In recent decades, the chemistry of functionally substituted 1,2,4-triazoles and the fused heterocyclic systems based on them have received significant attention due to their structural and biological characteristics. The 1,2,4-triazole ring is a fragment of many fungicides such as fluconazole, intraconazole, and voriconazole.Citation1 3-R-4-Amino-1,2,4-triazole-5-thiones were found to exhibit antimicrobial and anti-inflammatory activity.Citation2–5 Fused heterocyclic triazoles also possess important clinical applications; thus, 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles were reported as antiviral, antifungal, and antitumor agents.Citation6–8 Anti-HIV activity was also found.Citation9 Sterically hindered phenols were reported to possess anti-inflammatory and antimicrobial properties,Citation10,Citation11 in addition to their well-known antioxidant activity.Citation12
As a continuation of our studies on the synthesis of azoles containing a hindered phenol moiety,Citation13,Citation14 in the present work we have synthesized a number of novel compounds: substituted arylideneamino-2,4-dihydro-3H-1,2,4-triazole-3-thiones, 1,2,4-triazolo[3,4-b][1,3,4]-thiadiazoles and 6-aryl/alkyl-amino[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazoles, based on 3-(4-hydroxy-2,6-di-tert-butylphenyl) propanoic acid hydrazide, in search of new structures potentially exhibiting useful properties.
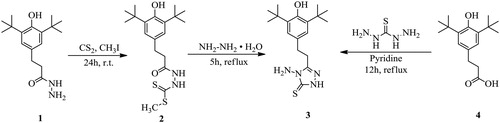
In this study two different approaches were used to prepare the starting 4-amino-3-[2-(4-hydroxy-3,5-di-tert-butylphenyl)ethyl]-1,2,4-triazole-5-thione 3 (Scheme 1). In the first case,Citation15,Citation16 the reaction of the hydrazide 1 with carbon disulfide and methyl iodide took place in ethanol to give the methyl ester of dithiocarbazic acid 2 in two stages over 24 hours (see Experimental section). Heterocyclization of the compound 2 with hydrazine hydrate lead to the formation of 3-R-4-amino-1,2,4-triazol-5-thione 3 in 55% yield. In the second case the 1,2,4-triazole 3 was obtained by the interaction of equimolar amounts of 3-(4-hydroxy-3,5-di-tert-butylphenyl)propanoic acid 4 and thiocarbohydrazide. According to the published data,Citation17,Citation18 this approach involves, in most cases, a short-term fusion of the initial reagents, but, in our case, even short heating of the reaction mixture above the melting point led to the destruction of the starting acid. So the reaction was carried out by refluxing the starting reagents in pyridine for 12 hours, and the desired product was isolated in 35% yield.
3-R-4-Amino-1,2,4-triazole-5-thiones, as it was estimated, can exist both in thionic and mercapto-forms.Citation19 The 1H NMR spectrum of compound 3 contains a signal in the 9.5-10 ppm region corresponding to the proton in the triazole ring, with no peak at 6 ppm corresponding to the proton of the mercapto-group.Citation20 In the IR spectrum there are medium intensity bands at 1350-1330 and 1530-1520 cm−1, corresponding to the C = S bond vibrations, and there are no bands in the 2610-2530 cm−1 region characterizing the vibrations of the C-SH unit.Citation21 Thus, the spectral data suggest that compound 3 in the crystalline state, as well as in the DMSO solution, exists in a thionic form.
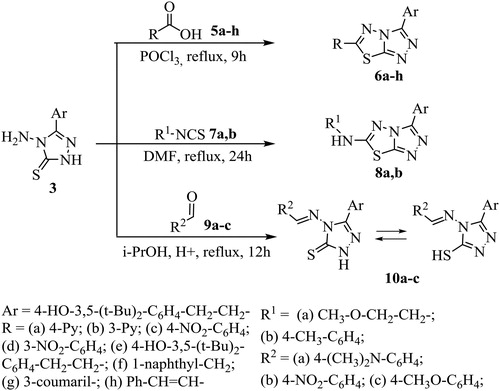
Compound 3 reacted further with a number of carboxylic acids, with aryl/alkyl isothiocyanates and several aldehydes (Scheme 2).
