Abstract
The emergence of microreactor technology has helped to drive supporting nuclear materials qualification and acceptance processes. One essential component in these small reactors is a solid moderator, which typically consists of metal hydride and cladding. While the behavior and performance of metal-hydride moderators go back to early advanced reactor development for nuclear-powered aviation and space propulsion, there remains a knowledge gap in the understanding of hydrogen transport–related phenomena and irradiation performance for hydride moderators. This impacts the acceptance/qualification of hydride moderators for microreactors.
The goal of this technical note is to lay out a potential path forward for advanced moderator qualification and acceptance for designers and developers of microreactors. The proposed approach has benefited from a model microreactor core with the design parameters of a hydride moderator. Based on the model core and design parameters, a simple chart was developed for the major challenges of hydride moderators where potential incidents, causes, effects, and resolutions are described. The relation between the offered resolutions and the maturity of the metal-hydride moderator technology was emphasized using technological readiness. Technological readiness levels (TRLs) were clustered to three sets: physical phenomena related, reactor irradiations, and system demonstration. Some essential needs to fill the knowledge gaps are discussed for physical phenomena–related TRLs. For reactor irradiations, the importance of identifying goals and priorities is stressed to reach certain TRLs. For system demonstration, it is noted that metal-hydride moderator qualification must comply with the overall microreactor design.
I. INTRODUCTION
Microreactors are mobile nuclear reactors operating at low power [less than approximately 30 MW(thermal)] and high temperatures (>600°C). These reactors aim to provide energy in the form of electricity and heat where a secure energy supply can face challenges, such as remote communities, mining sites, disaster regions, dispersed soft or focused industrial locations, and other potential use areas. To meet such a wide spectrum of use, these reactors must be easily operated (e.g., plug and play) and transportable via current transportation infrastructure. Therefore, the main prerequisites of such reactors are to be passively safe, lightweight, and compact.Citation1 Among the main prerequisites, compactness is challenging unless a thermal spectrum reactor is considered where dense neutron moderators are used. By using dense neutron moderators, such as metal hydrides, compactness can be attained to enable transportability.
Metal-hydride moderators were studied for nuclear-powered aviation and space applications from the early 1950s throughout the late 1960s (CitationRefs. 2, Citation3, and Citation4). Much of the focus was on the hydrides of zirconium and yttrium as a neutron moderator, but other options were investigated as well.Citation3,Citation5 Extensive thermodynamic, fabrication, and material property data were generated, and several patents were claimed on the fabrication of hydrides.Citation6–10 A variety of cladding alloys for metal hydrides were developed and investigated to mitigate the potential adverse effects of hydrogen ingress to the reactor system components. Furthermore, irradiation campaigns were executed to support reactor demonstrations, but post-irradiation examinations (PIEs) were curtailed short of full characterization.Citation11,Citation12
With the reemergence of modern microreactor concepts, metal hydrides have recouped their significance as neutron moderators to satisfy the technical requirements of transportable reactor designs. Although a vast amount of data is available on the moderator and candidate moderator cladding materials, technological/knowledge gaps still exist that must be resolved for the neutron-irradiated moderators to ensure reliable and safe operation. In addition, a forward-looking strategy for stakeholders is also needed to facilitate the qualification of these materials to help enable microreactor demonstrations and deployment.
This technical note suggests a strategy for a potential path forward for metal-hydride moderators where designers and developers of microreactor technology can benefit.Footnotea To serve this purpose, metal hydrides and hydrogen migration characteristics are briefly introduced. Hydrogen transport characteristics are considered as the main subject of interest for moderator acceptance in this layout. After that, a hypothetical reactor core is illustrated. Based on the illustrative reactor, the moderator design expectations are decided. Metal-hydride moderator challenges are identified using a simple incident-cause-effect-resolution chart where potential incidents and resolutions are listed. The resolutions are related to the technological readiness levels (TRLs). Finally, readiness for the metal-hydride moderator is discussed in the context of fabrication and TRL. These process steps are suggested as a layout framework for a potential path forward for dense hydride moderator qualification for use in microreactors.
