ABSTRACT
In Australia, cultured greenlip abalone (Haliotis laevigata) are fed formulated diets that lack macroalgae. This has resulted in a pale lip colour in contrast to the vivid green lip colour of wild abalone. We evaluated the effects of supplementing 15% dried macroalgae meal, using either commercially available macroalgae meal (MSM) or harvested Gracilaria cliftonii, on colour change in greenlip abalone. Abalone were fed the respective diets in a three month laboratory trial and an additional four month on-farm trial. The inclusion of MSM into formulated abalone diet did not change lip, foot, or shell colour, whereas the G. cliftonii meal treatment changed lip and shell colour. The laboratory study was extended for another three months. Greenlip abalone either: (1) continued to be fed their experimental diet; or (2) the abalone were fed a diet with no dried algal meal. The colour changes observed in the first study were retained for one month, after which the colour declined. The addition of 15% G. cliftonii meal to formulated diets can be used to manipulate greenlip abalone lip and shell colour. A minimum of three months is recommended and abalone should be harvested within one month to maintain the desirable colour changes.
Introduction
Abalone production was valued at $140 million over the 2019–2020 financial year, making it one of Australia’s top five seafood commodities. A large proportion of this (84%) came from wild capture fisheries, with aquaculture production making up the remainder (ABARE Citation2022). Many of Australia’s wild catch abalone fisheries have a status of depleted or depleting with the remainder undefined (FRDC Citation2022). An increasing demand in the Asian market provides an opportunity for abalone aquaculture to fill Australia’s top three abalone export markets: Vietnam, Hong Kong and China since 2014–2015 (Savage Citation2015). Australian abalone is seen as a high-quality product in these markets (Clark Citation2004; Brown et al. Citation2008), and this perception must be maintained to achieve maximum return now and in the future. Colour, along with taste and texture, is an important influential factor often attributed to the freshness and quality of a product by the consumer (Baker and Günther Citation2004; Font-i-Furnols and Guerrero Citation2014). Abalone colour has three components of relevance to marketing: foot colour, lip colour and shell colour. In the Asian market, foot colour can distract consumer perception and consequentially the cost of the product. Typically, abalone with a lighter foot are preferred, while a darker foot is considered low quality, which can result in further processing to lighten foot colour prior to sale (Oakes and Ponte Citation1996; Brown et al. Citation2008). However, wider market research on consumer perception is these areas is relatively limited.
In the wild, abalone consume a variety of different micro and macroalgae species depending on their life stage, location and seasonal availability (Leighton and Boolootian Citation1963; Shepherd and Cannon Citation1988). Diet type can affect the shell colour in marine gastropods including abalone (Leighton Citation1961; Underwood and Creese Citation1976; Ju et al. Citation2016; Hoang et al. Citation2016). Feeding red macroalgae (Rhodophyta) to aquacultured Pacific (Haliotis discus hannai) and blackfoot (Haliotis iris) abalone influenced shell and foot colour, however, feeding brown (Phaeophyta) or green (Chlorophyta) macroalgae did not (Allen et al. Citation2006; Qi et al. Citation2010; Ju et al. Citation2016). The shell colour of wild greenlip abalone (Haliotis laevigata) can vary from green to red depending on the location and food source, where foot colour is generally light in colour with a green fringing lip and mantle (Mayfield et al. Citation2014).
In Australian abalone aquaculture systems, they are fed formulated, pelleted diets, producing greenlip abalone with pale milky lips, green/blue shells and darker feet (Hoang et al. Citation2016). As a consequence, greenlip abalone colour differs more between cultured and wild animals than in the variation among those from wild caught fisheries. Hoang et al. (Citation2016) demonstrated that feeding juvenile aquacultured greenlip abalone fresh Gracilaria cliftonii (Rhodophyta) resulted in a greener lip, redder shell and lighter coloured foot.
Unfortunately there are some limitations to the utilisation of fresh macroalgae including G. cliftonii for greenlip abalone aquaculture. These include the lower nutrient density of the macroalgae compared to formulated feeds, inconsistent supply, unsustainable wild harvesting, and biosecurity concerns by introducing pests and pathogens when feeding a live product (Bansemer et al. Citation2014). Growth performance of greenlip abalone when fed fresh or nutrient-enriched G. cliftonii (Bansemer et al. Citation2016a) was not comparable to formulated diets. For this reason, dried macroalgae meal inclusions to formulated abalone diets provide the combined nutritional benefits of fresh macroalgae and formulated feeds. For juvenile greenlip abalone, a formulated diet containing ≥ 10% dried G. cliftonii meal inclusion levels produced comparative growth performance when compared to that of formulated diets (Bansemer et al. Citation2016b). In the same study, Hoang et al. (Citation2017) demonstrated that the addition G. cliftonii meal in a formulated diet also altered abalone colour. This was demonstrated by an intensification of the green lip colour and red/brown colouration to the shell compared with abalone fed formulated diets. The results from the same study also determined that live or dried Ulva sp. (Chlorophyta) meal has no effect on abalone colour. To investigate the colour change of near-market size 3-year-old greenlip abalone lip, foot and shell, a series of three experiments were conducted using digital photographic colour analysis to progressively measure the colour change in the lip, foot and shell when fed two types of dried macroalgae meals in formulated diets.
Methods
The present study was divided into three interrelated parts:
A 3 month laboratory based trial was conducted using three-year-old greenlip abalone to evaluate the effectiveness of a commercially available mixed species macroalgae (MSM) meal incorporated into a formulated abalone diet. In addition, a 15% dried G. cliftonii meal diet was tested, as the diet had been previously proven to change the colour of greenlip abalone (Hoang et al. Citation2016, Citation2017).
As an extension of the 3 month laboratory trial, the colour changes of greenlip abalone fed G cliftonii meal were investigated by evaluating the effects of G. cliftonii meal withdrawal on the retention period of colour changes from experiment 1. This extended study did not assess MSM meal, based on the first 3 month study, as the continuation of evaluating the diet based on results obtained was deemed unnecessary.
A parallel three month on-farm feed trial was conducted using three-year-old greenlip abalone to evaluate the effectiveness of a commercially available MSM meal which had been sourced by the farm and incorporated into a formulated abalone diet. This trial was run concurrently with experiment 1. The use of G cliftonii meal was not possible for the on-farm trial, as the meal is not in commercial production.
Experiment 1: laboratory trial evaluating the effects of dried macroalgae meal on abalone colour
Laboratory experimental animals and system
Experimental animals were purchased from South Australian Mariculture (Boston Point, Port Lincoln, S.A., Australia) and maintained at the South Australian Research and Development Institute (SARDI) Aquatic Sciences (West Beach, S.A., Australia) facility. Prior to stocking experiments, abalone were held in 170 L tanks supplied with flow-through UV-treated seawater and fed Abgrow Premium 5 mm chip ad libitum. Abalone stocked in both laboratory experiments were randomly selected, individually weighed (whole wet weight ±0.01 g), measured, (shell length ±0.01 mm), then systematically transferred into the experimental system at 10 abalone per culture tank. Three tank replicates were allocated for each dietary treatment, and treatments were randomly assigned to the culture tanks. Animals were acclimated to the experimental system for one week and fed their respective diets. Water temperature was then increased by 1°C day−1 until 22°C was reached. Dead abalone were weighed, measured, recorded and replaced with abalone of similar weight. Feed rates were adjusted in response.
All laboratory experiments were conducted in a photoperiod and temperature-controlled laboratory previously described in Stone et al. (Citation2013). Briefly, abalone were maintained in one of sixteen 12.5 L blue plastic culture tanks (Nally IH305, Viscount Plastics Pty Ltd.; 39.2 × 28.8 × 11.0 cm) and were supplied with sand filtered, UV treated, flow through sea water at a rate of 300 mL min−1. Water depth was maintained at 2.5 cm (tank water volume 2.8 L) using a stand pipe with a mesh screen (0.8 mm) to retain uneaten feed. Water temperature was maintained at 22 ± 1°C using an immersion heater (240 V, 3 kW; JQ20, Austin & Cridland., Carlton, NSW, Australia) in the system sump for optimal growth of this species (Stone et al. Citation2013). The photoperiod was controlled at 12 h of low intensity fluorescent lighting at 3.4 lux (0700-1900) and 12 h of darkness for the remainder of each day.
