ABSTRACT
Estuaries are important recruitment sites for many fish species, with structurally-complex habitats such as subtidal seagrass beds on soft sediments particularly valuable due to their provision of food and shelter. Soft sediments dominate most estuaries, but where hard-bottom reefs are present they have the potential to act as nursery habitats for juvenile fish. We document recruitment of juvenile Australasian snapper (Chrysophrys auratus) to a shallow linear sandstone reef in Whangateau Harbour, a small estuary in north-eastern New Zealand. Up to several hundred 0 + juveniles (∼20–70 mm fork length) were present on the reef during early summer to mid-late autumn each year from 2018 to 2021. Numbers were consistently highest at the seaward end of the reef, facing into incoming tidal currents, and lowest in the deeper central part of the reef, where larger, potentially cannibalistic, snapper were common. Juveniles disappeared from the reef fairly abruptly at the end of each season, with departure in at least two of the years appearing to follow a sudden decrease in water temperature rather than being associated with body size or absolute temperature. Although relatively small in area, estuarine reefs may be increasingly important nursery habitats in the future given the widespread loss of subtidal seagrass beds.
Introduction
Estuaries are important nurseries for many New Zealand fish species (Francis et al. Citation2005; Morrison et al. Citation2014a, Citation2014b, Citation2016; Jones et al. Citation2015). Due to extensive catchment modification by humans in the last one to two hundred years, with native forest cleared, and extensive development of rural and urban land uses, most estuaries are now dominated by soft sediments such as mud and sand (Hume et al. Citation2007). As a consequence, they lack shelter for small demersal fish except where it is provided by structurally-complex biogenic habitats such as subtidal seagrass meadows and horse mussel beds (Morrison et al. Citation2009, Citation2014b, Citation2014c, Citation2014d; Parsons et al. Citation2016). Since the arrival of Europeans, land-use changes have significantly reduced these biogenic habitats, primarily through increased sedimentation and, to a lesser extent, eutrophication (Morrison et al. Citation2009, Citation2023; Zabarte-Maeztu et al. Citation2021). In northern-most New Zealand, several large estuaries still act as strongholds for extensive subtidal seagrass beds, which support very high densities of juvenile Australasian snapper (Chrysophrys auratus) and other species (Morrison et al. Citation2014c, Citation2019). Unfortunately, aside from these few estuaries, most New Zealand estuaries now hold only intertidal beds, which are unsuitable for juveniles of snapper and other fish species due to their exposure at low tide (Morrison et al. Citation2014c, Citation2014d). It has been suggested that insufficient nursery habitat may be hindering the recovery of overfished adult snapper stocks (Parsons et al. Citation2014a), which, despite recent fisheries management, have remained well below management targets in the Hauraki Gulf and elsewhere (Francis and McKenzie Citation2015).
Rocky reefs provide shelter and food to numerous juvenile fishes on open coasts (e.g. Jones Citation1988) but are a rare habitat in most estuaries, typically being restricted to estuary mouths, headlands and occasional outcrops surrounded by soft sediments (e.g. Morrison Citation1990; Hartill et al. Citation2000; Shankar et al. Citation2000; Morrison et al. Citation2000a, Citation2000b; Parsons et al. Citation2016). Rocky reefs have been overlooked as potential fish nursery sites in estuaries, perhaps due to their low coverage and their unsuitability to sampling by trawls or seine nets. However, the structural complexity of rocky reefs and associated epibiota such as seaweeds and sponges could make them valuable refuges for small fish, particularly given the decline in subtidal seagrass. Additionally, the tendency of estuarine rocky reefs to occur in areas where strong tidal currents prevent deposition of soft sediments means that they may receive a steady supply of larval fish, and of plankton for those fish to eat following settlement.
The potential of such estuarine rocky reefs to be relevant to juvenile snapper is clear given the life history of Chrysophrys auratus. Snapper larvae are pelagic for around 28 days, settling into demersal habitats in early-mid summer when 9–14 mm long (Miskiewicz Citation1986; Trnski Citation2002; Parsons et al. Citation2014b). Juveniles remain in inshore, shallow nursery grounds until autumn, when they are usually about 50–70 mm long, (Francis Citation1994; Usmar Citation2009; Parsons et al. Citation2013; Parsons et al. Citation2014b). During this time, they consume a variety of small invertebrates, with planktonic copepods being particularly important (Usmar Citation2012; Lowe Citation2013; Lohrer et al. Citation2018). While the seasonality of juvenile fish movements is well-documented (Francis Citation1994; Miller and Sadro Citation2003; Usmar Citation2009), the triggers governing their arrival at and departure from nursery grounds remain more obscure. Previous studies have suggested that snapper may depart their nursery grounds upon reaching a certain size (Parsons et al. Citation2013), however, given the limited seasonal scope of monitoring, it is unclear whether other environmental factors are involved.
