Abstract
In the event of a severe nuclear accident, one major concern is the release of radioactive material into the environment causing potential exposure of the general public to radiation. Among the volatile radionuclides are a range of tellurium isotopes. Due to the radioactivity and the volatility of tellurium, it has to be taken into account when assessing the overall effects of an accident. The interest in tellurium is not limited only to its release but also to the fact that some tellurium isotopes decay to iodine, and thus affect the iodine release behavior. The release and transport behavior of tellurium has been investigated over the past decades, however, the aqueous chemistry of tellurium in the complex containment sump system is still unclear. This study presents the behavior of tellurium dioxide in simplified containment sump conditions in relation to dissolution, redox reactions, and interactions with water radiolysis products. The results indicate that radiolysis products have a significant effect on tellurium chemistry in both a reducing and oxidizing manner depending on the solution composition. The redox reactions also affect the solubility of tellurium. The results show that the current information used to assess tellurium source term needs to be reevaluated for both severe accident management and for code validation purposes.
I. INTRODUCTION
During a severe nuclear accident, the most significant radionuclides released are the most volatile fission products (FPs). In addition to noble gases, cesium, and iodine, there is a variety of tellurium isotopes (e.g., 132Te, 129mTe) released. Due to the relatively short half-lives of the released tellurium isotopes, the long-term effects originating from tellurium releases are negligible. However, they are still among the most significant radionuclides released in terms of activity during an accidentCitation1,Citation2 and are important in the midterm stage of an accident. It is estimated that during the Chernobyl accident around 1400 PBq of tellurium, mostly 132Te [1150 PBq (CitationRef. 3)], was released to the environment.Citation4 Compared to the release of 131I and 137Cs from the Chernobyl accident, 1760 PBq3 and 85 PBq (CitationRef. 3), respectively, the magnitude of the tellurium releases is considerable. In comparison, the accident in Fukushima resulted in releases of around 180 PBq (CitationRef. 5), 150 PBq (CitationRef. 6), and 12 PBq (CitationRef. 6) of 132Te, 131I, and 137Cs, respectively. In addition, according to the International Atomic Energy Agency, the multiplication factor for the radiological impact of 132Te releases is 0.3 compared to the radiological equivalence of 131I, which is considered one of the most significant isotopes released.Citation7 Therefore, considering the high releases and the resulting radiological impact of 132Te, the releases should be considered significant. Consequently, it is necessary to assess tellurium effects in the early and mid stage of an accident.
Another significant factor to consider in terms of tellurium release is that many tellurium isotopes decay to iodine, and therefore, tellurium can act as a delayed source for iodine release. Iodine is considered very radiotoxic since it effectively accumulates in the thyroid gland and results in increased dose to those exposed.Citation8,Citation9 Even if the toxicity of tellurium itself would not be of concern, it is still important to consider due to the decay to iodine.
The amount and the time of the release of FPs to the containment and further on to the environment can be referred to as source term. This is governed by the chemical and physical form of the FPs as well as the effectiveness of the mitigation actions.Citation10 The tellurium source term has been investigated in terms of release from the fuel and transport into the containment over the past decades.Citation11–Citation13 However, the knowledge of tellurium behavior in the containment and especially in the sump remains unclear. The information about the behavior of tellurium in solution that has been applied for severe accident scenarios has been obtained mostly from geological studies.Citation14 However, the aqueous chemistry related to severe accident conditions is extremely complex and different compared to environmental conditions. Therefore, experimental data are necessary to improve the knowledge of tellurium behavior.
Tellurium behavior has been widely studied in the past in terms of its release from the fuel and parameters affecting the timing and speciation of tellurium isotopes. One key parameter related to the release from the reactor core that sets tellurium apart from the other volatile FPs (e.g., cesium, iodine) is its interactions with the cladding. Tellurium vapor species react with inner surface zirconium forming zirconium tellurides until no metallic zirconium is left due to sufficient oxidation.Citation1,Citation11 This results in the delayed release of tellurium species.
One of the most comprehensive accident research programs is the PHÉBUS FP Severe Accident Experimental Program. The PHÉBUS experiments were performed in Commissariat à l’Energie Atomique at Cadarache, and the project was a collaboration between the Institut de Radioprotection et de Sûreté Nucléaire and the European Commission. The PHÉBUS experiments included studies of the release of FPs from the core, their transport in the reactor coolant system, and their behavior in the containment. In terms of tellurium, the PHÉBUS provided information about its behavior in the reactor and the containment. In addition, tellurium was used as an analogue for nonsoluble elements when analyzing their behavior in the sump.Citation15 The PHÉBUS experiments indicate that the majority of tellurium enters the containment as part of an FP aerosol. After entering the containment, the tellurium species will be washed from the condensing surfaces and walls into the sump. In addition, the PHÉBUS experiments suggest that a negligible amount of tellurium in the sump is present as soluble species. Although, the experiments were performed in both acidic and alkaline media,Citation16 no significant differences were observed concerning the tellurium behavior.Citation15 However, the chemistry phase in the PHÉBUS experiments was focused mainly on iodine, not tellurium.Citation16
In addition to the PHÉBUS experiments, some observations of tellurium behavior, mainly solubility, were also made from Three Mile Island aqueous samples,Citation17 but these cannot be used to estimate the overall tellurium behavior. It is therefore necessary to perform more comprehensive and focused experiments to improve the knowledge of tellurium chemistry. The main parameters influencing tellurium speciation are pH, redox, and radiolysis, all of which can have a significant effect on tellurium solubility and consequently can promote more tellurium species to take part in potential further reactions in the sump.