Laboratory experimental feeding regime
Animals were fed to excess (0.8% biomass day −1) daily at ∼1500 h, and feed rates were adjusted monthly based on bulk weight checks taken during sampling. Uneaten feed was collected at 0900 h daily throughout the trial in the morning following feeding by straining the entire contents of the culture tanks through a fine screen (500 µm) while faeces were rinsed away. Collected uneaten feeds were stored at −22 °C and later dried at 105 °C for 16 h (or until constant weight).
Experimental design and diets
A 92-day feed trial was conducted at SARDI Aquatic Sciences trial to evaluate the effectiveness of dried macroalgal meal addition, in changing the colour of greenlip abalone lip, foot and shell under laboratory conditions. Greenlip abalone (3-year-old age class) were fed one of 3 diets:
a formulated diet (Abgrow Premium 5 mm chip) as a commercial control (Comm)
a 5 mm diet formulated to contain 15% G. cliftonii meal and 85% commercial diet (Abgrow Premium) (GM)
a 5 mm diet formulated to contain 15% MSM and 85% commercial diet (Abgrow Premium; ).
Table 1. Proximate composition of mixed species macroalgae (MSM) meal and Gracilaria cliftonii meal and diets used in experimental treatments.
Sampling occurred at monthly intervals (three samplings in total), where all abalone were carefully removed from the culture tanks using a spatula and placed on a pre-moistened cloth. In addition, digital photographs of the first 7 animals removed from each culture tank were taken. Animals were then returned to their respective culture tanks and monitored to ensure recovery.
Experiment 2: laboratory trial evaluating the effects of phase feeding a diet containing Gracilaria cliftonii meal on the kinetics of abalone colour change
A 175-day phase feeding trial was conducted at SARDI Aquatic Sciences to evaluate colour change in 3-year-old greenlip abalone lip, foot and shell. The phase feeding trial was conducted in two parts: part (A) months one to three (Experiment 1; conditioning phase) and part (B) months four to six (diet withdrawal phase). Three dietary treatments were used:
commercial diet (Comm)
15% G. cliftonii meal (15% dried G. cliftonii meal and 85% commercial diet (positive colour control in experiment 2) ()) (GM)
Diet switch, which involved abalone being fed the GM diet for three months followed by the Comm diet for a further 3 months (GM-S)
Each dietary treatment was made up of three replicate culture tanks. For part A, 10 abalone (n = 10) were used per replicate tank. For part B, seven abalone (n = 7) were used per replicate tank. Sampling occurred at monthly intervals (six samplings in total), where all abalone were carefully removed from the culture tanks using a spatula and placed on to a pre-moistened cloth. For part A, digital photographs were taken of the first 7 animals removed from the culture tanks. For part B, all seven animals were photographed. Animals were then returned to their respective culture tanks and monitored to ensure recovery.
Towards the end of experiment 3, dissolved oxygen (DO) levels began to drop below 75% saturation (5.8 mg L−1). To avoid growth reductions known to occur due to lower DO (Harris et al. Citation1999), water flow rates were increased from 300 mL min−1 to 350 mL min−1 in all culture tanks on day 158 of experiment 3, while feed rates were reduced from 0.8% to 0.7% BW day−1. This latter feed rate was still over what was being consumed daily.
Growth performance
Growth parameters were determined in each experiment as follows:
SGR (% day−1) = ([ln final weight − ln initial weight]/days) × 100.
Shell growth rate (μm day−1) = (final shell length − initial shell length)/days.
Biomass gain (experiments 1 and 2) (g tank−1) = (final weight + Σ mortality weight) − (initial weight + Σ replacement weight).
Biomass gain (experiment 3 only) (g tank−1) = (final weight + Σ mortality weight + removal at 3 months weight) − (initial weight + Σ replacement weight).
Feed intake
Daily feed intake was calculated by the difference in feed offered and uneaten feed, on a dry weight basis. Collected uneaten feed was stored at 20 °C and was later dried at 105 °C for 16 h. To account for leaching loss, experimental diets were added to culture tanks at 22 °C without animals at 1500 h and collected the following morning at 0900 h. The dry weight difference between feed added and feed collected was used with the leaching correction factor to calculate apparent feed intake. Feed conversion ratios in experiments two and three were calculated in each treatment using the following, as per Stone et al. (Citation2013):
Apparent feed intake = feed offered − uneaten feed collected − ([total feed offered × % leaching loss without animals] + [uneaten feed collected / % retained without animals × % leaching loss without animals])/2.
Apparent feed conversion ratio (FCR) = feed consumed/abalone weight gain.
Proximate composition
Samples were then analysed for crude protein and crude lipid by the National Measurement Institute (Melbourne, Vic., Australia) using Kjeldahl and Mojonnier extractions, respectively. Ash content was evaluated by ashing 0.5 g of tissue at 600 °C, until a constant weight was achieved. Carbohydrate content was calculated by difference (carbohydrate (%) = 100% – (protein % + lipid % + ash %)), and energy content by calculation using the values of 17.2, 23.6 and 39.5 MJ kg−1 for carbohydrate, protein and lipid (Glencross et al. Citation2012).
Laboratory water quality monitoring
Water quality parameters were measured daily. Water temperature and DO (DO; mg L−1 and % saturation) were measured using a hand held DO metre (Handy Polaris 2, Oxyguard International A/S., Birkerod, Denmark) calibrated daily in 100% water-saturated air. The pH was measured using a metre (Oakton pHtestr 20; Oakton Instruments, Vernon Hills, IL, U.S.A.). Salinity was measured using a portable salinity refractometer (model RF20, Extech Instruments, Nashua, NH, U.S.A.).
Experiment 3: on-farm trial – evaluating the effects of mixed species macroalgae meal on abalone colour
Experimental animals and system
An on-farm feed trial was run with South Seas Abalone Group at Coastal Sea Farms (Portland, Vic., Australia). Greenlip abalone (three-year-old age class) were stocked into eight concrete production raceways (∼400 kg raceway−1) supplied with flow through sea water. Each raceway was 16.1 × 2.5 m wide with a laminar flow water depth of 4.6 cm at the inlet end and 1.5 cm depth at the outlet end. During a one-month acclimation period, abalone were fed a single commercially formulated diet (Abgrow Premium, Eyre Peninsula Aquafeed (EPA) Pty. Ltd., Lonsdale, S.A., Australia).
Diets and experimental design
Prior to diet formulation the MSM was harvested from Beachport and processed into a dried (< 10% moisture) meal by Australian Kelp Products Pty. Ltd. (Beachport, S.A., Australia).
Treatment 1 – MSM diet which contained 15% MSM meal and 85% formulated abalone diet (Abgrow premium; ).
Treatment 2 – commercial control Abgrow premium by EPA.
Greenlip abalone were fed at an estimated rate of 1% total body weight per day at ∼16:00 h each afternoon using standard on-farm procedures.
To evaluate the rate and degree of colour change in greenlip abalone lip, foot and shell during on-farm production, two diets (n = 4 raceways per treatment) were fed to greenlip abalone for 137 days, beginning mid-summer and ending in winter.
Sampling was conducted on days 29, 71, 107, and 137. At each sampling event, ten abalone were randomly selected and removed from each of the eight raceways, placed into labelled mesh bags and held in a tank supplied with flow-through sea water at 16°C. Within 2 h, abalone were removed from the mesh bags and placed onto a pre-moistened cloth, then individually weighed on a balance (wet whole weight ± 0.01 g) and measured with digital callipers (shell length ± 0.01 mm). Digital photographs were taken (see Digital photography) of the lip, foot and shell to evaluate colour change. Biomass gain, Specific Growth Rate (SGR) and shell growth rate were calculated (see Growth performance).
Digital photography and image analysis
Digital photography
To analyse the colour of greenlip abalone lip, foot and leading shell edge (new growth), digital photographs were taken of experimental animals using a custom-built light table with a white surface, and two parallel white fluorescent lights mounted to either side of the table. A compact all-in-one digital camera (Panasonic Lumix DMC FZ50) was attached to an adjustable arm between the two lights. The camera height was set at 35 cm above the base of the light table. No more than four animals were placed on the light table at a time, and images were captured alongside a reference colour card (X-rite; colour checker passport, Munsell Colour Services laboratory, Rochester Institute of Technology, Rochester, NY, U.S.A.).
