Abstract
The article reports the outcome of long-term demographic monitoring of elevational treeline ecotonal stands of Scots pine (Pinus sylvestris L.) in the southern Swedish Scandes. Annual censuses were undertaken of recruitment, mortality, growth, seed viability, and causes of mortality during the period 1973–2012 in a set of 18 permanent plots. A net gain in total population size occurred over the study period as the result of periodic fluctuations in recruitment and mortality, broadly congruent with annual variations in summer and winter temperatures. Summer temperatures affected seed viability, establishment, and height growth. Winter temperatures exerted an impact foremost by changing the incidence of winter desiccation injury. As a result of infilling, pine has become a more prominent and vital component of the landscape around the forest limit, which has advanced insignificantly into the bordering mountain birch forest. The results highlight a rigid climate-forest disequilibrium and falsify models suggesting extensive and swift expansion of the treeline ecotone and closed forest at the expense of alpine tundra in response to anticipated future warming during the present century. The balance of evidence suggests that patchy forest expansion is likely to occur at a very slow (plurisecular) pace, given that climate warming prevails.
Introduction
Dynamic performance of mountain treelines is a central aspect of global change ecology. One reason for concern is the role of treelines as sensitive bellwethers of large-scale ecologically relevant climate change and variability in high-mountain regions (Kullman Citation1998; Holtmeier & Broll Citation2005; Kharuk et al. Citation2009). A prevailing, although empirically unsupported paradigm is that a hypothetically warmer climate will induce a swift advance of the treeline ecotone into alpine and arctic tundra during the present century (ACIA Citation2005; Kaplan & New Citation2006).
Due to their stature, trees affect the local climate and capture a large proportion of available resources (light, nutrients, and water). Hence, their presence or absence may exert an impact on ground floor vegetation and biodiversity in general in high mountain regions (Camarero & Gutiérrez Citation2004; Lloyd Citation2005; Holtmeier Citation2009; Hofgaard et al. Citation2013a). Thus, realistic projections of future treeline trajectories are essential for proper landscape management.
A coupling between elevational treeline shifts and climate change and variability (mainly temperature) since the early 1910s has been inferred from retrospective studies in different parts of the world, based on various approaches such as remote sensing, age structure analyses, and observations in permanent transects (Kullman Citation1979; Danby & Hik Citation2007; Shiyatov et al. Citation2007; Batllori & Gutiérrez Citation2008; Harsch et al. Citation2009; Kharuk et al. Citation2009; Kullman & Öberg Citation2009; Elliott Citation2011). Moreover, the recognition of a treeline as ultimately a heat deficiency phenomenon (cf. Körner Citation2007) is supported by paleoecological data showing treelines positioned substantially higher and further north during parts of the Holocene and in much warmer conditions than today (Kremenetski et al. Citation1998; Nicolussi & Patzelt Citation2000; Helama et al. Citation2004; Bergman et al. Citation2005; Tinner & Kaltenrieder Citation2005; Paus Citation2010; Öberg & Kullman Citation2011).
A deeper comprehension of treelines and their ecological complexities, as a basis for meaningful projections into the future, is conditional on further progress within the fields outlined above and particularly for the generation of predictive climate-treeline models. This goal can only be achieved by focusing on widely different spatial and temporal scales as well as experimental and observational studies of natural systems (cf. Holtmeier Citation2009; Leonelli et al. Citation2011; Malanson et al. Citation2011).
Long-term and high-resolution demographic monitoring, particularly aiming at early life stages are recognized by several authors as an avenue to gain a firmer understanding of cause and effect and the ways in which annual climate (weather) variations and other agents influence and constrain treeline life and performance (cf. Juntunen et al. Citation2002; Graumlich et al. Citation2005; Holtmeier & Broll Citation2005; Batllori & Gutiérrez Citation2008; Walker et al. Citation2012). Although highly important in many respects, virtually all existing studies within this field are based on censuses, separated by years or decades. This leaves uncertainties as to potentially important mortality mechanisms, particularly infrequently occurring ones and their timing. A demographic approach, accounting annually for major stages of the regeneration process (i.e. recruitment, mortality, and growth) would ideally contribute to improve the interpretability of studies of static age structure (cf. Ågren & Zackrisson Citation1990), a common method for reconstructing treeline performance in the recent past and its association with climate variability (e.g. Batllori & Gutiérrez Citation2008; Elliott Citation2011). Some studies of this character have produced ambiguous results because they are solely based on more or less arbitrary assumptions concerning the relative importance of mortality and recruitment in the past (e.g. Van Bogaert et al. Citation2011), which may represent different periods of time in a way that is not easily understood in retrospect (cf. Johnson et al. Citation1994; Villalba & Veblen Citation1997).
Direct demographic and long-term temporally tight monitoring in treeline ecosystems is rarely undertaken (for an exception see Dobbertin & Rigling Citation2006). The present study updates such a monitoring demographic project concerning the treeline of Scots pine (Pinus sylvestris L.) in the southern Swedish Scandes (Kullman Citation2007a). The baseline inventory was made in 1973 and subsequently annual censuses were undertaken by the present author until 2012. This was an open-ended observational study without preconceived hypotheses. The primary objective was to increase the understanding of how treelines are formed and maintained. This aim was achieved by monitoring seed-based regeneration, growth, and mortality processes over a long period of time at the level of individuals and thereby to identify possible relationships with climatic and other environmental variables (e.g. herbivory and land use). A secondary objective was to view the monitoring results from the perspective of the history of the pine treeline ecotone since at least as early as the early 11th century, as it has emerged from other studies in the same region (reviewed by Kullman Citation2013a). In this way, a virtually new dimension was added to the monitoring endeavour. With empirical data now covering 40 years, and viewed in a historical perspective, there is a firm and truly unique basis for discussing the future performance of the treeline ecotone in hypothetical cases of future climate warming.
Study area
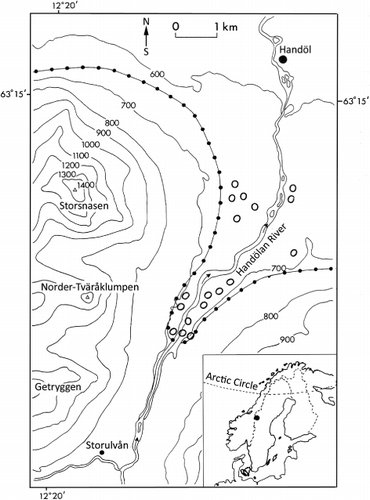
The study was carried out in the Handölan valley in the southern Swedish Scandes (64°14′ N; 12°25′ E) (). The valley floor is at 650–710 m a.s.l. and the surrounding mountains rise to 1100–1500 m a.s.l. Selective logging of large pines in the early 20th century occurred to a minor extent. Sites with traces of such activities are disregarded in this article. The climate is intermediate between oceanic and maritime types. The nearest meteorological station is Storlien-Visjövalen) (642 m a.s.l., 20 km to the north-west). Mean temperatures for January, July, and the year in the period 1961–1990 were −7.6 °C, 10.7 °C, and 1.1 °C, respectively. The annual precipitation is 850 mm, of which 45% falls as snow (all meterological data sourced from Swedish Meteorological and Hydrological Institute (SMHI Citation2012)). The Storlien-Visjövalen weather station is located at virtually the same elevation as the permanent plots. A more detailed account of the present-day landscape and its plant cover is provided by Kullman (Citation2007a).
