ABSTRACT
The endemic, green-lipped mussel (Perna canaliculus), trademarked as Greenshell™ mussel, contributes most to the New Zealand aquaculture industry based on tonnage and export value. Research on mussel immunity is motivated greatly by economical and biosecurity necessities. Indeed, mussel aquaculture is threatened by pathogenic micro-organisms and environmental stressors. As such there is a need to understand the mechanisms that drive mussel immune responses and the associated interactions with the environment. Specifically, this review (1) analyses the existing immunological studies conducted on P. canaliculus, (2) evaluates the literature pertaining to mussel immunity at the cellular and humoral levels, (3) identifies and discusses pathogens that are relevant to P. canaliculus, (4) focuses on the virulent factors employed by mussel pathogens likely to induce diseases, (5) provides a comprehensive analysis of the response mechanisms employed by mussels to various stressors, and (6) explores omics applications and future perspectives in mussel immunology. Finally, this review highlights various strategies from immunological research, such as gene rearrangement, probiotics, immunostimulants, and selective breeding, promising to enhance mussel health and resilience in aquaculture. By exploring these immunological findings and their practical applications, this review contributes to sustainable mussel aquaculture, improving productivity and disease management in the industry.
Introduction
The GreenshellTM mussel farming industry is leading the New Zealand aquaculture sector in terms of production volumes and export revenue (Lane et al. Citation2022 ). Compared to other commercially cultivated bivalve species, Perna canaliculus farmed stocks have experienced relatively few disease outbreaks to date (Castinel et al. Citation2019). However, GreenshellTM mussels are not immune to disease threats, necessitating awareness, and baseline health parameters as precautionary measures (Rolton and Ragg Citation2020). More efforts are needed to acquire a detailed understanding of the immunological pathways implemented by P. canaliculus when exposed to biotic and abiotic factors. Stressors, such as pathogens and environmental stressors are being investigated using multiple levels of biological organisation (Waller and Cope Citation2019). The impacts of multi-stressors on the marine environment, and the combination of omics research approaches along with histopathological assessments, is seen as a unique opportunity to quantify and identify aspects of disease physiology. As downstream tools, omics applications can be combined with upstream phenotyping tools to characterise the profiles of different cells and tissues (Nguyen and Alfaro Citation2020a). The new knowledge generated from these emerging approaches will no doubt improve our understanding of bivalve physiology when exposed to pathogens and environmental stresses. Additionally, proactive disease prevention strategies, along with risk analyses of current pathogens within farmed shellfish, and surveillance and mitigation measures will be key in maintaining sustainable aquaculture farms (Fox et al. Citation2020).
Collectively, this review showcases the current knowledge on the New Zealand Greenshell™ mussel (P. canaliculus) immunology and summarises the key immunological responses of this species to the presence of stressors. The focus of this review is placed on pathogens and diseases reported within P. canaliculus, encapsulating factors that determine the prevalence of bacterial pathogens. Furthermore, this review explores the diverse modes of transmission employed by bacterial pathogens, unravels the underlying mechanisms driving their virulence, and provides elucidation on the intricate interplay between the host and pathogens. The effect of biotic and abiotic stressors on P. canaliculus immunity is also reported, along with an overview of omics approaches to study bacterial pathogens. Understanding the fundamental mechanisms that govern the immune response and stress adaptation in mussels holds significant scientific implications. This knowledge not only contributes to our comprehension of mussel biology but also provides crucial insights for future immunological studies across various mussel species. Moreover, it enables us to anticipate and predict potential community changes. Finally, this review synthesises the practical applications of mussel immunity knowledge within the industry and discusses some potential next steps necessary to facilitate the development of effective measures for mitigating mussel diseases and safeguarding existing healthy populations.
Perna canaliculus
Green-lipped mussels, trademarked as Greenshell™ mussels (Perna canaliculus) are endemic to the inshore coastlines of New Zealand (NZ) and are classified as the most important aquaculture species in NZ. Approximately 303 million NZD was generated from Greenshell™ mussel exports in 2021 (Miller et al. Citation2023). These mussels are typically farmed for food and nutraceutical products in various forms, such as oil extracts and powders. From an indigenous Māori context, P. canaliculus is a treasured species, with an active role as a guardian entity in the natural environment (Castinel et al. Citation2019; Miller et al. Citation2023). P. canaliculus is also used as a model environmental indicator species in NZ, where it serves as a reliable bioindicator of coastal contamination and supports biomarker research on the effects of multiple environmental stressors (Webb et al. Citation2020). In aquaculture production, P. canaliculus is grown in shallow coastal waters, on longline systems for approximately 15–18 months until a harvestable size of 90–100 mm is achieved (Stenton-Dozey et al. Citation2021). In these culture systems, an increasingly diverse range of biofouling organisms (i.e. algae and worms) and potential pathogens are present, leading to increased production costs (Soliman and Inglis Citation2018) and possible threats to the aquaculture industry (Georgiades et al. Citation2020).
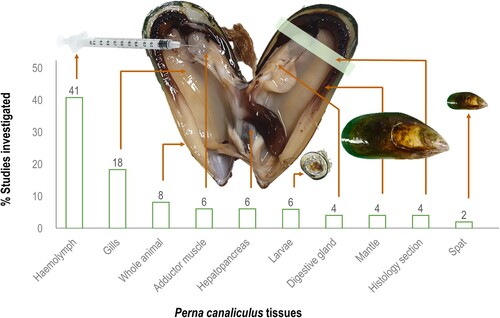
As a suspension-feeding bivalve, mussels feed by pumping water through their gill filaments (i.e. ctenidia) to capture food particles and eliminate non-food particles as pseudofaeces. The gills are the main site of interaction with the surrounding environment, acting as an important organ for oxygen uptake, bioaccumulation of contaminants or bacteria, and evacuation of waste (Gui et al. Citation2016). The gills are composed of various epithelial cells, mucous glands, cuboidal respiratory epithelium, trabecular cells, and infiltrated haemocytes, which contribute to the recognition or agglutination of filtered pathogens (Saco et al. Citation2020). Like the gills, the mantle is covered by mucus and is constantly exposed to microbes from the external environment (Gerdol Citation2017). The digestive gland is composed of basophilic cells supporting enzyme production and secretion and digestive cells with lysosomal content important for intracellular digestion, detoxification (Dimitriadis et al. Citation2004), and immunity (Allam and Raftos Citation2015). Mussel tissues, such as gills, mantle, hepatopancreas (digestive gland), and biofluids (e.g. haemolymph) are frequently utilised to monitor immune function as they serve as major sources of immune molecules (Nguyen et al. Citation2019c). Furthermore, mussel haemolymph has been well-studied for understanding aspects of cellular and humoral immunity (Green et al. Citation2019). Haemolymph contains haemocytes, which are responsible for the main cellular defence mechanisms, such as phagocytosis, encapsulation, and infiltration of pathogens, as well as the production of reactive oxygen and nitrogen species. Additionally, haemocytes are rich in hydrolytic enzymes and express proteins involved in pathogen recognition and agglutination (Campos et al. Citation2015) and involved in biomineralisation and shell formation (Song et al. Citation2019). Haemocytes are found in all internal spaces of mussels, circulating in the haemolymph, surrounding all tissues, and migrating into the pallial and extrapallial spaces (Saco et al. Citation2020). Considering the ease of haemolymph extraction and its crucial role in innate immunity, most immunological studies, or research focusing on bacterial infections in P. canaliculus have utilised haemolymph as the primary biological sample tissue (). Then again, responses from mussels from various life stages, such as larvae and spat, as well as sections of the whole animal itself (histological sections) have been document within immune studies.
Figure 1. Summary of the different tissues and samples utilised in Perna canaliculus research when investigating mussel immunity, environmental stressors, or associated factors or threats.
In this review, a comprehensive literature review was conducted on factors electing an immune response in P. canaliculus () with the following criteria implemented: (1) studies had to measure an immune response following the presence of a stressor, (2) in either field or laboratory study; (3) the methods had to clearly indicate which sample and tests (assays) were used to detect the immune response; and (4) studies had to evaluate Perna canaliculus. Interestingly, immunological studies on P. canaliculus have increased in the last five years (30 out of the 36 studies were published between 2018 and 2023). Method-wise the use of bioassays, flow cytometry and gas chromatography-mass spectrometry (GC-MS) have been mainly used to investigate P. canaliculus immunology, while microscopy, in situ hybridisation, polymerase chain reactions (PCR), and genotyping have also been incorporated in various studies. Compared to other mytilid species, data from genomic and protein databases, are largely unavailable for P. canaliculus. Yet, the molecular data from other species enables comparative immunological studies to confer findings and update knowledge on P. canaliculus immunity. Significant research efforts characterising immune systems of bivalves (Allam and Espinosa Citation2016; Zannella et al. Citation2017; Grinchenko and Kumeiko Citation2022) and particularly oyster immunity (Wang et al. Citation2018; Adzigbli et al. Citation2020; Petton et al. Citation2021), have enriched our understanding of immunological processes implemented by mussels. Next, mussel immunity is briefly discussed, with a focus on the functions of the immune processes, key genes, and pathways relating to significant diseases and stressors. Importantly, we also highlight the application of immunological research or the lack thereof in P. canaliculus.
Table 1. Immunological studies performed on P. canaliculus electing an immune response following exposure to a stressor or detection of a pathogen.
Mussel immunity
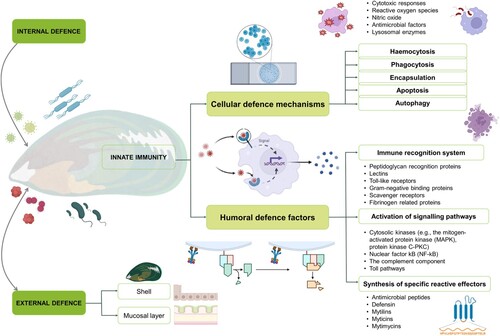
Mussel immunity refers to the complex defence mechanisms and responses exhibited by mussels, which enable them to protect themselves against various stressors and maintain overall health. The mussel immune system outlined in consists: (1) an efficient cellular and humoral innate immune system (internal defence), (2) physical barriers (shell and mucus), and (3) behavioural avoidance (external defence). These are generally implemented during unfavourable conditions or in the face of pathogen infections (Gerdol et al. Citation2018). The first part of the external defence system is the shell, protecting soft tissue from physical–chemical threats. Next, the skin and mucosal layer trap microbes and facilitie the elimination via ciliary activity (Allam and Raftos Citation2015). Mucosal surfaces play a key role in activating systemic immune responses, containing various cells and bioactive molecules that trap foreign invaders before they reach the soft tissue (Allam and Espinosa Citation2016). From an internal defence system point of view, cellular and humoral defence mechanisms, along with innate immunity help to control the proliferation of pathogens in mussels (Gerdol and Venier Citation2015; Bouallegui Citation2019). The cellular and humoral defence systems will be discussed separately along with their various sub-components in the following sections.
Figure 2. An overview of bivalve immunity, considering external and internal defences, along with cellular and humoral defence factors relating to mussel species.
Cellular immunity
The bivalve cellular immune response is achieved by haemocytes, which are present in haemolymph in an open vascular system. In P. canaliculus, haemocytes can be divided into granulocytes and hyalinocytes (Rolton and Ragg Citation2020), with various sub-types often classified as eosinophilic granulocytes, basophilic granulocytes, and small and large hyalinocytes (De La Ballina et al. Citation2022; Muznebin et al. Citation2022c). Granulocytes typically represent the major cells involved during defence reactions (Bouallegui Citation2019). In the presence of pathogens (foreign particles) bivalve haemocytes implement different cellular defence mechanisms, such as haemocytosis, phagocytosis, encapsulation, apoptosis, and autophagy (Nguyen and Alfaro Citation2020a).
During haemocytic infiltration (haemocytosis), haemocytes are activated, which leads to an observable increase in their circulation and subsequent movement towards infected or injured tissues, guided by chemo-attractant substances (Labreuche et al. Citation2006). Chemo-attractants, such as cytokines, chemokines, and other soluble molecules, are chemical signals released by damaged tissues, immune cells, or pathogens. These substances create a concentration gradient in the surrounding environment, to guide immune cells (such as haemocytes), to migrate towards the site of injury or infection (Labreuche et al. Citation2006). In P. canaliculus, haemocytosis has been reported in the mantle, the connective tissue around digestive tubules, digestive epithelium, and gonads as a response to the presence of the parasite Perkinsus olseni (Muznebin et al. Citation2022b).
Phagocytosis is the process by which phagocytic cells recognise and ingest nonself molecules (e.g. microbial pathogens and foreign organisms) and cell debris. During phagocytosis, the phagocyte usually attaches to the target particle with specialised receptors on its surface, facilitating adherence. This initial attachment is mediated by receptor–ligand interactions, enabling the phagocyte to recognise and bind to the pathogen or foreign material with specificity and selectivity. Within the phagosomes, cytoskeleton modification, internalisation, and destruction occur, whereafter the phagosome and lysosome fuse together and destroy the target particle using lysosomal enzymes, reactive oxygen species (ROS), nitric oxide or antimicrobial factors (Song et al. Citation2010). Using microscopic observations, as well as flow cytometry analyses, the process of phagocytosis has been reported within P. canaliculus following exposure to stressors (Rolton and Ragg Citation2020; Muznebin et al. Citation2022b). Additionally, the production of ROS has been seen in P. canaliculus studies in response to Vibrio sp. (Nguyen and Alfaro Citation2019), thermal stress (Delorme et al. Citation2021b), and immunostimulant exposure (Muznebin et al. Citation2022a).
When foreign bodies are too large to be phagocytosed, they will be encapsulated. Here, a capsule of haemocytes encloses the pathogen and cytotoxic products are released by the haemocytes in an attempt to destroy the invader (Allam and Raftos Citation2015). The process of encapsulation to eliminate foreign particles has not been reported within P. canaliculus studies. Yet, linkage to the phenoloxidase pathway responsible for melanisation activation following recognition and encapsulation of pathogens (Coaglio et al. Citation2018) has been reported in P. canaliculus exposed to an endotoxin (Muznebin et al. Citation2022a).
Apoptosis is the final defence response for an infected cell that is unable to clear the infectious agent. This programmed cell death involves a series of coordinated events that lead to cell morphological alterations and biochemical changes (Romero et al. Citation2015). Generally during apoptosis, the cell shrinks, rapid blebbing occurs, the nucleus collapses, deoxyribonucleic acid (DNA) fragmentation takes place, and the cell breaks into apoptotic bodies, which are often phagocytised before they lyse in an attempt to prevent further spread (Sunila and Labanca Citation2003). In bivalves, two major apoptotic pathways exist. In the first pathway, the intrinsic apoptotic signalling pathway is triggered by the initiator caspase 9, which is activated by the mitochondrial release of cytochrome c. The second, the extrinsic or death receptor pathway, involves initiator caspase-8, which is activated by binding of several death receptors (Wang et al. Citation2018). In P. canaliculus, apoptosis has been induced by lipopolysaccharides’ (LPS) (Nguyen et al. Citation2019a), heat stress (Ericson et al. Citation2023b), and cadmium exposure (Chandurvelan et al. Citation2013).
Autophagy is characterised by the presence of many autophagosomes, which fuse with the cellular lysosome system and initiate the degradation of the phagocytised material (Carella et al. Citation2015). Different autophagy categories have been described, such as microautophagy, chaperone-mediated autophagy, and macroautophagy, regulated by serval autophagy-related proteins (ATGs) (Picot et al. Citation2020). Various autophagy-related genes have been described in Crassostrea gigas (Liu et al. Citation2022), the formation of autophagosomes and autolysosomes (proof that the autophagic pathway was affected) have been reported in Mytilus galloprovincialis (Balbi et al. Citation2018), and autophagic enzymes were stimulated in M. edulis (Falfushynska et al. Citation2019). Data linking autophagy processes with P. canaliculus are lacking, creating an interesting opportunity for future research.
Humoral immunity
In synchronisation with behavioural and cellular defence systems, humoral immunity is the molecular system, which can be triggered by physical injury, pathogens, or biochemical compounds (Bassim et al. Citation2015). The humoral components of immunity are carried out by humoral defence factors, produced by haemocytes, which are the key players of mussel defence mechanisms when released into the haemolymph (Allam and Raftos Citation2015; Gerdol and Venier Citation2015). In broad terms, the humoral immunity consists of (1) the pathogen associated recognition system, (2) activation of signalling pathways, and (3) the synthesis of specific reactive effectors (Bassim et al. Citation2015). Each of these has their own components () and will be discussed next.