According to the literature data, the reaction with acids is usually carried out by allowing the starting materials to reflux in phosphorus oxychloride or thionyl chloride.Citation22 In our case the reaction of 4-amino-3-R-1,2,4-triazole-5-thione 3 with a series of carboxylic acids 5a-h was carried out by the reflux of the starting reagents in POCl3 for 9 hours, and new 1,2,4-triazolo [3,4-b][1,3,4]thiadiazoles 6a-h were obtained in yields of 35-81%.
In the IR spectra of the obtained compounds, there are no absorption bands at 3335 and 3269 cm−1, indicating that there is no NH group, and no band in the region of 1530-1520 cm−1 related to the C = S bond, but there is a band at 750 cm−1 corresponding to the deformation vibrations of the C-S-C unit. There are no peaks at 9.5-10 ppm in the 1H NMR spectra, indicating the absence of N-H protons.
One-pot reaction of the triazole 3 with aryl/alkyl isothiocyanates was carried out by the reflux of the starting reagents in DMF during 24 hours and triazolothiadiazoles 8a,b were obtained in yields of 35-41%. In the 1H NMR spectra of compounds 8a,b the signals of NH2 and NH (triazole) protons disappeared, but a singlet for the alkyl/aryl amino proton appeared in the range of 5.47-5.57 ppm, confirming that the triazoles had converted into triazolothiadiazoles.
Schiff bases 10a-c were obtained by the condensation of 1,2,4-triazole 3 with the appropriate aldehydes in the presence of a catalytic amount of concentrated sulfuric acid in refluxing i-PrOH in yields of 22-29%. The IR data for the compounds 10a-c revealed bands at 1344 cm−1 (C = S stretching) and the absence of absorption in the region of ∼2600-2550 cm−1 cited for an SH group, indicating that these compounds are in the thionic form. In the 1H-NMR spectra of the compounds 10a-c in DMSO-d6, the N = CH proton appeared near 8-10 ppm as a singlet.
In summary, 4-amino-1,2,4-triazol-5-thiones were used as convenient synthons for the preparation of 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. It was established that the best yields (55%) of the 4-amino-3-R-1,2,4-triazol-5-thione 3 were achieved by heterocyclization of methylcarbodithionate 2. A number of novel condensed derivatives and Schiff bases with hindered phenol fragments were obtained starting from the 4-amino-3-R-1,2,4-triazole-5-thione. They were characterized using IR, 1H-NMR, 13C-NMR, elemental analysis and mass spectroscopy methods. We hope that our study will stimulate further research on these fascinating heterocyclic systems.
Experimental section
The IR spectra were obtained on an Agilent Cary 660 FTIR spectrometer using an ATR cell with ZnSe crystal. 1H- and 13C-NMR spectra of solutions in DMSO-d6 were recorded on a Bruker AM-300 spectrometer. All experiments were performed according to the standard methods of Bruker. Chemical shifts were reported relative to Me4Si. The values of coupling constants are given in Hz. The mass spectra were recorded on an MS-30 Kratos device (Eu, 70 eV). A peak of the molecular ion M+ was observed for all synthesized compounds. The melting points of the compounds obtained were determined in open capillary tubes. Elemental analysis was carried out using a Vario Microcube analyzer. The course of reactions and purity of the compounds obtained was monitored by TLC on silica gel plates in a 10:1 benzene-ethanol solvent system, unless otherwise noted.
Methyl 2-(3-(4-hydroxy-3,5-di-tert-butylphenyl)propanoyl)hydrazine-1-carbodithionate (2)
A solution of 0.84 g (15 mmol) of KOH in 5 ml of water was added to a solution of 2.92 g (10 mmol) of hydrazide 1 and 3.8 g (50 mmol) of carbon disulfide in ethanol at 0° C. The reaction mixture was stirred at 0° C for 4 hours, then an additional 24 hours at room temperature. A solution of 2.84 g (20 mmol) of CH3I in 10 ml of ethanol was added, and the mixture was stirred for 6 hours. The precipitate was filtered off and crystallized from a mixture of benzene:hexane 5:1 to give 3.13 g, 82%, colorless crystals, m.p. 54-56° C. 1H NMR (DMSO-d6): δ 1.56 (18Н, s, t-Bu), 2.44 (2Н, t, J = 6.6, СН2-СН2), 3.67 (3Н, s, СН3-S-), 7.02 s (2Н, s, Нar), 7.28 (1Н, s, ОН), 8.11 (1Н, s, NH). MS: (M+), m/z 382.