When modeling tellurium with severe accident computer codes (e.g., MAAP-CANDU, MELCOR) the focus is mostly on the release from the core, and especially, the degree of oxidation in the cladding has been assessed.Citation18,Citation19 However, the modeling inside the containment and in the sump seems to be lacking information. Currently, the tellurium data for containment and aqueous modeling are taken from geological systems that have been assumed analogous to the containment.Citation20 The equilibrium constants are taken from hydrothermal and aqueous tellurium reactions.Citation21 However, the effect of radiolysis products on tellurium speciation and solubility is not considered but should be taken into account since tellurium is a relatively reactive element and can take part in further reactions in the sump. Therefore, it is important to provide more detailed information about the behavior and chemistry of tellurium in the sump to improve the chemical and containment modeling in the codes.
Beyond the core and containment, tellurium distribution in the environment has also been studied previously. The distribution of tellurium isotopes after Fukushima has been investigated via environmental samplingCitation22 as well as through simulations.Citation23 Due to the relatively short half-life of 132Te, 129mTe has been used to estimate the activity of 132Te. The estimated distribution of 129mTe after Fukushima largely coincides with that of 131I and 137Cs (CitationRef. 24).
However, in terms of areal distribution after Fukushima, there seems to have been some differences between the behavior of tellurium and iodine compared to cesium. By comparing 131I/137Cs and 129mTe/137Cs, it was found that both ratios were higher to the south of Fukushima than to the northwest.Citation25 This might indicate differences in timing of the release or in the transport behavior. However, 129mTe/137Cs ratios were more consistent compared to 131I/137Cs, indicating greater differences in iodine transport behavior. Nevertheless, this raises uncertainties about what happens during an accident and whether the timing and pathway of the releases are accurately known.
The behavior of tellurium in the environment has also been investigated after deposition. Due to the short half-lives of the tellurium isotopes released, there are less available data for tellurium than for example 137Cs with a long half-life. However, tellurium activity has been compared with that of cesium months after the Fukushima accident. It was concluded from the consistent 129mTe/137Cs ratio from collected environmental samples that the vertical mobility of both elements in the soil was negligible.Citation22 This is supported by the findings from research on mine tailings that show tellurium in oxidation states +IV and +VI is retained by Fe(III) hydroxides in the soil via surface and inner-sphere complexation.Citation26 However, there is also evidence that the complex chemistry and formation of ionic species in the environment can mobilize tellurium and result in the transfer to edible plants and cause dose by ingestion after an accident.Citation27
I.A. Containment Sump
The containment sump is a complex mixture of components originating either from accident management systems, e.g., the containment spray system (CSS), or from events that take place during the accident, e.g., leaks from the primary circuit; radiolysis of structural materials, water, and air; and corrosion and dissolution of materials from the surfaces. Potential elements and their sources present in the containment sump solution are presented in . Due to the complexity of the sump, it is difficult to predict the behavior of FPs and possible release by revolatilization. However, this must be considered since the sump formed during an accident will stay inside the containment and could be subjected to further chemical effects.Citation28
TABLE I Possible Corrosion and Debris Materials in the Containment Sump*
The main components in the sump of a pressurized water reactor in the early stages of an accident are boric acid from both the primary system and containment spray and a base (sodium hydroxide, potassium hydroxide, trisodium phosphate) from pH adjustment, originating either from the refueling water storage tank or placed on the bottom of the containment [trisodium phosphate (TSP)] (CitationRef. 29). Some plants also use a reducing additive (thiosulfate, hydrazine) in the spray system, which is used to decompose iodine compounds and manage the water chemistry. The pH of the sump is targeted to stay above 7 in accident conditions.Citation29 More precisely, the target pH is generally around 9.3 (CitationRef. 30). The pH is maintained alkaline with a base in order to keep iodine as a soluble species and thus minimize the possible iodine revolatilization from the sump back into the containment atmosphere. However, the radiolysis and pyrolysis of air and cables produce HCl and HNO3, respectively, which can lower the pH of the sump during the accident.Citation31 In addition, reactions of different FPs, including tellurium,Citation20,Citation32 with organic compounds originating from paint, ion exchange resins, or insulation materials in the sump can lead to more volatile species and increase the source term.Citation33,Citation34
I.B. Water Radiolysis
In addition to the aforementioned materials, the effect of water radiolysis products caused by radiation from the decay of FPs in the sump must also be considered. This is especially important as the radiolysis products can have a significant effect on the redox chemistry of the radionuclides. Consequently, this can lead to further reactions and possibly revolatilization from the sump.Citation35 Radiolysis of water produces both oxidizing (•OH, H2O2) and reducing (e−, H•) species that are extremely efficient reactants.