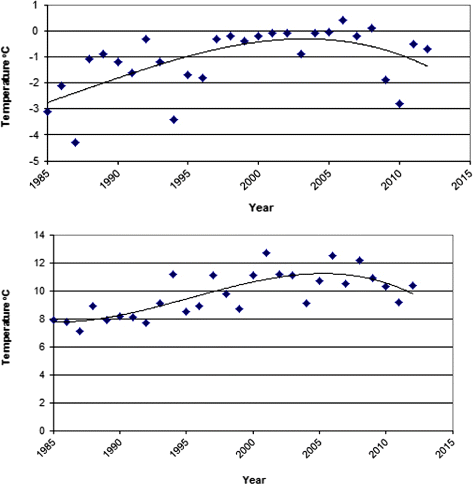
Climate evolution in the study area since 1902 is represented by data from the Storlien-Visjövalen weather station. During the period 1902–2012, the summer (June–August) and winter temperatures (December–February) rose by 1.5 °C and 0.8 °C respectively. However, that long-term sequence also embraced shorter periods with cooling climate. Since 2002 and 1992, summer and winter temperatures have declined significantly from a fairly high level (Kullman & Öberg Citation2009; Kullman Citation2013b). Precipitation in the study region has increased by 5–10% since the early 20th century, and most significantly by 10–20% during the summers since the mid-1990s. Concurrently, winter precipitation remained fairly stable (Alexandersson Citation2006; Eklund Citation2012).
Materials and methods
Permanent plots
The study reported in this article was based on a set of 18 permanent plots that were established in 1973 in the Handölan valley, within the southern part of the Swedish Scandes () and are within the Northern Boreal Zone (Ahti et al. Citation1968). The plots (680–715 m a.s.l.) were distributed on the southern fringe of a pine penetration wedge southwards and upwards into the valley, 0–5 km south of the nearest continuous stands of pine and spruce (Picea abies), positioned at virtually the same elevation. The location is a few tens of metres below the upper treeline of pine, which is 760 m a.s.l., and 200–250 m below the mountain birch (Betula pubescens ssp. czerepanovii) treeline on adjacent mountainsides. For both species, the treeline is defined as the uppermost trees that are at least 2 m tall.
The ground-floor vegetation within the plots at the study site is composed predominantly of mat-forming dwarf shrubs, Empetrum hermaphroditum, Vaccinium vitis-idaea, Calluna vulgaris, and Vaccinium myrtillus. Characteristically, the maximum snow depth rarely exceeds 0.2 m and the snow disappears much earlier than in most parts of the surrounding mountain birch forest.
At the onset of the project, widely scattered veteran pines, 250–500 years old and mostly moribund, prevailed in a matrix of relatively open subalpine heath birch forest (Betula pubescens ssp. czerepanovii) and subalpine mires. Those trees were the trailing edge of a long-term and virtually continuous pine treeline retraction, which had prevailed with only short-term reversals since the early Holocene thermal optimum c.9500 years ago (Kullman Citation2013a). Most of the plots embraced small pine stands, where most existing trees were gradually extirpated during the Little Ice Age (AD 1300–1900), without leaving any persistent offspring (Kullman Citation1987; Citation2005). Accordingly, botanists working in the study area by the turn of the 19th century reported a prevalence of dead and dying pine stands (reviewed by Kullman Citation1986).
Conspicuously, by the early 1970s, the old remnant pines were surrounded, within a circumference of a few tens of metres, by cohorts of saplings and young trees, presumably their offspring. Most of the juvenile pines were between 0.5 m and 1 m high and, based on counting of branch whorls, appeared to have established predominantly between the late 1930s and mid-1950s (Kullman Citation2007a). The strikingly unbalanced age structure, displaying plenty of young offspring and the virtual absence of effective regeneration for centuries, indicated that a fundamental environmental shift had been initiated during the preceding few decades, presumably in response to climate warming, as suggested also by observations of analogous pine treeline performance in northern Finland (e.g. Hustich Citation1958). These circumstances highlighted the need for implementation of a continuous monitoring effort.
Each of the plots (10 × 10 m) contained one old pine and a major part of the surrounding young-growth cohort. For each year (mid-July) between 1973 and 2012, all pines were carefully mapped (presence/absence) and tallied with respect to their size and vitality. A particularly intense search was made in order detect possible new recruits, although some tiny 1st-year seedlings may still have been overlooked. The botanical nomenclature in this article refers to Mossberg & Stenberg (Citation2003).
Foliage vigour
Each annual survey included a visual assessment of the percentage of needle/shoot mortality relative to the total needle mass of each pine. These data were presented as the annual frequency of saplings with more than 20% of the foliage dead since the previous census. When possible, a diagnosis of the cause of death was made (e.g. winter desiccation, fungi infection, or mechanical injuries imposed by reindeer or elk). The records enabled an individual anamnesis for the pines that had died. Thereby, some comprehension of ultimate and proximate death causes could be gained.
Seed germinability tests
Each year during the study period, in late winter 25 cones were collected from the south-facing side of the oldest pine tree in all plots. Germinability was tested in the laboratory according to a procedure described by Kullman (Citation1984).
Ground temperature records
Ground temperatures during both summer and winter are supposed to affect both the establishment of pine seedlings as well as their winter survival over the sapling stage (e.g. Kellomäki et al. Citation1997; Juntunen et al. Citation2002). Since 1985, root zone temperatures (30 cm below ground surface) have been recorded by resistance thermistors at two spots (averaged) in one of the sample plots. At this depth in the ground, short-term temperature variations are significantly damped (cf. Harris Citation2001; Körner Citation2007), as evidenced also by daily measurements during some shorter periods in all seasons. Thus, reading one or two times per month should give a fairly true representation of ecologically relevant annual variations in soil temperature regimes. For the purpose of this article, the highest and lowest records for July–August and February–March respectively are presented. A more detailed account of these measurements and their premises and settings is provided by Kullman (Citation2007a).