II. METAL HYDRIDES FOR NUCLEAR APPLICATIONS
An immense amount of fabrication and thermophysical data is available related to metal hydrides for nuclear applications via aerospace nuclear propulsion research,Citation3,Citation13–15 the Space Nuclear Auxiliary Power (SNAP) program,Citation2 and the use of Training Research Isotopes General Atomics (TRIGA) reactor fuel elements.Citation16 Furthermore, recent efforts by the Microreactor and Transformational Challenge Reactor programs manufactured yttrium hydrides and reproduced some thermophysical data, as reported in their handbooks.Citation17,Citation18 A comprehensive review is beyond the scope of this technical note. For more detailed information, see Mueller et al.’s milestone book entitled, Metal Hydrides.Citation19 In this section, we provide very targeted information as follows.
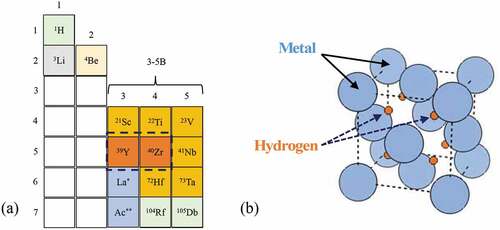
Hydrogen interacts with matter in two ways: (1) it can be occluded in metals such as iron Fe(H) or nickel Ni(H), or (2) it can form a compound by making a stable covalent, ionic, or metallic bond. Excluding the exceptions,Footnoteb metal hydrides relevant to nuclear applications belong to Groups 3B to 5B elements, such as zirconium (Zr) and yttrium (Y). These elements can form a stable compound where hydrogen occupies the interstitial positions in the crystal structure, as shown in .
Fig. 1. (a) Portion of the periodic table of interest for metal-hydride moderators (104Rf and 105Db are artificially synthesized), and (b) crystal structure of MH2 (M = Y or Zr).
Owing to hydrogen’s high mobility in condensed matter, hydrogen can escape from the metal hydride via disassociation reactions depending on the partial pressure of hydrogen at a specific temperature. For instance, hydrogen leaves the Group 5B hydride at temperatures less than 200°C at ~1 atm, and H2 gas is formed in the parent metal’s crystal structure, as indicated in their phase diagram.Citation20–22 Therefore, these hydrides are not suitable for neutron moderation at elevated temperatures. On the other hand, Group 3B to 4B hydrides, including lanthanide and actinide hydrides, have a high hydrogen retention capacity in the hydride phase, as shown in .
Fig. 2. Hydrogen retention of different metal hydrides compared to water at 1 atm (picture source is CitationRef. 18).
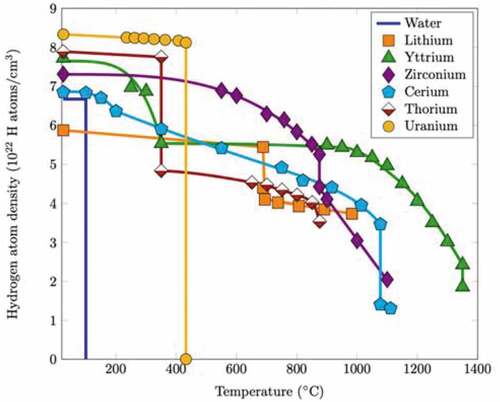
shows the hydrogen atom density as a function of temperature for various materials at ~1 atm. Uranium has the highest hydrogen retention capability up to 400°C, but it drastically decreases to zero above 400°C. At elevated temperatures, zirconium, yttrium, cerium, and lithium (Group 2A elements) exhibit extended hydrogen retention capacity at microreactor-relevant temperatures (>600°C). Importantly, highlights the relative hydrogen stability of various metal hydrides for microreactor operating temperatures.
The metal hydride chosen for a specific microreactor design may vary based on the requirements of that design. For example, if the operating temperature is less than 800°C, ZrHx might be chosen over YHx, since the former can retain more hydrogen than the latter at lower temperatures. However, if an operating temperature of >800°C is desired, the enhanced stability of YHx may make its selection preferable.
