Abstract
Life history characteristics of snapper (Pagrus auratus) were found to vary at fine spatial scales (less than tens of kilometres) in the sub-tropical waters of Shark Bay, Western Australia, from research undertaken between 1997 and 2004. Differences in the timing and duration of the spawning season, length and age at maturity, and maximum age and growth between snapper from the Eastern Gulf, Denham Sound and Freycinet Estuary were attributed to spatial differences in environmental conditions and density-dependent responses to fishing-induced changes in spawning biomass. Such fine-scale spatial variation in the biological characteristics of snapper is consistent with results of previous stock identification studies and further supports the geographic scale at which local snapper stocks are managed to protect both stock productivity and genetic diversity.
Introduction
Individual fish stocks typically demonstrate intrinsic life history characteristics such as length and age at maturity, maximum length and age, and rates of growth and mortality that reflect the underlying population dynamics and the complex interaction of biological, ecological and environmental processes. Differences in biological characteristics have long been used to identify separate fish stocks for management purposes (Begg Citation2005); however, whether such variation is genetic or environmental in origin can be difficult to determine (Trippel Citation1995; Law Citation2000).
Snapper, Pagrus auratus (Bloch & Schneider 1801), are widely distributed throughout warm temperate and sub-tropical waters of the western Indo-Pacific where they typically support important coastal fisheries (Paulin Citation1990). Around Australia, snapper inhabit waters out to depths of c. 250–300 m from Hinchinbrook Island in Queensland (18°S) to north of Barrow Island in Western Australia (21°S) (Kailola et al. Citation1993). State-managed commercial and recreational fisheries in Queensland, New South Wales, Victoria, South Australia and Western Australia contribute to a national snapper catch of c. 3000 tonnes/year (Henry & Lyle Citation2003; Anon. Citation2006). In Western Australia, c. 75% of the total commercial catch historically came from the oceanic waters adjacent to Shark Bay (26°S, 113°E, ) (Moran et al. Citation2005) while snapper in the inner gulfs support an important recreational boat-based fishery (Stephenson & Jackson Citation2005).
Fig. 1 Denham Sound, Freycinet Estuary and Eastern Gulf areas of inner Shark Bay, Western Australia. Boundaries represent recreational fishing areas for management purposes and are taken to delineate the approximate distributions of the three Pagrus auratus stocks in this study. Hamelin Pool is a marine nature reserve and closed to all fishing.
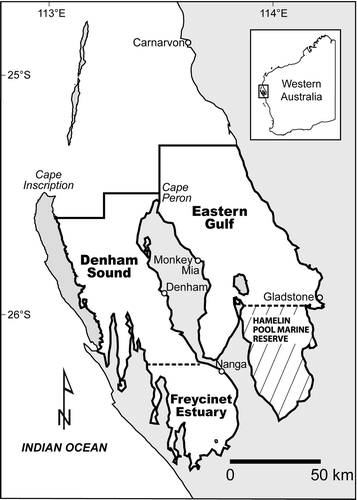
Shark Bay has a unique marine environment resulting from the combined physical and biological processes that operate within its waters and the surrounding environs (Wyatt et al. Citation2005). The bay is a large, semi-enclosed marine embayment and is comprised of open, deeper waters to the north and two shallower inner gulfs to the south (Eastern Gulf, Western Gulf = waters of Denham Sound and Freycinet Estuary combined) (). The climate is arid (average rainfall 200–220 mm/year) with minimal terrestrial runoff (Logan & Cebulski Citation1970). Limited tidal exchange combined with persistent winds and high evaporation rates (average 2000–2200 mm/year) have resulted in highly elevated salinities and marked environmental gradients (Nahas et al. Citation2005). Salinities in the inner gulfs consistently exceed the oceanic level (35 PSU) with metahaline conditions maintained in the Eastern Gulf and southern waters of Denham Sound (38–48 PSU) and Freycinet Estuary (45–48 PSU), whereas hypersaline conditions (50–65 + PSU) persist in Hamelin Pool (Logan & Cebulski Citation1970). Shark Bay was inscribed on the World Heritage List in 1991 for the high conservation value of features of its marine environment including: the distinct biological zones produced by the salinity gradients; the highly restricted marine communities adapted to the hypersaline conditions; and the significant genetic variability in some taxa including P. auratus (Environment Australia Citation1999).
Shark Bay is near the northern extent of the geographic range of snapper on the west coast of Australia (Kailola et al. Citation1993). A number of independent genetic (allozymes) studies indicate that snapper in the oceanic waters off Shark Bay, in the Eastern Gulf and Freycinet Estuary, are reproductively isolated from each other, whereas fish in Denham Sound are partially isolated from the other populations (MacDonald Citation1980; Johnson et al. Citation1986; Whitaker & Johnson Citation1998; Baudains Citation1999). Tagging (Moran et al. Citation2003; G. Jackson, unpublished data) and otolith chemistry (Edmonds et al. Citation1999; Bastow et al. Citation2002; Gaughan et al. Citation2003) indicate little or no mixing of juvenile, sub-adult and adult snapper between oceanic waters and the inner gulfs, or between the gulfs. Eggs and larvae inside Shark Bay are retained within meso-scale eddies (kilometres in diameter) effectively isolating the main spawning grounds from each other (Nahas et al. Citation2003). Given the complex snapper population structure in the region, four unit stocks are currently recognised for fisheries management purposes: an oceanic stock found along the continental shelf outside Shark Bay and, in the inner gulfs, separate stocks in the Eastern Gulf, Denham Sound and Freycinet Estuary (Stephenson & Jackson Citation2005).