Results
Demographic trends in relation to thermal variations
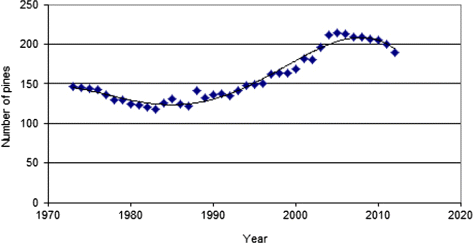
Winter and summer temperature evolution recorded at the Storlien-Visjövalen weather station during the monitoring period is presented in . Over the entire interval 1973–2012, the total population size increased by 28.9%, although the distinctly rising trend between 1988 and 2005 flattened or even turned down slightly thereafter (). Broadly, the form of the population curve for the period 1972–2012 is congruent with the curves of summer and winter temperatures as they appear in . The population trend correlates positively and significantly with the course of summer temperatures (r2 = 0.19, p < 0.01) and winter temperatures (r2 = 0.12, p < 0.01).
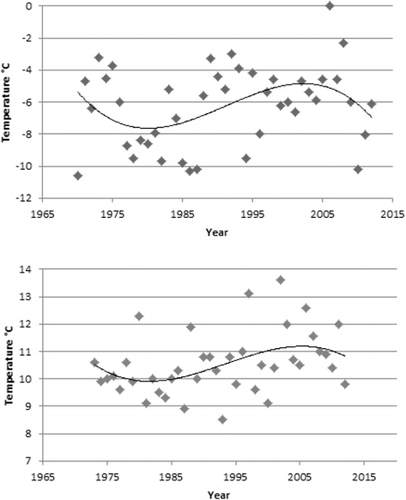
Of the initial cohort, from which a census was taken in 1973, 56% of pines were still alive in 2012. Despite the different densities of young pines, all plots showed virtually the same demographic trends as the composite of all plots. In detail, three distinct and contrasting phases could be discerned in the process (). The three phases are defined in , together with corresponding regional mean air temperatures for the summer and winter periods. Clearly, the periods were too short to test for statistically significant differences.
Table 1. Summer and winter air temperatures (°C ± SD) for periods with different population trends
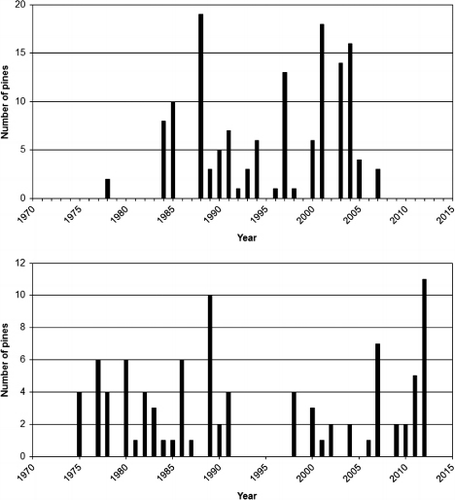
When viewing the three periods separately with respect to recruitment versus mortality, as show in , it appears that the periods of decline (1973–1987 and 2006–2012) where characterized by both relatively high mortality and low recruitment, while the expansion period (1988–2005) displayed high recruitment and low mortality.
Tree population expansion during 1988–2005 coincided with a period of exceptionally high winter temperatures and the second highest summer temperatures recorded. The other two periods, characterized by decline, experienced relatively low winter temperatures and quite different peaks in the summer temperatures. Notably, the period 2006–2012, with the marginally highest mean summer temperature, was not accompanied by population expansion.
With the exception of one of the old mother trees, all trees in each of the sample plots survived and retained or slightly improved their vitality (measured by their needle mass) during the monitoring period. However, during the winter of 2005–2006, the most marginal tree (aged c.450 years) in this category of mother trees was broken and succumbed during a severe storm.
Net population increase in the period 1988–2005 and decline in the period 2006–2012 () were roughly congruent with the course of the recorded root zone temperatures during the period 1985–2012 ().
Seed germinability
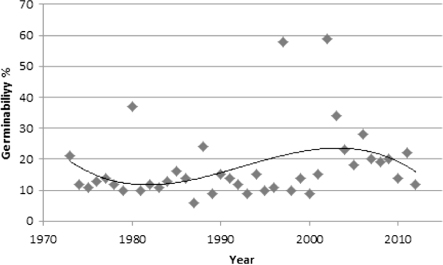
The germinability percentage of annually tested pine seeds shows a slight downturn in the period 1980–1987 (i.e. during the first period of decline). An upturn during the expansion period 1988–2005 was followed by a downturn during the period 2006–2012 (). Throughout the entire study period (1973–2012), viability correlated significantly (r2 = 0.75, p < 0.001) with the course of the current year's summer (June–August) air temperature as recorded at Storlien-Visjövalen weather station.
Foliage vigour and dieback
The main type of foliage dieback, as recorded in mid-summer each year, was asymmetric (south-southwest aspect) needle ‘reddening’ of needles, which was diagnosed in accordance with Tranquillini (Citation1979) as being a consequence of winter desiccation. During the period 1973–1994, the incidence of this type of injury was very high and removed a large proportion of the annual biomass production. Quite characteristically, by the early summer, the reddish pines constituted a discernible feature of the treeline landscape (Kullman Citation1993). A significant trend break occurred 1995, after which the destructive phenomenon was insignificant with the exception of a minor peak in 2010 ().
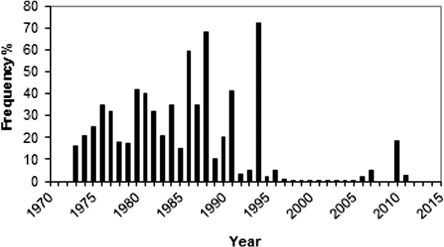
Winter desiccation was judged to have been the ultimate cause of death for 93% of the pines that died during the monitoring period. In most cases, the death occurred after repeated damages during a period of 3–5 years following the first severe needles losses (> 20% of the foliage), which would have increased the susceptibility to other stressors (e.g. snow blight (Phacidum infestans Karsten)). The large majority of pines that had died due to winter desiccation during the study period ranged in height between 0.3 and 1.0 m. This circumstance highlights the hazardous zone just above the wintertime snow-air interface, where the radiation heating of tissues (a prerequisite for winter desiccation) usually is most intense and injurious (cf. Tranquillini Citation1979; Holtmeier Citation2009).
A minor, although recurrent, mortality agent was mechanical injuries caused by reindeer and elk when rubbing their antlers against young pines. Consistently, this type of impact was preceded by some years of substantial winter desiccation. Thus, it appears that these animals prefer pines (1–2 m high) with these symptoms of major weather damages. Some of the largest saplings growing immediately beneath the often wide canopies of the old parent pines were mechanically damaged by the lower branches of the mother trees, which rendered their future quite uncertain.
Defoliation by severe winter desiccation did not necessarily resulted in the death of individual saplings. During the latest year (in the study period) with frequent winter desiccation, 1994, also large trees were affected with 50–70% needle mortality (Kullman Citation1997). The majority had recovered by 2013 (), although some old large trees had died beyond the study plots.