Here we describe the use by juvenile (0+) snapper of a natural reef fringing a tidal channel in the Whangateau Harbour, a small estuary in north-eastern New Zealand. We quantify the distribution, abundance, and habitat use of juvenile snapper on the reef at fine temporal and spatial scales over four years and relate the patterns to environmental factors such as position in the tidal stream, water temperature and the presence of potential predators.
Materials and methods
The study site was a sandstone reef running along the southern side of Horseshoe Island within Whangateau Harbour, which lies ∼60 km north of Auckland on the north-eastern coast of the North Island of New Zealand (36° 19.168’S, 174° 45.862’E) (). The reef at Horseshoe Island is ∼2 km upstream of the harbour entrance, and is ∼270 m long by ∼5–15 m wide, being composed of dissected soft sandstone topped by the brown seaweed Hormosira banksii in the intertidal (). It transitions from a flat upper intertidal area to a maximum depth of ∼5–m (at high tide) before merging into a sandy channel. There is another much smaller sandstone reef in the upper harbour south of the causeway to Omaha Beach, but no other natural rocky reefs are present once past the harbour entrance, and there are no significant beds of subtidal seagrass or other natural nursery habitats (Hartill et al. Citation2000).
Figure 1. Map of the study site showing the location of Horseshoe Island within the Whangateau estuary on the North Island of New Zealand. Aerial image shows the sandstone reef running along the southern side of Horseshoe Island marked by the red box. Numbers 1 and 10 show the position of the reef sections with section 1 at the south-eastern most end. Aerial image taken from https://geomapspublic.aucklandcouncil.govt.nz.
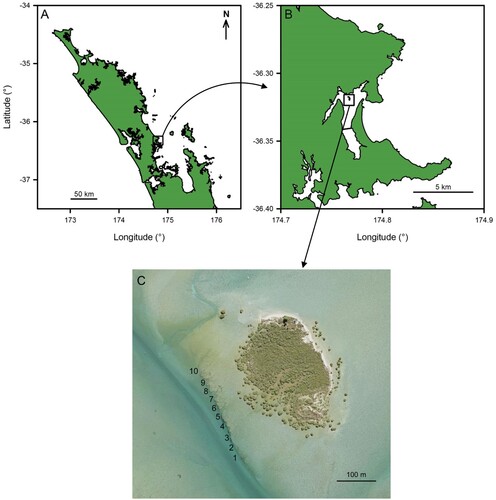
Figure 2. Reef at Horseshoe Island within the Whangateau estuary, north-eastern New Zealand [Photo: Richard Taylor].
![Figure 2. Reef at Horseshoe Island within the Whangateau estuary, north-eastern New Zealand [Photo: Richard Taylor].](/cms/asset/36d26560-afbc-4f1a-9a22-6130b26417e1/tnzm_a_2366022_f0002_oc.jpg)
The reef was marked out into ten sections using metal stakes with small numbered fishing floats attached to aid orientation. This facilitated recording of fish locations during SCUBA observations. The stakes were spaced ∼27 m apart on average, although their exact placement was based on landscape features, such as breaks in the reef, rather than fixed distances. Section one was at the reef's seaward-most (south-eastern) end, while section ten was furthest upstream at the north-westernmost end.
Snapper were surveyed every year from January to mid-April/late May in 2018–2021, except that observations started late in 2018 due to logistical constraints, making it difficult to document the arrival of juveniles that year. Observations finished early in 2020 due to the COVID 19 pandemic. Juvenile snapper ∼15–20 mm long began to appear on the reef in January (early austral summer) and were present until mid-April/early May (austral autumn). They were surveyed ∼weekly, as weather and logistics allowed, between 0630 and 1900h New Zealand Daylight Time and around high tide (when water was clearest). On each occasion a SCUBA diver zigzagged slowly along the entire length and width of the reef, starting at the south-eastern end (section one). When juvenile or adult snapper were encountered on the reef or in the immediately adjacent channel, the diver recorded their size, position on the reef (section number and whether they were on the reef crest, reef edge or adjacent sand), and group composition (if in a school). Individuals were assigned to five size classes (), following training in size estimation using 30–115 mm long plastic pipes (Bell et al. Citation1985). Monitoring ceased each year when juvenile snapper were no longer present on the reef.