The best-known metal hydride, which remains widely used, is the uranium-zirconium hydride (U-ZrH1.6) for TRIGA reactor fuel.Citation2,Citation16,Citation23 The U-Zr hydride fuel is composed of two-phase mixtures of α U and δ ZrH1.6 where the δ-hydride slows fast neutrons down that are emitted from α U via nuclear fission reactions. Thus, the U-Zr hydride fuel is a dual-purpose metal hydride with fission and moderation functions. The moderator function of the hydride fuel is currently qualified for TRIGA reactor conditions,Citation24 as well as its fission function. Because the U-Zr hydride fuel is currently being used in research reactors, the hydride moderator fabrication qualification can benefit from the U-Zr hydride fuel experience.
III. METAL-HYDRIDE MODERATOR CHALLENGES
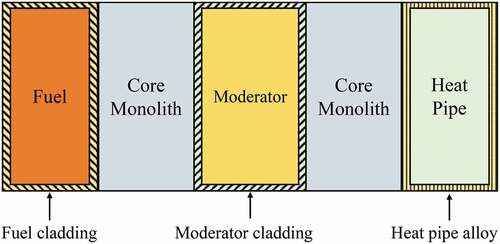
Metal-hydride challenges are defined with a specific microreactor design since hydride moderators can be in different geometric shapes with or without cladding. Furthermore, the operating temperature can vary for different reactors. These design unknowns make it difficult to determine the anticipated challenges that can be experienced with metal-hydride moderators. Therefore, a hypothetical reactor core design is illustrated to predict the technical considerations (see ). This hypothetical reactor core benefits from a monolithic core structure where fuel, a moderator, and a heat pipe are placed. The fuel element and heat pipe are separated from the moderator by the core monolith. A metal hydride is encapsulated with cladding, which forms the moderator element (e.g., metal hydride and cladding). The moderator cladding is in contact with the core monolith. This type of reactor design is selected because moderator-related challenges can be easily identified. These challenges almost cover real microreactor designs, which use a metal-hydride moderator. The reactor is anticipated to operate above 800°C.
Fig. 3. Illustration of a hypothetical microreactor core arrangement, including fuel, moderator, heat pipe, and the monolith that holds the fuel, moderator, and heat pipe components.
The design of the moderator can follow several options. The metal hydride can be a zirconium- or yttrium-base material or their alloys. The metal hydride can be bonded with the cladding or vice versa. Furthermore, a plenum volume can be present with hydrogen and noble gases. All these combinations affect the hydrogen transport characteristics and the expected hydrogen loss behavior from the moderator. The fundamental design expectations from the hydride moderator are as follows:
Maintaining the moderating power at the design limits over the lifetime of the reactor is the fundamental function of the moderator. However, hydrogen loss over time (chronic hydrogen loss) from the intact cladding may cause loss of moderation power, which disables the normal operation characteristic of the reactor.
Ensuring the predictable and stable behavior of the moderator during normal and transient conditions is critical to evaluating the moderator’s performance for long-term operation. Accurate prediction of the hydrogen inventory in the moderator establishes the reactor design parameters. In particular, hydrogen transport under temperature gradients can cause neutronic instability due to thermal transport and irradiation-enhanced diffusion. Hydrogen accumulates at lower temperatures due to its high mobility and thermal diffusion (e.g., Soret effect). This may cause mechanical failure of the moderator due to embrittlement of the metal hydride and extensive volumetric expansion if yttrium trihydride is formed.
Based on the design expectations from the moderator and the given reactor design, a simple chart, shown in , can be established to identify the potential key challenges for the hydride moderator. The chart includes key events for the hydride moderator, including incidents and their root cause(s) with potential effects to the reactor system. Furthermore, the potential resolutions for mitigation or prevention of these incidents are also listed in the chart. For a real microreactor, this simple chart transforms to the failure modes and effects analysis (FMEA) and the phenomena identification and ranking tables (PIRT) approaches for a moderator. Note that the FMEA and PIRT approaches also include the importance of incidents on the reactor system and current knowledge levels or uncertainties of occurring physical phenomena.Citation25,Citation26 The current chart does not include this information. Overall, this simple chart (or FMEA and PIRT for a real microreactor system) will comply with design expectations and inform the research and development strategy. This dual-purpose use of such a chart helps to identify the challenges and knowledge gaps where stakeholders can develop mitigation strategies, including research and development needs.