It has been proposed that tellurium speciation could be affected by the radiolysis products, mainly by oxidative radicals (H2O2, •OH) (CitationRefs. 12]\+ and Citation33). Previous studies support this assumption,Citation36–Citation38 however, experimental data involving severe accident conditions have not been presented previously.
I.C. Tellurium Chemistry
In aqueous solution, tellurium speciation is highly dependent on redox and pH conditions.Citation21 Tellurium can exist in oxidation states −2, +2, +4, and +6, of which +4 and +6 are the most abundant in natural waters.Citation39 Solid elemental tellurium is stable in water or aqueous solution but not in extremely alkaline solution (pH > 10) or in the presence of an oxidizing agent. If elemental tellurium is brought to an aerated solution, it is covered by tellurium dioxide, TeO2 (CitationRef. 40), which is an amphoteric compound and thus can act as an acid or a base depending on the prevailing conditions. The dominant Te(IV) species in neutral aqueous solution is tellurous(IV) acid H2TeO3, which can undergo either protonation to form a cationic product or deprotonation to form anionic products, depending on the pH. The solubility of TeO2 is relatively low with a minimum at around 2.1 × 10−10 mol/dm3 at pH 5.5 (CitationRef. 41). However, the solubility increases in both extremes of the pH scale when TeO2 dissolves as an ionic species. This is a result of the amphoteric nature of TeO2. In a hydrated form, TeO2•H2O, the solubility increases significantly and reaches a maximum at 1.6 × 10−2 mol/dm3 in alkaline solution.Citation41 Chemical equilibrium and thermodynamic data for Te(IV) species are presented in (CitationRefs. 14 and Citation21).
TABLE II Thermodynamic Data for Tellurium Solubility*
In an oxidizing environment Te(IV) can oxidize to Te(VI). The main form of Te(VI) is telluric(VI)acid, H2TeO4 or H6TeO6, which is soluble in water. Telluric acid itself is also a powerful oxidizing agent and can take part in redox reactions in aqueous solutions. One of the most interesting reactions concerning accident scenarios and sump chemistry is the possible redox reaction between telluric acid and iodide [Reactions (1) and (2)]42:
and
Telluric acid has the potential to oxidize iodide to volatile molecular iodine I2, and thus increase the expected source term by revolatilization from the sump.Citation12 The reaction has previously been proposed but supporting experimental data have not yet been presented.
Due to its complex chemistry, tellurium species can take part in multiple redox reactions depending on the prevailing conditions. Standard reduction potentials of several tellurium(IV) and tellurium(VI) reactions relevant to the study are presented in . The aforementioned radiolysis products can have a significant effect on tellurium redox chemistry. In addition, different oxidizing and reducing agents can be of importance as well. Most additives used in the accident management systems are designed to decompose iodine species. The main additives are sodium thiosulfate and hydrazine, N2H4, which are both found to be effective in removing organic iodide from the containment atmosphere.Citation43 The effect of any additive on tellurium chemistry relevant to nuclear accident scenarios has not previously been investigated.
TABLE III Standard Reduction Potentials for Possible Te(IV) and Te(VI) Redox Reactions*
II. EXPERIMENTAL
The behavior of tellurium dioxide (TeO2) in the containment sump simulant was investigated in terms of solubility, redox, and radiolysis. The changes in speciation and behavior were studied in both aqueous and solid forms. Tellurium dioxide was chosen as the tellurium precursor because it is one of the potential species forming in the sump during an accident. In addition, it has a complex chemistry and possible redox reactions affecting the overall source term that need to be considered.
II.A. Materials
Tellurium dioxide solubility and behavior were investigated in alkaline borate solution (ABS) with and without sodium thiosulfate (Na2S2O3). The solutions were prepared with 0.23 M H3BO3 (Merck, >99.8%), 0.15 M NaOH (EMPLURA®, 99%), and 0.064 M Na2S2O3 (Sigma Aldrich). All solutions were prepared with 18 MΩ deionized MilliQ water (Millipore). The pH of both solutions was around 9.3. Samples were irradiated in Gammacell 220 Co-60 source (MDS Nordion, Atomic Energy of Canada Ltd) with a dose rate of 5 kGy/h. The temperature in the gamma source was around 313 K.
Tellurium concentration in the samples was determined by inductively coupled plasma mass spectrometry (ICP-MS; Thermo Fisher), aqueous speciation was investigated with ion chromatography (IC; Dionex DX-100, IonPac AS4A-SC 4 × 250 mm), and solid speciation with X-ray diffraction (XRD; Siemens D5000 diffractometer with Cu Kα- radiation). Interpretation of the diffractograms was done with DIFFRAC.EVA 4.1.1. software using The International Center for Diffraction Data® database.
II.B. Methods
The samples for irradiation were prepared by weighing 30 mg of solid TeO2 (Sigma Aldrich) into a glass vial and adding 5 mL of ABS with or without sodium thiosulfate. The air-to-liquid ratio was 0.4. Samples were irradiated for a period of time ranging from 1 to 10 days. The maximum dose delivered to the sample was approximately 1.2 MGy. The reference samples were prepared in the same way but instead of irradiation, the samples were kept in a heating cabinet at 313 K. All samples were prepared as triplicates to obtain statistical significance.