Anal. Calcd for C19H30N2O2S2: C, 59.65; H, 7.90; N, 7.32; S, 16.76. Found: C, 59.78; H, 7.99; N, 7.18; S, 16.67.
4-Amino-3-[2-(4-hydroxy-3,5-di-tert-butylphenyl)ethyl]-1,2,4-triazol-5-thione (3)
Method A. A mixture of 1.91 g (5 mmol) methyl 2-[3-(4-hydroxy-3,5-di-tert-butylphenyl)propanoyl]hydrazine-1-carbodithionate 2 and 1.25 g (25 mmol) of hydrazine hydrate in 20 ml of ethanol was refluxed for 5 hours. The reaction mixture was cooled to room temperature and poured into water. Next, 10% HCl was added dropwise until the pH of the mixture reached 8, and the precipitate formed was filtered off. The purification was carried out using a column of silica gel, eluent benzene:ethanol 15:1, to give 0.96 g, 55%, colorless crystals, m.p. 155-156° C.
Method B. A mixture of 2.78 g (10 mmol) 3-(4-hydroxy-3,5-di-tert-butylphenyl)propanoic acid 4 and 1.06 g (10 mmol) of thiocarbodihydrazide in 15 ml of pyridine was heated under stirring for 18 hours. After cooling, the reaction mixture was poured into water and acidified with 10% HCl to a slightly acidic reaction, the solid filtered and crystallized from ethanol to give 1.6 g, 46%, colorless crystals, m.p. 155-157° C. 1H NMR (DMSO-d6): δ 1.37 (18Н, s, t-Bu), 2.86 (4H, s, СН2-СН2), 5.52 (2Н, s, NH2), 6.75 (1Н, s, ОН), 6.93 (2Н, s, Нar), 13.46 (1Н, s, NH). 13C NMR (DMSO-d6): δ 166.2, 152.0, 139.6, 131.66, 128.7, 124.9, 34.9, 32.0, 30.9, 27.1. MS: (M+), m/z 348.
Anal. Calcd for C18H28N4OS: C, 62.03; H, 8.10; N, 16.08; S, 9.20. Found: C, 62.21; H, 8.25; N, 16.01; S, 9.11.
General procedure for preparation of 3-[2-(4-hydroxy-3,5-di-tert-butylphenyl) ethyl]-6-R-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles (6a-h)
A mixture of 1.74 g (5 mmol) of 4-amino-1,2,4-triazole 3 and 5 mmol of the corresponding carboxylic acid 5a-h were refluxed under stirring in 15 ml of POCl3 for 9 hours. The reaction mixture was cooled, poured into ice water, a cooled 10% NaOH solution was added until the pH of the mixture reached 6, the precipitate formed was filtered off and purified using a silica gel column (toluene:ethanol 15:1).
3-[2-(4-Hydroxy-3,5-di-tert-butylphenyl)ethyl]-6-(4-pyridyl)-1,2,4-triazolo[3,4-b] [1,3,4]thiadiazole (6a)
Colorless crystals, yield: 0.84 g, 39%, m.p. 122-124 °С (dioxane:H2O 2:1). FT-IR cm−1: 3624, 1596, 1474, 1434, 765. 1H NMR (DMSO-d6): δ 1.31 (18Н, s, t-Bu), 3.05 (2H, t, J = 6.6, СН2-СН2), 3.34 (2Н, t, J = 6.9, СН2-СН2), 6.87 (2Н, s, Наr), 6.91 (1Н, s, ОН), 7.84 (2Н, d, J = 4.1, Нar), 8.83 (2Н, d, J = 3.9, Нar). 13C NMR (DMSO-d6): δ 164.20 (S-C = N in triazole), 152.6, 151.4, 148.1, 139.43, 136.7, 131.2, 128.7, 124.8, 121.1, 34.8, 30.9, 30.8, (2(CH3)3), 27.2. MS: (M+), m/z 435.
Anal. Calcd for C24H29N5OS: C, 66.18; H, 6.71; N, 16.08; S, 7.36. Found: C, 66.31; H, 6.90; N, 16.15; S, 7.42.