Table 1. Recorded size classes of juvenile snapper with the corresponding age that each size class represents. Age data are taken from a survey of snapper in the Hauraki Gulf in 2018 (J. Campbell unpublished data).
Horizontal water visibility was estimated on each dive by reference to familiar reef structures. Sea surface temperatures were taken from the nearby, long-term monitoring station at Cape Rodney-Okakari Point Marine Reserve (see Cook et al. Citation2022).
Results
Notwithstanding survey gaps in early 2018 and late 2020, 0 + juveniles consistently arrived in Whangateau Harbour in January of each year, remained on the reef for several weeks to months and were gone by May (A–D). Recruitment varied significantly between years, with 2018 having the highest peak population of juvenile snapper at 436 individuals, and 2021 having the lowest at 84 (Kruskal–Wallis, H = 19.22, df = 3, p < 0.01). In addition to this, there was considerable variation in when 0 + snapper disappeared each year, the earliest being in 2021, when snapper had left the reef by the end of March, and the latest being in 2019 when the final few fish left in early May. In 2018 and 2019 the disappearance of juveniles occurred shortly after a sustained drop in sea temperature over several days (Figure S1). No such pattern was observed with respect to time of year or any absolute temperature value across all three years for which we were able to establish a disappearance date.
Figure 3. A–D, Monthly average number of juvenile (0+) snapper (Chrysophrys auratus) recorded per size class, per dive on the reef at Horseshoe Island within the Whangateau estuary, north-eastern New Zealand. E, Mean percentage of snapper per size class, per month across all years. n.d. = no data.
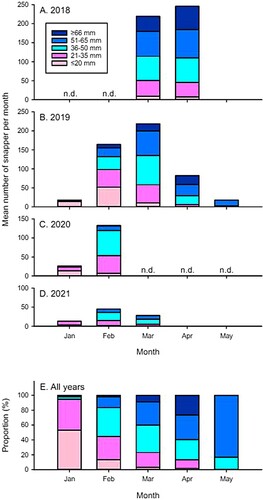
Although juvenile snapper began arriving in January of each year, they continued to settle onto the reef throughout the season and usually reached peak numbers in February to March (A–D). The newly-settled recruits (≤20 mm long), represented a smaller proportion of total 0 + juveniles as the season progressed, comprising almost 55% of individuals in January but only 2.4% in April (E). Correspondingly, the mean size of juvenile snapper increased significantly (Two-way Analysis of Variance (ANOVA) on log-transformed data, F = 37.26, df = 4, p = 0.02), from January (23.5 mm) to April (55.5 mm). This pattern was similar across all years, with no significant difference in the mean size of snapper between years (Two-Way ANOVA, F = 0.74, df = 3, p = 0.58). Larger size classes of fish made up a greater proportion of snapper in March, April and May in particular. This shift in percentage, however, masks the fact that new recruits ≤20 mm long were still arriving on the reef as late as April.
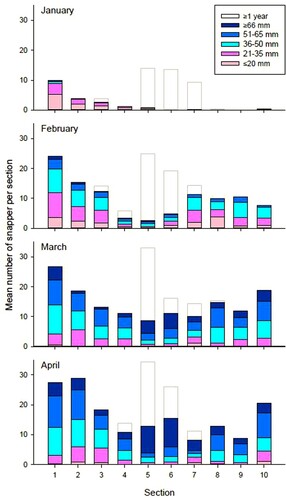
In all years (Figure S2), 0 + snapper were non-randomly distributed among the 10 reef sections, with significant differences between sections (Kruskal–Wallis, H = 49.41, df = 9, p < 0.01). The most seaward locations (sections one and two), were the most densely populated overall, with a mean number of 24.9 (SE ± 3.67) and 17.1 (SE ± 2.36) snapper recorded per dive, across all years. Sections four and five had means of 6.7 (SE ± 1.49 and 1.59) snapper per dive in both sections. The middle sections of the reef were, in general, under-utilised by all but the ≥66 mm fish, and very few snapper <35 mm were present there (). In contrast, ≥ 66 mm fish tended to inhabit sections five and six, along with ∼50 larger, > 1year old snapper. In January, almost all of the juvenile snapper were observed in sections one, two and to a lesser extent, three (the most seaward sections of the reef), with almost no recruits further down the reef; there was a steep decline in mean snapper abundance going from section one to ten. This pattern was remarkably consistent in each year of the study (Figure S3). As more and more juvenile snapper recruited onto the reef in February, this pattern was replaced by a U-shape with high numbers of snapper at either end, but few in the middle sections (). This general pattern was maintained but became less pronounced in March and April as individuals grew, with fish in the larger size classes becoming more abundant in the mid sections of the reef.