Beyond Shark Bay, three other snapper stocks are currently recognised around Australia: an ‘East Coast’ stock off southern Queensland, NSW, and eastern Victoria; a ‘Western Bass Straight’ stock off central and western Victoria; and a ‘Great Australian Bight–South Australian’ stock between the mouth of the River Murray and eastern Western Australia (Donnellan & McGlennon Citation1996; Meggs et al. Citation2003; Sumpton et al. Citation2008). No attempt has previously been made to investigate variation in life history characteristics amongst these Australian snapper stocks.
In this study, we compared some life history characteristics of snapper from closely adjacent areas of inner Shark Bay, which is located at the northern extent of the species range on the Australian west coast. We investigated the hypothesis that the growth and reproductive characteristics of snapper varies at a spatial scale of tens of kilometres given: (1) the complexity of regional population structure; (2) Shark Bay's marked environmental gradients; and (3) historic differences in exploitation amongst the three areas.
Materials and methods
Snapper were collected from the Eastern Gulf, Denham Sound and Freycinet Estuary () between 1997 and 2004, principally using rod and line fishing. In addition, smaller fish (c. 50–200 mm, fork length, FL) were obtained during research trawl (twin otter trawls, 45-mm cod-end mesh; Moran & Kangas Citation2003) and trap surveys (Antillean-Z fish traps, 12-mm mesh; Jackson et al. Citation2007) that were conducted in all three study areas. All fish were measured (FL, nearest 1 mm) and a random sub-sample weighed (whole wet weight, nearest 10 g). Data were logarithmically (natural ln) transformed and used to derive an allometric length:weight relationship for each area; differences between areas were tested using analysis of covariance and where non-significant, the corresponding data were pooled. After adjustment for log-bias (Quinn & Deriso Citation1999), the resulting relationship was used to estimate the weights of all unweighed fish. Gonads were sexed, weighed (nearest 0.01 g) and staged macroscopically. For females, a six-stage classification system (1 = immature, 2 = resting, 3 = developing, 4 = developed, 5 = spawning, 6 = spent) was used, whereas for males, a five-stage classification system (1 = immature, 2 = resting, 3 = developed, 4 = spawning, 5 = spent) was used (Mackie et al. Citation2009). A random selection of ovaries (n=1497) that had been fixed and used to prepare histological sections was examined microscopically and staged based on histological characteristics (Mackie et al. Citation2009). Sagittal otoliths were sectioned and individual ages determined using methods described by Jackson (Citation2007). The periodicity of increment formation was validated using marginal increment analysis and oxytetracycline (or calcein) marking of otoliths (Jackson Citation2007).
Length and age at maturity
Logistic regression analysis was used to determine the parameters α and β of the logistic relationship between the probability, p, that an individual is mature and the individual's length, L, i.e.
Timing and duration of spawning season
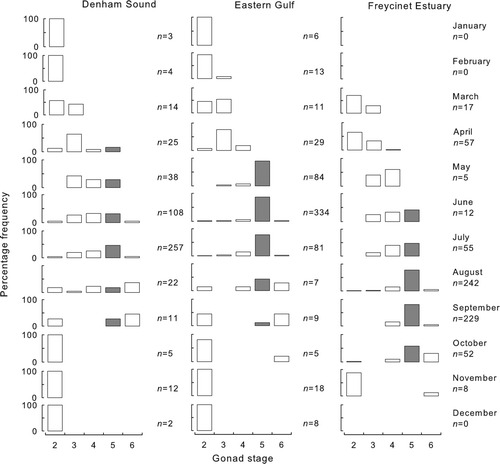
Mean monthly gonadosomatic indices (GSIs) for fish with lengths of the estimated L 50 or greater were determined for females and males within each area using the equation GSI = 100×Wg/(WW − Wg), where WW = whole wet weight of fish and Wg=wet weight of gonads. To determine the seasonal patterns of gonadal development, the monthly percentage frequency for each gonad maturity stage was calculated for females and males with lengths equal to or greater than the estimated L 50, and plotted as frequency histograms.
Growth
Growth curves were fitted to the lengths at age for females and males separately and for all individuals combined, for each area, using non-linear least-squares regression (S-Plus, v. 7.0, Insightful Corp., Seattle, WA, USA) and the von Bertalanffy growth model:
Juveniles for which sex could not be determined were randomly assigned to either the male or female sets of length-at-age data. Adequacy of fit for all cases was investigated by plotting standardised residuals against observed ages. Growth curves for both sexes were compared, both within and amongst the areas, using a likelihood-ratio test under the assumption that the residual variances differed (Cerrato Citation1990), with significance level adjusted using the Bonferroni method.
Results
The relationships of natural ln-transformed whole wet weight (WW, g) against FL (mm) for snapper from Denham Sound and Eastern Gulf (insufficient length:weight data were available for Freycinet Estuary to allow statistical comparison) were not significantly different (df = 315, between slopes P=0.608, between intercepts P=0.732). After these data were pooled, the relationship ln WW = 2.6703(ln FL) − 8.817 (r 2=0.99) was derived, which, after adjustment for log bias resulted in the non-linear relationship, WW = 0.000148FL2.6703.