3-[2-(4-Hydroxy-3,5-di-tert-butylphenyl)ethyl]-6-(3-pyridyl)-1,2,4-triazolo[3,4-b] [1,3,4]thiadiazole (6b)
Colorless crystals, yield: 0.83 g, 38%, m.p. 160-162 °С (benzene:hexane 5:1). FT-IR cm−1: 3624, 1602, 1474, 1435. 765. 1H NMR (DMSO-d6): δ 1,28 (18Н, s, t-Bu), 3.00 (2H, t, J = 7.4, СН2-СН2), 3.29 (2Н, t, J = 9.0, СН2-СН2), 6.70 (1Н, s, ОН), 6.81 (2Н, s, Нar), 7.60-7.65 (1H, m, Har), 8.25 (1Н, d, J = 11.9, Нar), 8.81 (1Н, d, J = 6.2, Нar), 9.06 (1H, s, Har). 13C NMR (DMSO-d6): δ 163.7 (S-C = N in triazole), 153.6, 152.6, 148.0, 139.4, 135.3, 131.2, 126.0, 124.9, 124.8, 34.8, 33.1, 30.8 (2(CH3)3), 27.2. MS: (M+), m/z 435.
Anal. Calcd for C24H29N5OS: C, 66.18; H, 6.71; N, 16.08; S, 7.36. Found: C, 66.02; H, 6.85; N, 16.24; S, 7.21.
3-[2-(4-Hydroxy-3,5-di-tert-butylphenyl)ethyl]-6-(4-nitrophenyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole (6c)
Yellow crystals, yield: 0.79 g, 33%, m.p. 124-126 °С (benzene: heptane 5:1). FT-IR cm−1: 3627, 1602, 1525, 1347, 752. 1H NMR (DMSO-d6): δ 1.32 (18Н, с, t-Bu), 3.05 (2H, t, J = 7.4, СН2-СН2), 3.34 (2Н, t, J = 7.1, СН2-СН2), 6.72 (1Н, s, ОН), 6.87 (2Н, s, Нar), 8.15, (2Н, d, J = 9.0, Нar), 8.39 (2Н, d, J = 9.0, Нar). 13C NMR (DMSO-d6): δ 164.13 (S-C = N in triazole), 152.6, 150.1, 148.1, 139.5, 135.1, 131.26, 129.0, 125.1, 124.8, 34.8, 30. 8, 33.0, 27.2. MS: (M+), m/z 479.
Anal. Calcd for C25H31N5O3S: C, 62.61: H, 6.09; N, 14.60; S, 6.69. Found: C, 62.78; H, 6.22; N, 14.75; S, 6.51.
3-[2-(4-Hydroxy-3,5-di-tert-butylphenyl)ethyl]-6-(3-nitrophenyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole (6d)
Yellow crystals, yield: 1.17 g, 49%, m.p. 116-118 °С. FT-IR cm−1: 3633, 1537, 1344, 771. 1H NMR (DMSO-d6): δ 1.31 (18Н, s, t-Bu), 3.04 (2H, t, J = 7.4, СН2-СН2), 3.34 (2Н, t, J = 7.2, СН2-СН2), 6.59 (1Н, s, ОН), 6.87 (2Н, s, Нar), 7.88-7.94 (1Н, т, J = 8.0, Нar), 8.33 (1Н, d, J = 8.7, Нar), 8.47 (1Н, d, J = 14.88, Нar), 8.63 (1Н, s, Нar). 13C NMR (DMSO-d6): δ 164.1 (S-C = N in triazole), 152.6, 148.82, 139.5, 133.8, 131.9, 131.3, 130.9, 127.4, 124.8, 121.8, 34.8, 33.0, 30.8, 27.1. MS: (M+), m/z 479.
Anal. Calcd for C25H31N5O3S: C, 62.61; H, 6.09; N, 14.60; S, 6.69. Found: C, 62.51; H, 6.28; N, 14.69; S, 6.78.