Pathogen-associated molecular patterns (PAMPS) via pathogen-associated pattern recognition receptors (PRRs)
Pathogen recognition receptors (PRRs) are the molecular motifs found in haemocytes and tissues of bivalves. PRRs detect potentially harmful material or organisms, activate intracellular signalling pathways, and finally react by synthesising the immune effective molecules, including the release of effector molecules, mediators, and intermediate elements (Kaloyianni et al. Citation2009; Burgos-Aceves and Faggio Citation2017). In mussels, there are several homologue genes related to key immune functions, such as peptidoglycan recognition proteins, lectins, toll-like receptors, Gram-negative binding proteins, and scavenger receptors, as showcased below.
Peptidoglycan recognition proteins (PGRPs) selectively bind to peptidoglycans (PGNs), aiding in the recognition of bacteria (Venier et al. Citation2016). These proteins can be classified into three classes based on their characteristics (short/extracellular PGRP-S, intermediate/transmembrane PGRP-I, and long/intercellular PGRP-L). PGRPs play roles in immune signal transduction, non-self-peptidoglycan recognition, agglutination, and phagocytosis (Dziarski and Gupta Citation2006; Liao et al. Citation2022). In Mytilus mussels, at least 35 PGRPs have been identified (Liao et al. Citation2022). It remains unclear if Perna species share the same PGRPs and genetic characteristics as other mussel genera, as only a draft genome of GreenshellTM mussel is currently available (Ashby Citation2019).
Lectins are sugar-binding proteins that interact with bacterial membrane glycoproteins or glycolipids (Chellapackialakshmi and Ravi Citation2022). They are involved in recognising and eliminating microorganisms. Various lectin families, such as C-type lectins (CTLs) and fibrinogen-related proteins (FREPs), have been associated with agglutination, opsonisation, antibacterial effects, and developmental processes in M. galloprovincialis mussels (Venier et al. Citation2009; Gerdol and Venier Citation2015; Gerdol et al. Citation2018). Currently, data concerning lectins in P. canaliculus and Perna species in general, are absent, with no data reported to confirm the presence or describe the functions of lectins in Perna species. . Even though there have been many studies that identify TLRs in other species of mussels, there is still a gap in the understanding of whether Perna species possess these.
Toll-like receptors (TLRs) are membrane-spanning proteins involved in detecting pathogens and activating immune responses (Brennan and Gilmore Citation2018). They can recognise pathogen-associated molecular patterns (PAMPs) like LPS and flagellin, leading to the production of pro-inflammatory cytokines and chemokines (Gerdol and Venier Citation2015). Also, different myeloid-differentiation primary response genes 88 (MyD88) used by TLRs to activate transcription factors via a complex cascade have been identified in M. galloprovincialis and M. edulis (Toubiana et al. Citation2013). Mussels, such as M. coruscus have been found to possess multiple TLRs (Li et al. Citation2019), but the presence of TLRs in P. canaliculus remains unclear.
Glucan (or gram)-negative binding proteins (GNBPs) are proteins that bind to gram-negative bacteria, such as LPS, and ß−1,3-glucan. They initiate defence reactions and immune signalling pathways (Song et al. Citation2010). Some studies have characterised glucan-binding proteins in P. viridis and M. edulis mussels (Jayaraj et al. Citation2008; Philipp et al. Citation2012), but specific information for P. canaliculus is lacking.
Scavenger receptors (SRs) are endocytic receptors with various functions, including lipoprotein binding, cellular transport, and clearing pathogens (Canton et al. Citation2013). They are poorly characterised in mussels, but transcriptome studies have detected scavenger-like receptors in M. chilensis and M. galloprovincialis (Detree et al. Citation2016; Moreira et al. Citation2015). These receptors have been found in other invertebrate models (e.g. Drosophila melanogaster and Caenorhabditis elegans) (Gerdol and Venier Citation2015), but have not been extensively studied in Perna species.
Activate intracellular signalling pathways
Once foreign compounds have been successfully recognised, signalling cascades initiate cellular defence, to enable transmission signals to move from extracellular to intracellular targets (Bassim et al. Citation2015; Wang et al. Citation2018). Typically, phagocytosis is activated, followed by the release of ROS, enzymes, and antimicrobial molecules, along with transcription of immune and stress response genes (Canesi and Pruzzo Citation2016). Several immune signalling pathways have been investigated in bivalves, such as mitogen-activated protein kinase (MAPK), janus kinase/signal transducer and activator of transcription (JAK-STAT), nuclear factor kB (NF-kB), toll-signalling pathways and complement component pathways (Song et al. Citation2010; Gerdol and Venier Citation2015), as briefly discussed below.
Mitogen-activated protein kinases (MAPK) are proteins which can transduce extracellular stimuli into cellular responses important for immune response, cell damage, and apoptosis (Tian et al. Citation2020). MAPKs are grouped into three sub-families, including, extracellular signal-regulated kinases (ERKs), c-Jun amino-terminal kinases (JNKs), and p38-MAPKs (Bassim et al. Citation2015). Typically, ERKs are activated by mitogens and differentiation signals, while the JNK and p38 MAPKs are activated by stress stimuli. Tumour necrosis factor α (TNFα) can activate all three MAPK groups, with specific responses observed in M. galloprovincialis (Betti et al. Citation2006); Furthermore, the activation of p38-MAPK and JNK has been confirmed in M. galloprovincialis, when exposed to temperatures above 24°C, highlighting the involvement of these signalling cascade during thermal stress (Anestis et al. Citation2007). Research on M. californianus and M. galloprovincialis has demonstrated that heat and cold stress-activated JNK and p38-MAPK signalling, which might be important for subsequent molecular responses to stress (Yao and Somero Citation2012). In P. viridis, MAPK was annotated following transcriptome sequencing while studying the effects of endocannabinoids on mussel attachment (Dai et al. Citation2021). In P. perna, phosphorylated p38 MAPK was activated following heat, osmotic, and hypoxic stress (Zilberberg et al. Citation2011). To date, no research has confirmed MAPKs in P. canaliculus.
Janus kinase/signal transducer and activator of transcription (JAK/STAT) is an important pathway for intracellular signal transduction of cytokine receptors and is known to be triggered as an immune response in bivalves (Bassim et al. Citation2015). Three types of STATS have been identified in the shell pearl mussels (Hyriopsis cumingii) and showed expression following a challenge experiment with Staphylococcus aureus or Aeromonas hydrophilia, linking STATS to defence functions following bacterial infection (Dai et al. Citation2017). Cytokine interferon gamma (IFNγ) stimulated tyrosine phosphorylation of STAT-like proteins in M. galloprovincialis (Canesi et al. Citation2003). The roles of the JAK/STAT pathway and their newly identified cytokines from P. canaliculus in future research will be an interesting field with promise to expand our understanding of mussel immunity.
Nuclear factor ĸB (NF-ĸB) pathway regulates cell differentiation and immunity (Bassim et al. Citation2015). The signalling mechanism for NF-ĸB activation is crucial for controlling several cellular and organismal processes, such as cellular growth, apoptosis, and inflammatory responses, and provides an immediate cellular immune defence (Li et al. Citation2015). Genes associated with NF-ĸB activity have been previously reported in mussels. For example, a study on the deep sea mussel (Bathymodiolus azoricus) confirmed the presence of the Rel-homology domain, a conserved motif present in all members of the Rel/nuclear-factor NF-κB family (Bettencourt et al. Citation2007). The NF-κB gene pathway was also affected in M. galloprovincialis following exposure to ibuprofen to infer the pharmacological effects and the possible implications on the non-target organism performance and ecological risk assessment (Maria et al. Citation2016). In M. edulis, a NF-κB transcript was used as an inflammatory marker of salinity stress (Falfushynska et al. Citation2023), and in B. azoricus as a target for copper exposure (Martins et al. Citation2017). Proteomics analysis of M. galloprovincialis during V. splendidus waterborne infection revealed that proteins enhancing the NF-kB pathway generally increased in expression, while inhibitory proteins decreased (Saco et al. Citation2021). The NF-kB pathway has been described in mussel species, other than P. canaliculus, and there is still lack of functional evidence of their implication in immune gene regulation.
Toll-signalling pathways are activated by all toll-like receptors (TRLs) which accumulate in the activation of NF-kB transcription factors and MAPKS (Leulier and Lemaitre Citation2008). TLR signalling is largely divided into two pathways: the myeloid differentiation primary response 88 (MyD88)-dependent and Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathways (Kawasaki and Kawai Citation2014), which play roles in limiting pathogenic infections and promoting tissue repair (Rauta et al. Citation2014). Multiple toll signalling pathways have been identified in various mussel species including M. galloprovincialis and M. edulis expressed sequence tags (ESTs) (Toubiana et al. Citation2014; Xu et al. Citation2019), but their exploration in P. canaliculus is limited.
Complement component pathway (system) depends on many interacting proteins to recognise and eliminate foreign microorganisms. When activated, the complement pathway promotes proteolytic reactions that function in the same way as lectins. This system partakes in the initiation of defence mechanisms, including immune cell homing and trafficking, agglutination, adhesion, opsonisation, and cell lysis (Bassim et al. Citation2015). While extensively studied in other mussel species (Gerdol and Venier Citation2015), including the mussel species, Hyriopsis cumingii (Huang et al. Citation2016), M. galloprovincialis (Gerdol et al. Citation2011; Venier et al. Citation2011) and M. coruscus (Chen et al. Citation2018; Han et al. Citation2021). Despite the extensive research on these immune signalling pathways, it remains uncertain whether all these pathways are conserved across P. canaliculus and how they are regulated in different environments.
Synthesis of antimicrobial effectors
Antimicrobial peptides (AMPs) are a group of molecules that form part of the humoral innate immune system, which contributes to the first line of defence against pathogens (Leoni et al. Citation2017; Bouallegui Citation2019). Antimicrobial activity obtained by AMPs derives from disruption of the membrane and osmotic lysis of bacteria. Some AMPs are also said to be efficient in inhibiting viral infections (Zannella et al. Citation2017). Generally, AMPs include (but are not limited to) defensins, mytilins, myticins, mytimysins, and mytimacins based on primary structure (Wang et al. Citation2013; Gerdol and Venier Citation2015). Both the diversity and structural features of mussel peptides (Mitta et al. Citation2000), and the potential application of AMPs in aquaculture (Cheng-Hua et al. Citation2009), have been reviewed elsewhere, and will not be discussed here as they go beyond the scope of this review.
With the focus on mussel AMPs, a review by Zannella et al. (Citation2017) describes the isolated peptides along with the mussel species of interest. In brief, Mytilus galloprovincialis defensin (MGD) 1 and 2; myticin A, B, and C; mytimycin; mytimacin and big-defensin have been linked to antimicrobial activity. Then again, mytilin A and B have been found in M. edulis and myticusin-1 in M. coruscus (Zannella et al. Citation2017). Additional research on, M. galloprovincialis, found a potential new family of AMPs, called myticalins. In vitro confirmations against a broad range of Gram-positive and Gram-negative bacteria, confirmed the antimicrobial properties of seven chemically synthesised myticalins (Leoni et al. Citation2017). Moreover, another type of AMP linked to M. coruscus was described as myticusin-beta, which is suggested to be an effective alternative to antibiotics (Oh et al. Citation2020). More recently, twelve AMPs representing the main AMP families were found in the following characterisation of the haemocyte transcriptome (Yang et al. Citation2022). Research on P. viridis identified four mytilin-like antimicrobial peptides (called pernalins), evident by the highest transcription levels in haemocytes. Moreover, pernalin genes were down regulated as immune response after bacterial infection with Vibrio parahaemolyticus (Zeng et al. Citation2022). Advances have clearly been made in the field of mussel AMPs, yet their presence in P. canaliculus has not been described. Future research endeavours should focus on characterising AMPs as part of the Greenshell™ mussels’ immune system and determining the molecular mechanisms involved in protecting them from pathogenic microorganisms.
Mussel diseases
In the aquaculture sector, disease outbreaks can hinder production resulting in significant losses (Naylor et al. Citation2021). There are three major pathogenic agents that cause diseases in mussels namely, viruses, protistans, and bacteria (Travers et al. Citation2015; Webb and Duncan Citation2019). There are also other diseases that may be caused by fungi (Aspergillus, Penicillium, and Fusarium), porifera (Cliona sp.), and helminth parasites, such as trematodes, cestodes, and nematodes (Gagné et al. Citation2008; Carver et al. Citation2010; Santos et al. Citation2017). It is believed that the most significant protozoan pathogens come from the genera Perkinsus, Haplosporidium, and Marteilia. Several diseases caused by Perkinsus olseni, Haplospordian tumefacientis, Marteilia refringens, and the parasite Apicomplexan X (APX) are under surveillance and require mandatory notifications to the World Organisation for Animal Health if detected (Georgiades et al. Citation2016; Muznebin et al. Citation2022a). Marine mussels, which are filter feeders, concentrate diverse and rich bacterial commensal microbiota made up of different Gram-negative and Gram-positive bacteria species from different genera, such as Vibrio, Pseudomonas, Acinetobacter, Photobacterium, Moraxella, Aeromonas, Micrococcus, Bacillus, and Nocardia (Sugumar et al. Citation1998; Garnier et al. Citation2008; Biel et al. Citation2014; Prado et al. Citation2014; Kwan and Bolch Citation2015). The effects of bacteria such as Vibrio species, Photobacterium, Pseudomonas, and Aeromonas on mussels are well documented (Travers et al. Citation2015) and the reported responses can be useful to describe biomarkers concerning bacterial-mussel interactions (Azizan et al. Citation2023d)
A range of endemic threats have been documented in GreenshellTM mussels, such as APX, digestive epithelial virosis (DEV), rickettsia-like organisms/chlamydia-like organisms/endozoicomonas-like organisms (RLO/CLO/ELO), Perkinsus olseni, Vibrio splendidus, Tergestia agnostomi, and Enterogonia orbicularis (Castinel et al. Citation2019; Webb and Duncan Citation2019). The presence of Vibrio sp. within P. canaliculus populations has gained considerable attention as resilience to climate change stressors is being placed at the forefront of research. Vibrio sp., which include V. splendidus and V. coralliilyticus/neptunis-like isolate (Kesarcodi-Watson et al. Citation2009), V. parahaemolyticus (He et al. Citation2022), V. mediterranei (Andree et al. Citation2021) and Photobacterium swingsii related to the Vibrio genus (Azizan et al. Citation2022) are largely found in GreenshellTM mussel populations. Because of Vibrio’s extensive metabolic diversity and genetic variation, they have a high colonisation potential and can lead to dangerously high accumulations (Le Roux et al. Citation2015; Le Roux and Blokesch Citation2018). For the accurate identification of Vibrio-like species, appropriate bacterial typing, including phenotypic and genotypic methods, is usually required. Vibrio 16S ribosomal ribonucleic acid (rRNA) gene sequences contain variations specific to Vibrio-like species, which serves as an important tool for phylogenetic identification, taxonomic classification, and evolutionary studies of Vibrio bacteria (Baker-AUSTIN et al. Citation2018). Vibrio sp. can be primary pathogens, responsible for pathological changes in healthy bivalves, or opportunistic pathogens, which cause disease when the protective barrier is breached or immunity is suppressed (Destoumieux-Garzón et al. Citation2020). In addition, the genus Photobacterium belongs to the family of Vibrionaceae, one of the oldest established genera. Primarily, this bacterial genus consists of marine luminescent Gram-negative bacteria found worldwide in marine ecosystems (Romalde et al. Citation2014). Several species of Photobacterium were isolated from different marine animals, including bivalves and fish (Urbanczyk et al. Citation2011). Fish were found to harbour Photobacterium damselae subspecies damselae (Lozano-León et al. Citation2003). P. swingsii, P. galatheae, and P. rosenbergii have been shown to be responsible for disease in corals, oysters, and mussels (Thompson et al. Citation2005; Gomez-Gil et al. Citation2011; Machado et al. Citation2015; Eggermont Citation2017).