Image analysis
Digital image analysis was performed using Image J (Schindelin et al. Citation2012). The average colour properties red, green and blue (RGB), for areas of interest in the digital image, were converted to hue, saturation and brightness (HSB). A detailed explanation of the HSB model can be seen in Georgieva et al. (Citation2005) and Yasir and Qin (Citation2009). Briefly, hue (actual colour) can be described by its position in degrees around an inverse cone, with red at 0°, green at 120° and blue at 240°; saturation (colour intensity) ranges from 0% (no saturation; colours may appear grey at a low saturation) – 100% (fully saturated; full colour intensity); and brightness from 0% (black) – 100% (white). Both the hue and saturation become meaningless at 0% brightness.
Statistical analyses
All statistical analyses were performed using SPSS (version 23 for Windows; IBM SPSS Inc., Chicago, IL, U.S.A.). Homogeneity of variances and normality among means were tested using Levene's test of equality of error variances and Shapiro–Wilk test, respectively and transformed where necessary. A two-factor ANOVA was performed to determine the effects of treatment and time on colour properties (HSB) of abalone lip, foot and shell. Where significant main effects were found, the Student–Newman–Keuls (SNK) post hoc test was used to determine significant differences between treatment means at a significance level of P < 0.05. For growth data, one-factor ANOVA was used to determine effects between treatments and where significant effects (P < 0.05) were found, SNK post hoc analysis was used to determine the differences between treatment means. All values are represented as means ± SE unless stated otherwise.
Results
Experiment 1: laboratory trial evaluating the effects of dried macroalgae meal on abalone colour
Growth and visual observations
Initial weight (55.56 ± 0.45 g) and shell length (76.41 ± 0.19 mm) of greenlip abalone were not significantly different between treatments (P = 0.958 and P = 0.976, respectively). At the end of the trial, biomass gain and SGR were not significantly different between treatments (P = 0.104 and P = 0.064, respectively; ). Shell growth rate was significantly higher in abalone in the 15% GM treatment (P = 0.037) than both other treatments but not significantly different between the commercial and 15% MSM treatments (P = 0.727). Apparent feed intake and apparent FCR were not significantly different between treatments (P = 0.645 and P = 0.414, respectively; ). Water quality parameters were maintained at levels ideal for greenlip abalone (means ± standard deviation, range; n = 3) in terms of temperature (21.5 ± 0.31 °C, 20.1–22.5 °C), DO (83.7 ± 1.40%, 76–93.8% saturation; 7.44 ± 0.20 mg L−1, 6.6–8.5 mg L−1), pH (8.08 ± 0.10, 7.67–8.30) and salinity (35 ± 1 ppt, 34–36 ppt).
Table 2. Experiment 1 growth performance and feed utilisation for greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% Gracilaria cliftonii meal or a diet formulated to contain 85% of the commercial diet and 15% mixed species macroalgae (MSM) meal. The three-month laboratory feed trial was carried out to validate experiment 1 on farm feed trial.
As per Buss et al. (Citation2015), all animals exhibited normal feeding behaviour and fed actively within each treatment. The overall mortality for the trial was 0.86% and occurred primarily during the 2 week acclimation period at the commencement of the trial and not due to dietary treatments.
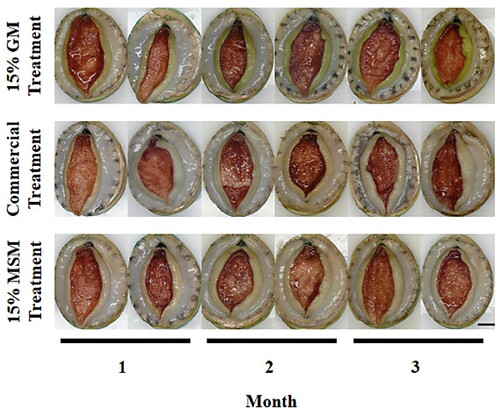
Lip colour of greenlip abalone in the Comm treatment and the MSM treatment was a milky white to light brownish/yellow (). There was no visual change in the lip colour of abalone in the Comm or MSM treatments throughout the trial. However, abalone in the Comm treatment showed a visual colour change in the lip by month one, taking on a more yellow colour which increased to yellow/green by month three (). Foot colour of greenlip abalone was deep red/brown; there was no visual change in foot colour for abalone in any of the three dietary treatments throughout the trial (). Shell colour in greenlip abalone in the GM treatment showed visual colour change after one month. The leading edge of the shell had taken on a deep red/brown colour which continued to widen with shell growth throughout the trial. Shell colour of ∼50% of the abalone in both the Comm treatment and the MSM treatment began to take on a light yellow/brown colour to the leading edge of the shell by month two with the remainder keeping the blue/green shell colour.
Figure 1. Experiment 1 lip and foot colour of greenlip abalone in either the 15% Gracilaria cliftonii meal treatment (formulated to contain 15% G. cliftonii meal (GM) and 85% of commercial diet) or the commercial treatment or the 15% MSM treatment (formulated to contain 15% mixed species macroalgae meal (MSM) and 85% of commercial diet) during a three-month laboratory feed trial. Bar = 1 cm.
Lip colour properties
There were no significant interactions for any lip colour properties (P > 0.05). For lip hue there were significant effects for treatment (P < 0.001) and time (P < 0.001). Abalone in the GM treatment significantly changed towards yellow/green compared to both the Comm and MSM treatments (P < 0.001 and P < 0.001, respectively). The MSM treatment also significantly changed lip hue value over the commercial treatment (P < 0.001). In all treatments, abalone lip hue value significantly increased (P < 0.001) from months one to two to three ().
Table 3. Experiment 1 colour properties of lip, foot and shell of greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% mixed species macroalgae (MSM) meal or a diet formulated to contain 85% of the commercial diet and 15% Gracilaria cliftonii meal during a three-month laboratory feed trial.
Lip saturation for greenlip abalone was significantly affected by treatment (P < 0.001) and time (P = 0.001). Abalone in the GM treatment increased lip saturation and were significantly different to abalone in both the Comm and MSM treatments (P < 0.001), which were not significantly different to each other (P = 0.582). In addition, lip saturation in months two and three (P = 0.219) was significantly higher than lip saturation in month one (P = 0.001; ).
Lip brightness for green lip abalone was significantly affected by treatment (P = 0.006) and time (P < 0.001). Abalone in the GM treatment had significantly lower lip brightness than abalone in both the Comm and MSM treatments (P = 0.006). Lip brightness for abalone in both the Comm and MSM treatments was not significantly different (P = 0.126). Abalone lip brightness significantly decreased from months one to two (P < 0.001) but did not change significantly between months two and three (P = 0.811; ).
Foot colour properties
There were no significant interactions for any lip colour properties (P > 0.05). Foot hue value for greenlip abalone was not affected by treatment (P = 0.982) or time (P = 0.233) For all treatments, foot hue value ranged between 14.5° and 17.5° (red/orange) ().
Foot saturation for greenlip abalone was not affected by treatment (P = 0.648) but was affected by time (P = 0.014). A significant increase in abalone foot saturation occurred from months one to two (P = 0.014), but not between months two to three (P = 0.699; ).
Foot brightness for greenlip abalone was not affected by treatment (P = 0.288) but was affected by time (P < 0.001). Abalone foot brightness significantly decreased from months one to two (P < 0.001), then significantly increased from months two to three (P < 0.001; ).
Shell colour properties
Shell hue value for greenlip abalone was significantly affected by treatment (P < 0.001) and time (P < 0.001), but there was no significant interaction (P = 0.146). Abalone shell hue value significantly changed (from green towards yellow/red) from months one to two and months two to three (P < 0.001 and P < 0.001, respectively). The degree of change was greatest in abalone in the GM treatment, which had shell hue values significantly different from abalone in both the Comm and MSM treatments (P < 0.001 and P < 0.001, respectively). Shell hue values for abalone in the Comm and MSM treatments were not significantly different (P = 0.796; ).