3-[2-(4-Hydroxy-3,5-di-t-butylphenyl)ethyl]-6-[2-(4-hydroxy-3,5-di-tertbutylphenyl) ethyl]-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole (6e)
Colorless crystals, yield: 2.51 g, 85%, m.p. 118-120 °С (EtOH:H2O 5:1). FT-IR cm−1: 3636, 1624, 1474, 1435, 768. 1H NMR (DMSO-d6): δ 1,32 (18Н, s, t-Bu), 1,35 (18Н, s, t-Bu), 2.85-2-96 (2H, m, СН2-СН2), 3.16-3.25 (2Н, m, СН2-СН2), 6.70 (1Н, s, ОН), 6.80 (2Н, s, Нar), 6.98, (2Н, s, Нar). 13C NMR (DMSO-d6): δ 170.0 (S-C = N in triazole), 152.8, 152.6, 147.2, 139.7, 139.5, 131.2, 130.5, 125.0, 124.7, 34.9 (CH2), 34.8, 34.1 (CH2), 30.9, 30.3, 27.1. MS: (M+), m/z 590.
Anal. Calcd for C35H50N4O2S: C, 71.15; H, 8.53; N, 9.48; S, 5.43. Found: C, 70.98; H, 8.71; N, 9.29; S, 5.35.
3-[2-(4-Hydroxy-3,5-di-t-butylphenyl)ethyl]-6-(1-naphthylmethyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole (6f)
Colorless crystals, yield: 2.01 g, 81%, m.p. 220-222 °С (dioxane:H2O 4:1). FT-IR cm−1: 3621, 1610, 1474, 1434, 752. 1H NMR (DMSO-d6): δ 1,29 (18Н, s, t-Bu), 2.99 (2H, t, J = 7.4, СН2-СН2), 3.24 (2Н, t, J = 7.2, СН2-СН2), 4.49 (2H, s, CH2), 6.78 (1Н, s, ОН), 6.87 (2Н, s, Нar), 7.09 (1H, d, J = 7.8, Har), 7.45-7.64 (1H, m, Har), 7.91-7.98 (1H, m, Har), 8.14 (1Н, d, J = 6.1, Нar). 13C NMR (DMSO-d6): δ 170.1 (S-C = N in triazole), 153.1, 152.7, 147.2, 139.6, 134.1, 131.7, 131.3, 129.2, 129.1, 128.6, 127.8, 127.2, 126.6, 126.2, 124.7, 124.0, 35.8 (CH2), 34.8, 32.5, 30.827.2. MS: (M+), m/z 498.
Anal. Calcd for C30H34N4OS: C, 72.26; H, 6.88; N, 11.24; S, 6.43. Found: C, 72.15; H, 6.96; N, 11.17; S, 6.28.
3-[2-(4-Hydroxy-3,5-di-t-butylphenyl)ethyl]-6-coumaryl-1,2,4-triazolo[3,4-b][1,3, 4]thiadiazole (6g)
Yellow crystals, yield: 0.93 g, 37%, m.p. 154-156 °С (EtOH:H2O 2:1). FT-IR cm−1: 3571, 1709, 1602, 1474, 1434, 762. 1H NMR (DMSO-d6): δ 1.32 (18Н, с, t-Bu), 3.14 (2H, t, J = 9.1, СН2-СН2), 3.34 (2Н, t, J = 7.5, СН2-СН2), 6.60 (1Н, s, ОН), 6.85 (2Н, с, Нar), 7.34 (1H, s, Har), 7.46 (1H, t, Har), 7.55 (1H, d, J = 8.1, Har), 7.77 (1H, t, Har), 8.01 (1H, d, J = 7.9, Har) 9.03 (1H, s). 13C NMR (DMSO-d6): δ 159.7 (S-C = N in triazole), 159.4 (C = O), 154.1, 152.6, 143.7, 139.6, 135.2, 131.4, 131.0, 126.0, 124.7, 118.8, 117.0, 116.6, 34.8, 32.7, 30.9, 27.1. MS: (M+), m/z 502.
Anal. Calcd for C28H30N4O3S: C, 66.91; H, 6.02; N, 11.15; S, 6.38. Found: C, 67.02; H, 6.20; N, 11.01; S, 6.26.