Pathogenicity and virulence factors
The ability of Vibrio sp. to cause disease or mortality in a host is correlated with its pathogenicity. A bacterium is pathogenic when (1) it is present in large quantities within the host organism, (2) it is capable of being isolated and re-cultured from the host organism, (3) experimental reproduction is feasible in a healthy host organism, and (4) it is possible to isolate and identify the same pathogenic bacterium following use in an experiment as aligned with the principles outlined in Koch’s postulates (Paillard et al. Citation2004). Virulence factors encoded by virulence genes, generally give rise to the pathogenicity of Vibrio strains (Deng et al. Citation2020). A virulence factor is a specific molecule secreted by a bacterium that promotes the bacterium’s ability to be virulent (Lam et al. Citation2014). Mostly, virulence factors allow pathogens to infect and damage the host, by enabling pathogenic adherence, entrance, and establishment. Generally, the pathogen will multiply, avoid the hosts defence mechanisms, cause damage to the host, and finally exit from the infected host (Deng et al. Citation2020; Muznebin et al. Citation2022b). Bacterial virulence factors, measured from pathogen infections within aquatic organisms with respect to motility, biofilm formation, lytic enzymes, bacterial heat-shock proteins, membrane regulatory proteins, and quorum sensing follow next (, ).
Figure 3. Schematic overview of different virulence factors produced by pathogenic bacteria potentially attributing to mussel mortality. The virulence factors are divided into three main categories, namely bacterial surface structures, secreted factors, and bacterial cell-to-cell interaction. The bacterial surface structures include surface appendages like (1) motility (pili and flagella), (5) membrane localised regulatory proteins, (4) bacterial heat-shock proteins. The secreted factors include various lytic enzymes. As for the bacterial cell-to-cell interaction, (2) bacterial biofilm and (6) quorum sensing.
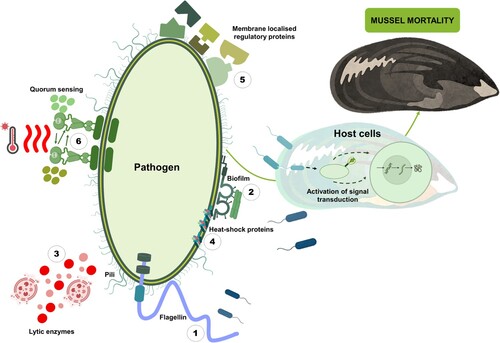
Table 2. Summary of bacterial virulence factors reported to contribute to mussel mortalities.
Motility
Bacterial motility is vital for pathogenic bacteria during infection, facilitating attachment, colonisation, nutrient acquisition, and biofilm formation (Johnson Citation2013). Vibrio species rely on specialised flagella, including a sheathed polar flagellum for swimming in water and a lateral flagellum for navigating mucus or biofilms (Defoirdt Citation2014). Flagellar motility is observed in Vibrio spp. infections. Motility genes have been identified in bacterial isolates from M. edulis mussel larvae (Eggermont et al. Citation2017). Furthermore, the sheathed flagellum helps bacteria evade immune responses during oyster challenges (Yoon et al. Citation2008).
Biofilm formation
Biofilms are matrix-like polysaccharides that form on surfaces to withstand environmental stresses (Yildiz and Visick Citation2009). Bacteria use extracellular polysaccharides to attach to host cells and create a protective matrix (Defoirdt Citation2014). Biofilm formation has been reported in V. parahaemolyticus isolates from contaminated mussel seafood and Asian green mussels, P. viridis (Ashrafudoulla et al. Citation2019; Palamae et al. Citation2022). Mannose sensitive haemagglutinin (mshA) gene found in bacterial isolates from GreenshellTM mussels (Azizan et al. Citation2022) has also been linked to biofilm formation (Johnson Citation2013), but exploration in P. canaliculus is limited.
Lytic enzymes
Lytic enzymes are crucial for tissue damage, nutrient acquisition, and the spreading of pathogenic bacteria (Johnson Citation2013). These enzymes encompass haemolysins, proteases, chitinases, and lipases. Haemolysins are toxic substances found in pathogenic Vibrio sp., while proteases hydrolyse peptide bonds in proteins (Zhang and Austin Citation2005). Chitinases degrade chitin, a prevalent molecule in the ocean used by Vibrio sp. for energy (Aunkham et al. Citation2018). Lipases and phospholipases play roles in disrupting host cell membranes (Silva et al. Citation2018; Wan et al. Citation2019). These enzymes have been associated with bacterial virulence in mussels, including P. canaliculus, but further research is required (Hossain et al. Citation2020; Azizan et al. Citation2022).
Haemolysins
Toxic substances called haemolysins, found in pathogenic Vibrio sp., play a crucial role in infections. With relation to mussel research, genes associated with haemolysins, such as thermostable haemolysin (tdh) and TDH-related haemolysin (trh) genes were detected in V. parahaemolyticus isolated from M. galloprovincialis following genomic analyses (Ottaviani et al. Citation2005).
Proteases
Proteases can include metalloproteases (proteases that need metal ions), serine proteases (proteases where serine is the nucleophilic amino acid), cysteine proteases (proteases with nucleophilic cysteine thiols at their active site), collagenases (proteinases that degrade collagen), caseinases (proteinases that degrade casein), and gelatinases (they degrade gelatine) (Shinoda and Miyoshi Citation2011). These enzymes break down peptide bonds in proteins and help bacteria evade the host immune system, for example, ROS production in host cells was inhibited by vam, the zinc metalloprotease obtained from V. aestuarianus (Johnson Citation2013). Secretion of extracellular protease (gelatinase and caseinase) has been positively identified in Vibrio sp. and Photobacterium sp. isolated from the blue mussel larvae, however, no correlation between in vitro expression levels of virulence-related genes in Vibrio was found (Eggermont et al. Citation2017). Zinc-metalloprotease (zm) and single-zinc metalloprotease (vcpA) genes were identified following qPCR analysis of Photobacterium sp. and V. celticus isolated from the infected GreenshellTM mussels (Azizan et al. Citation2022).
Chitinases
Among the most abundant molecules in the ocean is chitin, a polymer made of N-acetylglucosamine (GlcNac) monomers, which Vibrio sp. use as a source of cellular energy (Aunkham et al. Citation2018). Chitinases degrade chitin into smaller chitooligomers (Kumar et al. Citation2022). Typically, Vibrio sp. typically senses chitin, attaches to it, and produces enzymes to break it down into GlcNac and oligosaccharides, which are then catabolised (Souza et al. Citation2011). Chitinases-producing bacteria has been detected in M. trossulus (Beleneva and Maslennikova Citation2005), P. viridis (Khantavong et al. Citation2009), and P. canaliculus (Azizan et al. Citation2022).
Lipases and phospholipases
Lipases are lipolytic enzymes that cleave long-chain triacylglycerols into fatty acids and glycerol molecules at the water–lipid interface (Adetunji and Olaniran Citation2021). Phospholipases directly rupture host cells by damaging the phospholipid membrane, and the resulting products can trigger apoptosis or inflammation signalling (Wan et al. Citation2019). Both of these lipolytic enzymes have been associated with bacterial virulence linked to mussels. For example, phospholipase was detected as a potential virulence phenotype in V. parahaemolyticus isolates obtained from mussel samples (Mytella guyanensis), also lipase was detected, but not reported as significant (Silva et al. Citation2018). Another study identified four Vibrio spp. (V. diabolicus, V. alginolyticus, V. parahaemolyticus, and V. harveyi) from M. coruscus, whereafter pathogenic virulence factors were analysed among the tested isolates (Hossain et al. Citation2020). The results showed that all Vibrio spp. isolates were positive for phospholipase, and 87.5% of the isolates were positive for lipase (Hossain et al. Citation2020). Also in P. swingsii isolated from P. canaliculus the phospholipase (plp) gene was detected, which potentially attributed towards virulence in GreenshellTM mussels (Azizan et al. Citation2022).
Bacterial heat-shock proteins
Heat-shock proteins (HSPs) aid protein folding, protect against stress, and maintain cellular stability (Roncarati and Scarlato Citation2017). Bacterial HSPs, including chaperones and proteases, combat protein denaturation (Maleki et al. Citation2016). Molecular chaperones are surface-expressed and released into extracellular spaces, influencing virulence indirectly (Henderson et al. Citation2006). Protease HSPs remove damaged polypeptides, allowing bacteria to thrive at sub-optimal temperatures (Maleki et al. Citation2016). The hsp60 gene, found in bacterial isolates during a summer mortality outbreak in GreenshellTM mussels, may relate to infection factors (Azizan et al. Citation2022).
Membrane-localised regulatory proteins
Membrane regulatory proteins are crucial for adhesion, invasion, toxin release, and gene regulation in pathogens. Toxin R (ToxR), embedded in the inner membrane, controls virulence genes, biofilm formation, and outer membrane protein expression in V. cholera (Dirita et al. Citation1991; Provenzano and Klose Citation2000; Johnson Citation2013). High toxr gene expression in pathogenic P. swingsii and P. rosenbergii may impact pathogenicity in P. canaliculus (Azizan et al. Citation2022). Additionally, toxR and ompK genes were detected in V. alginolyticus isolated from mussels (Najwaa et al. Citation2015).
Quorum sensing
Quorum sensing (QS) is a cell-to-cell signalling process used by bacteria to regulate communal behaviour and gene expression based on cell density and chemical signals (Srivastava and Waters Citation2012). N-acyl homoserine lactones (AHLs) serve as autoinducers for QS in many Gram-negative bacteria, including Vibrio sp. (Defoirdt Citation2014; Islam et al. Citation2022). QS controls virulence-related traits, such as motility, biofilm formation, and protease production. For example, V. tasmaniensis and V. crassostreae, and their multi-channel quorum sensing mutants were characterised in vitro and challenged in vivo in blue mussel larvae to investigate their impact on virulence (Islam Citation2016). In a follow-up study, the AI-2 autoinducer-mediated QS was found to reduce virulence of V. crassostreae. Also, cinnamaldehyde was used to demonstrate that QS does not control the virulence of V. tasmaniensis and V. crassostreae in blue mussel larvae (Islam et al. Citation2022). Aromatic signalling molecules like indole have potential as anti-virulence therapy in aquaculture, targeting virulence-related phenotypes (Zhang et al. Citation2023).
In summary, understanding bacterial virulence factors is of paramount importance due to its multifaceted implications. Firstly, it allows us to comprehend how bacteria cause diseases and the specific mechanisms they employ to evade the mussel immune system. This knowledge paves the way for the development of highly effective strategies for combating and preventing bacterial infections. Secondly, investigation of bacterial virulence factors helps in identifying potential targets for therapeutic interventions. Insight into the key factors responsible for bacterial pathogenicity of mussel species empowers researchers to engineer targeted therapies or formulate vaccines which selectively neutralise these factors, resulting in more refined, efficient, and precise treatments. Furthermore, unravelling bacterial virulence factors aids in the development of diagnostic tools. By identifying and characterising these factors, scientists can devise diagnostic tests that detect their presence or activity, enabling early and accurate identification of bacterial infections.
Factors compromising Perna canaliculus immunity
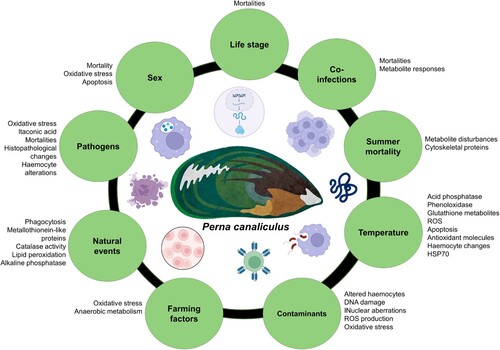
Stressors, both biotic and abiotic, trigger a costly cascade of cellular and molecular processes to maintain cellular homeostasis, which in time weakens biological defence mechanisms (Coates and Söderhäll Citation2021). Pathogen and parasite infections in mussels result from a combination of biotic and abiotic drivers. Among biotic drivers, such as host size, immunity status, density of the host population, physical injury, predators, other pathogens, malnutrition, etc., have been demonstrated to facilitate the evolution and spread of pathogens and the occurrence of disease outbreaks due to immune-compromised animals (Bondad-Reantaso et al. Citation2005; Skein et al. Citation2018; Bommarito et al. Citation2022). Abiotic drivers, including several environmental factors, influence pathogen prevalence and abundance, while climatic events, such as storms, droughts, aquatic, and atmospheric heatwaves, have a significant impact on water quality. These events cause changes in salinity, pH, introduce pollutants (chemicals, pharmaceuticals, and plastics), and lower dissolved oxygen levels. Consequently, these factors contribute to animal stress and compromise their immune systems (Babarro and de Zwaan Citation2002; Mydlarz et al. Citation2006; Lane et al. Citation2022; Reverter et al. Citation2021; Bommarito et al. Citation2022). Most studies in P. canaliculus within the last 14 years have investigated the influence of biotic or abiotic factors on mussel immunity (either directly measured specific immune parameters or detected changes in immune-related markers) in either wild population of mussels (field study) or in a laboratory experiment (). These biotic or abiotic factors are discussed next.
Pathogens
As mussels lack immunological memory, the innate immunity has the responsibility to secure protection against pathogenic microorganisms (Campos et al. Citation2015). Yet, the effects of pathogens on mussel health (immunity) have been understudied (Waller and Cope Citation2019). Although Greenshell™ mussels cultured in NZ are generally free from significant production diseases (Lane et al. Citation2022), a number of pathogens with effects on the immune system have been reported in P. canaliculus. For example, the use of Vibrio sp. DO1 (V. coralliilyticus/neptunius-like isolate), to challenge adult P. canaliculus in various laboratory studies resulted: (1) altered metabolites suggestive of oxidative stress and changes in protein synthesis linked to immune function (Nguyen et al. Citation2019b); (2) changes in metabolites involved in major perturbations on the host’s innate immune system (Nguyen et al. Citation2018c); (3) increased itaconic acid as antimicrobial metabolite and anti-inflammatory marker (Nguyen and Alfaro Citation2019) and (4) alterations within the hosts’ oxidative stress and inflammation processes and disruption of the tricarboxylic acid cycle (Nguyen et al. Citation2018b). Vibrio splendidus and a V. coralliilyticus/neptunius-like isolate were reported as pathogenic to GreenshellTM mussel larvae, causing mortalities, and histopathological changes in the digestive system (Kesarcodi-Watson et al. Citation2009). The use of this same V. coralliilyticus/neptunius-like isolate in a subsequent P. canaliculus experiment showed a higher proportion of dead haemocytes and lower overall haemocyte counts than uninfected controls (Ericson et al. Citation2022). An increase in metabolites linked to the immune-supportive metabolite pathways (glutathione pathway and branched-chain amino acids) were observed, when infecting mussels with V. mediterranei (Azizan et al. Citation2023c). Reports on other pathogenic micro-organisms, threatening P. canaliculus immunity included: the protozoan parasites Toxoplasma gondii and Giardia duodenalis from commercially-sourced mussels, detected via specific gene targets (Coupe et al. Citation2018). Also detected in P. canaliculus is, Perkinsus olseni, APX (78%), copepods (Pseudomyicola spinosus or Lichomolgus uncus), and Microsporidium rapuae collected from a commercial mussel farm, characterised by histology and confirmed by in situ hybridisation (Muznebin et al. Citation2022b). Disease dynamics are constantly changing, necessitating continued research on host–parasite interactions. Moreover, the empirical data generated can be combined with modelling methods to assist in forecasting future disease events (Lane et al. Citation2022).
Pathogen co-infections
Co-infection is defined as the simultaneous infection of one host with multiple pathogens which may be causative agents of different diseases or variants of the same microbes (Martcheva and Pilyugin Citation2006). Many pathogens, such as bacteria, microparasites, and viruses often co-occur within the same individual host, largely with a harmful outcome to the host (Toews et al. Citation1993; Morley Citation2010; Dong et al. Citation2015; Figueroa et al. Citation2017; Shen et al. Citation2019). It is notable that the co-infecting pathogens can be homologous (interactions occur between pathogens of the same types or species i.e. two different strains of bacteria) or heterogeneous (interactions occur between pathogens of different types or species i.e. involving a bacteria and a virus or parasite) (Kotob et al. Citation2017). In mussels, co-infected pathogens may interfere with the host immune response and compete for nutrients, either by synergistic or antagonistic actions between the pathogens (Künili et al. Citation2021). In P. canaliculus, a laboratory-based bacterial co-infection study, using Vibrio mediterranei and Photobacterium swingsii, resulted in higher mortalities, increased bacterial colonies for a longer period, and a decreased metabolite response largely influencing amino and fatty acid metabolism (compared to mussels receiving a single pathogen) (Azizan et al. Citation2023b). Co-infections on P. canaliculus larvae using V. splendidus and V. coralliilyticus/neptunius-like strains resulted in high mortality rates (Kesarcodi-Watson et al. Citation2009). Co-infections can have an important impact on the development and severity of disease and more research is needed to improve our understanding of the interactions between pathogens and how they interact with the immune response of the mussel host.