Height growth
Disregarding saplings lower than 0.1 m, the height of all living pines (mean ± SD) in 1973, 1981, 1991, 2005, and 2012 was 0.8 ± 1.4, 1.6 ± 1.2, 2.0 ± 1.7, 2.3 ± 2.1, and 4.5 ± 4.0 m respectively. Thus, it appears that the growth of surviving pines accelerated between 2005 and 2012, when terminal leaders of the largest saplings and small trees frequently attained annual increments of c.30–50 cm. The average time required to reach a size of 2 m was c.30 years. Characteristically, the tallest (oldest) members of the sapling cohorts overtopped the ‘mother trees’ in each of the plots. Although no precise data exist, it is striking that the needles were exceptionally long after the mid-1990s ().
Landscape change
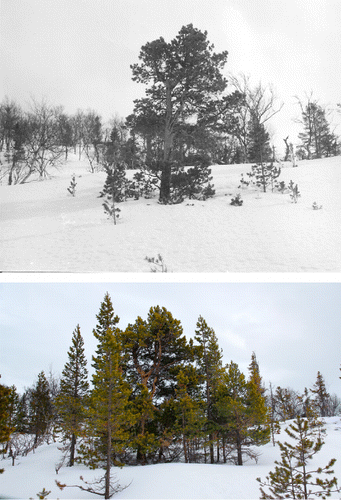
Rapid height growth, which transformed small saplings into trees during the study period, implies that pine has become more prominent in the treeline ecotone (i.e. the tension zone with more or less closed forest and the mountain birch forest). This phenomenon is highlighted more generally by some repeat photos of the permanent plots and other outlier stands in the Handölan valley (–).


The overall progressive trend of marginal pine vegetation is further substantiated by advancement of the treeline, both altitudinally and southwards towards the head of the Handölan valley. Previous studies comparing treeline positions in 1915 and 2007 at five locations in the same valley revealed an average upshift by 65 m (Kullman & Öberg Citation2009). Over the same period of time, pine penetrated deeper into the valley, and by the end of the study period solitary well-grown pine trees and occasional saplings could be found growing along c.10 km stretch to the south of the position held by the mid-1970s and at an elevation 140 m higher (). Notably, the treeline shifts were based on few and widely scattered pines, most of which were healthy and had grown quite rapidly since the mid-1990s (Kullman Citation2010a). Some of these solitary and outlying trees had produced offspring in their immediate vicinity (Kullman Citation2010a). Predominantly, the new individuals had established at sites with particularly sparse birch tree cover and on open mires.

In contrast to the above-defined dynamic treeline, the position of the ‘forest limit’ (i.e. stands with a size > 0.25 ha) has remained virtually unchanged since the first decade of the 20th century (Kullman Citation1986; Citation2010a). Climate-driven tree-population growth has prevailed as infilling in a narrow zone around the forest limit. Earlier studies have shown that the process was initiated already by the early 20th century (Kullman Citation1986; Citation2005). Currently, the landscape has the character of a mosaic of healthy pine forest stands, mires, heaths, and patches with mountain birch forest, with pine advancing into the latter vegetation types (). In great contrast, extensive pine deforestation took place there during the cold centuries AD 1300–1900 (the Little Ice Age), as evidenced by numerous radiocarbon-dated pine megafossils prevailing in currently more or less treeless parts of the landscape (Kullman Citation1987; Citation2005).
Discussion
The population context
The findings from the 1973–2012 study sustain the traditional view that the pine treeline ecotone is shaped and maintained by demographic processes (tree establishment) that are ultimately driven by temperature and involve virtually all life stages. The generic nature of this finding (cf. Elliott Citation2012) is further supported by a treeline positioned as much as 600 m higher during the warmest epoch of the Holocene than the present-day treeline in the study region (Kullman & Öberg Citation2012; Kullman Citation2013a).
The close relationship between pine performance and temperature variability is evident when the 40-year monitoring period is viewed in detail. Both mortality and recruitment have varied substantially over time. The resulting demographic evolution is formed in a wave-like pattern by episodic recruitment and mortality pulses, a common feature of marginal populations responding to variable climatic conditions (e.g. Peet Citation1981; Payette & Filion Citation1985; Batllori & Gutiérrez Citation2008). Clearly, periodic population ‘upsurges’ have been the combined outcome of high recruitment and low mortality, while decline has bee related to low recruitment and relatively high mortality. By extension, this implies that detailed interpretations of static treeline age structures may be misleading if they are based on an assumption that mortality has been constant over time and that obtained age structures predominantly represent the recruitment history of a specific population (cf. Batllori & Gutiérrez Citation2008; Elliott Citation2012).
Pine recruitment may be quite a complex process, depending in principle on seed viability and seed bed conditions as well as patterns and processes in the forest floor vegetation (e.g. Pearce et al. Citation1988; Ågren & Zackrisson Citation1990). In the present case study, it appears that the net population curve () broadly corresponds with the curve for annual seed viability (). This argues for a certain climate-driven impact of this parameter, particularly as seed viability displayed a clear and positively significant relationship with the summer temperature (June–August) of the current year, consistent with earlier findings (cf. Henttonen et al. Citation1986). A more rigorous statistical test of this relationship would not be meaningful because germinability data are annual whereas population size integrates environmental variability over several years.
The overriding cause of mortality was judged to be winter desiccation of needles and shoots, prevailing just above the snow surface. This is a well-known hazard in general to young conifers growing close to the treeline (Tranquillini Citation1979; Frey Citation1983; Veijola Citation1998; Holtmeier Citation2009), which is usually interpreted as caused by frozen soil and strong solar heating of needles in the late winter and spring (i.e. frost desiccation). In the 1973–2012 study, the incidence of this type of injury was almost entirely confined to the period 1973 to 1994 (). Soil temperatures recorded after 1985 showed an all-time-low during the period.
Overall, the course of population expansion over the entire period 1973–2012 showed a positive relationship with winter temperatures as well as summer temperatures. It therefore appears that treeline performance integrates both summer and winter climatic conditions, which appear as controlling factors driving population dynamics (cf. Kullman Citation1997; Citation2007a; Sturm et al. Citation2005; Payette Citation2007; Rickebusch et al. Citation2007; Harsch et al. Citation2009; Kharuk et al. Citation2009; Elliott Citation2012).
Population declines, particularly during cold winter periods, with preconditions for winter desiccation, seemed to be enhanced by mechanical damage caused by reindeer and elk. These suggested findings are consistent with earlier findings suggesting that such impacts may retard pine stand-level expansion in response to more congenial climatic conditions (e.g. Stöcklin & Körner Citation1999; Holtmeier & Broll Citation2011).
A notable feature emerging from the results of the study is that height growth accelerated during the period 2006–2012, while the total size of the population declined. Most likely, decreased seed viability () is part of the reason behind this discrepancy. The production of large seed crops with high viability depends on at least two consecutive relatively warm summers (Kellomäki et al. Citation1997). However, this prerequisite was not fulfilled during the period in question, despite the fact that the average summer temperature was relatively high. On the contrary, height growth responds positively to just one warm summer (Junttila Citation1986), which matches the actual course of summer temperature during the period 2006–2012 (). Thus, the discrepancy in response between recruitment and height growth is a reflection of the fact that the threshold for growth and survival of trees is much lower than for recruitment (e.g. Helland Citation1912; Kullman Citation1979). Consequently, treeline rise may continue for decades after peak warming, by continued height growth of pre-established individuals (Kullman Citation1979).