TABLE I Incident-Cause-Effect-Resolution for the Hydride Moderator Based on the Model Reactor
Incidents listed in the simple chart focus on (1) hydrogen loss over time during normal operating conditions, (2) loss of moderating power during transient conditions caused by design-basis accidents (DBAs) or beyond-design-basis accidents (BDBAs), (3) hydrogen redistribution under a temperature gradient, and (4) loss of pressure boundary of the moderator cladding due to thermal cycling and hydrogen-assisted failure. These incidents can be expanded depending on the microreactor design as well.
Continuous hydrogen loss from the moderator over time can cause chronic loss of moderating power. As a result, the reactor’s operational functions can be degraded. Furthermore, hydrogen can induce embrittlement in the moderator cladding material if hydrogen is adsorbed by the cladding. Additionally, hydrogen can interact with the core monolith, and it can cause deleterious effects on the core monolith or auxiliary system components over time, yielding a potential failure of these components. To resolve such an incident, the fabrication of moderators with better hydrogen retention and cladding candidates that are resistant to hydrogen permeation are to be considered. Designers can also identify the cladding options via materials selection.
Immediate hydrogen loss from the moderator can only occur if the cladding failure happened because of a transient incident, such as a DBA or BDBA event. In these cases, the reactor is expected to shut down. In an event where the core coolability is limited, the temperature can rise. Hydrogen starts to leave the metal hydride and increases the internal pressure of the cladding if no bonding exists between the cladding and the metal hydride as the temperature rises above 1000°C. With the loss of cladding mechanical strength and biaxial loading conditions, burst rupture along the axial direction can occur for the illustrated reactor. For mitigation, a reactor can be designed to avoid a moderator cladding rupture during a DBA or BDBA event. Furthermore, mechanical properties and failure modes of the cladding under relevant conditions must be known and implemented into fuel performance codes.
Hydrogen redistribution in the metal hydride can occur if the metal hydride experiences a temperature gradient along the axial or radial directions. A temperature gradient can occur due to highly skewed heat removal in the reactor where hydrogen redistributes in the metal hydride because of the thermal diffusion (e.g., the Ludwig-Soret or Soret effect) of the hydrogen. For shallow temperature gradients, hydrogen redistribution will be slow, but it will be fast for the sharp temperature gradients. The thermal diffusion characteristics of hydrogen also will be affected by the initial hydrogen number density as targeted during the moderator fabrication. Due to thermal diffusion, hydrogen tends to accumulate at the cold end of the moderator where metal hydride can fracture. To mitigate potential thermal diffusion–induced neutronic instabilities and metal-hydride failures, the Soret effect for the moderator must be resolved and implemented in fuel performance codes. Alternatively, reactor designs that inherently mitigate or avoid the Soret effect can be investigated, or a hydrogen distribution control mechanism can be included in the reactor design. The Soret effect in metal hydrides is currently unknown. The infrastructure to elucidate the Soret effect is not mature.
Loss of the pressure boundary of the moderator is directly related to the high temperature strength of the moderator cladding material during long-term reactor operation. The expected mechanical failure modes are creep rupture and/or creep/fatigue rupture, including hydrogen-induced embrittlement at lower operating temperatures. In addition to these, one potential mechanism can be strain-driven loading due to temperature ramps while load-following, particularly during the reactor’s end-of-life (EOL) period. With the occurrence of a cladding breach, hydrogen depressurization will happen. Depending on the rupture size, the metal hydride will be exposed to a core monolith, as observed in , and it will interact with the core monolith via species transport where the compositional integrity of the core may change. This incident can be avoided by the materials selection process for the moderator. If materials selection cannot meet the requirement, alloy development will be needed. For the alloy development case, current experience from the U.S. Department of Energy (DOE) programs may be beneficial. Finally, the materials behavior must be implemented into fuel performance codes. Another mitigation, not included in the simple chart, could be allowing the failure of a certain number of moderators over time (e.g., close to EOL), ensuring the reactor’s safe operation envelope is intact.
Incidents listed in the simple chart in only provide basic considerations related to the use of a metal-hydride moderator. Foreseeing this, more incidents will be present for an actual microreactor, and the number of resolutions will likely be greater by providing flexibility in the moderator design and qualification. These example resolutions are highly correlated with the readiness of the moderator technology where technological maturity is measured. Thus, resolutions in the simple chart (or FMEA and PIRT for a real system) will connect the design expectations with the technological readiness of metal-hydride moderators.