Temperature
Fluctuations in water temperature can significantly alter immune functions in mussels (Rahman et al. Citation2019), as demonstrated within P. canaliculus (Ericson et al. Citation2023a). In a study where Greenshell™ mussels were subjected to 26°C for 48 h, increased acid phosphatase and phenoloxidase activity was reported, along with increased metabolites linked to the glutathione metabolism and an increased realise of ROS by the haemocytes (Muznebin et al. Citation2022a). Chronic exposure (13 months) of P. canaliculus to 24°C resulted in 100% mortality towards the end of the experiment, and haemocytes showed increased respiratory burst (superoxide-positive) and apoptosis after 6 months (Ericson et al. Citation2023b). Furthermore, P. canaliculus subjected to a severe heat shock (30°C for 60 min) showed increases in non-viable haemocytes and metabolites which support antioxidant molecules. Decreases in the generation of ROS production and total antioxidant capacity were also observed (Delorme et al. Citation2021b). During a marine heatwave experiment (18–24°C, using a + 2°C per week ramp) on P. canaliculus, the metabolomics findings indicated the activation of molecular defence mechanisms, along with an increase in antioxidant metabolites. Additional evidence for immune functions was seen within the cytology results where high-temperature stress affected the haemocyte counts and the percentage of superoxide-positive haemocytes (Venter et al. Citation2023). Mussels stressed at 33°C showed reduced GABAergic synapse activity after 3 h (Dunphy et al. Citation2018) and mortality within two days (Dunphy et al. Citation2015). Larvae of the GreenshellTM mussel induced significant amounts of HSP70 when experiencing temperatures of 40°C or more (Dunphy et al. Citation2013).
Environmental conditions, such as temperature, usually do not occur in isolation. The collective effect of multiple drivers can be either a simple addition of the effects from individual drivers, greater (synergistic) or less (antagonistic) than the sum of isolated effects (Baag and Mandal Citation2022). Immune parameters of P. canaliculus have been affected due to temperature in combination with bacterial infections and food limitations. Mussels infected with Vibrio sp. had less haemocytes and lower antioxidant capacity when kept at a higher temperature (24°C) for 24 h (Ericson et al. Citation2022). Lower haemocyte counts were also seen when infecting P. canaliculus with P. swingsii at 24°C. Moreover, higher total antioxidant counts and lipid peroxidation levels were seen at 24°C (Azizan et al. Citation2023b). When subjecting P. canaliculus to 54 h of fasting followed by heat stress (27°C), an increase in oxidative damage and a decrease in antioxidant enzymes were seen (Delorme et al. Citation2020). It is crucial to have a robust and accurate understanding of the effects of temperature (and associated stressors) on mussel immunity to be able to predict the future effects of rising temperatures.
Contaminants
Contaminants are often detected in coastal areas and represent a potential threat to bivalves, from direct toxic actions or from alterations of the homeostatic mechanisms, including the immune system (Renault Citation2015). Environmental pollutants can suppress mussel immunity, resulting in an elevated parasite infection rate. Additionally, parasites can interact with both natural and anthropogenic stressors, compromising mussel health and increasing mortality rates (Sures et al. Citation2017). The effects of heavy metals, pesticides, polycyclic aromatic hydrocarbons, nanoparticles, polychlorinated biphenyls, and pharmaceuticals on mussel immunity have been previously reviewed (Renault Citation2015). In P. canaliculus, the effects of cadmium, copper, microplastics, and triclosan on the immune system have been investigated. Cadmium significantly altered the proportional composition of haemocytes, induced DNA damage in haemocytes, and increased nuclear aberrations in P. canaliculus (Chandurvelan et al. Citation2013). P. canaliculus exposed to copper resulted in increased haemocyte production, production of ROS, and haemocyte apoptosis. Additionally, metabolites linked to oxidative stress and apoptosis were affected (Nguyen et al. Citation2018a). Triclosan increased mussel oxidative stress markers including superoxide dismutase (SOD) and lipid peroxidation (LPO), while microplastics enhanced the uptake of triclosan within the tissue of mussels (Webb et al. Citation2020). Ultimately, it has been demonstrated that mussels living in contaminated areas are more vulnerable to infections due to immunosuppression caused by pollution (Ordás et al. Citation2007), highlighting contaminants as an important variable to examine in the evaluation of mussel immune responses.
Natural events
Mussel immunity can show seasonal fluctuations, driven by complex interactions between endogenous host factors and environmental factors (Balbi et al. Citation2017). Differential haemocyte counts of P. canaliculus reported higher phagocytosis in summer and lowest in winter months (Muznebin et al. Citation2022c). Post-earthquake biomarker measurements of P. canaliculus showed reduced metallothionein-like protein and catalase activity levels and increased lipid peroxidation and alkaline phosphatase levels in mussel gill and digestive gland tissues collected from affected sites post-earthquake period (in relation to a reference site) (Chandurvelan et al. Citation2016). These biomarkers have been suggested as indicators of general stress and could be used to follow the recovery of mussels following exposure to natural disasters.
Farming factors
Biomarker approaches have been proposed as measurable indicators for the quality control of farmed shellfish (Matozzo et al. Citation2018). The quality of aquaculture products is an outcome of the positive interactions between good environmental factors and correct farming procedures (Moschino et al. Citation2010). Immune parameter-based biomarkers in P. canaliculus have been used in connection to various farming practises on larvae, spat, juveniles, and adults. For example, elevation of the aragonite saturation state to 4.5 Ωarag, to enrich pre-veliger incubation water, increased superoxide dismutase, glutathione reductase, and peroxidase levels, thereby minimising oxidative stress during this process (Ragg et al. Citation2019). Seeding density had no effect on oxidative stress markers (total antioxidant capacity and lipid peroxidation) in spat during summer months (Reyden et al. Citation2023). Additionally, subjection to low relative humidity during emersion, followed by re-immersion, as a medium of transport or shoreline exposure, resulted in increased oxidative damage biomarkers (protein carbonyls, lipid hydroperoxides, 8-hydroxydeoxyguanosine) in juvenile P. canaliculus (Delorme et al. Citation2021a). Metabolite biomarkers of cultured P. canaliculus showed that mechanical harvesting associated with commercial processing of farmed mussels resulted in anaerobic metabolism and affected amino and fatty acid metabolism, which plays vital roles in mussel immunity (Nguyen et al. Citation2020). This research area aims to provide farmers with an understanding of the importance of minimising intense physical stress, such as handling, to reduce potential immunosuppression in farmed mussels.
Sex
For the most part animals of opposing sex respond differently to stressful environments due to energetic trade-offs between reproduction (e.g. gamete production, mating behaviours, parental care, and offspring development) and stress resistance (e.g. changes in temperature, salinity, pH, nutrient availability, or exposure to toxins or pathogens) (Petes et al., Citation2008). This has been seen in the blue mussel Mytillus edulis with males being more affected by reduced seawater pH, increased temperature, and a bacterial challenge than the females (Ellis et al. Citation2014). In P. canaliculus, males showed higher mortality, oxidative stress, and apoptosis after pathogen exposure, in comparison with females (Nguyen et al. Citation2018c). As a result, sex also affects disease susceptibility and immunological function within the host (Klein and Flanagan Citation2016). This observation emphasises the need to consider sex-differences when investigating environmental stress and immunological studies.
Mussel life stage
Mussel life stage has been shown to have a significant effect on the expression of stress-related biomarkers, highlighting the importance of including life stage as a parameter when performing an experiment (Zilberberg et al. Citation2011). It is believed that juvenile mussels are more susceptible to pathogenic infections (Benabdelmouna et al. Citation2018), as smaller mussels have a lower tolerance to pathogens, due to undeveloped immune systems (Pruzzo et al. Citation2005; Lattos et al. Citation2020). However, haemocyte parameters were not influenced by the size of green mussels, Perna viridis (Donaghy and Volety Citation2011). In a study on P. canaliculus, juvenile mussels showed higher mortalities, compared to adults when infected with V. mediterranei, P. swingsii, and a combination of both (Azizan et al. Citation2023c). Additional research is also needed to determine if juvenile mussels exhibit an inefficient immune response when subjected to other stressors, leading to higher mortalities than the adult counterparts.
Applications, future perspectives, and conclusions
Research on GreenshellTM mussels showcases the substantial worth of this marine organism across diverse scientific domains, encompassing aquaculture, human nutrition, environmental monitoring, and medicine (). Despite the lessons learned from pathogen infections around the globe, scientific gaps still exist, especially when considering P. canaliculus as a research model. The direct impact of diseases (e.g. mortality) is easy to monitor or quantify for farmers. However, indirect effects, such as a sublethal cellular response, are potentially more significant, but more difficult to measure (Castinel et al. Citation2019). Utilisation of multi-omics approaches allows researchers to obtain more inclusive information on the indirect effects of the disease. Omics approaches involve high throughput techniques that have greatly increased the ability to characterise the function and dynamics of genes (genomics), expressed genes (transcriptomics), proteins (proteomics), and metabolites (metabolomics) (Alfaro and Young Citation2018). The sensitivity and specificity of most omics techniques make them powerful tools in immune studies. Characterisation of bivalve haemocytes and tissues via transcriptome, proteome, and metabolome measures, in response to pathogenic infections and/or environmental stressors have provided useful information on the mechanisms that drive the innate immune system following stress challenges. Additionally, the field of omics research enabled the characterisation of complex host-pathogenic-environmental interactions across bivalve species (Nguyen and Alfaro Citation2020a).
Omics potential and constraints
In brief, genomics technology (e.g. whole genome sequencing) can identify pathogens associated with disease, and or assist in characterising a particular aetiology. Additionally, sequence analyses (i.e. sequence-dependent, e.g. 16S clone or amplicon analysis or metagenomics), detection or quantification of a target sequence [sequence-independent; e.g. in situ hybridisation (ISH) and quantitative polymerase chain reaction (qPCR)] and multiple target sequences (e.g. DNA microarrays) can be used to generate a complete catalogue of genes that are involved in host–pathogen interactions (Burge et al. Citation2016). Transcriptomic research can play a significant role in mussel research, as a tool to assess mechanisms involved in biological processes, such as responses to environmental stressors, new diets, or pathogens (Chandhini and Rejish Kumar Citation2019). Expressed sequence tags can be utilised for the detection of differential expression and regulation of certain genes (Tanguy et al. Citation2008). RNA-Seq using next-generation sequencing allows exploration of the transcriptome of non-model organisms (Rey-Campos et al. Citation2019), such as the GreenshellTM mussel, to better understand pathogenic evasion strategies. Proteomics allows for the identification, localisation, and quantification of proteins, as well as the analysis of protein modifications and the elucidation of protein–protein networks (Carrera et al. Citation2020). Techniques, such as two-dimensional gel electrophoresis and mass spectrometry (MS) are mostly used to provide valuable information for protein analysis in proteomics research (Campos et al. Citation2012). Proteomic approaches can be widely used in aquaculture to support the identification of new biomarkers and assess mechanisms involved in the responses of mussels, for example, to environmental stressors (Tomanek and Zuzow Citation2010) or pathogens and accumulation of algal toxins (Puerto et al. Citation2011). Metabolomics analyses show how metabolic entities within a cell, tissue, or biofluid respond to external stressors or stimuli, at a certain time (Alfaro and Young Citation2018). Utilisation of nuclear magnetic resonance (NMR) and MS-based techniques allows for the measurement of changes in metabolites due to the presence of stressors (Young and Alfaro Citation2018). In P. canaliculus, metabolomics studies have been previously used to investigate pathogen infections (Nguyen et al. Citation2019c; Ericson et al. Citation2022; Azizan et al. Citation2023c). Lipidomic analyses play a pivotal role in understanding the physiological responses of aquaculture species to environmental stressors. By analysing lipid profiles, researchers can unravel how stressors like temperature fluctuations or pollution affect lipid metabolism and composition in species including abalone (Zhang et al. Citation2019), clams, oysters, and mussels (Cajka and Fiehn Citation2016; Balbi et al. Citation2021). On the other hand, metagenomics provides a window into the complex microbial communities of bivalves within aquaculture systems. Environmental stressors can disrupt these communities, potentially leading to harmful shifts in the genetic content of microbiota composition (Paillard et al. Citation2022). Metagenomic analyses using 16S rRNA sequencing and whole-genome sequencing techniques can detect these changes, highlighting the presence of pathogens or the decline of beneficial microbes as seen in oyster (Kobiyama et al. Citation2018) and the purple-hinge rock scallop (Alma et al. Citation2020). Currently, there are no lipidomics or metagenomic studies on P. canaliculus, indicating a notable research gap and potential for future investigation. Consequently, the integration of omics technologies together with physiology, behaviour, and biology research for stressor-specific, species-specific, and tissue-specific studies remains valuable for advancing immune research in mussels. The research findings can be significantly amplified if adequate tools are used for monitoring and results are combined to understand the links between different pathways of biological organisation (Eissa and Wang Citation2016).
Multi-omics approaches, while powerful, present several challenges, for example, integrating data from diverse omics platforms can be complex and computationally demanding. To create precise models for health-related effects linked to bacterial composition, researchers need to integrate omics datasets encompassing multiple variables from both the host and microbial aspects. This integration allows for a deeper understanding of the interactions involved. Consequently, it becomes necessary to obtain supplementary omics data to characterise microbial functions. This includes data on RNA abundances (metatranscriptome), proteins (metaproteome), and metabolites (metabolome) (Kwoji et al. Citation2023). Additionally, the biological interpretation of multi-omics findings in bivalves remains a significant hurdle, as it involves relating the intricate interactions between genes, proteins, metabolites, and the environment to meaningful biological outcomes. The costs and resources required for multi-omics research can be substantial, and achieving adequate sample sizes for statistical power can be limiting (Hasin et al. Citation2017). Ensuring data quality and reproducibility across different layers of omics data is paramount, and researchers often face ethical and privacy concerns in data collection (Hasin et al. Citation2017).
Using immune responses to manage disease occurrences and potential application to the aquaculture industry
The lack of existing knowledge on the basic immune defences of marine invertebrates is highlighted when problems arise in the aquaculture industry (Mydlarz et al. Citation2006). By investigating mussel immunity, relevant information on the quality of the marine environment is obtained, while also facilitating the understanding of occurring infectious diseases (Burgos-Aceves and Faggio Citation2017). To this end, knowledge of immune responses is being applied to manage disease occurrences. Discoveries, such as gene rearrangement mechanisms are implemented to protect mussels against infectious diseases (Gestal et al. Citation2008). Probiotics have also been applied as a means to protect mussels against disease (Kesarcodi-Watson et al. Citation2012). The addition of immunostimulants to diets of farm-raised invertebrates has been shown to promote immune function and prevent disease (Mydlarz et al. Citation2006). While the use of probiotics and immunostimulants in aquaculture is generally regarded as safe and can enhance the health of mussels, their application must be carefully monitored to ensure they do not introduce harmful microorganisms or contaminants into the aquatic environment. Learning from past events also allows for valuable insight. For example, the natural selection of shellfish which survived a mortality event can be implemented to produce seeds with greater resistance to disease by utilising genome-based biotechnologies (Fox et al. Citation2020). Moreover, the implementation of a standard set of biomarkers or diagnostic tools (obtained from immunological studies) can help to assess mussel health facilitate the rapid detection of pathogens, and provide early warnings of unfavourable farm conditions (Aldridge et al. Citation2023).
Greater knowledge of mussel immunity can serve the aquaculture industry in various ways. For instance, a strong scientific basis can be used to support biosecurity programmes, enable risk assessments, accompany policy development, and identify priorities for future research (Bondad-Reantaso et al. Citation2005). Surveillance (confirming that a population is free from a disease) and monitoring (determining the level of disease) programmes will also benefit from immunological research, as it informs on responses implemented by the host (Cameron Citation2002), and highlights which factors should be closely investigated. Potential candidates for biomarkers might include various immune-related proteins, gene expression patterns associated with health and stress responses, or specific metabolites that could be valuable in surveillance and monitoring programmes for assessing the well-being of mussel populations. However, a comprehensive investigation, including omics studies (genomics, transcriptomics, proteomics, and metabolomics), would be necessary to pinpoint reliable biomarkers indicative of immune and overall health in this species. Ultimately, the implementation of aquatic health programmes will lead to the establishment of diagnostic services, such as reference laboratories. With such advances also come opportunities where these centres can facilitate research and collaboration, develop quality control programmes and act as contact centres for advice and training to regional experts who can provide diagnostic assistance and answer the technical question in the field (Bondad-Reantaso et al. Citation2005).