Figure 4. Along-reef distribution of juvenile (0+) and older snapper (Chrysophrys auratus) on the reef at Horseshoe Island within the Whangateau estuary, north-eastern New Zealand displayed per month, pooled across all years. Section 1 is at the seaward-most (south-eastern) end of the reef, while section 10 is furthest upstream at the north-westernmost end.
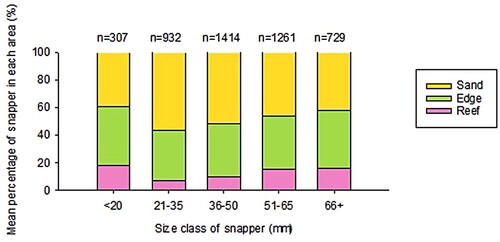
Juvenile snapper spent significantly more time on the sand and at the edge of the reef than on the reef crest (; permutational MANOVA, 999 permutations, F = 26.687, df = 2, p < 0.01). Indeed, on many dives, individuals were observed several metres out from the reef (J. Campbell, pers. obs.). Some size classes were more numerically dominant (permutational MANOVA, 999 permutations, F = 9.042, df = 4, p < 0.01). There was however, no difference in habitat use between different size classes (permutational MANOVA, 999 permutations, habitat*size class, F = 1.403, df = 8, p = 0.195); all size classes spent roughly equivalent amounts of time on the reef, around the edge of the reef and out on the sand. Although there was some variation from year to year, overall, the similarity in habitat across size classes was maintained (two-way ANOVA, habitat*size, p > 0.1 in all cases).
Discussion
We found that a small estuarine rocky reef consistently held in the order of one hundred to several hundred 0 + snapper each summer and autumn. The reef also supported large numbers of juvenile fishes of other species, including spotty (Notolabrus celidotus), parore (Girella tricuspidata) (Morrison Citation1990) and trevally (Pseudocaranx georgianus) (authors, pers. obs.). While the numbers of juvenile snapper on the reef were relatively small, and the densities much lower than found in subtidal seagrass beds (∼0.1 individuals.m−2 cf. ∼1.6 individuals.m−2 in subtidal seagrass at Rangaunu Harbour (Morrison et al. Citation2014c)), numbers are nevertheless higher than in unstructured habitats (Francis et al. Citation2005, Citation2011; Morrison et al. Citation2014c) and the reef is a potentially important nursery habitat in an estuary lacking other natural structurally-complex habitats.
Newly-settled fish (≤20 mm long) were first observed in January and continued to arrive until April in most years, with the total number of individuals peaking in March, and disappearing by May. Recruitment varied five-fold across years, with this level of variability being typical of many fish species (Aburto-Oropeza et al. Citation2007) including snapper (McGlennon et al. Citation2000; Fowler and Jennings Citation2003; Zeldis et al. Citation2005). The 2018 peak reflected an exceptionally strong recruitment year around the north-east of New Zealand’s North Island (Campbell Citation2023). There was no obvious relationship with variation in seawater temperatures over the period of the study (Cook et al. Citation2022) that may have correlated with recruitment strength (Francis Citation1993).