The landscape context
Concurrent with a dominant trend of summer and winter climate warming, cold-marginal pine populations in the study area have increased in size and density, at the same time as the treeline has expanded against the general landscape-scale thermal gradient. The low incidence of frost desiccation injury after 1994 implies a general vitalization of the treeline ecotone, since reddish discoloured pines have virtually disappeared in favour of fresh green and rapidly growing saplings and young trees. Hence, pine has gained a more prominent role in the marginal forest landscape as previous sparse stands with predominantly old-growth senescent pines have proliferated.
The current course of pine progression has contributed to a certain ‘greening’ of the mountain landscape, which has been discerned since the 1950s as being in response to a general growth-promoting climate change (Smith Citation1957; Veijola Citation1998; Kullman Citation2010a; Citation2010b). This change counters a previous trend of elevational pine recession in response to orbitally forced climate cooling (Neoglacial) since the early Holocene and until the early 20th century. During that interval, the treeline of pine was depressed by at least 600 m, and vast areas in the Scandes were transformed into alpine tundra, peatlands, and mountain birch forest (Bergman et al. Citation2005; Paus Citation2010; Kullman Citation2013a).
The trajectories outlined above are broadly paralleled in pine treelines in northern Finland (Veijola Citation1998; Juntunen et al. Citation2002; Holtmeier & Broll Citation2011). This unifying inter-regional pattern further stresses the role of climate warming as the driving factor for tree population growth. In addition, and from the perspective of a wealth of supporting literature (e.g. Kellomäki et al. Citation1997; Ainsworth & Long Citation2005; Martinez-Vilalta et al. Citation2008; Parn Citation2012), it should be contemplated whether ‘CO2 fertilization’ in combination with climate warming has contributed to the recent pine expansion, in particular the increased vigour and rapid height growth of young pines (cf. ).
It is important to stress that current pine progression and increased pine prominence since the early 1970s is most discernible in the close vicinity of old-established solitary trees and small outlier stands in the lower mountain birch forest, as reported also from treelines in other parts of northern Fennoscandia (e.g. Stöcklin & Körner Citation1999). Possibly, this phenomenon depends on particularly conducive preconditions for growth and survival at these specific sites, as indicated by the long-term persistence of the ‘mother trees’, while pines in the surrounding landscape perished in response to late Holocene cooling. Since the wide-crowned old pines intercept much of the falling snow, the ground snow cover is usually relatively thin at these specific sites (cf. Säppänen Citation1961), which appears as a general prerequisite for pine growth and survival at the treeline (Aas & Faarlund Citation2000; Kullman Citation2010a). In addition, most of the seed fall takes place in the near circumference of the solitary veteran trees (cf. Holtmeier Citation1974), which further restricts their dispersal in a landscape where subalpine birch forest hampers the dispersal of pine seeds. In addition, increasing stand density may promote favourable conditions for growth and survival in a feedback process, which implies alleviation from wind stress and frost-desiccation.
Notably, not one spruce seedling became established in any of the plots during the study period, which adds to the contention that outlier pines and pine regeneration at the margin of distribution are bound to habitats with qualities that are particularly suitable for pines. In addition to a sparse snow cover, a relatively thin humus layer may be part of the explanation for the lack of spruce seedlings. In this context, it may be of some interest that occasional saplings of the non-native Pinus cembra have emerged in some of the plots since the mid-1990s, presumably spread by the Siberian nutcracker (Nucifraga caryocatactes macrorhynchus) from commercial or ornamental plantations, of which the nearest are 5–10 km distant. In one of the plots (680 m a.s.l.) emerged a young specimen of Betula pendula (1.7 m high), a relatively warmth-demanding birch species that until then had not been recorded that close to the treeline.
Reasonably, the old mother pines and their current offspring may be functionally important as dispersal nodes if preconditions for landscape-scale pine expansion should occur (cf. Slot et al. Citation2005; Trant et al. Citation2011). To some extent, this option has been sustained since the early 1990s, when scattered young pine saplings started to appear sparsely but quite regularly in the alpine tundra, 600–700 m above the permanent plots (Kullman Citation2007b; Citation2007c). However, climate cooling since the early 2000s has exterminated a large proportion of the most ‘high-flying’ saplings (Kullman Citation2013b).
Treeline versus forest-limit: sensitivity versus inertia
Despite the post Little Age warming and the associated pine progression, the landscape has far from recovered to the more luxuriant and productive character that prevailed during the Medieval Warm Period, 900–1100 years ago (). The pine forest limit and treeline (defined above) are still lower than they were at that time (Kullman Citation2013a), which appears to be a general phenomenon (e.g. Payette Citation2007; MacDonald Citation2010). Substantial treeline upshift since c.1915, but a virtually stable forest line, indicate that a more massive altitudinal forest expansion of pine against a thermal and/or wind gradient up to the new treeline may occur, although it will probably be delayed for several centuries, presumably due to seed shortage, dispersal constraints, and other local circumstances, rather than heat deficiency at these elevations (cf. Shiyatov Citation2003; Holtmeier Citation2009; Kullman Citation2010a). Particularly the broad and dense mountain birch belt appears to be a main dispersal obstacle. Thus, a major and broad-scale elevational expansion of pine forest can only be achieved in a climate that substantially reduces the vigour of the mountain birch (Kullman Citation2010a). Although not anticipated at the present, this might happen quite rapidly if a certain threshold in gradual climate evolution is passed. The Holocene history of the treeline ecotone contains indications of such abrupt shifts in the balance between pine and birch as treeline dominants (Kullman Citation2013a). Furthermore, drawing on paleoecological evidence (Kullman & Öberg Citation2012; Kullman Citation2013a), any future pine forest upslope expansion will take the form of emerging patches in particularly congenial habitats.

The inference of a substantial forest-limit and/or climate disequilibrium concurs with general modelling results concerning shifts of closed forest in upper marginal situations (e.g. Campbell & McAndrews Citation1993; Körner Citation2005) and real world reconstructions (e.g. Hiller et al. Citation2001; Payette Citation2007; MacDonald Citation2010; Paus Citation2013; Hofgaard et al. Citation2013b). Experiences concerning mountain birch forest limit dynamics have demonstrated an analogous disequilibrium (Kullman Citation2010a). This implies that monitoring programmes to detect early signs of ecologically important climatic changes should focus on the more synchronously responding treeline (Kullman & Öberg Citation2009) rather than on any types of ‘forest limit’ (Körner Citation2007; Kullman Citation2010a; Harsch & Bader Citation2011).