Shell saturation for greenlip abalone was significantly affected by treatment (P < 0.001), time (P < 0.001) and their interaction (P = 0.002). The interaction was driven by the significant increase in shell saturation values between months one and two (P < 0.001) for the GM treatment compared to both the Comm and MSM treatments, which did not significantly increase (P = 0.429 and 0.213, respectively). Shell saturation continued to increase in abalone in the GM treatment from months two to three and was significantly higher than abalone in both the Comm and MSM treatments (P < 0.001 and P < 0.001, respectively). Shell saturation values in the MSM treatment increased from months one to three and were significantly higher than values from the Comm treatment (P < 0.001; ).
Shell brightness for greenlip abalone was not affected by treatment (P = 0.375) but was affected by time (P = 0.002); there was no significant interaction (P = 0.824). Abalone shell brightness decreased significantly from months one to two (P = 0.002) but was not significantly different between months two and three (P = 0.552; ).
Experiment 2: laboratory trial evaluating the effects of phase feeding a diet containing Gracilaria cliftonii meal on the kinetics of abalone colour change
Part A: Conditioning phase
Growth and visual observations
Initial weight (55.24 ± 2.01 g) and shell length (75.81 ± 0.37 mm) of abalone were not significantly different between treatments (P = 0.809 and P = 0.076, respectively). At the end of the conditioning phase, biomass gain and SGR were not significantly different between treatments (P = 0.162 and P = 0.130; ). Shell growth rate for abalone in both the GC and GM treatments were significantly higher compared to the Comm treatment (P = 0.002 and P = 0.026, respectively). Apparent feed intake and apparent FCR were not significantly different between all treatments (P = 0.824 and P = 0.450, respectively; ). Water quality parameters were maintained at levels ideal for greenlip abalone (means ± standard deviation, range; n = 3) in terms of water temperature (21.5 ± 0.31 °C, 20.1–22.5 °C), DO (83.7 ± 1.40%, 76–93.8% saturation; 7.44 ± 0.20 mg L−1, 6.6–8.5 mg L−1), pH (8.08 ± 0.10, 7.67–8.30), and salinity (35 ± 1 ppt, 34–36 ppt).
Table 4. Growth performance and feed utilisation for greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of commercial diet and 15% Gracilaria cliftonii meal during the preconditioning phase (months one to three) of a six-month phase feeding trial. During this phase the switched treatment was identical to the 15% G. cliftonii meal.
There was no visual change in the lip colour of abalone in the Comm treatment throughout the preconditioning phase of the trial. Lip colour of greenlip abalone in the Comm treatment was a milky white to light brownish/yellow (). Abalone in both the GM-S and GM treatments showed visual changes in lip colour by month one taking on a more yellow colour which increased to yellow/green by month three (). Foot colour of greenlip abalone was deep red-brown. There was no visual colour change in foot colour for abalone in any of the three dietary treatments throughout the preconditioning phase of the trial (). Shell colour in greenlip abalone in both the GM-S and GM treatments showed visual colour change by month one. The leading edge of the shell had taken on a deep red/brown colour which continued to widen with shell growth throughout the preconditioning phase of the trial. Shell colour of some abalone in the Comm treatment began to take on a light yellow/brown colour to the leading edge of the shell by month two with the remainder keeping the blue/green colour.
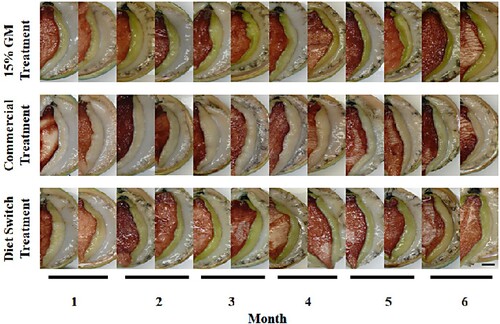
Figure 2. Experiment 2 lip colour for greenlip abalone in either the 15% Gracilaria cliftonii meal treatment (formulated to contain 15% G. cliftonii meal (GM) and 85% of commercial diet) or the commercial treatment or the diet switch treatment during a six-month phase feeding trial. During the first phase (months one to three) of the trial, the diet switch treatment was the 15% G. cliftonii meal diet, and switched to the commercial diet during the second phase (months four to six). Bar = 1 cm.
Lip colour properties
Lip hue value for abalone was significantly affected by treatment (P < 0.001), time (P < 0.001) and their interaction (P = 0.005). The interaction was driven by a significant reduction in lip hue value (away from yellow) in abalone in the Comm treatment between months two and three (P = 0.014), along with a significant increase in lip hue value (towards yellow) from months one to three in abalone in both the GM-S and GM treatments (P < 0.001 and P < 0.001, respectively). For abalone in both the GM-S and GM treatments, there was no significant difference in lip hue value (P = 0.536). Lip hue value for abalone in the Comm treatment was significantly different from abalone in both the GM-S and GM treatments (P = 0.009 and P = 0.002, respectively; ).
Table 5. Experiment 2 colour properties of lip, foot and shell of greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% Gracilaria cliftonii meal during the preconditioning phase (months one to three) of a six-month phase feeding trial. The switched treatment was identical to the 15% G. cliftonii meal during this phase.
Lip saturation for abalone was affected by treatment (P < 0.001) and time (P < 0.001) but not their interaction (P = 0.189). Abalone lip saturation increased significantly from months one to two and again from months two to three (P < 0.001 and P < 0.001, respectively). Lip saturation values for abalone in both the GM-S and GM treatments were not significantly different (P = 0.882), but both were significantly different to the Comm treatment (P < 0.001 and P < 0.001, respectively; ).
Lip brightness for abalone was affected by treatment (P = 0.028) and time (P < 0.001) but not their interaction (P = 0.411). There was a significant decrease in lip brightness from months one to two (P < 0.001), but not between months two and three (P = 0.946). Lip brightness in abalone in the Comm treatment was not significantly different from abalone in the GM-S treatment (P = 0.079) but was significantly different from abalone in the GM treatment (P = 0.028). Lip brightness in abalone in the GM treatment was not significantly different from abalone in the GM-S treatment (P = 0.303; ).
Foot colour properties
There were no significant interactions for any foot colour properties (P > 0.05). Foot hue value for abalone was not significantly affected by treatment (P = 0.120) or time (P = 0.123). Foot hue value remained between 14.5° and 19° (red/orange) for all treatments during the preconditioning phase ().
Foot saturation for abalone was not affected by treatment (P = 0.576) but was significantly affected by time (P = 0.020). Foot saturation increased significantly from months one to two (P = 0.020), but not from months two to three (P = 0.471; ).
Foot brightness for abalone was not affected by treatment (P = 0.104) but was significantly affected by time (P < 0.001). There was a significant decrease in foot brightness from months one to two (P < 0.001), followed by a significant increase in foot brightness from months two to three (P < 0.001). Abalone foot brightness was significantly lower at month three compared to month one (P < 0.001; ).
Shell colour properties
Shell hue value for greenlip abalone was significantly affected by treatment (P = 0.002) and time (P < 0.001) but not their interaction (P = 0.380). Abalone shell hue value significantly decreased (from green towards yellow/red) from months one to two (P < 0.001) and from months two to three (P < 0.001). The degree of change in shell hue value was greatest in abalone in both the GM-S and GM treatments, which were significantly different to abalone in the Comm treatment (P = 0.002). Shell hue value for abalone in the GM treatment and the GM-S treatment were not significantly different (P < 0.647; ).
Shell saturation for abalone was significantly affected by treatment (P < 0.001), time (P < 0.001) and their interaction (P = 0.004). The interaction was driven by a significant increase in shell saturation in both the GM and the GM-S treatments from months one to three (P < 0.001 and < 0.001, respectively). Abalone shell saturation did not change from months one to three in the Comm treatment (P = 0.477). Shell saturation in abalone fed the GM and the GM-S treatments significantly differed from abalone in the Comm treatment (P = 0.002 and P = 0.009, respectively). There was no significant difference in shell saturation for abalone in either the GM or the GM-S treatments (P < 0.536; ).
Shell brightness for abalone was not affected by treatment (P = 0.631) but was affected by time (P = 0.003); there was no interaction (P = 0.929). There was a significant decrease in shell brightness from months one to two (P = 0.003), but not from months two to three (P < 0.778; ).