Regardless of year-class strength, new recruits were consistently most abundant at the seaward end of the reef, particularly in sections one and two, with the highest early-season densities observed in these areas. The seaward end may be prime habitat due to a greater supply of plankton, before it is depleted by the resident population of planktivorous fish (Bray Citation1981; Hamner et al. Citation1988; Kingsford and MacDiarmid Citation1988; Parsons et al. Citation2018). An alternative explanation is that the seaward end is the first suitable habitat encountered by larvae entering the estuary, resulting in a recruitment shadow downstream (Jones Citation1997), similar to the failure of damselfishes to settle into apparently suitable habitat down-current from other reefs (Jones Citation1997). Previous snapper studies have observed higher abundances of juveniles near harbour mouths (Parsons et al. Citation2013) but were unable to distinguish between the two theories. In all years, the distribution of juvenile snapper shifted from the seaward end of the reef in January, to a U-shape with more snapper at either end of the reef, and fewer in the mid-sections a few weeks later. This occurred even in years with very low recruitment, such as 2021, suggesting that the recruitment shadow hypothesis is the more likely reason for the initial gradient.
The middle sections of the reef predominantly hosted larger juveniles, likely because smaller individuals either avoid this area or fall prey to cannibalism by larger snapper, a phenomenon previously documented by Compton et al. (Citation2012).
Juvenile snapper occurred most commonly near the reef edge and adjacent sand, and less commonly on the reef crest, with this distribution pattern very similar across all size classes. It was surprising to observe snapper at times several metres away from the reef on the sand, or high in the water column feeding on plankton, but they were nevertheless only a few seconds away from the shelter of the reef. As part of a larger project run at the same site and time, action cameras deployed on the reef during daytime for a total of 53.6 recording hours, detected predators on 25 occasions, the most common of which was kingfish (Seriola lalandi) (11 occasions), followed by adult trevally (Pseudocaranx georgianus) and adult snapper (7 and 6 occasions, respectively). Whenever a predator was observed in the video footage, 0 + snapper swiftly sought refuge in the reef's vicinity, often sheltering in small dips close to reef structures (J. Campbell, pers. obs.), a behaviour also observed elsewhere (Parsons et al. Citation2018). Kahawai (Arripis trutta) have also been recorded on unbaited fixed cameras unsuccessfully hunting 0 + snapper over subtidal seagrass in Rangaunu Harbour (Morrison et al. Citationin press.). This underscores the importance of structure in providing a refuge, aligning with the findings of Ross et al. (Citation2008).
The disappearance of juvenile snapper from the reef did not occur at a certain body size (Parsons et al. Citation2013), water temperature, or time of year, but instead tended to follow a rapid decrease in temperature. We have no direct evidence to ascertain the fate of the juvenile snapper that disappear from the reef, in terms of whether they emigrate or are subjected to mortality. Seasonal (quarterly) surveys in a much larger harbour (Mahurangi Harbour, 21 kilometres to the south) which holds extensive subtidal flats (< 10 m) and deeper channel areas (>10–26 m) (see Hauraki Auckland Benthic Habitat Map bookmarks arcgis.com), recorded the apparent shift of 0 + snapper from widespread shallower harbour areas in summer/autumn, down into the main channel’s deeper area near the estuary mouth during winter/spring. This suggests that 0 + snapper move to deeper waters in the colder months (Morrison et al, Citationin press). For Whangateau Harbour, which has only limited shallow subtidal areas (maximum depth 7 m), it is probable that most of the reef individuals migrated out of the estuary to populate the adjacent open coast, which includes the Cape Rodney-Okakari Point and Tawharanui marine reserves. Others may have been taken by predators (Parsons et al. Citation2014b). Although the aforementioned daytime video recordings never recorded predator strikes on juvenile snapper, predators did initiate a flight response (Campbell,), suggesting they are a threat. Nocturnal predators such as conger eels (Conger spp.) or broad squid (Sepioteuthis australis) may also be important (Parsons et al. Citation2022), as well as soft-sediment camouflage predators such as estuarine (Leptoscopus macropygus) and spotted (Genyagnus monopterygius) stargazers.
In conclusion, the natural rocky reef we monitored appears to be a locally important nursery habitat for snapper, which inhabit it for the first few months of their post-settlement lives. Similar reefs holding 0 + snapper occur in many other northern estuaries, e.g. Mahurangi Harbour (Morrison et al. Citation2000a) on the east coast, and Aotea and Kaipara harbours on the west coast (Morrison et al., Citationin press.). The widespread loss of subtidal seagrass beds from northern estuaries increases the value of remaining nursery habitats like reefs, but the latter may also be under threat from anthropogenic sediment deposition (Thrush et al. Citation2004). Artificial analogues of natural reefs such as mooring blocks, jetties, marinas, training walls and causeways are present in many estuaries (Wetzel et al. Citation2014) and merit further investigation as potential nursery habitats.