Both the irradiated and reference samples were filtered with 0.45-μm polyethylene syringe filters (VWR®) and prepared for ICP-MS measurements by diluting with 0.5 M Suprapur® HNO3 (Merck). Rhodium (1 ppb) (Ultra Scientific) was used as an internal standard in the ICP-MS measurements due to its rarity in most samples and relatively inert coordination chemistry. The tellurium standards were prepared by diluting from 10 ppm standard solution (High-Purity Standards). The solid material remaining after the experiments in the samples was dried and ground to a powder before examination with XRD.
III. RESULTS
In the following sections, the results of the tellurium dioxide experiments are presented in terms of solubility and aqueous and solid speciation. The main results are also presented in .
TABLE IV Results for Tellurium Dioxide Solubility and Speciation
III.A. Solubility
The results of the solubility experiments with TeO2 in the ABS with and without sodium thiosulfate are presented in . Both irradiated and nonirradiated results are presented as well as the reference results. The main finding was that gamma radiation has a significant effect on tellurium solubility in the alkaline sump simulate solution. Without the sodium thiosulfate additive, the solubility increased with increasing radiation dose. The solubility of tellurium in the nonirradiated samples reached an equilibrium at around 16 mM. In comparison, the concentration of tellurium after 10 days (1.2 MGy) irradiation in ABS without the sodium thiosulfate additive was 26 mM and did not reach equilibrium during the experiment.
Fig. 1. The solubility of tellurium in ABS with and without the sodium thiosulfate additive. Irradiated and reference samples are all presented. Maximum dose received after 10 days of irradiation was around 1.2 MGy. The solid lines show the linear trend of the irradiated samples in the two different solutions
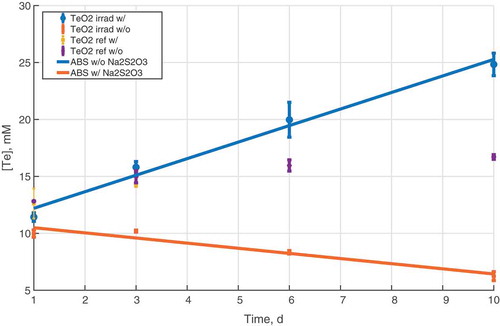
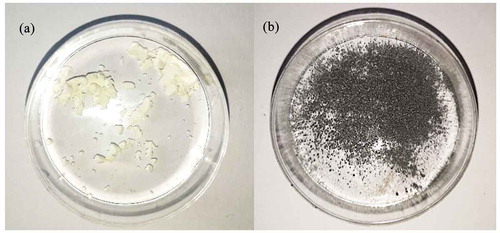
However, in the presence of the reducing thiosulfate additive, the concentration of tellurium decreased with increasing dose, reaching a concentration of 6 mM after an approximately 1.2-MGy dose. In addition, a change of color was observed in the solid material, from white to silvery black (), indicating possible reduction of initial precursor TeO2 to elemental tellurium had occurred. The nonirradiated reference samples behaved similarly to the ones without the thiosulfate additive; solubility reached an equilibrium at 16 mM and no color change occurred. No significant change in pH was observed in any of the samples.
III.B. Speciation Analysis
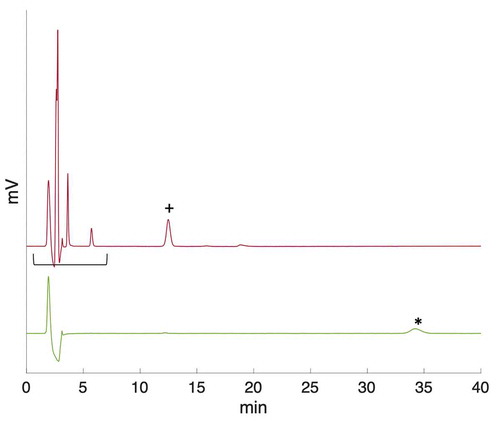
The speciation of tellurium in the simulated sump solutions was investigated with IC. This method is suitable for differentiating between tellurium in oxidation states +4 and +6. However, the exact speciation might be affected by matrix effects while the samples pass through the column, and thus the exact speciation might be different. Hence, additional methods may need to be used for complete speciation analysis. The samples were run untreated and undiluted since the concentration of tellurium in the solution was relatively low. However, this caused high peaks for other anions present in higher concentrations in the solution (OH−, B(OH)−4, SO42−, S2O32−). Samples were compared with standard solutions prepared from analytical-grade chemicals. The Te(IV) standard was prepared from sodium tellurite (Na2TeO3) (Sigma Aldrich) and the Te(VI) from H6TeO6 (Sigma Aldrich). presents the main results from the IC measurements. Tellurate, Te(VI), has a retention time of around 6 min. Tellurite, Te(IV), has a slightly longer retention time, however, in the IC system used, Te(IV) was determined indirectly from the negative peak in the chromatogram (III and IV in ). This is possibly due to the high positive hydration tendency of the TeO32− species resulting in lower conductivity.Citation44 Moreover, the method was suitable for speciation analysis since only one or the other of the species was present in the sample, and therefore, no separation was required.