Part B: Diet switch phase
Growth and visual observations
Initial weight (74.00 ± 1.74 g) and shell length (80.63 ± 0.56 mm) of abalone were not significantly different between treatments (P = 0.360 and P = 0.357, respectively). At the end of the diet switch phase, biomass gain, SGR and shell growth rate were not significantly different between treatments (P = 0.097, P = 0.194 and P = 0.295, respectively). Apparent intake and apparent FCR were not significantly different between treatments (P = 0.143 and P = 0.116, respectively; ). Water quality parameters were maintained at levels ideal for greenlip abalone (means ± standard deviation, range; n = 3) in terms of water temperature (21.7 ± 0.05 °C, 21.3–22.1 °C), DO (84.56 ± 1.36%, 74.7–89.7% saturation; 6.25 ± 0.18 mg L−1, 5.8–7.9 mg L−1), pH (8.08 ± 0.11, 7.92– 8.36), and salinity (35 ± 1 ppt, 34–36 ppt).
Table 6. Growth performance and feed utilisation for greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of commercial diet and 15% Gracilaria cliftonii meal during the second phase (months four to six) of a six-month phase feeding trial. During this phase the switched treatment was identical to the commercial diet.
All animals continued to exhibit normal feeding behaviour as per Buss et al. (Citation2015). Abalone in the GM-S treatment accepted the change in feed. There were no mortalities.
Lip and foot colour of abalone in the Comm treatment did not visually change over the six-month phase feeding trial. Shell colour along the leading edge of abalone in the Comm treatment changed from blue/green to a light yellow/brown colour. Lip colour for abalone in the GM treatment continued to increase in colour, becoming greener each month (). Lip colour for abalone in the GM-S treatment was not visually distinguishable from abalone in the GM treatment until month five and appeared to be maintaining a yellow/green colour. By month six, the lip of abalone in the GM-S treatment had a more yellow than green colour (). The leading edge of the shell of abalone in the GM treatment continued to take on a deep red colour. For abalone in the GM-S treatment the leading edge of the shell began to take on a lighter yellow/brown colour. This was noticeable by month four, one month after switching diets, and shell colour continued to lighten between months five and six. Changes in abalone foot colour were not visually distinguishable between treatments.
Growth and feed utilisation over six months
After six months, biomass gain, SGR, and shell growth rate were significantly higher for abalone in the GM treatment than abalone in the Comm treatment (P = 0.031, P = 0.049 and P = 0.037, respectively; ). Biomass gain and SGR for abalone in the GM-S treatment were not significantly different from abalone in the Comm treatment (P = 0.067 and P = 0.267, respectively), but did have a significantly higher shell growth rate (P = 0.030; ). Biomass gain, SGR and shell growth rate for abalone in the GM treatment were not significantly different to abalone in the GM-S treatment (P = 0.228, P = 0.098 and P = 0.777, respectively; ). There was no significant difference in apparent feed intake or apparent FCR between treatments (P = 0.611 and P = 0.106, respectively; ).
Table 7. Growth performance and feed utilisation for greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% Gracilaria cliftonii meal during a six month phase feeding trial. During the first phase (months one to three) of the trial, the diet switch treatment was in the 15% G. cliftonii meal diet and switched to the commercial diet during the second phase (months four to six).
Lip colour properties
Lip hue value for abalone was significantly affected by treatment (P < 0.001) and time (P = 0.020) but not their interaction (P = 0.057). Lip hue value did not significantly change from months three to five (P = 0.728) but significantly changed by month six (P = 0.020). Lip hue value for abalone in the GM treatment was significantly different (towards yellow/green) than the Comm and the GM-S treatments (P < 0.001 and P < 0.001, respectively). Abalone in the GM-S treatment had a significantly different lip hue value (towards yellow/green) to abalone in the Comm treatment (P < 0.001; ).
Table 8. Experiment 2 colour properties of lip, foot and shell of greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of commercial diet and 15% Gracilaria cliftonii meal during the diet switch phase (months four to six) of a six month phase feeding trial. During this phase the switched treatment was identical to the commercial diet treatment.
Lip saturation for abalone was significantly affected by treatment (P < 0.001), time (P = 0.030) and their interaction (P = 0.013). The interaction was driven by a reduction in the lip saturation of abalone in the GM-S treatment. Abalone maintained increased lip saturation for one month after the diet switch, with lip saturation level not significantly different from abalone in the GM treatment (P = 0.542) at month four. By month five, abalone in the GM-S treatment had a significantly reduced lip saturation compared with month four (P = 0.004) and was significantly less than abalone in the GM treatment (P = 0.006), but not significantly different from abalone in the Comm treatment by month six (P = 0.147). Abalone in the GM treatment continued to increase in lip saturation to month six and had significantly greater saturation levels than at month three (P = 0.006). Abalone in the GM treatment had significantly greater lip saturation than abalone in the Comm treatment (P < 0.001; ).
Lip brightness for abalone was not significantly affected by time (P = 0.165) but was significantly affected by treatment (P < 0.001) and their interaction (P = 0.001). The interaction was driven by an increase in lip brightness for abalone in the GM-S treatment. Abalone maintained lip brightness level for one month after the diet switch that was not significantly different to abalone in the GM treatment at month four (P = 0.869). By month five, abalone in the GM-S treatment had significantly increased lip brightness compared with month four (P = 0.004). Lip brightness also significantly increased compared to abalone in the GM treatment (P = 0.005) but was not significantly different to abalone in the Comm treatment (P = 0.503). By month six, abalone in the GM-S treatment had significantly increased lip brightness compared to abalone in both the GM and Comm treatments (P = 0.001 and P = 0.004, respectively). Lip brightness for abalone in the GM treatment was significantly lower compared to abalone in the C treatment (P < 0.001; ).
Foot colour properties
Foot hue value for abalone was significantly affected by treatment (P = 0.001) but was not affected by time (P = 0.249) or their interaction (P = 0.452). Foot hue value for abalone in both the Comm and GM meal treatments was not significantly different (P = 0.101). Foot hue value for abalone in the GM-S treatment was significantly different compared to abalone in both the C and constant GM treatments (P < 0.001 and P = 0.016, respectively; ). For all treatments, foot hue value ranged between 15 and 18 ° (red).
Foot saturation for abalone was not significantly affected by treatment (P = 0.248), time (P = 0.262) or their interaction (P = 0.424). All treatments’ foot saturations ranged between 62% and 72% ().
Foot brightness for abalone was significantly affected by treatment (P = 0.032) but was not significantly affected by time (P = 0.707) or their interaction (P = 0.386). Abalone in the GM-S treatment significantly increased foot brightness over abalone in both the Comm and GM treatments (P = 0.032). Foot brightness in abalone in the Comm treatment and GM treatment were not significantly different (P = 0.711; ).
Shell colour properties
Shell hue value for abalone was significantly affected by treatment (P < 0.001) and time (P = 0.002) but not by their interaction (P = 0.138). There was a significant change in shell hue value from months three to four (P = 0.002), but not between months four to six (P = 0.882). Shell hue value for abalone in the GM treatment was significantly different to abalone in both the Comm and the GM-S treatments (P < 0.001 and P < 0.001, respectively). Abalone in the Comm treatment had a significantly different shell hue value to abalone in the GM-S treatment (P < 0.001; ).
Shell saturation for abalone was significantly affected by the treatment (P < 0.001), time (P < 0.001) and their interaction (P = 0.002). The interaction was driven by a continued increase in shell saturation for abalone in both the Comm and GM treatments from months three to six. There was no significant change in shell saturation for abalone in the GM-S treatment between months three and six (P = 0.389). At months three and four there was a significant difference in shell saturation between abalone in the GM-S and the C treatment (P < 0.001 and P = 0.009, respectively), but not the GM treatment (P = 0.473 and P < 0.219, respectively). At months five and six, shell saturation for abalone in the GM-S treatment was significantly lower than in the GM treatment (P = 0.001 and P < 0.001, respectively), but not significantly different from abalone in the Comm treatment (P = 0.746 and P = 0.800, respectively). Abalone in the Comm treatment had significantly lower shell saturation than abalone in the constant GM treatment (P < 0.001; ).