Length and age at maturity
For females, mean length at 50% maturity decreased from 420 mm FL in the Freycinet Estuary to 401 mm FL in Denham Sound and to 348 mm FL in the Eastern Gulf (, ). Female mean age at 50% maturity was youngest at 3.2 years in the Eastern Gulf and increased to 4.5 years in the Freycinet Estuary and to 5.5 years in Denham Sound. Males were consistently smaller (by c. 90–120 mm) and younger (by 1.6–2.8 years) at 50% maturity compared with females in all three areas. Mean length and age for males at 50% maturity was smallest (243 mm FL) and youngest (1.6 years) in the Eastern Gulf, and was higher in Denham Sound (276 mm FL, 2.7 years) and the Freycinet Estuary (330 mm FL, 2.7 years).
Fig. 2 Proportions of mature female and male Pagrus auratus in each 50-mm length class (•, right y-axis) during the peak spawning period in Denham Sound, Eastern Gulf and Freycinet Estuary, Western Australia, 1997–2004. Relationship between the proportion of mature fish and length fitted using logistic regression analysis. The numbers of samples within each length class are indicated by the vertical bars and left y-axis (note different scale for females and males). Values of mean length at 50% maturity, L 50, ±95% confidence intervals are indicated by horizontal bars on the fitted curves. (Sample sizes are given in .)
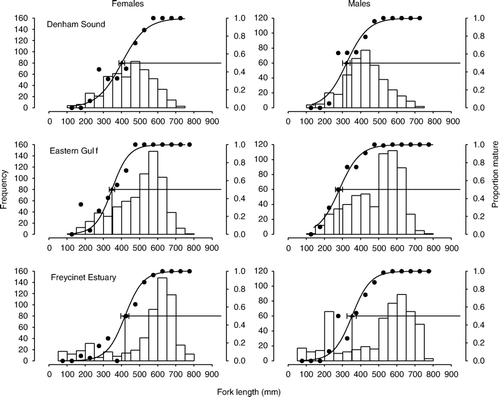
Table 1 Maturity and growth characteristics for Pagrus auratus and key environmental descriptors for the three study areas in Shark Bay, Western Australia
Likelihood-ratio tests indicated that female length at maturity in the Eastern Gulf was significantly different to that in Denham Sound (χ2=46.69, df = 2, P<0.001) and Freycinet Estuary (χ2=30.66, df = 2, P<0.001); however, differences between females in Denham Sound and Freycinet Estuary were not significant (χ2=5.64, df = 2, P=0.059). Male length at maturity in the Eastern Gulf was also significantly different to that in Denham Sound (χ2=11.33, df = 2, P=0.003) and Freycinet Estuary (χ2=22.73, df = 2, P<0.001), whereas differences between males in Denham Sound and Freycinet Estuary were again not significant (χ2=4.30, df = 2, P=0.117). Mean ages at maturity for both females and males differed significantly amongst the three areas (P<0.001 in all instances).
Timing and duration of spawning season
Mean monthly GSIs for both sexes rose sharply from April and peaked between May and July before declining to a base level in September–October in both Denham Sound and the Eastern Gulf (). In the Freycinet Estuary, GSIs rose sharply slightly later, from May, peaked around August–September and declined to a base level in November. Spawning females were recorded between April and September and were dominant in the respective samples between May and July in Denham Sound and the Eastern Gulf (). In the Freycinet Estuary, spawning females were not recorded until June, were present through to October and were dominant in the samples in August–October.
Fig. 3 Monthly gonadosomatic indices (mean±1 SE) for Pagrus auratus in Denham Sound, Eastern Gulf and Freycinet Estuary, Western Australia, 1997–2004, based on fish with lengths greater or equal to their length at 50% maturity. Closed rectangles on x-axis represent winter and summer months; open rectangles, spring and autumn months (numbers are sample sizes).
Growth
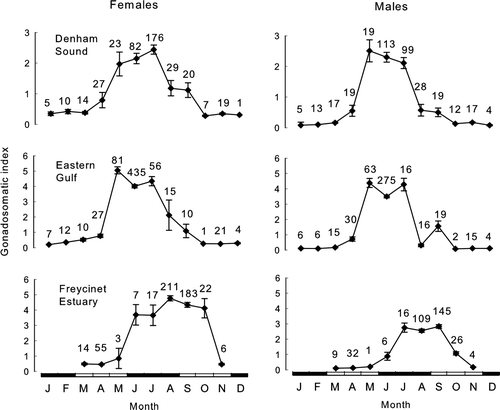
The von Bertlanffy growth curves fitted the length-at-age data from each area reasonably well; coefficients of determination (r 2) were generally high and the mean extrapolated ages at zero length (t 0) were close to zero in most instances (, ). Examination of the residuals indicated no systematic bias for fish from the Eastern Gulf and Denham Sound; however, some deviation was apparent with younger individuals (<3–4 years) of both sexes from the Freycinet Estuary. An a posteriori analysis indicated that the length-at-age data were fitted better by a Schnute & Richards (Citation1990) five-parameter growth model compared with the three-parameter von Bertalanffy model (P<0.001).