Going forward
Environmental stressors enable pathogenic bacteria to proliferate, resulting in bacterial infections of mussels (Babarro and de Zwaan Citation2002). Thus, the close link between mussels and their environment remains a key research area, as demonstrated in the examples provided above where temperature, contaminants, farming factors, and natural events have been shown to affect the immune system of Greenshell™ mussels. More can still be done to understand biological responses at physiological, evolutionary, and ecological levels (Boyd et al. Citation2018). The mechanisms underlying innate immunity depend on functional proteins, such as effector proteins, regulatory pathways and related genes, which are largely understudied (Renault Citation2009). In particular, factors within Greenshell™ mussels’ humoral immunity, are scarce. Specific information on the immune recognition system, activation of signalling pathways, and synthesis of specific effectors are needed for P. canaliculus, creating a research area with various opportunities. Moreover, virulence factors associated with pathogens of P. canaliculus are poorly recognised and not extensively examined. To improve our understanding of pathogenesis, more efforts will need to be invested into the characterisation of pathogen virulence factors. Additionally, investigating the pathogen itself also remains important to understand the pathogen life history (i.e. how they spread, or what time of the year the flourish), as this will be key to predict the risk of pathogens successfully establishing elsewhere (Brian et al. Citation2021). A further investigation is warranted to elucidate the impact of immune system variation on natural population susceptibility to infection (Mydlarz et al. Citation2006). To date, only a handful of studies have assessed natural field populations of Greenshell™ mussels. Yet, to meet the resilient outcomes of on-farm biosecurity plans, as outlined by the implementation plan of the NZ Governments aquaculture strategy (Fisheries New Zealand Citation2022), more efforts will be needed.
For the purpose of providing management tools and strategies for mussel diseases and/or protection of already healthy populations, it is necessary to understand how infections and diseases interact comprehensively. Consequently, an integrated approach that considers the entire ecosystem’s impact is needed. High-throughput technologies and rapid developments in bioinformatics and artificial intelligence are enabling omics tools to provide new methods and approaches for understanding these complex health processes. Furthermore, the ability to harness the potential of large-scale data sets effectively and derive comprehensive insights require the strengthening of collaborations between researchers from a variety of disciplines. Science remains a huge enabler of shellfish aquaculture success in New Zealand. Scientific advances relating to P. canaliculus immunology are a good example hereof, where great progress has been made in identifying and characterising various immune molecules and diseases from Greenshell™ mussels. These molecules act against various biotic and abiotic stressors and allow us as researchers to better understand immune response mechanisms implemented by the host and the relationship with the pathogen. Additionally, the understanding of immune responses in mussels holds substantial economic, ecological, and public health importance, both locally in New Zealand and worldwide.
Acknowledgements
This review was supported by the Aquaculture Health Strategies to Maximise Productivity and Security programme, funded by the New Zealand Ministry for Business, Innovation and Employment. We are thankful to the Aquaculture Biotechnology Research Group at AUT for ongoing support. We want to acknowledge the anonymous reviewers whose revisions improved this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adetunji AI, Olaniran AO. 2021. Production strategies and biotechnological relevance of microbial lipases: a review. Brazilian Journal of Microbiology. 52:1257–1269. doi:10.1007/s42770-021-00503-5.
- Adzigbli L, Hao R, Jiao Y, Deng Y, Du X, Wang Q, Huang R. 2020. Immune response of pearl oysters to stress and diseases. Reviews in Aquaculture. 12:513–523. doi:10.1111/raq.12329.
- Aldridge DC, Ollard IS, Bespalaya YV, Bolotov IN, Douda K, Geist J, Haag WR, Klunzinger MW, Lopes-Lima M, Mlambo MC. 2023. Freshwater mussel conservation: a global horizon scan of emerging threats and opportunities. Global Change Biology. 29:575–589. doi:10.1111/gcb.16510.
- Alfaro AC, Young T. 2018. Showcasing metabolomic applications in aquaculture: a review. Reviews in Aquaculture. 10:135–152. doi:10.1111/raq.12152.
- Allam B, Espinosa EP. 2016. Bivalve immunity and response to infections: are we looking at the right place? Fish & Shellfish Immunology. 53:4–12. doi:10.1016/j.fsi.2016.03.037.
- Allam B, Raftos D. 2015. Immune responses to infectious diseases in bivalves. Journal of Invertebrate Pathology. 131:121–136. doi:10.1016/j.jip.2015.05.005.
- Alma L, Kram KE, Holtgrieve GW, Barbarino A, Fiamengo CJ, Padilla-Gamiño JL. 2020. Ocean acidification and warming effects on the physiology, skeletal properties, and microbiome of the purple-hinge rock scallop. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 240:110579. doi:10.1016/j.cbpa.2019.110579.
- Andree KB, Carrasco N, Carella F, Furones D, Prado P. 2021. Vibrio mediterranei, a potential emerging pathogen of marine fauna: investigation of pathogenicity using a bacterial challenge in Pinna nobilis and development of a species-specific PCR. Journal of Applied Microbiology. 130:617–631. doi:10.1111/jam.14756.
- Anestis A, Lazou A, Pörtner HO, Michaelidis B. 2007. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 293:911–921. doi:10.1152/ajpregu.00124.2007.
- Ashby R. 2019. The development and implementation of genomic tools for the New Zealand Greenshell™ Mussel industry [Doctor of Philosophy PhD thesis], University of Otago.
- Ashrafudoulla M, Mizan MFR, Park H, Byun K-H, Lee N, Park SH, Ha S-D. 2019. Genetic relationship, virulence factors, drug resistance profile and biofilm formation ability of Vibrio parahaemolyticus isolated from mussel. Frontiers in Microbiology. 10:513. doi:10.3389/fmicb.2019.00513.
- Aunkham A, Zahn M, Kesireddy A, Pothula KR, Schulte A, Baslé A, Kleinekathöfer U, Suginta W, Van Den Berg B. 2018. Structural basis for chitin acquisition by marine Vibrio species. Nature Communications. 9:220. doi:10.1038/s41467-017-02523-y.
- Azizan A, Alfaro AC, Jaramillo D, Venter L, Young T, Frost E, Lee K, Van Nguyen T, Kitundu E, Archer SDJ, et al. 2022. Pathogenicity and virulence of bacterial strains associated with summer mortality in marine mussels (Perna canaliculus). FEMS Microbiology Ecology. 98:1–14. doi:10.1093/femsec/fiac140.
- Azizan A, Alfaro AC, Venter L, Jaramillo D, Bestbier M, Foxwell J, Bennet P, Young T. 2023a. Quantification of pathogenic Photobacterium swingsii and characterisation of disease progression in the New Zealand GreenshellTM mussel, Perna canaliculus. Journal of Invertebrate Pathology. Under review.
- Azizan A, Alfaro AC, Venter L, Zhang JJ, Ericson JA, Young T, Delorme NJ, Ragg NLC. 2023b. Interactive effects of elevated temperature and Photobacterium swingsii infection on the survival and immune response of marine mussels (Perna canaliculus). Science of The Total Environment, Submitted.
- Azizan A, Carter J, Alfaro AC, Venter L, Young T, Sharma SS, Chen T. 2023c. Investigating the effect of bacterial co-infections on juvenile and adult, green-lipped mussels (Perna canaliculus). Journal of World Aquaculture Society. 1–18. doi:10.1111/jwas.13009.
- Azizan A, Venter L, Alfaro AC.. 2023d. Physiological biomarkers of mussel Vibrionaceae: a review on the constraints and potentials. In-preparation.
- Azizan A, Venter L, Jansen Van Rensburg PJ, Ericson JA, Ragg NLC, & Alfaro AC. 2023e. Metabolite changes of Perna canaliculus following a laboratory marine heatwave exposure: insights from metabolomic analyses. Metabolites. 13:815–833. doi:10.1152/ajpregu.00124.2007.
- Baag S, Mandal S. 2022. Combined effects of ocean warming and acidification on marine fish and shellfish: a molecule to ecosystem perspective. Science of the Total Environment. 802:149807. doi:10.1016/j.scitotenv.2021.149807.
- Babarro JM, de Zwaan A. 2002. Influence of abiotic factors on bacterial proliferation and anoxic survival of the sea mussel Mytilus edulis L. Journal of Experimental Marine Biology and Ecology. 273:33–49. doi:10.1016/S0022-0981(02)00139-9.
- Baker-AUSTIN C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J. 2018. Vibrio spp. infections. Nature Reviews Disease Primers. 4:1–19. doi:10.1038/s41572-018-0005-8.
- Balbi T, Auguste M, Ciacci C, Canesi L. 2021. Immunological responses of marine bivalves to contaminant exposure: contribution of the-omics approach. Frontiers in Immunology. 12:618726. doi:10.3389/fimmu.2021.618726.
- Balbi T, Cortese K, Ciacci C, Bellese G, Vezzulli L, Pruzzo C, Canesi L. 2018. Autophagic processes in Mytilus galloprovincialis hemocytes: effects of Vibrio tapetis. Fish and Shellfish Immunology. 73:66–74. doi:10.1016/j.fsi.2017.12.003.
- Balbi T, Fabbri R, Montagna M, Camisassi G, Canesi L. 2017. Seasonal variability of different biomarkers in mussels (Mytilus galloprovincialis) farmed at different sites of the Gulf of La Spezia, Ligurian sea, Italy. Marine Pollution Bulletin. 116:348–356. doi:10.1016/j.marpolbul.2017.01.035.
- Bassim S, Genard B, Gauthier-Clerc S, Moraga D, Tremblay R. 2015. Ontogeny of bivalve immunity: assessing the potential of next-generation sequencing techniques. Reviews in Aquaculture. 7:197–217. doi:10.1111/raq.12064.
- Beleneva I, Maslennikova E. 2005. Hydrolytic activity of marine bacteria associated with the mussel Mytilus trossulus. Mikrobiolohichnyi Zhurnal (Kiev, Ukraine: 1993). 67:3–8.
- Benabdelmouna A, Garcia C, Ledu C, Lamy P, Maurouard E, Dégremont L. 2018. Mortality investigation of Mytilus edulis and Mytilus galloprovincialis in France: An experimental survey under laboratory conditions. Aquaculture. 495:831–841. doi:10.1016/j.aquaculture.2018.06.075.
- Bettencourt R, Roch P, Stefanni S, Rosa D, Colaço A, Santos RS. 2007. Deep sea immunity: unveiling immune constituents from the hydrothermal vent mussel Bathymodiolus azoricus. Marine Environmental Research. 64:108–127. doi:10.1016/j.marenvres.2006.12.010.
- Betti M, Ciacci C, Lorusso LC, Canonico B, Falcioni T, Gallo G, Canesi L. 2006. Effects of tumour necrosis factor α (TNFα) on Mytilus haemocytes: role of stress-activated mitogen-activated protein kinases (MAPKs). Biology of the Cell. 98:233–244. doi:10.1042/BC20050049.
- Biel FM, Allen FA, Häse CC. 2014. Autolysis in Vibrio tubiashii and Vibrio coralliilyticus. Canadian Journal of Microbiology. 60:57–63. doi:10.1139/cjm-2013-0654.
- Bommarito C, Wahl M, Thieltges D, Pansch C, Zucchetta M, Pranovi F. 2022. Biotic and abiotic drivers affect parasite richness, prevalence and abundance in Mytilus galloprovincialis along the Northern Adriatic Sea. Parasitology. 149:15–23. doi:10.1017/S0031182021001438.
- Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M. 2005. Disease and health management in Asian aquaculture. Veterinary Parasitology. 132:249–272. doi:10.1016/j.vetpar.2005.07.005.
- Bouallegui Y. 2019. Immunity in mussels: an overview of molecular components and mechanisms with a focus on the functional defenses. Fish & Shellfish Immunology. 89:158–169. doi:10.1016/j.fsi.2019.03.057.
- Boyd PW, Collins S, Dupont S, Fabricius K, Gattuso JP, Havenhand J, Hutchins DA, Riebesell U, Rintoul MS, Vichi M. 2018. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change – a review. Global Change Biology. 24:2239–2261. doi:10.1111/gcb.14102.
- Brennan JJ, Gilmore TD. 2018. Evolutionary origins of toll-like receptor signaling. Molecular Biology and Evolution. 35:1576–1587. doi:10.1093/molbev/msy050.
- Brian JI, Ollard IS, Aldridge DC. 2021. Don't move a mussel? Parasite and disease risk in conservation action. Conservation Letters. 14:e12799. doi:10.1111/conl.12799.
- Burge CA, Friedman CS, Getchell R, House M, Lafferty KD, Mydlarz LD, Prager KC, Sutherland KP, Renault T, Kiryu I. 2016. Complementary approaches to diagnosing marine diseases: a union of the modern and the classic. Philosophical Transactions of the Royal Society B: Biological Sciences. 371:20150207. doi:10.1098/rstb.2015.0207.
- Burgos-Aceves MA, Faggio C. 2017. An approach to the study of the immunity functions of bivalve haemocytes: physiology and molecular aspects. Fish & Shellfish Immunology. 67:513–517. doi:10.1016/j.fsi.2017.06.042.
- Cajka T, Fiehn O. 2016. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Analytical Chemistry. 88:524–545. doi:10.1021/acs.analchem.5b04491.
- Cameron A. 2002. Survey toolbox for aquatic animal diseases: a practical manual and software package, ACIAR Monograph.
- Campos A, Apraiz I, Da Fonseca RR, Cristobal S. 2015. Shotgun analysis of the marine mussel Mytilus edulis hemolymph proteome and mapping the innate immunity elements. Proteomics. 15:4021–4029. doi:10.1002/pmic.201500118.
- Campos A, Tedesco S, Vasconcelos V, Cristobal S. 2012. Proteomic research in bivalves: towards the identification of molecular markers of aquatic pollution. Journal of Proteomics. 75:4346–4359. doi:10.1016/j.jprot.2012.04.027.
- Canesi L Betti M, Ciacci C, Citterio B, Pruzzo C, Gallo G. 2003. Tyrosine kinase-mediated cell signalling in the activation of Mytilus hemocytes: possible role of STAT-like proteins. Biology of the Cell. 95:603–613. doi:10.1016/j.biolcel.2003.09.006.
- Canesi L, Pruzzo C. 2016. Specificity of innate immunity in bivalves: a lesson from bacteria. In: Ballarin L., Cammarata M., editors. Lessons in immunity. London (UK): Academic Press; p. 79–91. doi:10.1016/B978-0-12-803252-7.00006-0.
- Canton J, Neculai D, Grinstein S. 2013. Scavenger receptors in homeostasis and immunity. Nature Reviews Immunology. 13:621–634. doi:10.1038/nri3515.
- Carella F, Feist S, Bignell J, De Vico G. 2015. Comparative pathology in bivalves: aetiological agents and disease processes. Journal of Invertebrate Pathology. 131:107–120. doi:10.1016/j.jip.2015.07.012.
- Carrera M, Piñeiro C, Martinez I. 2020. Proteomic strategies to evaluate the impact of farming conditions on food quality and safety in aquaculture products. Foods. 9:1050. doi:10.3390/foods9081050.
- Carver CE, Thériault I, Mallet AL. 2010. Infection of cultured eastern oysters Crassostrea virginica by the boring sponge Cliona celata, with emphasis on sponge life history and mitigation strategies. Journal of Shellfish Research. 29:905–915. doi:10.2983/035.029.0423.
- Castinel A, Webb S, Jones J, Peeler E, Forrest B. 2019. Disease threats to farmed green-lipped mussels Perna canaliculus in New Zealand: review of challenges in risk assessment and pathway analysis. Aquaculture Environment Interactions. 11:291–304. doi:10.3354/aei00314.
- Chandhini S, Rejish Kumar VJ. 2019. Transcriptomics in aquaculture: current status and applications. Reviews in Aquaculture. 11:1379–1397. doi:10.1111/raq.12298.
- Chandurvelan R, Marsden ID, Gaw S, Glover CN. 2013. Waterborne cadmium impacts immunocytotoxic and cytogenotoxic endpoints in green-lipped mussel, Perna canaliculus. Aquatic Toxicology. 142:283–293. doi:10.1016/j.aquatox.2013.09.002.
- Chandurvelan R, Marsden ID, Glover CN, Gaw S. 2016. Biomarker responses of mussels exposed to earthquake disturbances. Estuarine, Coastal and Shelf Science. 182:98–111. doi:10.1016/j.ecss.2016.09.008.
- Chellapackialakshmi M, Ravi C. 2022. Investigation on Mollusc Lectins. In: Elumalai P, Baskaralingam B, Lakshmi S, editors. Aquatic lectins: immune defense, biological recognition and molecular advancements. Springer: Springer Nature Singapore; p. 81–95.