Fig. 3. Ion chromatograms: (I) 1.2 MGy irradiated TeO2 in ABS, (II) Te(VI) standard, (III) TeO2 in ABS nonirradiated reference, (IV) Te(IV) standard, and (V) ABS standard solution. Peaks: (A) NaOH, (B) borate, (C) tellurate(VI), and (D) tellurite(IV)
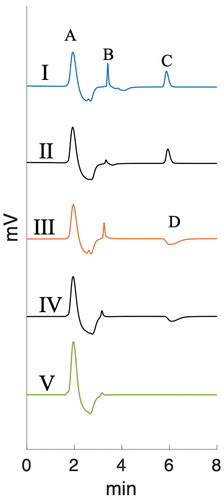
The results suggest that without Na2S2O3, Te(IV) is oxidized to Te(VI) species under irradiation. In the prevailing conditions, the species formed is most probably HTeO−4. The oxidation explains the increase in solubility, since the Te(VI) species have significantly higher solubility than TeO2. Oxidation is most likely due to the oxidizing water radiolysis products (e.g., H2O2, •OH) formed by the gamma radiation. In the reference samples, TeO2 dissolved as TeO32− species as expected in such alkaline pH.
With sodium thiosulfate present, TeO2 dissolves as the TeO32− species in both the irradiated and the reference samples. However, a change in the solid material was observed as a color change (). This was further investigated using XRD analysis.
The IC was also used to identify other species and changes in the solutions. Sodium thiosulfate was oxidized to sulfate under irradiation (). Thiosulfate and sulfate ions have retention times of 34 and 12 min, respectively. In addition, different borate species were detected in the chromatograms. However, due to the relatively high concentration of borate, the interpretation of the ion chromatograms becomes difficult due to possible formation of polyborates. Moreover, changes were observed in the borate/boric acid peaks before and after irradiation, which could require further investigation.
Fig. 4. (top line) Irradiated and (bottom line) nonirradiated ABS solution with sodium thiosulfate. Sulfate (+) was detected in the irradiated sample with a retention time of 12 min, whereas the thiosulfate (*) peak was detected in the reference sample after 33 min. The change in borate speciation is seen at the region between around 2 to 7 min
III.C. Solid Speciation
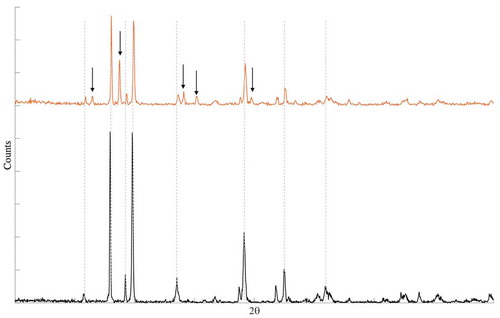
The aforementioned color change in the solid material was investigated using powder XRD. After solubility experiments, the irradiated and reference samples in ABS with sodium thiosulfate were evaporated to dryness and ground to a homogeneous consistency. The obtained diffractograms are presented in . The irradiated sample was interpreted to be a mixture of TeO2 and Te metal, as expected from the color change, whereas the reference sample was characteristic for TeO2. No quantification of the results was performed since the purpose was to analyze the speciation of the solid material.
IV. DISCUSSION
The oxidation of tellurium under irradiation from Te(IV) to Te(VI) can be presented as shown in Reaction (3):
In the prevailing alkaline conditions, TeO2 dissolves as TeO32− and reacts with oxidizing water radiolysis products (•OH, H2O2) via two-electron oxidation. Reaction (3) can be considered as the overall reaction, and there are possibly more complex intermediate radical reactions that take place under irradiation. However, the results obtained in the experiments after longer irradiation times suggest that the overall reaction from Te(IV) to Te(VI) can be simplified to the form in Reaction (3).
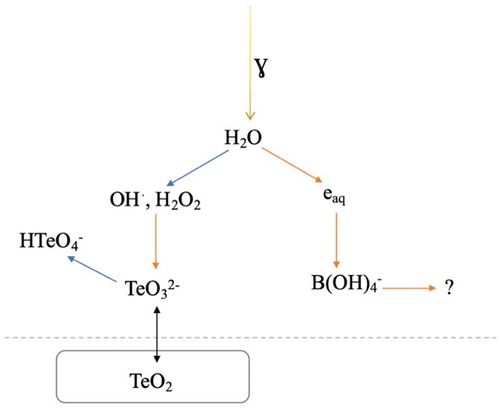
According to the results presented in , it can be concluded from the linear increase in the concentration of tellurium with increasing dose that the oxidative dissolution of tellurium follows zero-order kinetics. It can be assumed that TeO2 powder is in excess compared to the oxidants produced during irradiation since without applied irradiation the system reached maximum solubility and became saturated. Another assumption is that the oxidation reaction takes place at the solid-liquid interface since no mixing was used, and therefore, dissolution would continue until the solubility limit is reached or all of the precursor is dissolved. The slope (1.0 × 10−3 mmol/min) of the graph gives the rate of the solubility, and thereby, also the rate of oxidation. In this case, the rate can be considered as the rate of oxidative dissolution, assuming that the increased solubility is all due to reactions with oxidative radiolysis products. presents the schematics of the system and reactions with the radiolysis products. The aforementioned borate speciation cannot be determined as the IC measurements were not able to provide accurate speciation. This might be of interest for future work, however.