Supplementary material
Download MS Word (1.4 MB)Acknowledgements
We thank Lily Kozmian-Ledward, Errol Murray, Peter Browne, Charlie Bedford, Peter Schlegel, Esther Stuck, Luis Nahmad, Ohad Peleg, and Al Alder for help in the field. We are also grateful to the two anonymous reviewers for their constructive comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aburto-Oropeza O, Sala E, Paredes G, Mendoza A, Ballesteros E. 2007. Predictability of reef fish recruitment in a highly variable nursery habitat. Ecology. 88(9):2220–2228. doi:10.1890/06-0857.1.
- Bell JD, Craik G, Pollard DA, Russell BC. 1985. Estimating length frequency distributions of large reef fish underwater. Coral Reefs. 4(1):41–44. doi:10.1007/BF00302203.
- Bray RN. 1981. Influence of water currents and zooplankton densities on daily foraging movements of blacksmith, Chromis punctipinnis, a planktivorous reef fish. Fishery Bulletin 78(4):829–841.
- Campbell JL. 2023. The habitat use, diet and behaviour of juvenile snapper (Chrysophrys auratus) [PhD thesis]. Auckland: University of Auckland.
- Compton TJ, Morrison MA, Leathwick JR, Carbines GD. 2012. Ontogenetic habitat associations of a demersal fish species, Pagrus auratus, identified using boosted regression trees. Marine Ecology Progress Series. 462:219–230. doi:10.3354/meps09790.
- Cook F, Smith RO, Roughan M, Cullen NJ, Shears N, Bowen M. 2022. Marine heatwaves in shallow coastal ecosystems are coupled with the atmosphere: Insights from half a century of daily in situ temperature records. Frontiers in Climate. 4:1–21.
- Fowler AJ, Jennings PR. 2003. Dynamics in 0 + recruitment and early life history for snapper (Pagrus auratus, Sparidae) in South Australia. Marine and Freshwater Research. 54(8):941–956. doi:10.1071/MF02172.
- Francis M, Morrison M, Leathwick J, Walsh C, Middleton M. 2005. Predictive models of small fish presence and abundance in northern New Zealand harbours. Estuarine, Coastal, and Shelf Science. 64:419–435. doi:10.1016/j.ecss.2005.03.007.
- Francis MP. 1993. Does water temperature determine year class strength in New Zealand snapper (Pagrus auratus, Sparidae)? Fisheries Oceanography. 2(2):65–72. doi:10.1111/j.1365-2419.1993.tb00121.x.
- Francis MP. 1994. Growth of juvenile snapper, Pagrus auratus. New Zealand Journal of Marine and Freshwater Research. 28(2):201–218. doi:10.1080/00288330.1994.9516608.
- Francis MP, Morrison MA, Leathwick J, Walsh C. 2011. Predicting patterns of richness, occurrence and abundance of small fish in New Zealand estuaries. Marine and Freshwater Research. 62(11):1327–1341. doi:10.1071/MF11067.
- Francis RICC, McKenzie JR. 2015. Assessment of the SNA 1 stocks in 2013. New Zealand Fisheries Assessment Report 2015/76. 82pp.
- Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DM. 1988. Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bulletin of Marine Science. 42(3):459–479.
- Hartill B, Morrison M, Shankar U, Drury J. 2000. Whangateau Harbour habitat map. NIWA Information Series. 10.
- Hume TM, Snelder S, Weatherhead M, Liefting R. 2007. A controlling factor approach to estuary classification. Ocean and Coastal Management. 50:905–929. doi:10.1016/j.ocecoaman.2007.05.009.
- Jones E, Francis M, Paterson C, Morrison M. 2015. Habitats of particular significance for fisheries management: identification of threats and stressors to rig nursery areas. New Zealand Aquatic Environment and Biodiversity Report No. 150. 76pp.
- Jones GP. 1988. Ecology of rocky reef fish of north-eastern New Zealand: a review. New Zealand Journal of Marine and Freshwater Research. 22:445–462. doi:10.1080/00288330.1988.9516315.
- Jones GP. 1997. Relationships between recruitment and postrecruitment processes in lagoonal populations of two coral reef fishes. Journal of Experimental Marine Biology and Ecology. 213(2):231–246. doi:10.1016/S0022-0981(96)02763-3.