Shell brightness for abalone was significantly affected by treatment (P < 0.001) but was not affected by time (P = 0.119) or their interaction (P = 0.485). Abalone in the GM treatment had significantly lower shell brightness than abalone in both the Comm and the GM-S treatments (P < 0.001 and P < 0.001, respectively). Shell brightness in abalone in the GM-S treatment was not significantly different to abalone in the Comm treatment (P = 0.455; ).
Experiment 3: on-farm trial evaluating the effects of mixed species macroalgae meal on abalone colour
Growth and visual observations
Initial weight (55.8 ± 2.40 g) and shell length (73.9 ± 0.99 mm) of greenlip abalone were not significantly different between treatments (P = 0.986 and P = 0.952, respectively). At the end of the experiment, biomass gain, SGR, and shell growth rate of greenlip abalone in the MSM treatment were not significantly different to the Comm treatment (P = 0.670, P = 0.482 and P = 0.132, respectively; ). At all four sampling events, abalone appeared in good health and with visual signs of growth. Any colour change in greenlip abalone lip, foot and shell could not be visually distinguished between treatments; visual lip colours ranged from opaque light green/yellow though to more greyish greens and blues (). Foot colour ranged from lighter tan browns to deeper red/brown (). Old growth areas of the shells had high levels of biofouling; new shell growth along the leading edge was free of biofouling and mainly blue to green in colour.
Figure 3. Experiment 3 lip and foot colour of greenlip abalone in either the commercial treatment or the 15% MSM treatment (formulated to contain 15% mixed species macroalgae meal (MSM) and 85% of commercial diet) during a four-month on-farm feed trial. Bar = 1 cm.
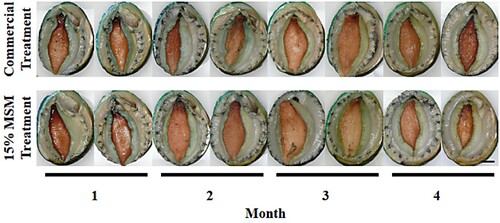
Table 9. Experiment 3 growth performance for greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% mixed species macroalgae (MSM) meal in a four-month on-farm feed trial.
Lip colour properties
There were no significant interactions for any lip colour properties (P > 0.05). Lip hue value for greenlip abalone was significantly affected by both treatment (P = 0.036) and time (P < 0.001). Lip hue values were lower in the MSM treatment compared to the Comm treatment. After month two, lip hue values in abalone in both treatments began to decrease towards yellow so that by months three and four, lip hue value was significantly different from months one and two (P < 0.001; ).
Table 10. Experiment 3 colour properties of lip, foot and shell of greenlip abalone fed either a commercial diet or a diet formulated to contain 85% of the commercial diet and 15% mixed species macroalgae (MSM) meal during a four-month on farm feed trial.
Lip saturation for greenlip abalone was not significantly affected by treatment (P = 0.185) however there was a significant effect of time (P < 0.001). Abalone lip saturation was not significantly different from months one to three (P = 0.225) but did significantly increase at month four (P < 0.001; ).
Lip brightness for greenlip abalone was not significantly affected by treatment (P = 0.568) but was significantly affected by time (P < 0.001). Abalone lip brightness in month one was significantly lower than three (P < 0.001), whereas lip brightness in month four decreased so that it was significantly different from month three (P < 0.001) but not significantly different from months one or two (P = 0.366 and P = 0.106, respectively; ).
Foot colour properties
There were no significant interactions for any lip colour properties (P > 0.05). Foot hue values for greenlip abalone were not significantly affected by treatment (P = 0.273), but there was a significant effect of time (P < 0.001). Foot hue values were significantly higher at months three and four (moving towards yellow) compared with months one and two (P < 0.001; ).
There were no significant effects of treatment (P = 0.108) or time (P = 0.889) on greenlip abalone foot colour saturation ().
Foot brightness for greenlip abalone was not significantly affected by treatment (P = 0.379), however there was a significant effect for foot brightness over time (P = 0.004). At month three, foot brightness increased significantly compared with months one, two and four (P = 0.004; ).
Shell colour properties
Shell hue value for greenlip abalone was not significantly affected by treatment (P = 0.092) but was significantly affected by time (P = 0.007). There was a significant change in hue values between months two and three, moving towards yellow from green (P = 0.007). There was no significant interaction for shell hue (P = 0.962; ).
Shell saturation for greenlip abalone was not significantly affected by treatment (P = 0.230) or time (P = 0.462) however there was a significant interaction (P = 0.016). The interaction was driven by the 4.9% decrease in shell saturation in the Comm treatment and a 4.6% increase in shell saturation in the MSM treatment ().
There was no significant interaction (P = 0.645) or effect of treatment (P = 0.107) on the shell brightness of greenlip abalone, but there was an effect of time (P = 0.002). Abalone maintained relatively stable shell brightness over the first three months, and treatments were not significantly different between months (P = 0.593). However, by month four, shell brightness had significantly decreased from month three (P = 0.002; ).
Discussion
The colour change kinetics of greenlip abalone were evaluated in three experiments based on previous studies that show the addition of macroalgae to diets of farmed abalone can result in a visible colour change to the lip (Hoang et al. Citation2016, Citation2017), foot (Allen et al. Citation2006; Hoang et al. Citation2016) and shell (Leighton Citation1961; Gallardo et al. Citation2003; Ju et al. Citation2016). This produces colour characteristics in farmed abalone closer in appearance to those from wild capture fisheries, which may better suit consumer preference and be beneficial for marketing (Oakes and Ponte Citation1996; Brown et al. Citation2008).
In experiment one, abalone in the GM treatment showed significant differences from abalone in the MSM and Comm treatments. At month one there was a 13.9% shift in lip hue value towards yellow, along with a 10.4% increase in lip colour saturation over the Comm treatment. By month three, lip hue value further increased by 21.1% over the Comm treatment and a 23.1% increase in lip colour saturation. This translated to a visual colour change, towards yellow by month one, and an intensification of lip colour, towards yellow/green by month three. These changes distinguished the abalone in the GM treatment from those in the Comm and MSM treatments. This finding agrees with results obtained Hoang et al. (Citation2016) and Hoang et al. (Citation2017), where greenlip abalone fed fresh G. cliftonii and G. cliftonii meal inclusions ≥ 10% also developed greener lip colour. There was also no visual difference in foot colour for greenlip abalone in the GM treatment and the MSM and Comm treatments. However, for shell colour there was a change from green/blue towards red, similar to previous findings with greenlip abalone fed dried G. cliftonii meal inclusions ≥ 10% in formulated feeds (Hoang et al. Citation2017).
In experiment two, abalone lip hue began to decrease by an average of 2.6% per month from months three to six, when the abalone were switched from the GM diet, back to a commercial diet (GM-S treatment). Notably during the three month period lip hue did not return to the same level as seen in the Comm treatment. A visual change in lip colour was not discernible between abalone in the GM treatment and abalone in the GM-S treatment until month five. At this point in time the lip colour saturation of abalone in the diet switch treatment had begun to reduce by an average of 8.25% per month, from months four to six. Abalone in the GM treatment peaked in lip hue value of 47.1 ± 0.58 ° (slightly below yellow at 60 °) at month three and decreased by 4.4% between months four and six. This suggests that a maximum change in lip hue towards green may be achieved in greenlip abalone after three months of being fed a formulated feed containing a 15% G. cliftonii meal inclusion. However, the peak lip hue value for this trial at three months was less than that achieved by Hoang et al. (Citation2017) of 52.5 ± 1.63 ° for one-year-old greenlip abalone with a 10% G. cliftonii meal inclusion to a formulated feed. However, in the current study abalone in the GM treatment continued to increase lip colour saturation by an average of 6% per month from months four to six, making them visually distinguishable from abalone in the GM-S treatment. By month six, abalone in the GM treatment had 20.3% greater lip colour saturation than abalone in the GM-S treatment and 26.7% greater lip colour saturation than abalone in the Comm treatment. Therefore, the visual difference in greenlip abalone lip colour in this trial is more likely the result of differences in lip colour saturation rather than differences in lip hue.