Fig. 6. Schematic presentation of the system with the TeO2 precursor in ABS without the sodium thiosulfate additive
Tellurium dioxide reduction to metallic tellurium is more complex since the matrix has more compounds. This means that the number of possible redox reactions increases. The observations made from the different analysis methods suggest that two separate reactions with water radiolysis products take place under irradiation. First, thiosulfate ion, a mild reducing agent, reacts with the oxidizing radiolysis product to form sulfate ions (). Second, tellurite ions in the solution react with the reducing species and reduce to tellurium metal and resolidify. Thiosulfate is known to be a relatively effective oxidation inhibitor and radical scavenger, which supports the assumption of two individual reactions. In addition, since the redox reactions do not occur in the nonirradiated reference samples, it is apparent that there are no direct interactions between thiosulfate and tellurium causing redox reactions.
The possible reduction reaction proceeds via four-electron reductive path [Reaction (4)] between the dissolved tellurite and the dissolved electrons from water radiolysis:
The reaction between the thiosulfate ions and oxidative radiolysis products producing sulfate ions is presented in Reaction (5):
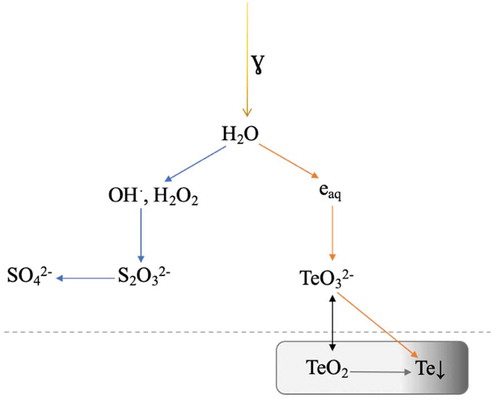
The reaction path has more intermediate radical steps, however, during long-term irradiation thiosulfate is fully oxidized and the intermediate species are not detected in the measurements. A schematic presentation of the system and reactions of tellurite and thiosulfate ions with the radiolysis products is presented in .
Fig. 7. Schematic presentation of the system with TeO2 as the precursor in ABS with the sodium thiosulphate
As in the samples without thiosulfate, it seems likely that the reduction also follows zero-order kinetics with a rate constant of 3.1 × 10−4 mmol/min. The difference between the rate of oxidation and the rate of reduction is due to the limiting scavenging reaction of thiosulfate ions. As demonstrated earlier, TeO32− has a tendency to preferentially oxidize in the presence of both oxidizing and reducing radiolysis products, the reason being the difference in the electrode potentials. The potential for oxidation reaction from TeO32− to HTe(VI)O4− is smaller than that of the reduction from TeO32− to elemental tellurium. Thus it is more favorable to follow the oxidative path. However, in the presence of thiosulfate the number of oxidizing products is limited. This means that only reducing products are readily available to react with. The possible limiting factors here are the scavenging rate of thiosulfate and the surface area of the solid precursor. Assuming the reduction reaction involves the dissolved tellurite species and proceeds to reprecipitation to metallic tellurium, the rate is limited by the ability of TeO2 to dissolve from the bulk.
V. CONCLUSION
Despite its relatively short half-life, tellurium needs to be taken into account when assessing severe accident consequences. The results presented here indicate that tellurium has an interesting chemistry in the sump that needs to be thoroughly investigated. In addition, the sump composition as well as radiolysis products have a significant effect on tellurium speciation, and by increasing the solubility, more tellurium compounds are available to take part in further reactions in the aqueous phase. Furthermore, tellurium, especially in the form of telluric acid, can have an effect on iodine source term via redox reaction resulting in the revolatilization of iodine from the sump. The results presented here indicate that in certain conditions the formation of telluric acid is feasible. However, more research needs to be performed with a more complex sump solution to fully understand tellurium behavior during a severe accident. In addition, interesting observations were made in terms of borate chemistry, as well as thiosulfate scavenging, that might be important and worth investigating. Comprehensive tellurium severe accident research provides important information that can be used in accident management, as well as for code validation purposes.
Acknowledgments
This work was supported by Swedish APRI-10 (Accident Phenomena of Risk Importance) and the Swedish Radiation Safety Authority.
References
- J. L. COLLINS, M. F. OSBORNE, and R. A. LORENZ, “Fission Product Tellurium Release Behavior Under Severe Light Water Reactor Accident Conditions,” Nucl. Technol., 77, 1, 18 (1987); https://doi.org/10.13182/NT87-A33948.
- R. A. LORENZ, E. C. BEAHM, and R. P. WICHNER, “Review of Tellurium Release Rates from LWR Fuel Elements Under Accident Conditions,” CONF-830816-51, Oak Ridge National Laboratory (1983).
- Sources and Effects of Ionizing Radiation (Annex D),” United Nations Scientific Committee, New York (2008).