2,6-di-tert-Butyl-4-(2-(6-styryl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-3-yl)ethyl) phenol (6h)
Colorless crystals, yield: 2.02 g, 88%, m.p. 108-110 °С (EtOH:H2O 3:1). FT-IR cm-1: 3612, 1628, 1474, 1434, 751. 1H NMR (DMSO-d6): δ 1.32 (18Н, с, t-Bu), 3.02 (2H, t, J = 7.3, СН2-СН2), 3.17 (2Н, t, J = 7.2, СН2-СН2), 6.74 (1Н, s, ОН), 6.85 (2Н, s, Нar), 7.44-7.47 (2H, m, Har), 7.48 (2H, d, J = 5.1, Har), 7.77 (1Н, d, J = 1.7, -CH = CH-), 7.79 (1Н, d, J = 2.5, -CH = CH-). 13C NMR (DMSO-d6): δ 170.8 (S-C = N in triazole), 157.4, 156.7, 152.5, 145.8, 144.3, 139.6, 136.0, 135.6, 134.2, 133.7, 129.5, 123.4, 39.6, 34.8, 31.9, 30.9. MS: (M+), m/z 460.
Anal. Calcd for C27H32N4OS: C, 70.40; H, 7.00; N, 12.16; S, 6.96; Found: C, 70.52; H, 7.19; N, 12.01; S, 7.25.
General procedure for preparation of 6-aryl/alkylamino-3-substituted [1,2,4] triazolo[3,4-b][1,3,4]- thiadiazoles (8a,b)
A mixture of 1.74 g (5 mmol) of 4-amino-1,2,4-triazole 3 and 5 mmol of the corresponding isothiocyanate 7a,b was refluxed under stirring in 20 ml of DMF for 24 hours. The reaction mixture was cooled, poured into ice water, the precipitate formed was filtered off and purified using a silica gel column (CH2Cl2:EtOH 10:1).
2,6-di-tert-Butyl-4-(2-(6-((2-methoxyethyl)amino)-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazol-3-yl)ethyl)phenol (8a)
Yellow crystals, yield: 0.75 g, 35%, m.p. 111-113 °С. FT-IR cm−1: 3625, 3348, 1601, 1120, 752. 1H NMR (DMSO-d6): δ 1.35 (18Н, s, t-Bu), 2.64 (1H, s), 2.73 (1H s), 3.32 (3H, s, O-CH3), 5.47 (1H, s, NH), 6.70 (1Н, s, ОН), 6.90 (2Н, s, Нar). 13C NMR (DMSO-d6): δ 166.1 (S-C = N in triazole), 165.2, 162.8, 152.6, 152.0, 139.6, 131.7, 131.0, 124.8, 34.9, 32.0, 30.9, 27.2. MS: (M+), m/z 431.
Anal. Calcd for C22H33N5O2S: C, 61.22; H, 7.71; N, 16.23; S, 6.91; Found: C, 61.36; H, 7.90; N, 16.14; S, 6.75.
2,6-di-tert-Butyl-4-(2-(6-(p-tolylamino)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-3-yl) ethyl)phenol (8b)
Yellow crystals, yield: 0.95 g, 41%, m.p. 95-97 °С. FT-IR cm−1: 3631, 3422, 1602, 752. 1H NMR (DMSO-d6): δ 1.35 (18Н, s, t-Bu), 2.24 (2H, s), 2.48-2.5 (1H, t), 2.72 (3H, s, -CH3), 5.47 (1H, s, NH), 6.70 (1Н, s, ОН), 6.90 (2Н, s, Нar), 7.09 (2H, d, J = 8.1, Har), 7.44 (2H, d, J = 8.3, Har). 13C NMR (DMSO-d6): δ 166.1 (S-C = N in triazole), 162.8, 159.8, 152.6, 152.0, 139.6, 131.7, 130.2, 129.7, 124.8, 119.6, 36.2 (CH3), 34.9, 31.8, 30.9, 27.15. MS: (M+), m/z 463.
Anal. Calcd for C26H33N5OS: C, 67.35; H, 7.17; N, 15.11; S: 6.91. Found: C: 67.18; H: 7.32; N, 15.02; S, 6.75.
