Jasus edwardsii. Marine and Freshwater Research. 52:323–331. doi: 10.1071/MF00028
- Kelly S, MacDiarmid AB, Babcock RC. 1999. Characteristics of spiny lobster, Jasus edwardsii, aggregations in exposed reef and sandy areas. Marine and Freshwater Research. 50:409–416. doi: 10.1071/MF98126
- Kendrick TH, Bentley N. 2003. Movements of rock lobsters (Jasus edwardsii) tagged by commercial fishers around the coast of New Zealand from 1993 (pp. 48). New Zealand Fisheries Assessment Report 2003/55. Ministry of Fisheries. Wellington, New Zealand.
- Linnane A, Crosthwaite K. 2009. Spatial dynamics of the South Australian rock lobster (Jasus edwardsii) fishery under a quota based system. New Zealand Journal of Marine and Freshwater Research. 43:475–484. doi: 10.1080/00288330909510016
- Linnane A, Dimmlich W, Ward T. 2005. Movement patterns of the southern rock lobster, Jasus edwardsii, off South Australia. New Zealand Journal of Marine and Freshwater Research. 39:335–346. doi: 10.1080/00288330.2005.9517314
- Linnane A, McGarvey R, McLeay L, Feenstra J, Reilly D. 2016. Victorian rock lobster and giant crab fisheries status report - 2014/2015 season. Fishery Status Report to Fisheries Victoria, Department of Economic Development, Jobs, Transport and Resources. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2012/000434-5. SARDI Research Report Series No. 908. 41pp.
- MacDiarmid AB. 1989. Moulting and reproduction of the spiny lobster Jasus edwardsii (Decapoda: Palinuridae) in northern New Zealand. Marine Biology. 103:303–310. doi: 10.1007/BF00397263
- MacDiarmid AB. 1991. Seasonal changes in depth distribution, sex ratio and size frequency of spiny lobster Jasus edwardsii on a coastal reef in northern New Zealand. Marine Ecology Progress Series. 70:129–141. doi: 10.3354/meps070129
- McGarvey R, Linnane A, Feenstra J, Punt A, Matthews J. 2010. Integrating recapture-conditioned movement estimation into spatial stock assessment: a South Australian lobster fishery application. Fisheries Research. 105:80–90. doi: 10.1016/j.fishres.2010.03.006
- McKoy JL. 1983. Movements of rock lobsters, Jasus edwardsii (Decapoda, Palinuridae), tagged near Stewart Island, New Zealand. New Zealand Journal of Marine and Freshwater Research. 17:357–366. doi: 10.1080/00288330.1983.9516011
- Moore R, MacFarlane J. 1984. Migration of the ornate rock lobster, Panulirus ornatus (Fabricius), in Papua New Guinea. Marine and Freshwater Research. 35:197–212. doi: 10.1071/MF9840197
- Pearn R. 1994. Rock lobster tagging shows movement occurs. Fishing Today, Tasmania. 7:27–29.
- Phillips B. 1983. Migrations of pre-adult western rock lobsters, Panulirus cygnus, in Western Australia. Marine Biology. 76:311–318. doi: 10.1007/BF00393034
- Street RJ. 1969. The New Zealand crayfish, Jasus edwardsii (Hutton 1875). New Zealand Marine Department Fisheries Technical Report 30. 29 p.
- Street RJ. 1971. Rock lobster migration off Otago. Commercial Fishing. 10:16–17.
- Walters CJ, Hall N, Brown R, Chubb C. 1993. Spatial model for the population dynamics and exploitation of the Western Australian rock lobster, Panulirus cygnus. Canadian Journal of Fisheries and Aquatic Sciences. 50:1650–1662. doi: 10.1139/f93-186
- Ziegler PE, Frusher SD, Johnson CR, Gardner C. 2003. Catchability of the southern rock lobster Jasus edwardsii. I. effects of sex, season and catch history. Marine and Freshwater Research. 53:1143–1148. doi: 10.1071/MF01243
- Ziegler PE, Haddon M, Frusher SD, Johnson CR. 2004. Modelling seasonal catchability of the southern rock lobster Jasus edwardsii by water temperature, moulting, and mating. Marine Biology. 145:179–190. doi: 10.1007/s00227-004-1298-6