- Chen Y, Xu K, Li J, Wang X, Ye Y, Qi P. 2018. Molecular characterization of complement component 3 (C3) in Mytilus coruscus improves our understanding of bivalve complement system. Fish & Shellfish Immunology. 76:41–47. doi:10.1016/j.fsi.2018.02.044.
- Cheng-Hua L, Jian-Min Z, Lin-Sheng S. 2009. A review of advances in research on marine molluscan antimicrobial peptides and their potential application in aquaculture. Molluscan Research. 29:17–26.
- Coaglio AL, Ferreira MAND, Dos Santos Lima W, De Jesus Pereira CA. 2018. Identification of a phenoloxidase-and melanin-dependent defence mechanism in Achatina fulica infected with Angiostrongylus vasorum. Parasites & Vectors. 11:1–8. doi:10.1186/s13071-018-2710-2.
- Coates CJ, Söderhäll K. 2021. The stress–immunity axis in shellfish. Journal of Invertebrate Pathology. 186:107492. doi:10.1016/j.jip.2020.107492.
- Coupe A, Howe L, Burrows E, Sine A, Pita A, Velathanthiri N, Vallée E, Hayman D, Shapiro K, Roe WD. 2018. First report of Toxoplasma gondii sporulated oocysts and Giardia duodenalis in commercial green-lipped mussels (Perna canaliculus) in New Zealand. Parasitology Research. 117:1453–1463. doi:10.1007/s00436-018-5832-8.
- Cruz C, Chycka M, Hedderley D, Fletcher G. 2016. Prevalence, characteristics and ecology of Vibrio vulnificus found in New Zealand shellfish. Journal of Applied Microbiology. 120:1100–1107. doi:10.1111/jam.13064.
- Dai Q, Wang Z-X, Sheng Y-Q, Wu Z-W, Qiu Y, Su P, Ke C-H, Feng D-Q. 2021. 2-Arachidonoylglycerol as an endogenous cue negatively regulates attachment of the mussel Perna viridis. Frontiers in Marine Science. 8:719781. doi:10.3389/fmars.2021.719781.
- Dai YJ, Hui KM, Zhang YH, Liu Y, Wang YQ, Zhao LJ, Lin I, Chai LQ, Wei S, Lan JF. 2017. Three STATs are involved in the regulation of the expression of antimicrobial peptides in the triangle sail mussel. Hyriopsis Cumingii. Fish & Shellfish Immunology. 63:181–188. doi:10.1016/j.fsi.2017.02.012.
- Defoirdt T. 2014. Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Reviews in Aquaculture. 6:100–114. doi:10.1111/raq.12030.
- De La Ballina NR, Maresca F, Cao A, Villalba A. 2022. Bivalve haemocyte subpopulations: a review. Frontiers in Immunology. 13:826255. doi:10.3389/fimmu.2022.826255.
- Delorme N, Biessy L, South P, Zamora L, Ragg N, Burritt D. 2020. Stress-on-stress responses of a marine mussel, Perna canaliculus: food limitation reduces the ability to cope with heat stress in juveniles. Marine Ecology Progress Series. 644:105–117. doi:10.3354/meps13375.
- Delorme NJ, Burritt DJ, Ragg NL, South PM. 2021a. Emersion and relative humidity modulate stress response and recovery dynamics in juvenile mussels (Perna canaliculus). Metabolites. 11:580. doi:10.3390/metabo11090580.
- Delorme NJ, Venter L, Rolton A, Ericson JA. 2021b. Integrating animal health and stress assessment tools using the green-lipped mussel Perna canaliculus as a case study. Journal of Shellfish Research. 40:93–112. doi:10.2983/035.040.0109.
- Deng Y, Xu L, Chen H, Liu S, Guo Z, Cheng C, Ma H, Feng J. 2020. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in south China. Scientific Reports. 10:14329–14336. doi:10.1038/s41598-020-71288-0.
- Destoumieux-Garzón D, Canesi L, Oyanedel D, Travers MA, Charrière GM, Pruzzo C, Vezzulli L. 2020. Vibrio–bivalve interactions in health and disease. Environmental Microbiology. 22:4323–4341. doi:10.1111/1462-2920.15055.
- Detree C, Núñez-Acuña G, Roberts S, Gallardo-Escarate C. 2016. Uncovering the complex transcriptome response of Mytilus chilensis against saxitoxin: implications of harmful algal blooms on mussel populations. PLoS One. 11:e0165231–e0165250. doi:10.1371/journal.pone.0165231.
- Dimitriadis V, Domouhtsidou G, Cajaraville M. 2004. Cytochemical and histochemical aspects of the digestive gland cells of the mussel Mytilus galloprovincialis (L.) in relation to function. Journal of Molecular Histology. 35:501–509. doi:10.1023/B:HIJO.0000045952.87268.76.
- Dirita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proceedings of the National Academy of Sciences. 88:5403–5407. doi:10.1073/pnas.88.12.5403.
- Donaghy L, Volety AK. 2011. Functional and metabolic characterization of hemocytes of the green mussel, Perna viridis: in vitro impacts of temperature. Fish & Shellfish Immunology. 31:808–814. doi:10.1016/j.fsi.2011.07.018.
- Dong HT, Nguyen VV, Phiwsaiya K, Gangnonngiw W, Withyachumnarnkul B, Rodkhum C, Senapin S. 2015. Concurrent infections of Flavobacterium columnare and Edwardsiella ictaluri in striped catfish, Pangasianodon hypophthalmus in Thailand. Aquaculture. 448:142–150. doi:10.1016/j.aquaculture.2015.05.046.
- Dunphy B, Ruggiero K, Zamora L, Ragg N. 2018. Metabolomic analysis of heat-hardening in adult green-lipped mussel (Perna canaliculus): a key role for succinic acid and the GABAergic synapse pathway. Journal of Thermal Biology. 74:37–46. doi:10.1016/j.jtherbio.2018.03.006.
- Dunphy BJ, Ragg NL, Collings MG. 2013. Latitudinal comparison of thermotolerance and HSP70 production in F2 larvae of the greenshell mussel (Perna canaliculus). Journal of Experimental Biology. 216:1202–1209. doi:10.1242/jeb.076729.
- Dunphy BJ, Watts E, Ragg NLC. 2015. Identifying thermally-stressed adult green-lipped mussels (Perna canaliculus Gmelin, 1791) via metabolomic profiling. American Malacological Bulletin. 33:127–135. doi:10.4003/006.033.0110.
- Dziarski R, Gupta D. 2006. The peptidoglycan recognition proteins (PGRPs). Genome biology. 7:232. doi:10.1186/gb-2006-7-8-232.
- Eggermont M. 2017. Toolbox development to study host-pathogen interactions in the blue mussel Mytilus edulis [Doctor of Philosophy PhD thesis]. University of Ghent.
- Eggermont M, Bossier P, Pande GSJ, Delahaut V, Rayhan AM, Gupta N, Islam SS, Yumo E, Nevejan N, Sorgeloos P, et al. 2017. Isolation of Vibrionaceae from wild blue mussel (Mytilus edulis) adults and their impact on blue mussel larviculture. FEMS Microbiology Ecology. 93:1–11. doi:10.1093/femsec/fix039.
- Eissa N, Wang HP. 2016. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Reviews in Aquaculture. 8:61–88. doi:10.1111/raq.12081.
- Ellis RP, Spicer JI, Byrne JJ, Sommer U, Viant MR, White DA, Widdicombe S. 2014. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environmental Science and Technology. 48:7044–7052. doi:10.1021/es501601w.
- Ericson JA, Delorme NJ, Ragg NLC. 2023a. Heat tolerance of GreenshellTM mussels (Perna canaliculus): collated research findings and implications for mussel farming. Nelson (NZ): Cawthron Institute.
- Ericson JA, Venter L, Copedo JS, Nguyen VT, Alfaro AC, Ragg NLC. 2023b. Chronic heat stress as a predisposing factor in summer mortality of mussels, Perna canaliculus. Aquaculture. 564:738986. doi:10.1016/j.aquaculture.2022.738986.
- Ericson JA, Venter L, Welford MRV, Kumanan K, Alfaro AC, Ragg NLC. 2022. Effects of seawater temperature and acute Vibrio sp. challenge on the haemolymph immune and metabolic responses of adult mussels (Perna canaliculus). Fish and Shellfish Immunology. 128:664–675. doi:10.1016/j.fsi.2022.08.015.
- Falfushynska H, Wu F, Sokolov EP, Sokolova IM. 2023. Salinity variation modulates cellular stress response to ZnO nanoparticles in a sentinel marine bivalve, the blue mussel Mytilus sp. Marine Environmental Research. 183:105834. doi:10.1016/j.marenvres.2022.105834.
- Falfushynska HI, Wu F, Ye F, Kasianchuk N, Dutta J, Dobretsov S, Sokolova IM. 2019. The effects of ZnO nanostructures of different morphology on bioenergetics and stress response biomarkers of the blue mussels Mytilus edulis. Science of the Total Environment. 694:133717. doi:10.1016/j.scitotenv.2019.133717.
- Figueroa C, Bustos P, Torrealba D, Dixon B, Soto C, Conejeros P, Gallardo JA. 2017. Coinfection takes its toll: Sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Scientific Report. 7:17817. doi:10.1038/s41598-017-18180-6.
- Fisheries New Zealand. 2022. The New Zealand goverment aquaculture strategy: 2022 implementation plan. Wellington: Ministry for Primary Industries.
- Fox M, Christley R, Lupo C, Moore H, Service M, Campbell K. 2020. Preventing and mitigating farmed bivalve disease: a northern Ireland case study. Aquaculture International. 28:2397–2417. doi:10.1007/s10499-020-00597-y.
- Gagné N, Cochennec N, Stephenson M, Mcgladdery S, Meyer GR, Bower SM. 2008. First report of a Mikrocytos-like parasite in European oysters Ostrea edulis from Canada after transport and quarantine in France. Diseases of Aquatic Organisms. 80:27–35. doi:10.3354/dao01922.
- Garnier M, Labreuche Y, Nicolas JL. 2008. Molecular and phenotypic characterization of Vibrio aestuarianus subsp. francensis subsp. nov., a pathogen of the oyster Crassostrea gigas. Syst Appl Microbiol. 31:358–365. doi:10.1016/j.syapm.2008.06.003.
- Georgiades E, Fraser R, Jones B. 2016. Options to strengthen on-farm biosecurity management for commercial and non-commercial aquaculture. Aquaculture Unit. Technical Paper No: 2016/47.
- Georgiades E, Kluza D, Bates T, Lubarsky K, Brunton J, Growcott A, Smith T, Mcdonald S, Gould B, Parker N. 2020. Regulating vessel biofouling to support New Zealand’s marine biosecurity system – a blue print for evidence-based decision making. Frontiers in Marine Science. 7:390. doi:10.3389/fmars.2020.00390.
- Gerdol M. 2017. Immune-related genes in gastropods and bivalves: a comparative overview. Invertebrate Survival Journal. 14:95–111. doi:10.1016/j.fsi.2015.02.013.
- Gerdol M, Gomez-Chiarri M, Castillo MG, Figueras A, Fiorito G, Moreira R, Novoa B, Pallavicini A, Ponte G, Roumbedakis K, et al. 2018. Immunity in molluscs: recognition and effector mechanisms, with a focus on bivalvia. In: Cooper EL, editor. Advances in comparative immunology. Cham: Springer.
- Gerdol M, Manfrin C, De Moro G, Figueras A, Novoa B, Venier P, Pallavicini A. 2011. The C1q domain containing proteins of the Mediterranean mussel Mytilus galloprovincialis: a widespread and diverse family of immune-related molecules. Developmental & Comparative Immunology. 35:635–643. doi:10.1016/j.dci.2011.01.018.
- Gerdol M, Venier P. 2015. An updated molecular basis for mussel immunity. Fish & Shellfish Immunology. 46:17–38. doi:10.1016/j.fsi.2015.02.013.
- Gestal C, Roch P, Renault T, Pallavicini A, Paillard C, Novoa B, Oubella R, Venier P, Figueras A. 2008. Study of diseases and the immune system of bivalves using molecular biology and genomics. Reviews in Fisheries Science. 16:133–156. doi:10.1080/10641260802325518.
- Gomez-Gil B, Roque A, Rotllant G, Peinado L, Romalde JL, Doce A, Cabanillas-Beltrán H, Chimetto LA, Thompson FL. 2011. Photobacterium swingsii sp. nov., isolated from marine organisms. International Journal of Systematic and Evolutionary Microbiology. 61:315–319. doi:10.1099/ijs.0.019687-0.
- Green DS, Colgan TJ, Thompson RC, Carolan JC. 2019. Exposure to microplastics reduces attachment strength and alters the haemolymph proteome of blue mussels (Mytilus edulis). Environmental pollution. 246:423–434. doi:10.1016/j.envpol.2018.12.017.
- Grinchenko A, Kumeiko V. 2022. Bivalves humoral immunity: key molecules and their functions. Russian Journal of Marine Biology. 48:399–417. doi:10.1134/S1063074022060062.
- Gui Y, Zamora LN, Dunphy B, Jeffs AG. 2016. Understanding the ontogenetic changes in particle processing of the greenshell™ mussel, Perna canaliculus, in order to improve hatchery feeding practices. Aquaculture. 452:120–127. doi:10.1016/j.aquaculture.2015.07.035.
- Han Y, Zhou W, Tang Y, Shi W, Shao Y, Ren P, Zhang J, Xiao G, Sun H, Liu G. 2021. Microplastics aggravate the bioaccumulation of three veterinary antibiotics in the thick shell mussel Mytilus coruscus and induce synergistic immunotoxic effects. Science of The Total Environment. 770:145273. doi:10.1016/j.scitotenv.2021.145273.
- Hasin Y, Seldin M, Lusis A. 2017. Multi-omics approaches to disease. Genome biology. 18:1–15. doi:10.1186/s13059-017-1215-1.
- He Z, He J, Wang J, Zhang X, Fan M, Buttino I, Qi P, Yan X, Liao Z. 2022. Comparative transcriptomic analysis of gill and gonad from Mytilus under antibiotics treatment followed by different bacteria challenge. Aquaculture. 547:737457. doi:10.1016/j.aquaculture.2021.737457.
- Henderson B, Allan E, Coates AR. 2006. Stress wars: the direct role of host and bacterial molecular chaperones in bacterial infection. Infection and immunity. 74:3693–3706. doi:10.1128/IAI.01882-05.
- Hossain S, Wickramanayake MVKS, Dahanayake PS, Heo G-J. 2020. Occurrence of virulence and extended-spectrum β-lactamase determinants in Vibrio spp. isolated from marketed hard-shelled mussel (Mytilus coruscus). Microbial Drug Resistance. 26:391–401. doi:10.1089/mdr.2019.0131.
- Huang Y, Wang W, Ren Q. 2016. Identification and function of a novel C1q domain-containing (C1qDC) protein in triangle-shell pearl mussel (Hyriopsis cumingii). Fish & Shellfish Immunology. 58:612–621. doi:10.1016/j.fsi.2016.10.010.
- Islam SS. 2016. Impact of quorum sensing on the virulence of Vibrio crassostreae and Vibrio tasmaniensis in vitro and in vivo in blue mussel larvae [Master of science MSc thesis]. Ghent University.
- Islam SS, Zhang S, Eggermont M, Bruto M, Le Roux F, Defoirdt T. 2022. The impact of the multichannel quorum sensing systems of Vibrio tasmaniensis and Vibrio crassostreae on virulence towards blue mussel (Mytilus edulis) larvae. Aquaculture. 547:737414. doi:10.1016/j.aquaculture.2021.737414.
- Jayaraj SS, Thiagarajan R, Arumugam M, Mullainadhan P. 2008. Isolation, purification and characterization of β-1, 3-glucan binding protein from the plasma of marine mussel Perna viridis. Fish & Shellfish Immunology. 24:715–725. doi:10.1016/j.fsi.2007.11.012.
- Johnson CN. 2013. Fitness factors in vibrios: a mini-review. Microbial Ecology. 65:826–851. doi:10.1007/s00248-012-0168-x.
- Kaloyianni M, Dailianis S, Chrisikopoulou E, Zannou A, Koutsogiannaki S, Alamdari D, Koliakos G, Dimitriadis V. 2009. Oxidative effects of inorganic and organic contaminants on haemolymph of mussels. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 149:631–639. doi:10.1016/j.cbpc.2009.01.006.
- Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Frontiers in Immunology. 5:461. doi:10.3389/fimmu.2014.00461.
- Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. 2009. Two pathogens of Greenshell™ mussel larvae, Perna canaliculus: Vibrio splendidus and a V. coralliilyticus/neptunius-like isolate. Journal of Fish Diseases. 32:499–507. doi:10.1111/j.1365-2761.2009.01006.x.
- Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. 2012. Performance of single and multi-strain probiotics during hatchery production of Greenshell™ mussel larvae, Perna canaliculus. Aquaculture. 354:56–63. doi:10.1016/j.aquaculture.2012.04.026.
- Khantavong A, Tunkijjanukij S, Patarajinda S, Kangsuwan A. 2009. Isolation and characterization of chitinase from marine bacteria. Journal of Fisheries and Environment. 33:8–21.
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nature Reviews Immunology. 16:626–638. doi:10.1038/nri.2016.90.
- Kobiyama A, Ikeo K, Reza MS, Rashid J, Yamada Y, Ikeda Y, Ikeda D, Mizusawa N, Sato S, Ogata T. 2018. Metagenome-based diversity analyses suggest a strong locality signal for bacterial communities associated with oyster aquaculture farms in Ofunato Bay. Gene. 665:149–154. doi:10.1016/j.gene.2018.04.073.
- Kotob MH, Menanteau-Ledouble S, Kumar G, Abdelzaher M, El-Matbouli M. 2017. The impact of co-infections on fish: a review. Veterinary research. 47:1–12. doi:10.1186/s13567-016-0383-4.
- Kumar M, Chakdar H, Pandiyan K, Thapa S, Shahid M, Singh A, Srivastava AK, Saxena AK. 2022. Bacterial chitinases: genetics, engineering and applications. World Journal of Microbiology and Biotechnology. 38:252. doi:10.1007/s11274-022-03444-9.
- Künili IE, Ertürk Gürkan S, Aksu A, Turgay E, Çakir F, Gürkan M, Altinağaç U. 2021. Mass mortality in endangered fan mussels Pinna nobilis (Linnaeus 1758) caused by co-infection of Haplosporidium pinnae and multiple Vibrio infection in Çanakkale Strait, Turkey. Biomarkers. 26:450–461. doi:10.1080/1354750X.2021.1910344.
- Kwan TN, Bolch CJS. 2015. Genetic diversity of culturable Vibrio in an Australian blue mussel Mytilus galloprovincialis hatchery. Diseases of Aquatic Organisms. 116:37–46. doi:10.3354/dao02905.
- Kwoji ID, Aiyegoro OA, Okpeku M, Adeleke MA. 2023. ‘Multi-omics’ data integration: applications in probiotics studies. NPJ Science of Food. 7:25–34. doi:10.1038/s41538-023-00199-x.
- Labreuche Y, Lambert C, Soudant P, Boulo V, Huvet A, Nicolas JL. 2006. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes and Infection. 8:2715–2724. doi:10.1016/j.micinf.2006.07.020.
- Lam O, Wheeler J, Tang CM. 2014. Thermal control of virulence factors in bacteria: a hot topic. Virulence. 5:852–862. doi:10.4161/21505594.2014.970949.
- Lane HS, Brosnahan CL, Poulin R. 2022. Aquatic disease in New Zealand: synthesis and future directions. New Zealand Journal of Marine and Freshwater Research. 1–42.
- Lane HS, Brosnahan CL, Poulin R. 2022. Aquatic disease in New Zealand: synthesis and future directions. New Zealand Journal of Marine and Freshwater Research. 56:1–42. doi:10.1080/00288330.2020.1848887.
- Lattos A, Giantsis IA, Karagiannis D, Michaelidis B. 2020. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Marine environmental research. 155:104889. doi:10.1016/j.marenvres.2020.104889.
- Leoni G, De Poli A, Mardirossian M, Gambato S, Florian F, Venier P, Wilson DN, Tossi A, Pallavicini A, Gerdol M. 2017. Myticalins: a novel multigenic family of linear, cationic antimicrobial peptides from marine mussels (Mytilus spp. Marine Drugs. 15:261. doi:10.3390/md15080261.
- Le Roux F, Blokesch M. 2018. Eco-evolutionary dynamics linked to horizontal gene transfer in Vibrios. Annual Review of Microbiology. 72:89–110. doi:10.1146/annurev-micro-090817-062148.
- Le Roux F, Wegner KM, Baker-Austin C, Vezzulli L, Osorio CR, Amaro C, Ritchie JM, Defoirdt T, Destoumieux-Garzón D, Blokesch M, et al. 2015. The emergence of Vibrio pathogens in Europe: ecology, evolution and pathogenesis (Paris, 11-12 March 2015). Frontiers in Microbiology. 6:830–838. doi:10.3389/fmicb.2015.00830.
- Leulier F, Lemaitre B. 2008. Toll-like receptors – taking an evolutionary approach. Nature Reviews Genetics. 9:165–178. doi:10.1038/nrg2303.
- Li R, Zhang R, Zhang L, Zou J, Xing Q, Dou H, Hu X, Zhang L, Wang R, Bao Z. 2015. Characterizations and expression analyses of NF-κB and Rel genes in the Yesso scallop (Patinopecten yessoensis) suggest specific response patterns against Gram-negative infection in bivalves. Fish & shellfish immunology. 44:611–621. doi:10.1016/j.fsi.2015.03.036.
- Li S, Alfaro AC, Nguyen TV, Young T, Lulijwa R. 2020. An integrated omics approach to investigate summer mortality of New Zealand Greenshell™ mussels. Metabolomics. 16:1–16. doi:10.1007/s11306-019-1621-3.
- Li YF, Liu YZ, Chen YW, Chen K, Batista FM, Cardoso JC, Chen YR, Peng LH, Zhang Y, Zhu YT. 2019. Two toll-like receptors identified in the mantle of Mytilus coruscus are abundant in haemocytes. Fish & Shellfish Immunology. 90:134–140. doi:10.1016/j.fsi.2019.05.001.
- Liao Z, Yang Z, Wang Y, He J, He Z, Zhang X, Buttino I, Qi P, Fan M, Guo B. 2022. Molecular characterization of peptidoglycan recognition proteins from Mytilus coruscus. Fish & Shellfish Immunology. 131:612–623. doi:10.1016/j.fsi.2022.10.018.
- Liu Y, Zhan X, Catalano SR, Qin J, Han J, Li X. 2022. Investigation on redox status and gene expression related to larval cryopreservation in the Pacific oyster Crassostrea gigas. Fisheries Science. 88:377–386. doi:10.1007/s12562-022-01594-1.
- Lozano-León A, Osorio CR, Nuñez S, Martínez-Urtaza J, Magariños B. 2003. Occurrence of Photobacterium damselae subsp. damselae in bivavlve molluscs from Northwest Spain. Bulletin of the European Association of Fish Pathologists. 23:40–44.
- Machado H, Giubergia S, Mateiu RV, Gram L. 2015. Photobacterium galatheae sp. nov, a bioactive bacterium isolated from a mussel in the Solomon Sea. International Journal of Systematic and Evolutionary Microbiology. 65:4503–4507. doi:10.1099/ijsem.0.000603.
- Maleki F, Khosravi A, Nasser A, Taghinejad H, Azizian M. 2016. Bacterial heat shock protein activity. Journal of clinical and diagnostic research: JCDR. 10:BE01–BE03. doi:10.7860/JCDR/2016/14568.7444.
- Maria VL, Amorim MJ, Bebianno MJ, Dondero F. 2016. Transcriptomic effects of the non-steroidal anti-inflammatory drug Ibuprofen in the marine bivalve Mytilus galloprovincialis Lam. Marine environmental research. 119:31–39. doi:10.1016/j.marenvres.2016.05.010.
- Martcheva M, Pilyugin SS. 2006. The role of coinfection in multidisease dynamics. SIAM Journal on Applied Mathematics. 66:843–871. doi:10.1137/040619272.
- Martins I, Goulart J, Martins E, Morales-Roman R, Marin S, Riou V, Colaco A, Bettencourt R. 2017. Physiological impacts of acute Cu exposure on deep-sea vent mussel Bathymodiolus azoricus under a deep-sea mining activity scenario. Aquatic toxicology. 193:40–49. doi:10.1016/j.aquatox.2017.10.004.
- Matozzo V, Ercolini C, Serracca L, Battistini R, Rossini I, Granato G, Quaglieri E, Perolo A, Finos L, Arcangeli G, et al. 2018. Assessing the health status of farmed mussels (Mytilus galloprovincialis) through histological, microbiological and biomarker analyses. Journal of Invertebrate Pathology. 153:165–179. doi:10.1016/j.jip.2018.02.018.
- Miller MR, Abshirini M, Wolber FM, Tuterangiwhiu TR, Kruger MC. 2023. Greenshell mussel products: a comprehensive review of sustainability, traditional use, and efficacy. Sustainability. 15:3912. doi:10.3390/su15053912.
- Mitta G, Vandenbulcke F, Roch P. 2000. Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Letters. 486:185–190. doi:10.1016/S0014-5793(00)02192-X.
- Moreira R, Pereiro P, Canchaya C, Posada D, Figueras A, Novoa B. 2015. RNA-Seq in Mytilus galloprovincialis: comparative transcriptomics and expression profiles among different tissues. BMC Genomics. 16:728. doi:10.1186/s12864-015-1817-5.
- Morley NJ. 2010. Interactive effects of infectious diseases and pollution in aquatic molluscs. Aquatic Toxicology. 96:27–36. doi:10.1016/j.aquatox.2009.09.017.
- Moschino V, Meneghetti F, Da Ros L. 2010. Use of biomarkers to assess the welfare of the edible clam, Ruditapes philippinarum: may it be a tool for proving areas of origin? Aquaculture international. 18:327–337. doi:10.1007/s10499-009-9246-6.
- Muznebin F, Alfaro AC, Venter L, Young T. 2022a. Acute thermal stress and endotoxin exposure modulate metabolism and immunity in marine mussels (Perna canaliculus). Journal of Thermal Biology. 110:103327. doi:10.1016/j.jtherbio.2022.103327.
- Muznebin F, Alfaro AC, Webb SC. 2022b. Perkinsus olseni and other parasites and abnormal tissue structures in New Zealand Greenshell™ mussels (Perna canaliculus) across different seasons. Aquaculture International. 31:1–36. doi:10.1007/s10499-022-00991-8.
- Muznebin F, Alfaro AC, Webb SC, Merien F. 2022c. Characterization of mussel (Perna canaliculus) haemocytes and their phagocytic activity across seasons. Aquaculture Research. 53:4288–4303. doi:10.1111/are.15926.
- Mydlarz LD, Jones LE, Harvell CD. 2006. Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst. 37:251–288. doi:10.1146/annurev.ecolsys.37.091305.110103.
- Najwaa MN, Danielb AMD, Amin K, Effendya A. 2015. Detection of virulence genes in Vibrio alginolyticus isolated from green mussel, P. viridis. Jurnal Teknologi. 77:19–23. doi:10.11113/jt.v77.6731.
- Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M. 2021. A 20-year retrospective review of global aquaculture. Nature. 591:551–563. doi:10.1038/s41586-021-03308-6.
- Nguyen TV, Alfaro AC. 2019. Targeted metabolomics to investigate antimicrobial activity of itaconic acid in marine molluscs. Metabolomics. 15:97. doi:10.1007/s11306-019-1556-8.
- Nguyen TV, Alfaro AC. 2020a. Applications of omics to investigate responses of bivalve haemocytes to pathogen infections and environmental stress. Aquaculture. 518:734488. doi:10.1016/j.aquaculture.2019.734488.
- Nguyen TV, Alfaro AC. 2020b. Metabolomics investigation of summer mortality in New Zealand Greenshell™ mussels (Perna canaliculus). Fish & Shellfish Immunology. 106:783–791. doi:10.1016/j.fsi.2020.08.022.
- Nguyen TV, Alfaro AC, Merien F, Lulijwa R, Young T. 2018a. Copper-induced immunomodulation in mussel (Perna canaliculus) haemocytes. Metallomics. 10:965–978. doi:10.1039/C8MT00092A.
- Nguyen TV, Alfaro AC, Merien F, Young T. 2019a. In vitro study of apoptosis in mussel (Perna canaliculus) haemocytes induced by lipopolysaccharide. Aquaculture. 503:8–15. doi:10.1016/j.aquaculture.2018.12.086.
- Nguyen TV, Alfaro AC, Merien F, Young T, Grandiosa R. 2018b. Metabolic and immunological responses of male and female new Zealand Greenshell™ mussels (Perna canaliculus) infected with Vibrio sp. Journal of Invertebrate Pathology. 157:80–89. doi:10.1016/j.jip.2018.08.008.
- Nguyen TV, Alfaro AC, Young T, Green S, Zarate E, Merien F. 2019b. Itaconic acid inhibits growth of a pathogenic marine Vibrio strain: a metabolomics approach. Scientific Reports. 9:5937. doi:10.1038/s41598-019-42315-6.
- Nguyen TV, Alfaro AC, Young T, Merien F. 2019c. Tissue-specific immune responses to Vibrio sp. infection in mussels (Perna canaliculus): a metabolomics approach. Aquaculture. 500:118–125. doi:10.1016/j.aquaculture.2018.09.061.
- Nguyen TV, Alfaro AC, Young T, Ravi S, Merien F. 2018c. Metabolomics study of immune responses of New Zealand Greenshell™ mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Marine Biotechnology. 20:396–409. doi:10.1007/s10126-018-9804-x.
- Nguyen TV, Ragg NL, Alfaro AC, Zamora LN. 2020. Physiological stress associated with mechanical harvesting and transport of cultured mussels (Perna canaliculus): a metabolomics approach. Aquaculture. 529:735657. doi:10.1016/j.aquaculture.2020.735657.
- Oh R, Lee MJ, Kim Y-O, Nam B-H, Kong HJ, Kim J-W, Park J-Y, Seo J-K, Kim D-G. 2020. Myticusin-beta, antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish & Shellfish Immunology. 99:342–352. doi:10.1016/j.fsi.2020.02.020.
- Ordás MC, Albaigés J, Bayona J, Ordas A, Figueras A. 2007. Assessment of in vivo effects of the prestige fuel oil spill on the Mediterranean mussel immune system. Archives of Environmental Contamination and Toxicology. 52:200–206. doi:10.1007/s00244-006-0058-7.
- Ottaviani D, Santarelli S, Bacchiocchi S, Masini L, Ghittino C, Bacchiocchi I. 2005. Presence of pathogenic Vibrio parahaemolyticus strains in mussels from the Adriatic Sea, Italy. Food Microbiology. 22:585–590. doi:10.1016/j.fm.2005.01.005.
- Paillard C, Gueguen Y, Wegner KM, Bass D, Pallavicini A, Vezzulli L, Arzul I. 2022. Recent advances in bivalve-microbiota interactions for disease prevention in aquaculture. Current Opinion in Biotechnology. 73:225–232. doi:10.1016/j.copbio.2021.07.026.
- Paillard C, Le Roux F, Borrego JJ. 2004. Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquatic Living Resources. 17:477–498. doi:10.1051/alr:2004054.
- Palamae S, Mittal A, Yingkajorn M, Saetang J, Buatong J, Tyagi A, Singh P, Benjakul S. 2022. Vibrio parahaemolyticus Isolates from Asian green mussel: molecular characteristics, virulence and their inhibition by chitooligosaccharide-tea polyphenol conjugates. Foods. 11:4048. doi:10.3390/foods11244048.
- Petes LE, Menge BA, Harris AL. 2008. Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecological Monographs. 78(3):387–402.
- Petton B, Destoumieux-Garzón D, Pernet F, Toulza E, De Lorgeril J, Degremont L, Mitta G. 2021. The Pacific oyster mortality syndrome, a polymicrobial and multifactorial disease: state of knowledge and future directions. Frontiers in Immunology. 12:630343. doi:10.3389/fimmu.2021.630343.
- Philipp EE, Kraemer L, Melzner F, Poustka AJ, Thieme S, Findeisen U, Schreiber S, Rosenstiel P. 2012. Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis. PloS one. 7:e33091. doi:10.1371/journal.pone.0033091.
- Picot S, Faury N, Arzul I, Chollet B, Renault T, Morga B. 2020. Identification of the autophagy pathway in a mollusk bivalve, Crassostrea gigas. Autophagy. 16:2017–2035. doi:10.1080/15548627.2020.1713643.