General procedure for preparation 5-substituted-4-arylidenylideneamino-)-2,4-dihydro-3H-1,2,4-triazole-3-thiones (10a-c)
A mixture of 1.74 g (5 mmol) of 4-amino-1,2,4-triazole 3 and 5 mmol of the corresponding aldehyde 9a-c was refluxed under stirring in 20 ml of i-PrOH and a few drops of H2SO4 for 10-12 hours. The reaction mixture was cooled, poured into ice water, the precipitate formed was filtered off and dissolved in CHCl3. The solution was washed with NaHCO3 solution and with water. The CHCl3 was evaporated under vacuum and the precipitate obtained was purified using a silica gel column (benzene:EtOH 10:1 or toluene:EtOH 10:1).
5-(3,5-di-tert-Butyl-4-hydroxyphenethyl)-4-((4-(dimethylamino)benzylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione (10a)
Orange crystals, yield: 0.62 g, 26%, m.p. 220-222 °С. FT-IR cm−1: 3641, 1597, 750. 1H NMR (DMSO-d6): δ 1.35 (18Н, s, t-Bu), 2.25 (6H, s, N(CH3)2), 2.70-2.75 (2H, t, СН2-СН2), 2.70-3.93 (2H, t, СН2-СН2), 6.71 (1Н, s, ОН), 6.82 (2Н, s, Нар), 7.83 (2H, d, J = 7.9, Har), 8.1 (1H, s, CH=), 8.27 (2H, d, J = 8.3, Har), 12.23 (1H, s, NH). MS: (M+), m/z 479.
Anal. Calcd for C27H37N5OS: C, 67.61; H, 7.78; N, 14.60; S, 6.68. Found: C, 67.45; H, 7.68; N, 14.43; S, 6.55.
5-(3,5-di-tert-Butyl-4-hydroxyphenethyl)-4-((4-nitrobenzylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione (10b)
Yellow crystals, yield: 0.70 g, 29%, m.p. 199-201 °С. FT-IR cm−1: 3628, 1541, 1604, 755. 1H NMR (DMSO-d6): δ 1.35 (18Н, s, t-Bu), 2.87 (2H, t, J = 7.4, СН2-СН2), 3.03 (2Н, t, J = 7.2, СН2-СН2), 6.71 (1Н, s, ОН), 6.84 (2Н, s, Нar), 8.10 (2H, d, J = 8.6, Har), 8.34 (2H, d, J = 8.9, Har), 10.25 (1H, s, CH=), 13.93 (1H, s, NH). 13C NMR (DMSO-d6): δ 161.8 (C = S), 158.9, 152.6, 151.7, 149.9, 138.3, 136.6. 131.2, 129.9, 124.9, 124.6, 34.8, 32.7, 30.7, 27.5. MS: (M+), m/z 481.
Anal. Calcd for C25H31N5O3S: C, 62.35; H, 6.49; N, 14.54; S, 6.66. Found: C, 62.47; H, 6.67; N, 14.38; S, 6.51.
5-(3,5-di-tert-Butyl-4-hydroxyphenethyl)-4-((4-methoxybenzylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione (10c)
Yellow crystals, yield: 0.51 g, 22%, m.p. 156-158 °С. FT-IR cm−1 3635, 1601, 1242, 761. 1H NMR (DMSO-d6): δ 1.35 (18Н, s, t-Bu), 2.87 (2H, t, J = 7.4, СН2-СН2), 2.96 (2Н, t, J = 7.2, СН2-СН2), 3.87 (3H, s, O-CH3), 6.71 (1Н, s, ОН), 6.84 (2Н, s, Нар), 7.06 (2H, d, J = 8.9, Har), 7.79 (2H, d, J = 8.9, Har), 9.57 (1H, s, CH=), 13.74 (1H, s, NH). 13C NMR (DMSO-d6): δ 168.30 (C = S), 168.07, 166.52, 165.51, 157.36, 155.88, 144.24, 136.02, 135.61, 129.56, 119.88, 60.77, 39.55, 37.4, 35.56,32.3. MS: (M+), m/z 466.
Anal. Calcd for C26H34N4O2S: C, 66.92; H, 7.34; N, 12.01; S, 6.87. Found: C, 66.74; H, 7.51; N, 11.86; S, 6.70.
References
- V. Courtney Broaddus, R. C. Mason, J. D. Ernst, T. E. King, S. C. Lazarus, J. F. Murray, Ja. A. Nadel, A. Slutsky, and M. Gotway, “Murray & Nadel's Textbook of Respiratory Medicine E-Book,” p. 2208, Elsevier Health Sciences, Amsterdam, 2015.