IV. TECHNOLOGICAL READINESS
To meet the design expectations, the technological readiness of the moderator needs to reach adequate completion for a specific reactor design. By taking nuclear fuel component qualification as the basis,Citation27,Citation28 this maturation level spans metal-hydride moderator fabrication, essential data production, irradiation performance, and post-irradiation property measurements. For the reactor shown in , the fabrication of a hydride moderator consists of three steps: (1) the fabrication of the metal hydride, (2) the fabrication of the cladding, and (3) the assembling/joining of the two components into a final hydride moderator shape. Metal-hydride fabrication is performed through massive hydriding or several powder compacting techniques.Citation3,Citation5,Citation18,Citation29,Citation30 Metal-hydride fabrication needs to include a certain quality level from initial feedstock material specifications to the manufacturing processes, as well as the post-manufacturing quality metrics.
Post-manufacturing quality metrics need to be set to meet the design-basis functionality of the moderator. Clearly, the most important metric is the hydrogen content after moderator fabrication. Independent of the fabrication method, mass measurements, X-ray diffraction volume fraction analysis, and hydrogen content measurements with analytical techniques (e.g., hot vacuum extraction or inert gas fusion) are vital techniques.Citation31 For post-fabrication hydrogen content characterization, applying more than one technique ensures the quality of the final fabricated metal-hydride product. Some morphological metrics may be important for a specific reactor design, such as the oxide layer after manufacturing, component shape, or surface and internal cracks. Once the selected metrics are evaluated with the allowable design requirements, metal-hydride fabrication can reach adequate maturation, as decided by the stakeholders.
While metal-hydride fabrication is relatively straightforward, cladding fabrication has diverse challenges, such as tube-making (e.g., a circular or rectangular profile), joining/welding capability, hydrogen permeation, and thermomechanical strength under the hydrogen environment. Identifying and meeting these essential metrics are directly related to the TRL of the cladding material and the materials selection. At this point, cladding manufacturing experience obtained in the Accident Tolerant Fuel (ATF) program may speed up the qualification processes for novel materials.Citation32 For instance, FeCrAl alloys and silicon carbide/silicon carbide (SiC/SiC) fiber composites may be viewed as being suitable for moderator cladding. The FeCrAl alloy family and the SiC/SiC have been investigated as light water reactor fuel cladding with respect to manufacturing,Citation33,Citation34 irradiation behavior,Citation35,Citation36 and mechanical behavior under transient loadings.Citation37–40 In the ATF program, various coating application techniques have also been developed and studied.Citation32 Using a different coating, hydrogen loss could be mitigated.
Assembling metal hydride and cladding as a single moderator component focuses on the joining and welding techniques of these components into the final shape. Some generic metrics for assembly are the long-term mechanical integrity at elevated temperatures and mechanical performance during temperature cycles or ramps.
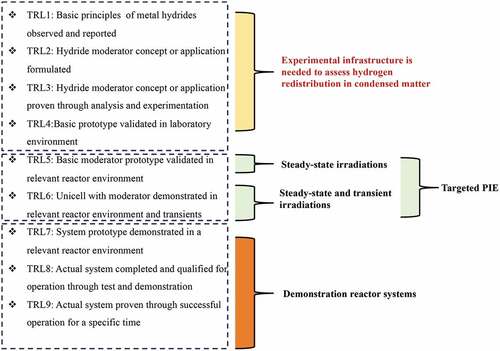
Beyond fabrication, there is a knowledge gap related to metal hydrides, although nuclear propulsion programs have demonstrated the use of metal hydrides as a neutron moderator. The essential knowledge gaps appear in understanding the hydrogen redistribution in the metal hydride under temperature gradient (e.g., thermal diffusion), irradiation performance of the metal hydrides, high-temperature mechanical response of the candidate cladding under a hydrogen gas environment for short- and long-term experiments under monotonic and cyclic loading conditions, and irradiation performance of the moderator (e.g., metal hydride + cladding). Using the TRL approach, as observed in , TRLs can be combined into three sets for metal-hydride moderators: (1) TRLs 1 through 4, (2) TRLs 5 and 6, and (3) TRLs 7, 8, and 9. The first part (e.g., TRL 1 through 4) describes the readiness correlated to the basic phenomena, like thermal diffusion of hydrogen or mechanical integrity of the cladding under a hydrogen gas environment.