- Prado S, Dubert J, Da Costa F, Martínez-Patiño D, Barja JL. 2014. Vibrios in hatchery cultures of the razor clam, Solen marginatus (Pulteney). Journal of Fish Diseases. 37:209–217. doi:10.1111/jfd.12098.
- Provenzano D, Klose KE. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proceedings of the National Academy of Sciences. 97:10220–10224. doi:10.1073/pnas.170219997.
- Pruzzo C, Gallo G, Canesi L. 2005. Persistence of vibrios in marine bivalves: the role of interactions with haemolymph components. Environmental Microbiology. 7:761–772. doi:10.1111/j.1462-2920.2005.00792.x.
- Puerto M, Campos A, Prieto A, Cameán A, De Almeida AM, Coelho AV, Vasconcelos V. 2011. Differential protein expression in two bivalve species; Mytilus galloprovincialis and Corbicula fluminea; exposed to Cylindrospermopsis raciborskii cells. Aquatic Toxicology. 101:109–116. doi:10.1016/j.aquatox.2010.09.009.
- Ragg NL, Gale SL, Le DV, Hawes NA, Burritt DJ, Young T, Ericson JA, Hilton Z, Watts E, Berry J. 2019. The effects of aragonite saturation state on hatchery-reared larvae of the Greenshell mussel Perna canaliculus. Journal of Shellfish Research. 38:779–793. doi:10.2983/035.038.0328.
- Rahman M, Henderson S, Miller-Ezzy P, Li X, Qin J. 2019. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish & Shellfish Immunology. 86:868–874. doi:10.1016/j.fsi.2018.12.017.
- Rauta PR, Samanta M, Dash HR, Nayak B, Das S. 2014. Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunology Letters. 158:14–24. doi:10.1016/j.imlet.2013.11.013.
- Renault T. 2009. Trends and perspectives in preventing and controlling infectious diseases in molluscs. In: Nakamura TK, editor. Aquaculture research progress. Lancaster: Nova Science Publishers; p. 99–126.
- Renault T. 2015. Immunotoxicological effects of environmental contaminants on marine bivalves. Fish & Shellfish Immunology. 46:88–93. doi:10.1016/j.fsi.2015.04.011.
- Reverter M, Tapissier-Bontemps N, Sarter S, Sasal P, Caruso D. 2021. Moving towards more sustainable aquaculture practices: a meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Reviews in Aquaculture. 13:537–555. doi:10.1111/raq.12485.
- Rey-Campos M, Moreira R, Valenzuela-Muñoz V, Gallardo-Escárate C, Novoa B, Figueras A. 2019. High individual variability in the transcriptomic response of Mediterranean mussels to Vibrio reveals the involvement of myticins in tissue injury. Scientific Reports. 9:1–15. doi:10.1038/s41598-019-39870-3.
- Reyden CA, Delorme NJ, South PM, Aguirre JD. 2023. Impacts of seeding density on the oxidative stress response of the Greenshell™ mussel, Perna canaliculus. Aquaculture International. 31:1–17. doi:10.1007/s10499-023-01078-8.
- Rolton A, Ragg NLC. 2020. Green-lipped mussel (Perna canaliculus) hemocytes: a flow cytometric study of sampling effects, sub-populations and immune-related functions. Fish and Shellfish Immunology. 103:181–189. doi:10.1016/j.fsi.2020.05.019.
- Romalde JL, Dieguez AL, Lasa A, Balboa S. 2014. New Vibrio species associated to molluscan microbiota: a review. Frontiers in Microbiology. 4:1–11. doi:10.3389/fmicb.2013.00413.
- Romero A, Novoa B, Figueras A. 2015. The complexity of apoptotic cell death in mollusks: an update. Fish & Shellfish Immunology. 46:79–87. doi:10.1016/j.fsi.2015.03.038.
- Roncarati D, Scarlato V. 2017. Regulation of heat-shock genes in bacteria: from signal sensing to gene expression output. FEMS Microbiology Reviews. 41:549–574. doi:10.1093/femsre/fux015.
- Saco A, Panebianco A, Blanco S, Novoa B, Diz AP, Figueras A. 2021. Integration of transcriptomics and proteomics improves the characterization of the role of mussel gills in a bacterial waterborne infection. Frontiers in Marine Science. 8:735309. doi:10.3389/fmars.2021.735309.
- Saco A, Rey-Campos M, Novoa B, Figueras A. 2020. Transcriptomic response of mussel gills after a Vibrio splendidus infection demonstrates their role in the immune response. Frontiers in Immunology. 11:1–18. doi:10.3389/fimmu.2020.615580.
- Santos A, Hauser-Davis RA, Santos MJS, De Simone SG. 2017. Potentially toxic filamentous fungi associated to the economically important Nodipecten nodosus (Linnaeus, 1758) scallop farmed in southeastern Rio de Janeiro, Brazil. Marine Pollution Bulletin. 115:75–79. doi:10.1016/j.marpolbul.2016.11.058.
- Shen SS, Qu XY, Zhang WZ, Li J, Lv ZY. 2019. Infection against infection: parasite antagonism against parasites, viruses and bacteria. Infectious Diseases of Poverty. 8:1–12. doi:10.1186/s40249-018-0513-5.
- Shinoda S, Miyoshi S. 2011. Proteases produced by vibrios. Biocontrol Science. 16:1–11. doi:10.4265/bio.16.1.
- Silva IP, De Souza Carneiro C, Saraiva MAF, De oliveira TAS, De sousa OV, Evangelista-Barreto NS. 2018. Antimicrobial resistance and potential virulence of Vibrio parahaemolyticus isolated from water and bivalve mollusks from Bahia, Brazil. Marine Pollution Bulletin. 131:757–762. doi:10.1016/j.marpolbul.2018.05.007.
- Skein L, Robinson TB, Alexander ME. 2018. Impacts of mussel invasions on the prey preference of two native predators. Behavioral Ecology. 29:353–359. doi:10.1093/beheco/arx172.
- Soliman T, Inglis GJ. 2018. Forecasting the economic impacts of two biofouling invaders on aquaculture production of green-lipped mussels Perna canaliculus in New Zealand. Aquaculture Environment Interactions. 10:1–12. doi:10.3354/aei00249.
- Song L, Wang L, Qiu L, Zhang H. 2010. Bivalve immunity. Advance Experimental Medical Biology. 708:44–65. doi:10.1007/978-1-4419-8059-5_3.
- Song X, Liu Z, Wang L, Song L. 2019. Recent advances of shell matrix proteins and cellular orchestration in marine molluscan shell biomineralization. Frontiers in Marine Science. 6:41. doi:10.3389/fmars.2019.00041.
- Souza CP, Almeida BC, Colwell RR, Rivera IN. 2011. The importance of chitin in the marine environment. Marine biotechnology. 13:823–830. doi:10.1007/s10126-011-9388-1.
- Srivastava D, Waters CM. 2012. A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP. Journal of bacteriology. 194:4485–4493. doi:10.1128/JB.00379-12.
- Stenton-Dozey JM, Heath P, Ren JS, Zamora LN. 2021. New Zealand aquaculture industry: research, opportunities and constraints for integrative multitrophic farming. New Zealand Journal of Marine and Freshwater Research. 55:265–285. doi:10.1080/00288330.2020.1752266.
- Sugumar G, Nakai T, Hirata Y, Matsubara D, Muroga K. 1998. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Diseases of Aquatic Organisms. 33:111–118. doi:10.3354/dao033111.
- Sunila I, Labanca J. 2003. Apoptosis in the pathogenesis of infectious diseases of the eastern oyster Crassostrea virginica. Diseases of aquatic organisms. 56:163–170. doi:10.3354/dao056163.
- Sures B, Nachev M, Selbach C, Marcogliese DJ. 2017. Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology’. Parasites and Vectors. 10:1–19. doi:10.1186/s13071-017-2001-3.
- Tanguy A, Bierne N, Saavedra C, Pina B, Bachère E, Kube M, Bazin E, Bonhomme F, Boudry P, Boulo V. 2008. Increasing genomic information in bivalves through new EST collections in four species: development of new genetic markers for environmental studies and genome evolution. Gene. 408:27–36. doi:10.1016/j.gene.2007.10.021.
- Thompson FL, Thompson CC, Naser S, Hoste B, Vandemeulebroecke K, Munn C, Bourne D, Swings J. 2005. Photobacterium rosenbergii sp. nov. and Enterovibrio coralii sp. nov., vibrios associated with coral bleaching. International Journal of Systematic and Evolutionary Microbiology. 55:913–917. doi:10.1099/ijs.0.63370-0.
- Tian Y, Liu J, Pan L. 2020. The mechanism of mitogen-activated protein kinases to mediate apoptosis and immunotoxicity induced by benzo[a]pyrene on hemocytes of scallop Chlamys farreri in vitro. Fish & shellfish immunology. 102:64–72. doi:10.1016/j.fsi.2020.04.006.
- Toews S, Beverley-Burton M, Lawrimore T. 1993. Helminth and protist parasites of zebra mussels, Dreissena polymorpha (Pallas, 1771), in the Great Lakes region of southwestern Ontario, with comments on associated bacteria. Canadian Journal of Zoology. 71:1763–1766. doi:10.1139/z93-250.
- Tomanek L, Zuzow MJ. 2010. The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: implications for thermal tolerance limits and metabolic costs of thermal stress. Journal of Experimental Biology. 213:3559–3574. doi:10.1242/jeb.041228.
- Toubiana M, Gerdol M, Rosani U, Pallavicini A, Venier P, Roch P. 2013. Toll-like receptors and MyD88 adaptors in Mytilus: complete cds and gene expression levels. Developmental & Comparative Immunology. 40:158–166. doi:10.1016/j.dci.2013.02.006.
- Toubiana M, Rosani U, Giambelluca S, Cammarata M, Gerdol M, Pallavicini A, Venier P, Roch P. 2014. Toll signal transduction pathway in bivalves: complete cds of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis. Developmental & Comparative Immunology. 45:300–312. doi:10.1016/j.dci.2014.03.021.
- Travers MA, Boettcher Miller K, Roque A, Friedman CS. 2015. Bacterial diseases in marine bivalves. Journal of Invertebrate Pathology. 131:11–31. doi:10.1016/j.jip.2015.07.010.
- Tuckey NP, Timms BA, Fletcher GC, Summers G, Delorme NJ, Ericson JA, Ragg NL, Miller P, Wibisono R, Taylor R. 2023. Examination of the potential of refrigerated seawater to improve live transport of the mussel Perna canaliculus: physiological responses, meat quality and safety implications under different chilled storage conditions. Aquaculture. 575:739794–739810. doi:10.1016/j.aquaculture.2023.739794.
- Urbanczyk H, Ast JC, Dunlap PV. 2011. Phylogeny, genomics, and symbiosis of Photobacterium. FEMS Microbiology Reviews. 35:324–342. doi:10.1111/j.1574-6976.2010.00250.x.
- Venier P, De Pittà C, Bernante F, Varotto L, De Nardi B, Bovo G, Roch P, Novoa B, Figueras A, Pallavicini A. 2009. Mytibase: a knowledgebase of mussel (M. galloprovincialis) transcribed sequences. BMC genomics. 10:1–16. doi:10.1186/1471-2164-10-72.
- Venier P, Domeneghetti S, Sharma N, Pallavicini A, Gerdol M. 2016. Immune-related signaling in mussel and bivalves. Lessons in Immunity. Elsevier.
- Venier P, Varotto L, Rosani U, Millino C, Celegato B, Bernante F, Lanfranchi G, Novoa B, Roch P, Figueras A, Pallavicini A. 2011. Insights into the innate immunity of the Mediterranean mussel Mytilus galloprovincialis. BMC Genomics. 12. doi:10.1186/1471-2164-12-69.
- Venter L, Alfaro AC, Ragg NL, Delorme NJ, Ericson JA. 2023. The effect of simulated marine heatwaves on green-lipped mussels, Perna canaliculus: A near-natural experimental approach. Journal of Thermal Biology. 117:103702. doi:10.1016/j.jtherbio.2023.103702.
- Venter L, Young T, Alfaro AC, Lindeque JZ. 2021. Establishing sampling confidence parameters: effect of sampling and transport conditions on haemocyte and metabolite profiles of Greenshell mussels. Aquaculture. 538:736538. doi:10.1016/j.aquaculture.2021.736538.
- Waller DL, Cope WG. 2019. The status of mussel health assessment and a path forward. Freshwater Mollusk Biology and Conservation. 22:26. doi:10.31931/fmbc.v22i2.2019.26-42.
- Wan Y, Liu C, Ma Q. 2019. Structural analysis of a Vibrio phospholipase reveals an unusual Ser–His–chloride catalytic triad. Journal of Biological Chemistry. 294:11391–11401. doi:10.1074/jbc.RA119.008280.
- Wang L, Qiu L, Zhou Z, Song L. 2013. Research progress on the mollusc immunity in China. Developmental & Comparative Immunology. 39:2–10. doi:10.1016/j.dci.2012.06.014.
- Wang L, Song X, Song L. 2018. The oyster immunity. Developmental & Comparative Immunology. 80:99–118. doi:10.1016/j.dci.2017.05.025.
- Webb S, Duncan J. 2019. New Zealand shellfish health monitoring 2007 to 2017: insights and projections. Cawthron Report No. 2568. Cawthron Report.
- Webb S, Gaw S, Marsden I, Mcrae N. 2020. Biomarker responses in New Zealand green-lipped mussels Perna canaliculus exposed to microplastics and triclosan. Ecotoxicology and Environmental Safety. 201:110871–11088010.1016/j.ecoenv.2020.110871.
- Xu K, Zhang Z, Xu Z, Tang Z, Liu L, Lu Z, Qi P. 2019. A novel invertebrate toll-like receptor is involved in TLR mediated signal pathway of thick shell mussel Mytilus coruscus. Developmental & Comparative Immunology. 97:11–19. doi:10.1016/j.dci.2019.03.012.
- Yang J, He J, Liu L, He M, Zhang X, Buttino I, Guo B, Yan X, Liao Z. 2022. Expression profiles of antimicrobial peptides in Mytilus coruscus. Aquaculture. 548:737709. doi:10.1016/j.aquaculture.2021.737709.
- Yao CL, Somero GN. 2012. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. Journal of Experimental Biology. 215:4267–4277. doi:10.1242/jeb.073577.
- Yildiz FH, Visick KL. 2009. Vibrio biofilms: so much the same yet so different. Trends in Microbiology. 17:109–118. doi:10.1016/j.tim.2008.12.004.
- Yoon K, Min K, Jung Y, Kwon K, Lee J, Oh S. 2008. A model of the effect of temperature on the growth of pathogenic and nonpathogenic Vibrio parahaemolyticus isolated from oysters in Korea. Food Microbiology. 25:635–641. doi:10.1016/j.fm.2008.04.007.
- Young T, Alfaro AC. 2018. Metabolomic strategies for aquaculture research: a primer. Reviews in Aquaculture. 10:26–56. doi:10.1111/raq.12146.
- Zannella C, Mosca F, Mariani F, Franci G, Folliero V, Galdiero M, Tiscar PG, Galdiero M. 2017. Microbial diseases of bivalve mollusks: infections, immunology and antimicrobial defense. Marine Drugs. 15:182. doi:10.3390/md15060182.
- Zeng Z, Wang Y, Anwar M, Hu Z, Wang C, Lou S, Li H. 2022. Molecular cloning and expression analysis of mytilin-like antimicrobial peptides from Asian green mussel Perna viridis. Fish & Shellfish Immunology. 121:239–244. doi:10.1016/j.fsi.2021.12.061.
- Zhang S, Yang Q, Eggermont M, Defoirdt T. 2023. Quorum-sensing interference in vibrios. Reviews in Aquaculture. 15:1452–1466. doi:10.1111/raq.12787.
- Zhang XH, Austin B. 2005. Haemolysins in Vibrio species. Journal of Applied Microbiology. 98:1011–1019. doi:10.1111/j.1365-2672.2005.02583.x.
- Zhang YY, Liu YX, Zhou Z, Zhou DY, Du M, Zhu BW, Qin L. 2019. Improving lipidomic coverage using UPLC-ESI-Q-TOF-MS for marine shellfish by optimizing the mobile phase and resuspension solvents. Journal of Agricultural and Food Chemistry. 67:8677–8688. doi:10.1021/acs.jafc.9b01343.
- Zilberberg C, Sereno D, Lima G, Custódio MR, Lôbo-hajdu G. 2011. Effect of mussel’s gender and size on a stress response biomarker. Water, Air, & Soil Pollution. 217:317–320. doi:10.1007/s11270-010-0589-4