Fig. 5 von Bertalanffy curves fitted to lengths at age of female and male Pagrus auratus from Denham Sound, Eastern Gulf and Freycinet Estuary in Shark Bay, Western Australia.
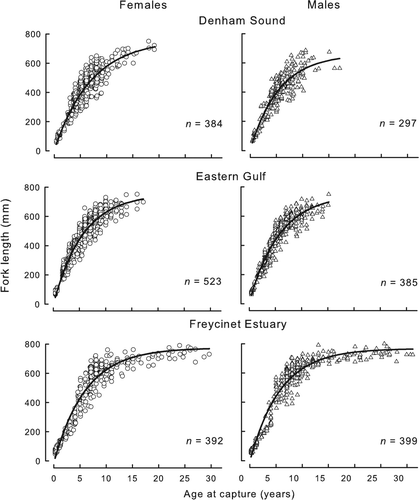
The maximum ages and corresponding fork lengths for both sexes were greatest in the Freycinet Estuary. Older fish (20 + years) were not represented in samples from the Eastern Gulf and Denham Sound in contrast with the Freycinet Estuary, where individuals approaching 30 years of age were sampled in most years of this study. Length at age estimates for both sexes were comparable among the three areas up to c. 3 years after which growth of both sexes in Denham Sound was slower compared with the Eastern Gulf and the Freycinet Estuary (). Likelihood-ratio tests indicated no significant difference in growth curves between females and males in the Eastern Gulf (χ2=4.70, df = 3, P=0.195) and Freycinet Estuary (χ2=3.73, df = 3, P=0.292) but a highly significant difference between sexes for fish from Denham Sound (χ2=11.42, df = 3, P=0.009). When both sexes were compared amongst the areas, growth of both females and males were not significantly different between the Eastern Gulf and Freycinet Estuary (females, χ2=9.48, df = 3, P=0.024; males, χ2=6.94, df = 3, P=0.074) but growth differed significantly for both sexes between these areas and Denham Sound (P<0.001 in all instances).
Table 2 Fork lengths (mm, mean±1 SE) at 5, 10 and 15 years of age for female and male Pagrus auratus from the three study areas inside Shark Bay, Western Australia, including size range of fish within each age group.
Discussion
Variation in life history characteristics of snapper, a large, potentially mobile sparid, was found to occur at a much finer spatial scale (less than tens of kilometres) inside Shark Bay than has been shown elsewhere within the species range or with any closely related marine sparid (e.g. Pagrus pagrus; Potts & Manooch Citation2002). This variation, while unusual for such a teleost inhabiting a marine environment with no obvious physical barriers, could be linked to Shark Bay's marked environmental gradients, historic differences in levels of fishing mortality and the nil or very low levels of mixing at any life-history stage between snapper inhabiting the closely adjacent areas of inner Shark Bay (Edmonds et al. Citation1999; Bastow et al. Citation2002; Gaughan et al. Citation2003; Moran et al. Citation2003; Nahas et al. Citation2003).
Snapper in the Eastern Gulf and Denham Sound mostly spawned in May–July, whereas fish in the Freycinet Estuary mostly spawned later in the year, between August and October. Monthly sea surface temperature data indicate that optimum water temperatures for spawning by snapper (c. 16–21°C, Scott & Pankhurst Citation1992) exist for c. 5–6 months in the Freycinet Estuary compared with only c. 3–4 months in Denham Sound and the Eastern Gulf. The longer spawning season in the Freycinet Estuary likely reflects both the duration of optimal water temperatures and the estuary's greater overall environmental variability. Waters in the Freycinet Estuary are more saline (c. 40–50 + PSU) and the annual temperature range is greater (c. 12°C) than in the Eastern Gulf and Denham Sound (). Seasonal spawning patterns inside Shark Bay are consistent with those observed in other lower latitude snapper populations such as in southern Queensland (Sumpton Citation2002) where environmental conditions are similar to Shark Bay. In contrast, snapper from higher latitudes such as South Australia, Victoria and New Zealand, spawn later in the year, in late spring to late summer (Scott & Pankhurst Citation1992; Coutin et al. Citation2003; McGlennon Citation2003).
There were significant differences in the mean length and age at 50% maturity between sexes with females being consistently larger and older than males in all three areas. Based on estimates derived using reproductive data pooled over the 8-year study, both sexes matured at significantly smaller lengths and younger ages in the Eastern Gulf compared with Denham Sound and the Freycinet Estuary. The smaller lengths and younger ages at maturity may reflect some phenotypic compensatory response to a significant decline in population size in the Eastern Gulf. Although there was no evidence of any major variation in environmental conditions (water temperature, salinity) unique to the Eastern Gulf over the study period, significant recruitment overfishing is acknowledged to have occurred there during the early to mid-1990s, when recreational boat-based fishers caught large numbers of spawning fish in the vicinity of the main spawning aggregation off Monkey Mia (Stephenson & Jackson Citation2005). An egg-production-based survey (Jackson & Cheng Citation2001) and age-based stock assessment modelling (Jackson et al. Citation2005) indicate that spawning biomass in the Eastern Gulf had declined to possibly as low as 10% of the unexploited level by c. 1997–1998. Following the introduction of a moratorium on catching snapper in the Eastern Gulf, which remained in place for almost 5 years (June 1998–March 2003), spawning biomass was estimated to have recovered to around 60% of the unexploited level by 2005 (Jackson et al. Citation2005).