Taken together, the circumstances highlighted above provide little factual evidence for a pending broad-scale forest encroachment on the alpine and arctic tundra, as frequently postulated (Moen et al. Citation2004; ACIA Citation2005; Kaplan & New Citation2006). The situation may be somewhat different at the southern and eastern margin of the Swedish Scandes, with relatively low and isolated mountains, a more continental and/or drier climate, and a less vigorously competing birch belt. In these landscapes, pine has frequently tended to replace the sparse and declining mountain birch belt since the early 20th century (Engelmark & Zackrisson Citation1985; Kullman Citation2012).
Conclusions
In summary, long-term demographic monitoring of elevational treeline ecotonal stands of Scots pine in the southern Swedish Scandes revealed the following:
In accordance with fluctuating summer and winter temperatures, altitudinal range-edge populations of pine increased by c.30% during the period 1973–2012.
The demographic dynamics were driven by periodically variable recruitment and mortality rates.
Mortality was accomplished primarily by winter conditions, foremost winter desiccation and mechanical injuries caused by reindeer and elk.
Recruitment success related mainly to variations in summer conditions (e.g. seed viability).
During the period 1973–2012, pine became a more prominent part of the landscape around the forest line.
The forest limit has shifted insignificantly during the observation period and throughout the past 100 years (i.e. since the early 20th century). This contrasts with the more responsive treeline, which has expanded substantially, both altitudinally and latitudinally.
Forest limit inertia relates to shortage of seed and dispersal obstacles, imposed by the dense mountain birch forest at higher elevations.
References
- Aas, B. & Faarlund, T. 2000. Forest limits and the subalpine birch belt in North Europe with focus on Norway. AmS-Varia 37, 103–147.
- ACIA 2005. Arctic Climate Impact Assessment. Cambridge University Press, Cambridge.
- Ågren, J. & Zackrisson, O. 1990. Age and size structure of Pinus sylvestris populations on mires in central and northern Sweden. Journal of Ecology 78, 1049–1062. 10.2307/2260951
- Ahti, T., Hämet-Ahti, L. & Jalas, J. 1968. Vegetation zones and their sections in northwestern Europe. Annales Botanici Fennici 5, 169–211.
- Ainsworth, E.A. & Long, S.P. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? New Phytologist 165, 351–372.
- Alexandersson, H. 2006. Klimat i förändring: En jämförelse av temperatur och nederbörd 1991–2005 med 1961–1990. SMHI Faktablad 29, 1–8.
- Batllori, E. & Gutiérrez, E. 2008. Regional tree line dynamics in response to global change in the Pyrenees. Journal of Ecology 96, 1275–1288.
- Bergman, J., Hammarlund, D., Hannon, G., Barnekow, L. & Wohlfarth B. 2005. Deglacial vegetation succession and Holocene tree-limit dynamics in the Scandes Mountains, west-central Sweden: Stratigraphic data compared to megafossil evidence. Review of Palaeobotany and Palynology 134, 129–151. 10.1016/j.revpalbo.2004.12.005
- Camarero, J.J. & Gutiérrez, E. 2004. Pace and pattern of recent treeline dynamics: Response of ecotones to climatic variability in the Spanish Pyrenees. Climatic Change 63, 181–200. 10.1023/B:CLIM.0000018507.71343.46
- Campbell, I.D. & McAndrews, J.H. 1993. Forest disequilibrium caused by rapid Little Ice Age cooling. Nature 366, 336–338. 10.1038/366336a0
- Danby, R.K. & Hik, D.S. 2007. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology 95, 352–363. 10.1111/j.1365-2745.2006.01200.x
- Dobbertin, M. & Rigling, A. 2006. Pine mistletoe (Viscum album ssp. austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. Forest Pathology 36, 309–322. 10.1111/j.1439-0329.2006.00457.x
- Eklund, D. 2012. Klimatsammanställning-Fjällkedjan. Länsstyrelsens rapportserie 13/2012. Länsstyrelsen i Norrbottens län, Norrköping.
- Elliott, G.P. 2011. Influences of 20th-century warming at the upper tree line contingent on local-scale interactions: Evidence from a latitudinal gradient in the Rocky Mountains, USA. Global Ecology and Biogeography 20, 46–57. 10.1111/j.1466-8238.2010.00588.x
- Elliott, G.P. 2012. Extrinsic regime shifts drive abrupt changes in regneration dynamics at upper treeline in the Rocky Mountains, USA. Ecology 93, 1614–1625. 10.1890/11-1220.1
- Engelmark, O. & Zackrisson, O. 1985. Utvecklingen av ett talldominerat skogsgränsbestånd i Pite lappmark efter brand 1711. Svensk Botanisk Tidskrift 79, 243–248.
- Frey, W. 1983. The influence of snow on growth and survival of planted trees. Arctic and Alpine Research 15, 241–251. 10.2307/1550925
- Graumlich, L., Waggoner, L.A. & Bunn, A.C. 2005. Detecting global change at alpine treeline: Coupling paleoecology with contemporary studies. Huber, U.M., Bugman, H.K.M. & Reasoner, M.A. (eds.) Global Change and Mountain Regions, 501–508. Springer, Dordrecht.
- Harris, S.A. 2001. Twenty years of data on climate-permafrost-active layer variations at the lower limit of alpine permafrost, Marmot Basin, Jasper National Park. Geografiska Annaler 83A, 1–14. 10.1111/1468-0459.00140
- Harsch, M.A. & Bader, M.Y. 2011. Treeline form – a potential key to understanding treeline dynamics. Global Ecology and Biogeography 20, 582–596. 10.1111/j.1466-8238.2010.00622.x
- Harsch, M.A., Hulme, P.E., McGlone, M.S. & Duncan, R.P. 2009. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12, 1040–1049. 10.1111/j.1461-0248.2009.01355.x
- Helama, S., Lundholm, M., Timonen, M. & Eronen, M. 2004. Dendrochronologically dated changes in the limit of pine in northernmost Finland during the past 7.5 millennia. Boreas 33, 250–259. 10.1080/03009480410001253
- Helland, A. 1912. Traegraendser og sommervarmen. Tidsskrift for Skogbruk 20, 131–146, 169–175, 303–313.
- Henttonen, M., Kanninen, M., Nygren, M. & Ojansuu, R. 1986. The maturation of Pinus sylvestris seeds in relation to temperature climate in northern Finland. Scandinavian Journal of Forest Research 1, 243–249. 10.1080/02827588609382415
- Hiller, A., Boettger, T. & Kremenetski, C. 2001. Mediaeval climate warming recorded by radiocarbon dated alpine tree-line shift on the Kola Peninsula. The Holocene 11, 491–497. 10.1191/095968301678302931
- Hofgaard, A., Harper, K.A. & Golubeva, E. 2013a. The role of circumarctic forest-tundra ecotone for Arctic biodiversity. Biodiversity 13, 174–181. 10.1080/14888386.2012.700560
- Hofgaard, A., Tømmervik, H., Rees, G. & Hanssen, F. 2013b. Latitudinal forest advance in northernmost Norway since the early 20th century. Journal of Biogeography 40, 938–949. 10.1111/jbi.12053
- Holtmeier, F.-K. 1974. Geoökologische Beobachtungen und Studien and der subarktischen und alpinen Waldgrenze in vergleichender Sicht. Erdwissenschaftliche Forschung 8, 1–130.