For instance, it is well known that hydrogen can thermally diffuse to colder zones, which may impact the neutron moderation function during steady-state operation. However, there are no available data that address hydrogen’s thermal transport in metal hydrides with quantified uncertainty at microreactor-relevant temperatures. The simple chart showing the FMEA/PIRT cannot quantitively prioritize the impact of the thermal diffusion of hydrogen in the moderator for the illustrative reactor without collecting the necessary experimental data. Therefore, testing infrastructure is needed to address the hydrogen’s thermal diffusion phenomenon in metal hydrides. Importantly, it must be ensured that the data be collected under defined quality assurance with reliability and reproducibility. The same situation occurs for the unknown phenomena for the cladding and assembled moderator where testing capabilities are needed to overcome the first part of the TRLs.
The second part (TRLs 5 and 6) is related to moderator readiness under a relevant environment (i.e., nuclear reactor irradiations). While the legacy PIE data can be found in CitationRefs.11, Citation12, and Citation41, these PIE data are inadequate to answer essential questions, such as the effect of fast neutron fluence on the hydrogen inventory for metal-hydride moderators. Therefore, steady-state and transient irradiations with identified and prioritized goals will be needed. Irradiations and PIEs focusing on the investigation of geometrical stability and the quantification of hydrogen loss after irradiation will be essential for qualification or acceptance.
The third part (TRLs 7, 8, and 9) is more related to overall system demonstration and deployment in a nuclear reactor where moderator qualification must comply. Moderator-initiated incidents must be evaluated or predicted. TRLs 7, 8, and 9 must include a reactor system design beyond the nuclear reactor, as illustrated in this study. Thus, this part surpasses the aim of this technical note.
V. SUMMARY
This technical note provides a potential layout that helps to establish a path forward for the acceptance or qualification of hydride moderators for microreactor use.Footnotec At first, the layout includes an illustrative reactor core with the presence of critical components. Based on the core design, essential design parameters were identified for a metal-hydride moderator by focusing on the hydrogen transport characteristics. Using these essential design parameters, a simple chart was introduced to determine several incidents, causes, impacts, and potential resolutions for the illustrative microreactor core.
These potential resolutions were directly connected to the TRL of the metal-hydride moderator. Therefore, maturity of the technology was briefly discussed at multiple levels, such as fabrication, hydrogen transport phenomena, and irradiation performance. From the moderator fabrication perspective, identifying the key fabrication metrics was emphasized to reach sufficient fabrication maturity. In general, TRL items were combined into three parts: (1) physical phenomena related, (2) irradiation performance, and (3) system demonstration. Some essential needs to fill the knowledge gaps were identified. For reactor irradiations, the importance of the identification of goals and prioritization was stressed to reach certain TRLs. A final TRL set was conducted at the system scale, which was beyond the present scope and more related to the stakeholders, but we noted that metal-hydride moderator qualification must comply with the overall microreactor design.
Acknowledgments
This work was supported by the DOE, Office of Nuclear Energy, under the Advanced Reactor Technology portfolio’s Microreactor Program. The content represents ongoing efforts by the Microreactor Program to enable the accelerated development and demonstration of microreactor technology.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Notes
a We do not intend to provide a specific framework that binds stakeholders and other institutions. Furthermore, topics covered in this technical note may not correspond to specific reactor types.
b Beryllium and lithium can also form hydrides, which have been used as a neutron moderator and as shielding and reflector components for aviation and space nuclear propulsion.
c This framework may not be the actual approach but provides a path forward for the acceptance of hydride moderators. This framework or variants may be applied to other components as well, as needed.
References
- “A Microreactor Program Plan for the Department of Energy,” INL/EXT-20-58191, p. 23, Idaho National Laboratory (2020).
- G. F. BURDI, “SNAP Technology Handbook, Volume II Hydride Fuels and Claddings SNAP Technology Handbook Volume II,” p. 129, Atomics International (1964).