While reduced intraspecific competition is a likely cause, the smaller lengths and younger ages at maturity in the Eastern Gulf may also be linked to some genotypic change resulting from heavy exploitation of larger, older spawners (Trippel Citation1995). Baudains (Citation1999) reported that a number of rare alleles that were present in snapper from the Eastern Gulf that were collected in 1984 (c. 10 years before the acknowledged period of recruitment overfishing), as part of an earlier allozyme-based study (Johnson et al. Citation1986), were absent in samples collected in 1997 (c. 12–13 years after the period of recruitment overfishing). Reasons for the loss of these alleles were unclear; however, evidence of loss of genetic diversity within another heavily fished snapper population has been shown in Tasman Bay, New Zealand (Hauser et al. Citation2002).
Snapper in inner Shark Bay are moderately long-lived with a maximum age of at least 31 years. This longevity is similar to that found in snapper from South Australia (McGlennon et al. Citation2000) and Victoria (Coutin et al. Citation2003), but significantly lower than for fish from the cooler waters of New Zealand (Francis et al. Citation1992). Growth rates were lower for both sexes in Denham Sound compared with the Eastern Gulf and Freycinet Estuary. Differences in growth rates among separate stocks of snapper in New Zealand have been attributed to water temperature, food availability and genetic diversity (Francis Citation1994). Sarre & Potter (Citation2000) found variation in growth rates with the closely related sparid, Acanthopagrus butcheri, among estuaries along the lower west coast of Western Australia, which was attributed to differences in salinity, diet and stock density. Research with juvenile snapper in Shark Bay (30–200 mm FL; Tapp Citation2003) and mature fish in South Australia (W. Hutchinson, South Australian Research and Development Institute, personal communication) found that growth was unaffected at salinities between 35–44 PSU but declined significantly at salinities of 48 PSU or greater. While salinities can exceed 50 PSU in waters at the southern margins of the Freycinet Estuary, most fish reside in waters of salinities c. 40–46 PSU (G. Jackson unpublished data). That growth rates were higher in the Freycinet Estuary and Eastern Gulf suggest that the differences in snapper growth are not wholly explained by differences in abiotic factors.
While there are genetic differences amongst the three areas (Johnson et al. Citation1986; Whitaker & Johnson Citation1998; Baudains Citation1999), differences in growth may be linked to density-dependent factors such as food availability or competition. Lower growth rates in Denham Sound snapper may reflect reduced prey biomass linked with commercial trawl fisheries for scallops and prawns that operate in these waters but not in the Eastern Gulf or Freycinet Estuary (Sporer et al. Citation2008). Such trawling has the capacity to remove large quantities of prey species taken by snapper and thereby potentially limit individual growth. Additionally, stock densities over the period of this study were estimated to be greater in Denham Sound compared with the other two areas (Jackson et al. Citation2005). Growth coefficients for Shark Bay (0.14–0.18/year) were higher than those reported for snapper elsewhere in Australia () with the exception of fish from southern Spencer Gulf, South Australia (0.18/year), where environmental conditions (water temperature, salinity, bathymetry, prey species) are comparable with Shark Bay. In contrast, growth coefficients were lower for snapper from New Zealand (0.10–0.16/year) where water temperatures are generally cooler.
Table 3 Comparison of von Bertalanffy growth parameters for Pagrus auratus from Australia and New Zealand estimated from lengths at age based on sectioned otoliths.
Fish of 15 years of age or more were more common in the Freycinet Estuary where fish of up to 25 years of age were consistently observed in recreational catches up to 2004. The presence of the older fish in this area reflects the greater survival of these fish when they first recruited to the fishery (around 3–4 years of age), when levels of exploitation were lower in 1970s and/or pulses of better than average recruitment over the last 20–30 years. While stocks in Shark Bay's inner gulfs appear to be self-recruiting (Nahas et al. Citation2003), and differences in annual recruitment strength among the three areas cannot be discounted, it is more likely that differences in observed maximum age and age composition amongst the three areas reflect historic differences in fishing pressure. Recreational catches of snapper are thought to have increased only gradually between the 1970s and early 1980s and then more rapidly, between the late 1980s and mid-1990s (Stephenson & Jackson Citation2005). This increase in recreational exploitation is attributed to improved vehicle access to the region and increased fishing efficiency linked to fish finding technology (colour sounders and GPS) (Jackson et al. Citation2005). The progressive introduction of stricter management from c. 1998 onwards, including a 5-year moratorium in the Eastern Gulf (1998–2003), a 6-week spawning closure in the Freycinet Estuary (from 2000 onwards), and the introduction of a Total Allowable Catch for each snapper stock in 2003, has resulted in significantly reduced recreational snapper catches in each area (Jackson et al. Citation2005).
Moderately long-lived species such as snapper can potentially have 20–25 age classes coexisting in a population such as in northern Spencer Gulf, South Australia (Fowler et al. Citation2005). In inner Shark Bay, fish of more than 20 years of age were consistently represented only in samples from the Freycinet Estuary. The lower maximum age and absence of older fish in both the Eastern Gulf and Denham Sound could possibly reflect the effects of higher exploitation during the 1970s–1980s compared with the Freycinet Estuary.