- M. DREICER et al., “Consequences of the Chernobyl Accident for the Natural and Human Environments,” UCRL-JC-125028, CONF-960404-3, Lawrence Livermore National Laboratory (1996).
- G. STEINHAUSER, A. BRANDL, and T. E. JOHNSON, “Comparison of the Chernobyl and Fukushima Nuclear Accidents: A Review of the Environmental Impacts,” Sci. Total Environ., 470–471, 800 (2014); https://doi.org/10.1016/J.SCITOTENV.2013.10.029.
- M. CHINO et al., “Preliminary Estimation of Release Amounts of 13Li and 137Cs Accidentally Discharged from the Fukushima Daiichi Nuclear Power Plant into the Atmosphere,” J. Nucl. Sci. Technol., 48, 7, 1129 (2011); https://doi.org/10.1080/18811248.2011.9711799.
- “The International Nuclear and Radiological Event Scale User’s Manual,” International Atomic Energy Agency (2008).
- G. STEINHAUSER et al., “Using Animal Thyroids as Ultra-Sensitive Biomonitors for Environmental Radioiodine,” Environ. Sci. Technol., 46, 23, 12890 (2012); https://doi.org/10.1021/es303280g.
- J. ROBBINS and A. B. SCHNEIDER, Thyroid Cancer Following Exposure to Radioactive Iodine, Kluwer Academic Publishers (2000).
- B. R. SEHGAL, Nuclear Safety in Light Water Reactors: Severe Accident Phenomenology, Academic Press (2011).
- R. D. E. BOER and E. H. P. CORDFUNKE, “Reaction of Tellurium with Zircaloy-4,” J. Nucl. Mater., 223, 2, 103 (1995); https://doi.org/10.1016/0022-3115(95)00005-4.
- J. McFARLANE and J. C. LEBLANC, “Fission-Product Tellurium and Cesium Telluride Chemistry Revisited,” AECL--11333, Atomic Energy of Canada Ltd. (1996).
- R. D. E. BOER and E. H. P. CORDFUNKE, “The Chemical Form of Fission Product Tellurium During Reactor Accident Conditions,” J. Nucl. Mater., 240, 2, 124 (1997); https://doi.org/10.1016/S0022-3115(96)00600-9.
- P. V. GRUNDLER et al., “Speciation of Aqueous Tellurium(IV) in Hydrothermal Solutions and Vapors, and the Role of Oxidized Tellurium Species in Te Transport and Gold Deposition,” Geochim. Cosmochim. Acta, 120, 298 (2013); https://doi.org/10.1016/J.GCA.2013.06.009.
- M. LAURIE et al., “Containment Behaviour in Phébus FP,” Ann. Nucl. Energy, 60, 15 (2013); https://doi.org/10.1016/J.ANUCENE.2013.03.032.
- P. MARCH and B. SIMONDI-TEISSEIRE, “Overview of the Facility and Experiments Performed in PHÉBUS FP,” Ann. Nucl. Energy, 61, 11 (2013); https://doi.org/10.1016/J.ANUCENE.2013.03.040.
- K. VINJAMURI et al., “Tellurium Chemistry, Tellurium Release and Deposition During the TMI-2 Accident,” EGG-M--23485, EG and G Idaho (1985).
- A. C. MORREALE, L. S. LEBEL, and M. J. BROWN, “Effects of Filtered Containment Venting on Fission Product Releases During CANDU Reactor Severe Accidents,” ASME J. Nucl. Eng. Radiat. Sci., 3, 2 (2017); https://doi.org/10.1115/1.4035434.
- R. O. GAUNTT et al., MELCOR Computer Code Manuals, Sandia National Laboratories, NUREG/CR, 6119 (2000).
- J. McFARLANE, “Fission Product Tellurium Chemistry from Fuel to Containment,” Proc. 4th OECD/CSNI Workshop on the Chemistry of Iodine in Reactor Safety, Würenlingen, Switzerland, June 10-12, 1996.
- D. C. McPHAIL, “Thermodynamic Properties of Aqueous Tellurium Species Between 25 and 350°,” Geochim. Cosmochim. Acta, 59, 5, 851 (1995); https://doi.org/10.1016/0016-7037(94)00353-X.
- K. TAGAMI et al., “Estimation of Te-132 Distribution in Fukushima Prefecture at the Early Stage of the Fukushima Daiichi Nuclear Power Plant Reactor Failures,” Environ. Sci. Technol., 47, 10, 5007 (2013); https://doi.org/10.1021/es304730b.
- S. TAKAHASHI et al., “Estimation of the Release Time of Radio-Tellurium During the Fukushima Daiichi Nuclear Power Plant Accident and Its Relationship to Individual Plant Events,” Nucl. Technol., 205, 5, 646 (2018); https://doi.org/10.1080/00295450.2018.1521186.
- N. KINOSHITA et al., “Assessment of Individual Radionuclide Distributions from the Fukushima Nuclear Accident Covering Central-East Japan,” Proc. Natl. Acad. Sci. USA, 108, 49, 19526 (2011); https://doi.org/10.1073/pnas.1111724108.