- R. VAN HOUTEN, “Selected Engineering and Fabrication Aspects of Nuclear Metal Hydrides (Li, Ti, Zr, and Y), ” Nucl. Eng. Des., 31, 3, 434 (1974); https://doi.org/10.1016/0029-5493(75)90178-8.
- G. THORNTON and A. ROTHSTEIN, “Comprehensive Technical Report General Electric Direct-Air-Cycle Aircraft Nuclear Propulsion Program: Program Summary and References,” APEX-901, General Electric (1962).
- J. B. VETRANO, “Hydrides as Neutron Moderator and Reflector Materials,” Nucl. Eng. Des., 14, 3, 390 (1971); https://doi.org/10.1016/0029-5493(70)90159-7.
- E. C. PHILLIPS JR., “Casting Hydrides,” p. 3, U.S. Patent 3,692,888 (1969).
- R. VAN HOUTEN, “Massive Metal Hydride Structures and Methods for Their Preparation,” U.S. Atomic Energy Commission (1967).
- J. W. RAYMOND and H. TAKETANI, “Hydriding Process,” p. 5, U.S. Atomic Energy Commission (1965).
- J. B. VETRANO, “Preparation of Metal Hydride Bodies by Improved Powder Metallurgy Process,” p. 7, Rockwell International Corporation (1964).
- R. W. SULLIVAN, “Method of Making Crack-Free Zirconium Hydride,” p. 6, U.S. Department of Energy (1966).
- J. C. MARSHALL, R. VAN HOUTEN, and W. G. BAXTER, “Yttrium Hydride Moderator Evaluation—In-Pile Thermal Stability,” General Electric Company (1962).
- J. C. MARSHALL, R. VAN HOUTEN, and W. G. BAXTER, “1000 Hour Demonstration of Clad Yttrium Hydride as a Neutron Moderator,” Proc. American Nuclear Society 1964 Annual Mtg., p. 2, M. FERRIER, Ed. (1964).
- R. VAN HOUTEN, “Recent Developments of Metallic Hydride Shielding Materials GEMP-518,” General Electric Company (1967).
- R. L. BECK, “Research and Development of Metal Hydrides,” University of Denver (1960).
- J. M. FACKELMANN, M. A. GEDWILL JR., and H. H. KRAUSE JR., “Survey of Zirconium, Yttrium, and Cerium Hydrides for Reflector Application in the GCRE,” p. 86, Battelle Memorial Institution (1960).
- M. T. SIMNAD, “The U-ZrHx Alloy: Its Properties and Use in TRIGA Fuel,” Nucl. Eng. Des., 64, 3, 403 (1981); https://doi.org/10.1016/0029-5493(81)90135-7.
- X. HU et al., “Handbook on the Material Properties of Yttrium Hydride for High Temperature Moderator Applications,” p. 36, Oak Ridge National Laboratory (2021).
- A. P. SHIVPRASAD et al., “Advanced Moderator Material Handbook,” p. 66, Los Alamos National Laboratory (2020).
- W. M. MUELLER, J. P. BLACKLEDGE, and G. G. LIBOWITZ, “Metal Hydrides,” in Metal Hydrides, W. M. MUELLER, J. P. BLACKLEDGE, and G. G. LIBOWITZ, Eds., Academic Press (1968).
- R. GRIFFITHS, J. PRYDE, and A. RIGHINI-BRAND, “Phase Diagram and Thermodynamic Data for the Hydrogen/Vanadium System,” J. Chem. Soc., Faraday Trans. 1 F, 68, 2344 (1972); https://doi.org/10.1039/f19726802344.
- H. OKAMOTO, ““H-Nb (Hydrogen-Niobium),” J. Phase Equilibria Diffus., 34, 2, 163 (2013); https://doi.org/10.1007/s11669-012-0165-2.
- A. SAN-MARTIN and F. D. MANCHESTER, “The H-Ta (Hydrogen-Tantalum) System,” J. Phase Equilib., 12, 3, 332 (1991); https://doi.org/10.1007/BF02649922.
- D. OLANDER et al., “Uranium-Zirconium Hydride Fuel Properties,” Nucl. Eng. Des., 239, 8, 1406 (2009); https://doi.org/10.1016/j.nucengdes.2009.04.001.
- “Reduced Enrichment for Research and Test Reactors Program, Currently Qualified Fuels,” Argonne National Laboratory; https://www.rertr.anl.gov/QualFuel.html (current as of May 14, 2022).