- H. Xiao, P. Li, R. Li, L. Wu, and D. Guo, Appl. Biochem. Biotechnol., 172, 2188 (2014). doi:10.1007/s12010-013-0657-5
- M.A. Al-Omar, Molecules, 15, 502 (2010). doi:10.3390/molecules15010502
- T. Önkola, D. S. Doğruera, L. Uzuna, S. Adaka, S. Özkanb and F. Şahina, J. Enzyme Inhib. Med. Chem., 23, 277 (2008). doi:10.1080/14756360701408697
- P. Karegoudar, D. J. Prasad, M. Ashok, M. Mahalinga, B. Poojary and B. S. Holla, Eur. J. Med. Chem., 43, 808 (2008). doi:10.1016/j.ejmech.2007.06.026
- L. X. Zhang, A. J. Zhang, X.X. Chen, X. X. Lei, X. Y. Nan, D. Y. Chen and Z. Y. Zhang, Molecules, 7, 681 (2002). doi:10.3390/70800681
- I. Khana, S. Zaibb, A. Ibrara, H. N. Ramaa, J. Simpsonc and J. Iqbal, Eur. J. Med. Chem., 78, 167 (2014). doi:10.1016/j.ejmech.2014.03.046
- T. Karabasanagouda, A. V. Adhikari and N. S. Shetty, Eur. J. Med. Chem., 42, 521 (2007). doi:10.1016/j.ejmech.2006.10.010
- I. A. Al-Masoudi, Y. A. Al-Soud, N. J. Al-Salihi and N. A. Al-Masoudi, Chem. Heterocycl. Comp., 42, 1377 (2006). doi:10.1007/s10593-006-0255-3
- A. L. Branen, P. M. Davidson and B. Katz, Food Technol., 34, 42 (1980).
- M. Raccach, J. Food Safety, 6, 141 (1984). doi:10.1111/j.1745-4565.1984.tb00479.x
- W. A. Yehye, N. A. Rahman, A. Ariffin, S. B. A. Hamid, A. A. Alhadi, F. A. Kadir and M. Yaeghoobi, Eur. J. Med. Chem., 101, 295 (2015). doi:10.1016/j.ejmech.2015.06.026
- R. A. Karakhanov, V. I. Kelarev, V. N. Koshelev, G. V. Morozova and D. Ammar, Chem. Heterocycl. Comp., 2, 238 (1995).
- M. A. Silin, V. I. Kelarev, V. Abu-Ammar, D. Kh. Putkaradze and I. A. Golubeva, Petr. Chem., 40, 392 (2000).
- L.-X. Zhang, A.-J. Zhang, X.-X. Chen, X.-X. Lei, X.-Y. Nan, D.-Y. Chen, and Z.-Y. Zhang, Molecules, 7, 681, (2002). doi:10.3390/70800681
- B. Balandis, K. Anusevičius, J. Šiugždaitė, K. Kantminienė, and V. Mickevičius, Research on Chemical Intermediates, 45, 5499 (2019). doi:10.1007/s11164-019-03916-y
- S. M. I. Badr and R. M. Barwa, Bioorg. Med. Chem., 19, 4506 (2011). doi:10.1016/j.bmc.2011.06.024
- K. H. M. E. Tehrani, V. Mashayekhi, P. Azerang, S. Minaei, S. Sardari and F. Kobarfard, Iranian J. Pharm. Res., 14, 59 (2015).
- R. M. Shaker and A. A. Aly, Phosphorus, Sulfur and Silicon, 181, 2577 (2006). doi:10.1080/10426500600775922
- E. Pretsch, P. Bühlmann and M. Badertscher, “Structure Determination of Organic Compounds. Tables of Spectral Data,” passim, Springer, Berlin, 2009.
- V. I. Kelarev, G. A. Shvehgeimer and A. F. Lunin, Chem. Heterocycl. Comp., 20, 1271 (1984).
- Z. K. Yang, Z. J. Fan, X. Zuo, Q. X. Zheng, H. B. Song, J. M. You, P. B. Natalia, and V. Bakulev, Chin. J. Struct. Chem., 29, 13 (2010).