- Kingsford MJ, MacDiarmid AB. 1988. Interrelations between planktivorous reef fish and zooplankton in temperate waters. Marine Ecology Progress Series. 48(2):103–117. doi:10.3354/meps048103.
- Lohrer AM, McCartain LD, Buckthought D, MacDonald I, Parsons DM. 2018. Benthic structure and pelagic food sources determine post-settlement snapper (Chrysophrys auratus) abundance. Frontiers in Marine Science. 5:427. doi:10.3389/fmars.2018.00427.
- Lowe M. 2013. Factors affecting the habitat usage of juvenile estuarine fish in northern New Zealand [PhD thesis]. Auckland: University of Auckland.
- McGlennon D, Jones GK, Baker J, Jackson WB, Kinloch MA. 2000. Ageing, catch-at-age and relative year-class strength for snapper (Pagrus auratus) in northern Spencer Gulf, South Australia. Marine and Freshwater Research. 51(7):669–677. doi:10.1071/MF98095.
- Miller BA, Sadro S. 2003. Residence time and seasonal movements of juvenile coho salmon in the ecotone and lower estuary of Winchester Creek, South Slough, Oregon. Transactions of the American Fisheries Society. 132(3):546–559. doi:10.1577/1548-8659(2003)132<0546:RTASMO>2.0.CO;2.
- Miskiewicz AG. 1986. The season and length at entry into a temperate Australian estuary of the larvae of Acanthopagrus australis, Rhabdosargus sarba and Chrysophrys auratus (Teleostei: Sparidae). In: Uyeno T, Arai R, Taniuch T, Matsuura K, Miskiewicz AG, editor. Proceedings of the Second International Conference on Indo-Pacific Fishes. Tokyo, Japan: Ichthyological Society of Japan; p. 740–747.
- Morrison M, Hartill B, Shankar U, Drury J. 2000b. Pahurehure Inlet, Manukau Harbour habitat map. NIWA information series. 12.
- Morrison M, Shankar U, Hartill B, Drury J. 2000a. Mahurangi Harbour habitat map. NIWA Information Series. 13.
- Morrison MA. 1990. Ontogenetic shifts in the ecology of the parore, Girella tricuspidata [Unpublished MSc thesis]. University of Auckland. 66p.
- Morrison MA, Elliot S, Hughes A, Kainamu A, Williams E, Lowe M, Lohrer D, Needham H, Semadeni-Davies A. 2023. Land-based effects on coastal fisheries and kaimoana and their habitats - a review. New Zealand Aquatic Environment and Biodiversity Report. 309:167.
- Morrison MA, Jones E, Consalvey M, Berkenbusch K. 2014b. Linking marine fisheries species to biogenic habitats in New Zealand: a review and synthesis of knowledge. New Zealand Aquatic Environment and Biodiversity Report 130. 156pp.
- Morrison MA, Jones E, Parsons DP, Grant C. 2014a. Habitats and areas of particular significance for coastal finfish fisheries management in New Zealand: A review of concepts and current knowledge, and suggestions for future research. New Zealand Aquatic Environment and Biodiversity Report 125. 202pp.
- Morrison MA, Lowe M, Walsh C, Middleton C, Jones EG, Williams J. In press. Habitat degradation impacts on fishery productivity and support in northern New Zealand harbours. Draft Aquatic Environment and Biodiversity Report. Fisheries New Zealand.
- Morrison MA, Lowe ML, Grant C, Reed J, Carbines G, Smith PJ, Bury SJ, Brown J. 2014c. Seagrass meadows as biodiversity and productivity hotspots. New Zealand Aquatic Environment and Biodiversity Report 137. 147pp.
- Morrison MA, Lowe ML, Jones EG, Makey L, Shankar U, Usmar N, Miller A, Smith M, Middleton C. 2014d. Habitats of particular significance for fisheries management: The Kaipara Harbour. New Zealand Aquatic Environment and Biodiversity Report 129. 169pp.
- Morrison MA, Lowe ML, Parsons D, Usmar N, McLeod I. 2009. A review of land-based effects on coastal fisheries and supporting biodiversity in New Zealand. Aquatic Biodiversity and Biosecurity Report No. 37. 100pp.