- Holtmeier, F.-K. 2009. Mountain Timberlines – Ecology, Patchiness, and Dynamics. Springer, Dordrecht.
- Holtmeier, F.-K. & Broll, G. 2005. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography 14, 395–410. 10.1111/j.1466-822X.2005.00168.x
- Holtmeier, F.-K. & Broll, G. 2011. Response of Scots pine (Pinus sylvestris) to warming climate at its altitudinal limit in northernmost subarctic Finland. Arctic 64, 269–280. 10.14430/arctic4118
- Hustich, I. 1958. On the recent expansion of the Scotch pine in northern Europe. Fennia 82, 1–25.
- Johnson, E.A., Miyaniski, K. & Kleb, H. 1994. The hazards of interpreting static age structures as shown by stand reconstructions in a Pinus contorta-Picea engelmannii forest. Journal of Ecology 82, 921–930. 10.2307/2261455
- Junttila, O. 1986. Effects of temperature on shoot growth in northern provenance of Pinus sylvestris. Tree Physiology 1, 185–192. 10.1093/treephys/1.2.185
- Juntunen, V., Neuvonen, S., Norokorpi, Y. & Tasanen, T. 2002. Potential for timberline advance in Northern Finland, as revealed by monitoring during 1983–99. Arctic 55, 348–361. 10.14430/arctic719
- Kaplan, J.O. & New, M. 2006. Arctic climate change with a 2 °C global warming: Timing, climate patterns and vegetation change. Climatic Change 79, 213–241. 10.1007/s10584-006-9113-7
- Kellomäki, S., Väisänen, H. & Kolström, T. 1997. Model computations on the effects of elevating temperature and atmospheric CO2 on the regeneration of Scots pine at the timberline in Finland. Climatic Change 37, 683–708. 10.1023/A:1005394616150
- Kharuk, V.I., Ranson, K.J., Im, S.T. & Dvinskaya, M.L. 2009. Response of Pinus sibirica and Larix sibirica to climate change in southern Siberian alpine forest-tundra ecotone. Scandinavian Journal of Forest Research 24, 130–139. 10.1080/02827580902845823
- Körner, C. 2005. The green cover of mountains in a changing environment. Huber, U.M., Bugman, H.K.M. & Reasoner, M.A. (eds.) Global Change and Mountain Regions, 367–375. Springer, Dordrecht.
- Körner. 2007. Climatic treelines: Conventions, global patterns, causes. Erdkunde 61, 316–324. 10.3112/erdkunde.2007.04.02
- Kremenetski, C.V., Sulerzhitsky, L.D. & Hantemirov, R. 1998. Holocene history of the northern range limits of some trees and shrubs in Russia. Arctic and Alpine Research 30, 317–333. 10.2307/1552004
- Kullman, L. 1979. Change and stability in the altitude of the birch tree-limit in the southern Swedish Scandes 1915–1975. Acta Phytogeographica Suecica 65, 1–121.
- Kullman, L. 1984. Germinability of mountain birch (Betula pubescens ssp. tortuosa) along two altitudinal transects downslope from the tree-limit in Sweden. Reports from the Kevo Subarctic Research Station 19, 11–18.
- Kullman, L. 1986. Late Holocene reproductional patterns of Pinus sylvestris and Picea abies at the forest limit in central Sweden. Canadian Journal of Botany 64, 1682–1690. 10.1139/b86-225
- Kullman, L. 1987. Little Ice Age decline of a cold marginal Pinus sylvestris forest in the Swedish Scandes. New Phytologist 106, 567–584. 10.1111/j.1469-8137.1987.tb00134.x
- Kullman, L. 1993. Pine (Pinus sylvestris) tree-limit surveillance during recent decades, Central Sweden. Arctic and Alpine Research 25, 24–31. 10.2307/1551476
- Kullman, L. 1997. Tree-limit stress and disturbance: A 25-year survey of geoecological change in the Scandes Mountains of Sweden. Geografiska Annaler 79A, 139–165. 10.1111/1468-0459.00012
- Kullman, L. 1998. Tree-limits and montane forests: Sensitive biomonitors of climate change and variability. Ambio 27, 312–321.
- Kullman, L. 2005. Pine (Pinus sylvestris) treeline dynamics during the past millennium – a population study in west-central Sweden. Annales Botanici Fennici 42, 95–106.
- Kullman, L. 2007a. Treeline population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: Implications for tree line theory and climate change ecology. Journal of Ecology 95, 41–52. 10.1111/j.1365-2745.2006.01190.x
- Kullman, L. 2007b. Long-term geobotanical observations of climate change impacts in the Scandes of West-Central Sweden. Nordic Journal of Botany 24, 445–467. 10.1111/j.1756-1051.2004.tb02209.x
- Kullman, L. 2007c. Modern climate change and shifting ecological states of the subalpine/alpine landscape in the Swedish Scandes. GeoÖko 28, 187–221.
- Kullman, L. 2010a. One century of treeline change and stability – experiences from the Swedish Scandes. Landscape Online 17, 1–31.
- Kullman, L. 2010b. A richer, greener and smaller alpine world: Review and projection of warming-induced plant cover change in the Swedish Scandes. Ambio 39, 159–169. 10.1007/s13280-010-0021-8
- Kullman, L. 2012. The alpine treeline ecotone in the southernmost Swedish Scandes: Dynamism on different scales. Myster, R.W. (ed.) Ecotones Between Forest and Grassland, 271–298. Springer, New York.
- Kullman, L. 2013a. Ecological tree line history and palaeoclimate – review of megafossil evidence from the Swedish Scandes. Boreas 42, 555–567. 10.1111/bor.12003
- Kullman, L. 2013b. Recent cooling and dynamic responses of alpine summit floras in the southern Swedish Scandes. Nordic Journal of Botany. doi:0.1111/j.1756-1051.2013.00229.x
- Kullman, L. & Öberg, L. 2009. Post-Little Ice Age tree line rise and climate warming in the Swedish Scandes: A landscape ecological perspective. Journal of Ecology 97, 415–429. 10.1111/j.1365-2745.2009.01488.x
- Kullman, L. & Öberg, L. 2012. Melting glaciers and ice patches in Swedish Lapland provide new insights into the Holocene arboreal history. GeoÖko 33, 121–146.