While the importance of understanding stock structure has been long recognised, stock identification is now receiving greater attention at smaller geographic scales for a number of reasons including increased emphasis on biodiversity and a shift towards more cautious management (Stephenson Citation1999). Fisheries management has typically assumed population homogeneity over large geographic scales (ocean-wide) with potentially mobile species. However, there is increasing evidence of population structuring for marine species at scales of hundreds of kilometres and less such as for red snapper, Lutjanus campechanus (Salliant & Gold Citation2006). Differences in life history characteristics such as growth and length/age at maturity are being used to support the management of fish populations at the finer spatial scale, e.g. orange roughy (Hoplostethus atlanticus) in the Tasman Sea off New Zealand (Smith et al. Citation2002), red snapper in the Gulf of Mexico (Fischer et al. Citation2004), carpenter (Argyrozona argyrozona) off South Africa (Brouwer & Griffiths Citation2005), and snapper and West Australian dhufish (Glaucosoma herbraicum) on the west coast of Western Australia (Wise et al. Citation2007).
The results of this study are consistent with those from previous stock identification research and add further support for the fine spatial scale at which local snapper stocks and the recreational fishery in the inner gulfs of Shark Bay are managed (Stephenson & Jackson Citation2005). The variation in important biological characteristics demonstrated in this study supports the need to manage snapper in the Eastern Gulf, Denham Sound and the Freycinet Estuary as separate management units, both to maintain stock productivity and genetic diversity, in particular given the region's World Heritage status.
Acknowledgements
The research was funded by the Natural Heritage Trust (Fisheries Action Program, Projects 973720 and 973340), the Fisheries Research and Development Corporation (Project 2003/066) and the Department of Fisheries, Western Australia. We are grateful to the many colleagues and recreational and commercial fishers of Shark Bay who helped to collect the snapper samples. Thanks to John McKinlay, Monty Craine and Adrian Thomson for assistance with the statistical analyses. This study represents part of PhD-thesis research at the Centre for Fish and Fisheries Research, Murdoch University, by GJ. The authors thank Rod Lenanton, Mike Moran and Stephen Newman, and many anonymous reviewers for constructive comments on earlier drafts of the manuscript.
References
- Anonymous 2006 . Australian fisheries statistics 2005 . Canberra : Australian Bureau of Agricultural and Resource Economics .
- Bastow , TP , Jackson , G and Edmonds , JS . 2002 . Elevated salinity and isotopic composition of fish otolith carbonate: stock delineation of snapper, Pagrus auratus, in Shark Bay, Western Australia . Marine Biology , 141 : 801 – 806 .
- Baudains , G . 1999 . Population genetic structure of pink snapper (Pagrus auratus) in the Eastern Gulf of Shark Bay, Western Australia. Report to Natural Heritage Trust (Canberra) , Perth : Department of Fisheries .
- Begg , GA . 2005 . “ Life history parameters ” . In Stock identification methods: applications in fishery science , Edited by: Cadrin , SX , Friedland , KD and Waldman , JR . 119 – 150 . Amsterdam : Elsevier Academic Press .
- Brouwer , SL and Griffiths , MH . 2005 . Stock separation and life history of Argyrozona argyrozona (Pisces: Sparidae) on the South African east coast . African Journal of Marine Science , 27 : 585 – 595 .
- Cerrato , RM . 1990 . Interpretable statistical tests for growth comparisons using parameters in the von Bertalanffy equation . Canadian Journal of Fisheries and Aquatic Sciences , 47 : 1416 – 1426 .
- Coutin PC , Cashmore S , Sivakumuran KP 2003 . Assessment of the snapper fishery in Victoria . Fisheries and Research Development Corporation final report, project 1997/128 . Victoria : Department of Primary Industries .
- Davies , NM , Hartill , B and Walsh , C . 2003 . A review of methods used to estimate snapper catch-at-age and growth in SNA 1 and SNA 8 , Wellington : Ministry of Fisheries .
- Donnellan SC , McGlennon D 1996 . Stock identification and discrimination in snapper (Pagrus auratus) in Southern Australia . Fisheries and Research Development Corporation final report, project 1994/168 . Adelaide : SARDI Aquatic Sciences .
- Edmonds , JS , Steckis , RA , Moran , MJ , Caputi , N and Morita , M . 1999 . Stock delineation of snapper and tailor from Western Australia by analysis of stable isotope and strontium/calcium ratios in otolith carbonate . Journal of Fish Biology , 55 : 243 – 259 .
- Environment Australia 1999 . Australia's World Heritage Canberra : Department of Environment and Heritage .
- Ferrell DJ 2004 . Young-of-the-year snapper and recruitment to the NSW fishery . Unpublished PhD thesis , University of Sydney , Sydney .
- Fischer , AJ , Baker , MS Jr and Wilson , CA . 2004 . Red snapper (Lutjanus campechanus) demographic structure in the northern Gulf of Mexico based on spatial patterns in growth rates and morphometrics . Fishery Bulletin , 102 : 593 – 603 .
- Fowler AJ , Gillanders BM , Hall KC 2004 . Adult migration, population replenishment and geographic structure for snapper in South Australia . Fisheries Research and Development Corporation final report, project 2002/001 . Adelaide : SARDI Aquatic Sciences .