- Morrison MA, McKenzie J, Bian R. 2019. Pre-recruit (0+) snapper (Chrysophrys auratus) beam trawl and beach seine surveys of East Northland and the Hauraki Gulf (SNA 1) New Zealand Fisheries Assessment Report 2019/72. 50 p.
- Morrison MA, McKenzie J, Gillanders B, Tuck I. 2016. Can otolith chemistry predict the natal origins of grey mullet (Mugil cephalus)? New Zealand Fisheries Assessment Report 2016/15. 68pp.
- Parsons D, Taylor R, Hughes R, Middleton C, Gublin Y, Levell D. 2022. Predators and habitat association of post-settlement snapper (Chrysophrys auratus). Journal of Fish Biology. 101:1509–1521. doi:10.1111/jfb.15222.
- Parsons DM, Buckthought D, Middleton C, MacKay G. 2016. Relative abundance of snapper (Chrysophrys auratus) across habitats within an estuarine system. New Zealand Journal of Marine and Freshwater research. 50(3):358–370. doi:10.1080/00288330.2016.1146310.
- Parsons DM, MacDonald I, Buckthought D, Middleton C. 2018. Do nursery habitats provide shelter from flow for juvenile fish? PLoS One. 13(1):1–20. doi:10.1371/journal.pone.0186889.
- Parsons DM, Middleton C, Smith MD, Cole RG. 2014a. The influence of habitat availability on juvenile fish abundance in a northeastern New Zealand estuary. New Zealand Journal of Marine and Freshwater Research. 48(2):216–228. doi:10.1080/00288330.2013.875927.
- Parsons DM, Morrison MA, Thrush SF, Middleton C, Smith M, Spong KT, Buckthought D. 2013. The influence of habitat structure on juvenile fish in a New Zealand estuary. Marine Ecology. 34(4):492–500. doi:10.1111/maec.12050.
- Parsons DM, Sim-Smith CJ, Cryer M, Francis MP, Hartill B, Jones EG, Le Port A, Lowe M, McKenzie J, Morrison M, et al. 2014b. Snapper (Chrysophrys auratus): a review of life history and key vulnerabilities in New Zealand. New Zealand Journal of Marine and Freshwater Research. 48(2):256–283. doi:10.1080/00288330.2014.892013.
- Ross PM, Thrush SF, Montgomery JC, Walker JW, Parsons DM. 2008. Habitat complexity and predation risk determine juvenile snapper (Pagrus auratus) and goatfish (Upeneichthys lineatus) behaviour and distribution. Marine and Freshwater Research. 58(12):1144–1151. doi:10.1071/MF07017.
- Shankar U, Morrison M, Hartill B, Drury J. 2000. Matakana Harbour habitat map. NIWA Information Series. 11.
- Thrush SF, Hewitt JE, Cummings VJ, Ellis JI, Hatton C, Lohrer A, Norkko A. 2004. Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment. 2:299–306. doi:10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2.
- Trnski T. 2002. Behaviour of settlement-stage larvae of fishes with an estuarine juvenile phase: in situ observations in a warm-temperate estuary. Marine Ecology Progress Series. 242:205–214. doi:10.3354/meps242205.
- Usmar NR. 2009. Ontogeny and ecology of snapper (Pagrus auratus) in an estuary, the Mahurangi Harbour [PhD thesis]. Auckland: University of Auckland.
- Usmar NR. 2012. Ontogenetic diet shifts in snapper (Pagrus auratus: Sparidae) within a New Zealand estuary. New Zealand Journal of Marine and Freshwater Research. 46(1):31–46. doi:10.1080/00288330.2011.587824.
- Wetzel MA, Scholle J, Teschke K. 2014. Artificial structures in sediment-dominated estuaries and their possible influences on the ecosystem. Marine Environmental Research. 99:125–135. doi:10.1016/j.marenvres.2014.04.008.
- Zabarte-Maeztu I, Matheson FE, Manley-Harris M, Davies-Colley RJ, Hawes I. 2021. Fine sediment effects on seagrasses: A global review, quantitative synthesis and multi-stressor model. Marine Environmental Research. 171:1–15. doi:10.1016/j.marenvres.2021.105480.
- Zeldis JR, Oldman J, Ballara SL, Richards LA. 2005. Physical fluxes, pelagic ecosystem structure, and larval fish survival in Hauraki Gulf, New Zealand. Canadian Journal of Fisheries and Aquatic Sciences. 62(3):593–610. doi:10.1139/f04-209.