- Leonelli, G., Pelfini, M., Morra di Cella, U. & Garavaglia, V. 2011. Climate warming and the recent treeline shift in the European Alps: The role of geomorphological factors in high-altitude sites. Ambio 40, 264–273. 10.1007/s13280-010-0096-2
- Lloyd, A.H. 2005. Ecological histories from Alaskan tree lines provide insight into future change. Ecology 86, 1687–1695. 10.1890/03-0786
- MacDonald, G.M. 2010. Some Holocene palaeoclimatic and palaeoenvironmental perspectives on Arctic and Subarctic climate warming and the IPCC 4th assessment report. Journal of Quaternary Science 25, 39–47. 10.1002/jqs.1307
- Malanson, G.P., Resler, L.M., Bader, M.Y., Holtmeier, F.-K., Butler, D.R., Weiss, D.J., Daniels, L.D. & Fagre, D.B. 2011. Mountain treelines: A roadmap for research orientation. Arctic, Antarctic, and Alpine Research 43, 167–177. 10.1657/1938-4246-43.2.167
- Martinez-Vilalta, J., Lopez, B.C., Adell, N., Badiella, L. & Niyerola, M. 2008. Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Global Change Biology 14, 2868–2881. 10.1111/j.1365-2486.2008.01685.x
- Moen, J., Aune, K., Edenius, L. & Angerbjörn, A. 2004. Potential effects of climate change on treeline position in the Swedish mountains. Ecology and Society 16, 1–10.
- Mossberg, B. & Stenberg, L. 2003. Den Nya Nordiska Floran. Wahlström & Widstarnd, Stockholm.
- Nicolussi, K. & Patzelt, G. 2000. Discovery of early Holocene wood and peat on the forefield of the Pasterze Glacier, Eastern Alps, Austria. The Holocene 10, 191–199. 10.1191/095968300666855842
- Öberg, L. & Kullman, L. 2011. Recent glacier recession – a new source of postglacial treeline and climate history in the Swedish Scandes. Landscape Online 26, 1–38.
- Parn, H. 2012. Changes in the radial growth of two consecutive generations of Scots pine (Pinus sylvestris) stands. Baltic Forestry 18, 12–24.
- Paus, A. 2010. Vegetation and environment of the Rødalen alpine area, Central Norway, with emphasis of the early Holocene. Vegetation History and Archaeobotany 19, 29–51. 10.1007/s00334-009-0228-4
- Paus, A. 2013. Human impact, soil erosion, and vegetation response lags to climate change: Challenges for mid-Scandinavian pollen-based transfer-function temperature reconstructions. Vegetation History and Archaeobotany 22, 269–284.
- Payette, S. 2007. Contrasted dynamics of northern Labrador tree lines caused by climate change and migrational lag. Ecology 88, 770–780. 10.1890/06-0265
- Payette, S. & Filion, L. 1985. White spruce expansion at the tree line and recent climate change. Canadian Journal of Forest Research 15, 241–251. 10.1139/x85-042
- Pearce, C.M., McLennan, D. & Cords, L.D. 1988. The evolution and maintenance of white spruce woodland on the Mackenzie Delta, N.W.T. Canada. Holarctic Ecology 11, 248–288.
- Peet, R.K. 1981. Forest vegetation of the Colorado Front Range: Composition and dynamics. Vegetatio 45, 3–75. 10.1007/BF00240202
- Rickebusch, S., Lischke, H., Bugmann, H., Guisan, A. & Zimmermann, N.E. 2007. Understanding the low-temperature limitations to forest growth through calibration of a forest dynamics model with tree-ring data. Forest Ecology and Management 246, 251–263. 10.1016/j.foreco.2007.04.030
- Säppänen, M. 1961. On the accumulation and the increasing of snow in pine dominated forest in Finland. Fennia 86, 1–51.
- Shiyatov, S.G. 2003. Rates of change in the upper treeline ecotone in the Polar Ural Mountains. PAGES News 11, 8–10.
- Shiyatov, S.G., Terent'ev, M.M., Fomin, V.V. & Zimmermann, N.E. 2007. Altitudinal and horizontal shifts of the upper boundaries of open and closed forests in the Polar Urals in the 20th century. Journal of Ecology 38, 223–227.
- Slot, M., Wirth, C., Schumacher, J., Mohren, G.M.J., Shibistova, O., Lloyd, J. & Ensminger, I. 2005. Regeneration patterns in boreal Scots pine glades linked to cold-induced photoinhibition. Tree Physiology 25, 1139–1150. 10.1093/treephys/25.9.1139
- SMHI. 2012. Juni 2012 – Lufttemperatur och vind. http://data.smhi.se/met/climate/time_series/month/vov_pdf/SMHI_vov_temperature_wind_jun12.pdf ( accessed 4 March 2014).
- Smith, H. 1957. En botanisk undersökning av Neans dalgång. Svenska Vetenskapsakademiens Avhandlingar i Naturskyddsärenden 16, 1–21.
- Stöcklin, J. & Körner, C. 1999. Recruitment and mortality of Pinus sylvestris near the Nordic treeline: The role of climatic change and herbivory. Ecological Bulletins 47, 168–177.
- Sturm, M., Schimel, J., Michaelson, G., Welker, J.M., Oberbauer, S.F., Liston, G.E., Fahnestock, J. & Romanovsky, V.E. 2005. Winter biological processes could help convert arctic tundra to shrubland. BioScience 55, 17–26. 10.1641/0006-3568(2005)055[0017:WBPCHC]2.0.CO;2
- Tinner, W. & Kaltenrieder, P. 2005. Rapid responses of high-mountain vegetation to early Holocene environmental changes in the Swiss Alps. Journal of Ecology 93, 936–947. 10.1111/j.1365-2745.2005.01023.x
- Tranquillini, W. 1979. Physiological Ecology of the Alpine Timberline. Springer-Verlag, Berlin.
- Trant, A.J., Jameson, R.G. & Hermanutz, L. 2011. Persistence at the treeline: Old trees as opportunists. Arctic 64, 367–370. 10.14430/arctic4126
- Van Bogaert, R., Haneca, K., Hoogesteger, J., Jonasson, C., De Dapper, M. & Callaghan, T.V. 2011. A century of tree line changes in sub-Arctic Sweden shows local and regional variability and only minor influence of 20th century warming. Journal of Biogeography 38, 907–921. 10.1111/j.1365-2699.2010.02453.x
- Veijola, P. 1998. The northern timberline and timberline forests in Fennoscandia. Finnish Research Institute Research Paper 672, 1–242.
- Villalba, R. & Veblen, T.T. 1997. Regional patterns of tree population age structures in northern Patagonia: Climate and disturbance influences. Journal of Ecology 85, 113–124. 10.2307/2960643
- Walker, X., Henry, G.H.R., McLeod, K. & Hofgaard, A. 2012. Reproduction and seedling establishment of Picea glauca across the northernmost forest-tundra region in Canada. Global Change Biology 18, 3202–3211. 10.1111/j.1365-2486.2012.02769.x