Foot colour for abalone in the GM-S treatment had slight but significant increases in hue and brightness after the switch to the commercial diet. However, there was no significant change in foot colour saturation between the treatments, and a visual colour change was not discernible. In H. iris the addition of Gracilaria sp. particles to stimulate feeding resulted in a darkening of the foot compared to abalone fed only the formulated diet (Allen et al. Citation2006). In greenlip abalone feeding fresh Ulva sp. or G. cliftonii resulted in a lightening of foot colour compared to formulated diets (Hoang et al. Citation2016). However, feeding dried Ulva sp. or G. cliftonii meal inclusions did not affect foot colour compared to formulated diets (Hoang et al. Citation2017). The lack of change noted in terms of foot colour in these experiments suggest that manipulation of foot colour in this species requires a different approach. It is also clear that there is flexibility in ingredient manipulation in terms of impact on foot colour.
Shell colour for all treatments reduced in hue value over the six-month trial with a visual change in colour in all treatments. Abalone in the Comm treatment took on a light yellow/brown colour; in contrast to juvenile greenlip abalone (Hoang et al. Citation2017) and Haliotis asinina (Gallardo et al. Citation2003) fed formulated diets which maintained green/blue shell colour. In contrast, H. discus hannai developed abnormally pink colour shells when fed two different formulated diets (Ju et al. Citation2016). One of the diets contained a pigment supplement, suggesting that this dietary pigment was not driving shell pigmentation. In the current study abalone in the GM treatment took on a darker red/brown shell colour. For abalone in the GM-S treatment, there were two distinct colour bands on the leading edge of the shell. Firstly, a dark red/brown colour developed while being in the GM treatment, and secondly, a light yellow/brown colour developed after abalone was switched to the Comm treatment. It is clear that further examination of formulated abalone diets is required to produce a commercially relevant change of shell colour in greenlip abalone.
It has been previously established that abalone fed formulated diets, brown macroalgae or green macroalgae including Laminaria sp., Macrocystis sp. or Ulva sp. produce greenish/blue coloured shells. Abalone fed red macroalgae including Palmaria sp., Porphyra sp., Gracilaria sp. or Gracilariopsis sp. produce shells ranging from brown to red depending on abalone species (Leighton Citation1961; Bautista-Teruel and Millamena Citation1999; Gallardo et al. Citation2003; Qi et al. Citation2010). For H. discus hannai fed fresh Gracilariopsis bailinae a red shell colour was produced, but when fed brown or green macroalgae a blue/green shell colour was produced; when fed a formulated diet which also contained G. bailinae a blue/green shell colour was still produced (Gallardo et al. Citation2003). This may have been caused by how G. bailinae was utilised in the formulated diet, as a binding agent rather than a dietary inclusion to induce colour change (Bautista-Teruel and Millamena Citation1999). Hoang et al. (Citation2017) showed that for greenlip abalone inclusions of G. cliftonii meal ≥ 10% are required to produce a significant change in shell colour. Therefore, the inclusion level of G. bailinae used as a feed binding agent for H. discus hannai is likely too low to produce a red-coloured shell (Gallardo et al. Citation2003). In Haliotis tuberculata, feeding with fresh red macroalgae resulted in brown shells, and feeding with brown and green macroalgae resulted in green shells (Marchais et al. Citation2017), except one species of brown macroalgae Laminaria hyperborea which produced a shell colour the same as H. tuberculata fed Gracilaria sp. (Marchais et al. Citation2017). In this study, the MSM used was harvested from the beach in Beachport, South Australia and contained a mixture of brown, red and green macroalgae (PIRSA Citation2014). It is quite possible that the proportion of red macroalgae in the MSM inclusion to the formulated diet was insufficient to produce a colour change in greenlip abalone compared to that of the G. cliftonii meal.
The biomass gain, SGR and shell growth rate of abalone was not negatively affected by the addition of 15% MSM to a commercial diet formulation in experiment three. Shell growth rate was significantly improved in abalone in the GM treatment over the equivalent Comm treatment and the MSM treatments, whereas neither apparent FCR nor apparent feed intake was different between treatments. The difference in growth performance of abalone in the MSM and Comm treatments compared to GM treatments may be due to several factors. These include the difference in carbohydrates, fatty acid and amino acid profiles (Mercer et al. Citation1993) and the use of protein-enriched G. cliftonii meal in experiments one and two. This increases the protein level of the macroalgae and results in better growth performance in greenlip abalone fed enriched compared to non-enriched macroalgae (Bansemer et al. Citation2016a). In experiment one, greenlip abalone in the GM treatment had a 48% increase in biomass gain and significant improvements in both SGR and shell growth rate over six months compared to abalone in the Comm treatment. Abalone in the GM-S treatment had numerically higher biomass gain, SGR and shell growth rate but was not significantly different to abalone fed either the GM treatment or the Comm treatment over six months. Neither apparent FCR nor apparent feed intake was different between treatments.
During experiments two and three, similar results were seen in regards to lip hue change for abalone in the MSM treatment. On-farm abalone lip hue increased towards green by 19%; while in the laboratory trial, it increased by 4.8%. In both cases, the change in lip hue was met with a corresponding low lip colour saturation. This means that although there was a significantly detectable shift in lip hue for abalone in the MSM treatment, a change in lip colour was not visually discernible between the treatments. An important aspect of abalone visual appeal to consumers is the foot colour; a lighter-pigmented foot being preferred over a darker-pigmented foot (Oakes and Ponte Citation1996; Brown et al. Citation2008). In both experiments two and three, abalone in the MSM treatments maintained foot hue between 21 and 25 °, placing the colour in the red/orange range. With a foot colour saturation of > 60%, the foot colour remained a deep red/brown colour. Allen et al. (Citation2006) also demonstrated that H. iris had darker foot colour when Gracilaria sp. particles were used to stimulate feeding.
To improve colour characteristics, for standardisation and marketability of aquacultured greenlip abalone prior to harvest, the inclusion of 15% MSM in formulated diets was not successful and cannot be recommended. In both on-farm and laboratory trials, there was no visual change in lip, foot or shell colour compared to currently formulated diets. The use of G. cliftonii meal inclusions to formulated diets, however, provides an avenue for improved colour characteristics and standardisation of aquacultured greenlip abalone. At a 15% inclusion level to a formulated diet there were significant visual changes in abalone lip hue value (towards yellow/green) and a monthly increase in lip colour saturation resulting in an intensification of the green lip colour. This trend would likely have continued as long as abalone were fed this diet. Returning greenlip abalone to a commercially formulated diet after three months resulted in the gradual loss of colour from one month after the diet switch, with a significant change in lip hue (away from yellow/green) and a reduction in lip colour saturation. Foot colour however was not affected by the 15% G. cliftonii meal inclusion and thus does not address the problem of altering abalone foot colour to meet the demands of an expanding Asian market. Increasing the inclusion level may solve this, but further trials would need to be conducted to confirm this approach. There are potential issues with diet water stability that would need to be addressed.
In conclusion, the MSM meal is not recommended to improve abalone colour characteristics. However, it does show potential as a bulk ingredient if economically viable. For colour manipulation of these animals, a minimum of 15% G. cliftonii meal to formulated diets can be fed to greenlip abalone to improve lip colour characteristics. A minimum of three months will see a significant improvement over currently formulated diets. However, the longer abalone are on the GM diet, the greater the lip colour improvement will be. Greenlip abalone should then be harvested within one month if the GM diet is stopped to maintain the maximum colour gain from the diet.
Acknowledgements
The authors would like to thank SARDI, Flinders University and Marine Innovation Southern Australia for their financial contribution towards this research. Thanks also to South Seas Abalone Group and Coastal Sea Farms for their hospitality and providing samples for the project. We would also like to thank and Joel Scanlon of Aquafeeds Australia for providing ingredients and manufacturing the diets, and Dr. Thomas Coote and Kym Heidenreich of Eyre Peninsula Aquafeeds for assistance in the manufacture and supply of abalone feed and ingredients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- ABARE. 2022. Australian fisheries and aquaculture statistics 2020. Australian Bureau of Agriculture and Resource Economics. [accessed 2022 March 10]. https://daff.ent.sirsidynix.net.au/client/en_AU/search/asset/1032481/2.