- Fowler , AJ , McGarvey , R , Feenstra , JE , Jackson , WB and Jennings , PR . 2005 . Snapper (Pagrus auratus) fishery , Adelaide : SARDI Aquatic Sciences .
- Francis , MP . 1994 . Growth of juvenile snapper, Pagrus auratus . New Zealand Journal of Marine and Freshwater Research , 28 : 201 – 218 .
- Francis , RICC , Paul , LJ and Mulligan , KP . 1992 . Ageing of adult snapper (Pagrus auratus) from otolith annual ring counts: validation by tagging and oxytetracycline injection . Australian Journal of Marine and Freshwater Research , 43 : 1069 – 1089 .
- Gaughan D , Moran M , Ranaldi M , Watling J 2003 . Identifying nursery areas used by inner bay and oceanic pink snapper (Pagrus auratus) stocks in the Shark Bay region, in relation to the effect of prawn trawling on inner bay snapper stocks . Fisheries and Research Development Corporation final report, project 2002/061 . Perth : Department of Fisheries .
- Gilbert , DJ and Sullivan , KJ . 1994 . Stock assessment of snapper for the 1992–93 fishing year , Wellington : Ministry of Fisheries .
- Hauser , L , Adcock , GJ , Smith , PJ , Bernal-Ramirez , JH and Carvalho , GR . 2002 . Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus) . Proceedings of the National Academy of Science of the United States of America , 99 : 11742 – 11747 .
- Henry GW , Lyle JM 2003 . The national recreational and indigenous fishing survey . Fisheries and Research Development Corporation final report, project 1999/158 . Cronulla : NSW Fisheries .
- Jackson , G and Cheng , YW . 2001 . Parameter estimation with egg production surveys to estimate snapper, Pagrus auratus, biomass in Shark Bay, Western Australia . Journal of Agricultural, Biological and Environmental Statistics , 6 : 243 – 257 .
- Jackson G , Sumner N , Cribb A , Norriss J 2005 . Comparing conventional social-based and alternative output-based management models for recreational fisheries using Shark Bay pink snapper as case study . Fisheries and Research Development Corporation final report, project 2003/066. Perth, Department of Fisheries .
- Jackson G 2007 . Fisheries biology and management of pink snapper, Pagrus auratus, in the inner gulfs of Shark Bay, Western Australia . Unpublished PhD thesis, Murdoch University, Perth .
- Jackson G , Burton C , Moran M , Radford B 2007 . Distribution and abundance of juvenile pink snapper, Pagrus auratus, in the gulfs of Shark Bay, Western Australia, from trap surveys . Fisheries Research Report No. 161. Perth, Department of Fisheries .
- Johnson , MS , Creagh , S and Moran , M . 1986 . Genetic subdivision of stocks of pink snapper, Chrysophrys unicolor, in Shark Bay, Western Australia . Australian Journal of Marine and Freshwater Research , 37 : 337 – 345 .
- Kailola PJ , Williams MJ , Stewart PC , Reichelt RE , McNeee A , Grieve C 1993 . Australian Fisheries Resources . Canberra, Bureau of Resource Sciences .
- Kimura , DK . 1980 . Likelihood methods for the von Bertalanffy equation . Fishery Bulletin , 77 : 765 – 776 .
- Law , R . 2000 . Fishing, selection and phenotypic evolution . ICES Journal of Marine Science , 57 : 659 – 668 .
- Logan , BW and Cebulski , DE . 1970 . Sedimentary environments of Shark Bay, Western Australia . Memoirs of the American Association of Petroleum Geologists , 13 : 1 – 37 .
- MacDonald CM 1980 . Population structure, biochemical adaption and systematics in temperate marine fishes of the Genera Arripis and Chrysophrys . Unpublished PhD thesis , Australian National University , Canberra .
- Mackie M , Jackson G , Tapp N , Norriss J 2009 . Macroscopic and microscopic description of snapper (Pagrus auratus) gonads from Shark Bay, Western Australia . Fisheries Research Report No. 184. Perth, Department of Fisheries .
- McGlennon , D , Jones , GK , Baker , J , Jackson , WB and Kinloch , MA . 2000 . Ageing, catch-at- age and relative year class strength for snapper (Pagrus auratus) in northern Spencer Gulf, South Australia . Marine and Freshwater Research , 51 : 669 – 677 .
- McGlennon D 2003 . The fisheries biology and population dynamics of snapper Pagrus auratus in northern Spencer Gulf, South Australia . Unpublished PhD thesis , University of Adelaide , Adelaide .
- Meggs , LB , Austin , CM and Coutin , PC . 2003 . Low allozyme variation in snapper, Pagrus auratus, in Victoria, Australia . Fisheries Management and Ecology , 10 : 155 – 162 .
- Moran , M , Burton , C and Jenke , J . 2003 . Long-term movement patterns of continental shelf and inner gulf snapper (Pagrus auratus, Sparidae) from tagging in the Shark Bay region of Western Australia . Marine and Freshwater Research , 54 : 913 – 922 .
- Moran , MJ and Kangas , M . 2003 . The effects of the trawl fishery on the stock of pink snapper, Pagrus auratus, in Denham Sound, Shark Bay. Fisheries Research Bulletin No. 31 , Perth : Department of Fisheries, Government of Western Australia .