- G. E. WILSON and B. E. BOYACK, “The Role of the PIRT Process in Experiments, Code Development and Code Applications Associated with Reactor Safety Analysis,” Nucl. Eng. Des., 186, 1, 23 (1998); https://doi.org/10.1016/S0029-5493(98)00216-7.
- B. BOYACK, “Phenomenon Identification Ranking Tables (PIRTs) for Power Oscillations Without Scram in Boiling Water Reactors Containing High Burnup Fuel,” NUREG/CR-6743, LA-UR-00-5079, U.S. Nuclear Regulatory Commission (2001).
- D. C. CRAWFORD et al., “An Approach to Fuel Development and Qualification,” J. Nucl. Mater., 371, 1, 232 (2007); https://doi.org/10.1016/j.jnucmat.2007.05.029.
- W. J. CARMACK et al., “Technology Readiness Levels for Advanced Nuclear Fuels and Materials Development,” Nucl. Eng. Des., 313, 177 (2017); https://doi.org/10.1016/j.nucengdes.2016.11.024.
- X. HU et al., “Fabrication of Yttrium Hydride for High-Temperature Moderator Application,” J. Nucl. Mater., 539, 152335 (2020); https://doi.org/10.1016/j.jnucmat.2020.152335.
- C. L. HUFFINE, “Chapter 13—Fabrication of Hydrides,” in Metal Hydrides, W. M. MUELLER, J. P. BLACKLEDGE, and G. G. LIBOWITZ, Eds., pp. 675–747, Academic Press (1968).
- C. N. TAYLOR, “Hydrogen and Its Detection in Fusion and Fission Nuclear Materials—A Review,” J. Nucl. Mater., 558, 153396 (2022); https://doi.org/10.1016/j.jnucmat.2021.153396.
- K. A. TERRANI, “Accident Tolerant Fuel Cladding Development: Promise, Status, and Challenges,” J. Nucl. Mater., 501, 13 (2018).
- Y. YAMAMOTO et al., “Report on Exploration of New FeCrAl Heat Variants with Improved Properties,” Oak Ridge National Laboratory (2019).
- Y. YAMAMOTO et al., “Optimized Properties on Base Metal and Thin-Walled Tube of Generation II ATF FeCrAl,” Oak Ridge National Laboratory (2015).
- K. G. FIELD, K. C. LITTRELL, and S. A. BRIGGS, “Precipitation of α′ in Neutron Irradiated Commercial FeCrAl Alloys,” Scr. Mater., 142, 41 (2018); https://doi.org/10.1016/j.scriptamat.2017.08.022.
- M. N. GUSSEV, E. CAKMAK, and K. G. FIELD, “Impact of Neutron Irradiation on Mechanical Performance of FeCrAl Alloy Laser-Beam Weldments,” J. Nucl. Mater., 504, 221 (2018); https://doi.org/10.1016/j.jnucmat.2018.03.036.
- N. R. BROWN et al., “Mechanical Failure of Fresh Nuclear Grade Iron-Chromium-Aluminum (FeCrAl) Cladding Under Simulated Hot Zero Power Reactivity Initiated Accident Conditions,” J. Nucl. Mater., 539, 152352 (2020); https://doi.org/10.1016/j.jnucmat.2020.152352.
- B. GARRISON et al., “Burst Characteristics of Advanced Accident-Tolerant FeCrAl Cladding Under Temperature Transient Testing,” J. Nucl. Mater., 560, 153488 (2022); https://doi.org/10.1016/j.jnucmat.2021.153488.
- M. N. CINBIZ et al., “Transient Mechanical Test Development for Accident Tolerant Cladding Candidates to Simulate PCMI-like Conditions,” Oak Ridge National Laboratory (2017).
- M. N. CINBIZ et al., “Report on Design and Failure Limits of SiC/SiC and FeCrAl ATF Cladding Concepts Under RIA,” Oak Ridge National Laboratory (2018).
- S. J. PAPROCKI, E. S. HODGE, and H. D. HANES, “Cladding of Yttrium Hydride with Iron-Chromium-Aluminum by Gas-Pressure Bonding,” Battelle Memorial Institute (1960).