- Allen VJ, Marsden ID, Ragg NL, Gieseg S. 2006. The effects of tactile stimulants on feeding, growth, behaviour, and meat quality of cultured blackfoot abalone, Haliotis iris. Aquaculture. 257:294–308. doi:10.1016/j.aquaculture.2006.02.070.
- Baker R, Günther C. 2004. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends in Food Science & Technology. 15:484–488. doi:10.1016/j.tifs.2004.04.0094.
- Bansemer MS, Qin JG, Harris JO, Duong DN, Currie KL, Howarth GS, Stone DAJ. 2016b. Dietary inclusions of dried macroalgae meal in formulated diets improve the growth of greenlip abalone (Haliotis laevigata). Journal of Applied Phycology. 28:3645–3658. doi:10.1007/s10811-016-0829-0.
- Bansemer MS, Qin JG, Harris JO, Duong DN, Hoang TH, Howarth GS, Stone DAJ. 2016a. Growth and feed utilisation of greenlip abalone ( Haliotis laevigata ) fed nutrient enriched macroalgae. Aquaculture. 452:62–68. doi:10.1016/j.aquaculture.2015.10.025.
- Bansemer MS, Qin JG, Harris JO, Howarth GS, Stone DAJ. 2014. Nutritional requirements and use of macroalgae as ingredients in abalone feed. Reviews in Aquaculture. 5:1–15.
- Bautista-Teruel MN, Millamena OM. 1999. Diet development and evaluation for juvenile abalone, Haliotis asinina: protein/energy levels. Aquaculture. 178:117–126. doi:10.1016/S0044-8486(99)00121-0.
- Brown MR, Sikes AL, Elliott NG, Tume RK. 2008. Physicochemical factors of abalone quality: a review. Journal of Shellfish Research. 27:835–842. doi:10.2983/0730-8000(2008)27[835:PFOAQA]2.0.CO;2.
- Buss JJ, Jones DA, Lumsden A, Harris JO, Bansemer MS, Stone DAJ. 2015. Restricting feed rate has more effect than diet type on the feeding behaviour of greenlip abalone Haliotis laevigata. Marine and Freshwater Behaviour and Physiology. 48(1):51–70. doi:10.1080/10236244.2014.990701.
- Clark S. 2004. Understanding pressures on fishery resources through trade statistics: a pilot study of four products in the Chinese dried seafood market. Fish and Fisheries. 5:53–74. doi:10.1111/j.1467-2960.2004.00137.x.
- Font-i-Furnols M, Guerrero L. 2014. Consumer preference, behavior and perception about meat and meat products: an overview. Meat Science. 98:361–371. doi:10.1016/j.meatsci.2014.06.025.
- FRDC. 2022. Status of Australian fish stocks. Fisheries Research and Development Corporation. [accessed 2022 March 10]. https://www.fish.gov.au/report/284-Greenlip-Abalone-2020.
- Gallardo WG, Bautista-Teruel MN, Fermin AC, Marte CL. 2003. Shell marking by artificial feeding of the tropical abalone Haliotis asinina Linne juveniles for sea ranching and stock enhancement. Aquaculture Research. 34:839–842. doi:10.1046/j.1365-2109.2003.00890.x.
- Georgieva L, Dimitrova T, Angelov N. 2005. RGB and HSV colour models in colour identification of digital traumas images. International Conference on Computer Systems and Technologies 12(1). Varna, Bulgaria: Technical University.
- Glencross B, Blyth D, Tabrett S, Bourne N, Irvin S, Anderson M, Fox-Smith T, Smullen R. 2012. An assessment of cereal grains and other starch sources in diets for barramundi (Lates calcarifer) - implications for nutritional and functional qualities of extruded feeds. Aquaculture Nutrition. 18:388–399. doi:10.1111/j.1365-2095.2011.00903.x.
- Harris JO, Maguire GB, Edwards SJ, Johns DR. 1999. Low dissolved oxygen reduces growth rate and oxygen consumption rate of juvenile greenlip abalone, Haliotis laevigata Donovan. Aquaculture. 174:265–278. doi:10.1016/S0044-8486(99)00022-8.
- Hoang TH, Qin JG, Stone DAJ, Harris JO, Duong DN, Bansemer MS. 2016. Colour changes of greenlip abalone (Haliotis laevigata Donovan) fed fresh macroalgae and dried algal supplement. Aquaculture. 456:16–23. doi:10.1016/j.aquaculture.2016.01.022.
- Hoang TH, Stone DAJ, Duong ND, Bansemer MS, Harris JO, Qin JG. 2017. Colour change of greenlip abalone (Haliotis laevigata Donovan) fed formulated diets containing graded levels of dried macroalgae meal. Aquaculture. 468:278–285. doi:10.1016/j.aquaculture.2016.10.027.
- Ju ZY, Viljoen C, Hutchinson P, Reinicke J, Horgen FD, Howard L, Lee CS. 2016. Effects of diets on the growth performance and shell pigmentation of Pacific abalone. Aquaculture Research. 47:4004–4014. doi:10.1111/are.12851.
- Leighton D, Boolootian RA. 1963. Diet and growth in the black abalone, Haliotis cracerodii. Ecology. 44:227–238. doi:10.2307/1932170.
- Leighton DL. 1961. Observations of the effect of diet on shell coloration in the red abalone, Haliotis rufescens Swainson. Veliger. 4:29–32.
- Marchais V, Jolivet A, Hervé S, Roussel S, Schöne BR, Grall J, Chauvaud L, Clavier J. 2017. New tool to elucidate the diet of the ormer Haliotis tuberculata (L.): Digital shell colour analysis. Marine Biology. 164:71–84. doi:10.1007/s00227-017-3103-3.
- Mayfield S, Miller KJ, Mundy CN. 2014. Towards understanding greenlip abalone population structure. Final report to the Fisheries Research and Development Corporation. Prepared by the South Australian research and development institute (aquatic sciences), Adelaide. FRDC Project No. 2010/013. p. 31.
- Mercer JP, Mai KS, Donlon J. 1993. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata Linnaeus and Haliotis discus hannai Ino. 1. Effects of algal diets on growth and biochemical composition. Invertebrate Reproduction & Development. 23:75–88. doi:10.1080/07924259.1993.9672298.
- Oakes FR, Ponte RD. 1996. The abalone market: opportunities for cultured abalone. Aquaculture. 140:187–195. doi:10.1016/0044-8486(95)01189-7.
- PIRSA. 2014. South Australian beach-cast seagrass and marine algae fishery assessment. Primary Industries and Regions South Australia. Adelaide. [accessed 2017 October 16]. https://www.environment.gov.au/system/files/pages/63ba70de-cdd0-4c55-8d3c-54200dc93fcd/files/sa-beach-cast-mini-assessment-report-2014.pdf.
- Qi Z, Liu H, Li B, Mao Y, Jiang Z, Zhang J, Fang J. 2010. Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus hannai Ino. Aquaculture. 300:189–193. doi:10.1016/j.aquaculture.2010.01.019.
- Savage J. 2015. Australian fisheries and aquaculture statistics 2015, Fisheries Research and Development Corporation project 2016-246. Canberra: ABARES.
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9:676–682. doi:10.1038/nmeth.2019.
- Shepherd SA, Cannon J. 1988. Studies on the southern Australian abalone (genus Haliotis) X. Food and feeding of juveniles. Journal of the Malacological Society of Australia. 9:21–26. doi:10.1080/00852988.1988.10673997.
- Stone DAJ, Harris JO, Wang H, Mercer GJ, Schaefer EN, Bansemer MS. 2013. Dietary protein level and water temperature interactions for greenlip abalone, Haliotis laevigata. Journal of Shellfish Research. 32:119–130. doi:10.2983/035.032.0118.
- Underwood AJ, Creese RG. 1976. Observations on the biology of the trochoid Gastropod Austrocochlea constricta (Lamarck) (Prosobranchia). II. The effects of available food on shell-banding pattern. Journal of Experimental Marine Biology and Ecology. 23:229–240. doi:10.1016/0022-0981(76)90022-8.
- Yasir I, Qin JG. 2009. Effect of light intensity on colour performance of false clownfish, Amphiprion ocellaris Cuvier. Journal of the World Aquaculture Society. 40:337-350. doi:10.1111/j.1749-7345.2009.00254.x