- Moran , M , Stephenson , P , Gaughan , D , Tapp , N and Moore , J . 2005 . Minimising the cost of future stock monitoring, and assessment of the potential for increased yields, from the oceanic snapper, Pagrus auratus, stock of Shark Bay. Fisheries and Research Development Corporation final report, project 2000/138 , Perth : Department of Fisheries .
- Nahas , EL , Jackson , G , Pattiaratchi , CB and Ivey , GV . 2003 . Hydrodynamic modelling of snapper (Pagrus auratus) egg and larval dispersal in Shark Bay, Western Australia: reproductive isolation at a fine spatial scale . Marine Ecology Progress Series , 265 : 213 – 226 .
- Nahas , EL , Pattiaratchi , CB and Ivey , GV . 2005 . Processes controlling the position of frontal systems in Shark Bay, Western Australia . Estuarine, Coastal Shelf Science , 65 : 463 – 474 .
- Paulin , CD . 1990 . Pagrus auratus, a new combination for the species known as snapper in Australasian waters (Pisces: Sparidae) . New Zealand Journal of Marine and Freshwater Research , 24 : 259 – 265 .
- Potts , JC and Manooch , CS III . 2002 . Estimated ages of red porgy (Pagrus pagrus) from fishery-dependent and fishery-independent data and a comparison of growth parameters . Fishery Bulletin , 100 : 81 – 89 .
- Quinn , TJ II and Deriso , RB . 1999 . Quantitative fish dynamics , New York : Oxford University Press .
- Quinn , GP and Keough , MJ . 2003 . Experimental design and data analysis for biologists , Cambridge, , UK : Cambridge University Press .
- Salliant , E and Gold , JR . 2006 . Population structure and variance effective size of red snapper (Lutjanus campechanus) in the northern Gulf of Mexico . Fishery Bulletin , 104 : 136 – 148 .
- Sarre , GA and Potter , IC . 2000 . Variation in age compositions and growth rates of Acanthopagrus butcheri (Sparidae) among estuaries: some possible contributing factors . Fishery Bulletin , 98 : 785 – 799 .
- Schnute , JT and Richards , LJ . 1990 . A unified approach to the analysis of fish growth, maturity and survivorship data . Canadian Journal of Fisheries and Aquatic Sciences , 47 : 24 – 40 .
- Scott , SG and Pankhurst , NW . 1992 . Interannual variation in the reproductive cycle of the New Zealand snapper Pagrus auratus (Bloch and Schneider) (Sparidae) . Journal of Fish Biology , 41 : 685 – 696 .
- Smith , PJ , Robertson , SG , Horn , PL , Bull , B , Anderson , OF , Stanton , BR and Oke , CS . 2002 . Multiple techniques for determining stock relationships between orange roughy, Hoplostethus atlanticus, fisheries in the eastern Tasman Sea . Fisheries Research , 58 : 119 – 140 .
- Sporer , E , Kangas , M and Brown , S . 2008 . “ Shark Bay prawn and scallop managed fisheries status report ” . In State of the fisheries report 2008/09 , Edited by: Fletcher , WJ and Santoro , K . 93 – 101 . Perth : Department of Fisheries, Government of Western Australia .
- Stephenson , P and Jackson , G . 2005 . “ Managing depleted snapper stocks in inner Shark Bay, Western Australia ” . In Fisheries assessment and management in data-limited situations , Edited by: Kruse , GH , Gallucci , VF , Hay , DE , Perry , RI , Peterman , RM , Shirley , TC , Spencer , PD , Wilson , B and Woodby , D . 31 – 50 . Fairbanks, AL : Alaska Sea Grant College Program .
- Stephenson , RL . 1999 . Stock complexity in fisheries management: a perspective of emerging issues related to population sub-units . Fisheries Research , 43 : 247 – 249 .
- Sumpton WD 2002 . Population biology and management of snapper (Pagrus auratus) in Queensland . Unpublished PhD thesis , University of Queensland , Brisbane .
- Sumpton , WD , Ovenden , JR , Keenan , CP and Street , R . 2008 . Evidence for a stock discontinuity of snapper (Pagrus auratus) on the east coast of Australia . Fisheries Research , 94 : 92 – 98 .
- Tapp N 2003 . Do size differences of juvenile snapper (Pagrus auratus) in two regions of Shark Bay, Western Australia, reflect different environmental conditions? Unpublished MSc thesis , Edith Cowan University , Perth .
- Trippel , EA . 1995 . Age at maturity as a stress indicator in fisheries . BioScience , 45 : 759 – 771 .
- Whitaker , K and Johnson , M . 1998 . Population genetic structure of Pagrus auratus in the Western Gulf of Shark Bay, Western Australia. Report to Natural Heritage Trust (Canberra) , Perth : Department of Fisheries .
- Wise BS , St John J , Lenanton R 2007 . Spatial scales of exploitation among populations of demersal scalefish: implications for management. Part 1: Stock status of the key indicator species for the demersal scalefish fishery in the West Coast bioregion. Fisheries Research Report No. 163 . Perth : Department of Fisheries, Government of Western Australia .
- Wyatt , ASJ , Hewitt , CL , Walker , DI and Ward , TJ. 2005 . Marine introductions in the Shark Bay World Heritage Property, Western Australia: a preliminary assessment . Diversity and Distributions , 11 : 33 – 44 .