- K. SAITO et al., “Detailed Deposition Density Maps Constructed by Large-Scale Soil Sampling for Gamma-Ray Emitting Radioactive Nuclides from the Fukushima Dai-ichi Nuclear Power Plant Accident,” J. Environ. Radioact., 139, 308 (2015); https://doi.org/10.1016/j.jenvrad.2014.02.014.
- H.-B. QIN et al., “Tellurium Distribution and Speciation in Contaminated Soils from Abandoned Mine Tailings: Comparison with Selenium,” Environ. Sci. Technol., 51, 11, 6027 (2017); https://doi.org/10.1021/acs.est.7b00955.
- G. YANG et al., “Soil-to-Crop Transfer Factors of Tellurium,” Chemosphere, 111, 554 (2014); https://doi.org/10.1016/j.chemosphere.2014.04.094.
- C. B. BAHN, “Chemical Effects on PWR Sump Strainer Blockage After a Loss-of-coolant Accident: Review on U.S. Research Efforts,” Nucl. Eng. Technol., 45, 3, 295 (2013); https://doi.org/10.5516/NET.07.2013.705.
- “Nuclear Safety Update Knowledge Base for Long-Term Core Cooling Reliability,” Nuclear Energy Agency (2013).
- R. SANDRINE et al., “Precipitate Formation Contributing to Sump Screens Clogging of a Nuclear Power Plant During an Accident,” Chem. Eng. Res. Des., 86, 6, 633 (2008); https://doi.org/10.1016/J.CHERD.2008.03.016.
- A. AUVINEN, R. ZILLIACUS, and J. JOKINIEMI, “Chlorine Release from Hypalon Cable Insulation During Severe Nuclear Reactor Accidents,” Nucl. Technol., 149, 2, 232 (2005); https://doi.org/10.13182/NT05-A3592.
- B. R. BOWSHER et al., “Chemical Forms of Fission Product Tellurium in a Severe Reactor Accident,” Proc. ACS Symp. on Chemical Phenomena Associated with Radioactivity Releases during Severe Nuclear Plant Accidents, NUREG/CP-0078, Anaheim, California, September 9–12, 1986, p. 3 (1987).
- E. C. BEAHM, “Tellurium Behavior in Containment Under Light Water Reactor Accident Conditions,” Nucl. Technol., 78, 3, 295 (1987); https://doi.org/10.13182/NT87-A15995.
- S. GUENTAY et al., “Radiochemical Studies of the Retention of Volatile Iodine in Aqueous Solutions,” J. Radioanal. Nucl. Chem., 273, 3, 557 (2007); https://doi.org/10.1007/s10967-007-0909-3.
- S. GUILBERT et al., “Radiolytic Oxidation of Iodine in the Containment at High Temperature and Dose Rate,” Nucl. Energy New Eur., p. 10 (2007).
- T. NISHI, I. FUJIWARA, and H. MORIYAMA, “γ-Radiolysis of Aqueous Solutions of Tellurium,” Bulletin of the Institute for Chemical Research, Kyoto University, 55, 1, 1 (1977).
- H. MORIYAMA, I. FUJIWARA, and T. NISHI, “Chemical Behavior of Antimony and Tellurium Fission Products in Aqueous Solutions,” J. Radioanal. Nucl. Chem., 55, 1, 45 (1980); https://doi.org/10.1007/BF02514538.
- F. S. DAINTON and R. RUMFELDT, “Radical and Molecular Yields in the γ-Radiolysis of Water. III. The Nitrous Oxide-Sodium Tellurite System,” Proc. R. Soc. London. Ser. A. Math. Phys. Sci., 287, 1411, 444 (1965); https://doi.org/10.1098/rspa.1965.0189.
- N. BELZILE and Y.-W. CHEN, “Tellurium in the Environment: A Critical Review Focused on Natural Waters, Soils, Sediments and Airborne Particles,” Appl. Geochem., 63, 83 (2015); https://doi.org/10.1016/J.APGEOCHEM.2015.07.002.
- M. BOUROUSHIAN, Electrochemistry of Metal Chalcogenides, Springer Science & Business Media (2010).
- M. POURBAIX, Atlas of Electrochemical Equilibria in Aqueous Solutions, Pergamon Press, Oxford, New York (1966).
- W. M. LATIMER, Oxidation Potentials, 2nd ed., Prentice-Hall, New York (1952).
- A. HABERSBERGEROVÁ and B. BARTONÍČEK, “Radiolysis of Iodine Compounds in Model Systems of PWR,” Radiat. Phys. Chem., 21, 3, 289 (1983); https://doi.org/10.1016/0146-5724(83)90157-7.
- L. T. VLAEV and V. G. GEORGIEVA, “Activation Energy for Electroconduction of Aqueous Solutions of Sulfuric and Selenic Acids and Potassium Tellurate,” Russ. J. Electrochem., 40, 6, 674 (2004); https://doi.org/10.1023/B:RUEL.0000032021.43984.d3.
- T. LAVONEN, “Chemical Effects in the Sump Water Pool During Post-LOCA Conditions—Literature Review,” VTT-R-01126-14, VTT Technical Research Centre of Finland (2014).