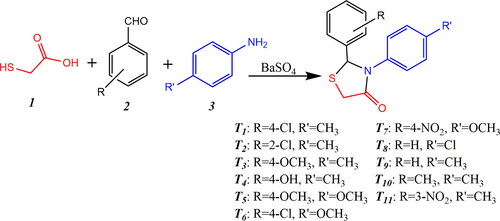
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Barium sulfate is an abundantly available material with numerous applications,Citation1,Citation2 and as a result there are several techniques for its preparation.Citation3–10 Even so, much work remains to be done on the evaluation of the catalytic properties of BaSO4 in organic transformations. The present work concentrates on the preparation of BaSO4 nano-powder and its use as an efficient catalyst in the synthesis of 2,3-diarylthiazolidin-4-ones. Derivatives containing thiazolidin-4-ones are of broad interest because of their diverse and beneficial biological activities, including anticancer,Citation11 anti-HIV,Citation12 antimalarial,Citation13 tuberculostatic,Citation14 antihistaminic,Citation15 anticonvulsant,Citation16 antibacterial,Citation17 and antiarrhythmicCitation18 properties; but surely, in light of the coronavirus pandemic, their significant antiviral characteristics make them of high current interest to the medical research community.Citation19–21 Their preparation is an important issue in synthetic organic chemistry, and among the existing methods, the cyclo-condensation reaction of aldehydes, amines and thioglycolic acid has been especially versatile.Citation22–32 In keeping with our interest in exploring synthetic methodologies for the production of heterocyclic compounds,Citation33–39 we now report the preparation of several thiazolidin-4-ones (Scheme 1, compounds T1-T11), using the cyclo-condensation reaction of aldehydes, amines and thioglycolic acid in the presence of BaSO4 nano-powder as a catalyst.
To search for the optimal conditions, the synthesis of 2-phenyl-3-p-tolylthiazolidin-4-one (Scheme 1, T9) from benzaldehyde, 4-methylaniline, and thioglycolic acid was selected as a model. This reaction was investigated in solvent-free conditions and in different solvents using a catalytic amount of barium sulfate nanoparticles (). We found that ethanol was the best solvent for the reaction. The maximum yield was obtained when 0.18 mmol of catalyst was used (, Entry 5). A further increase in the amount of BaSO4 nano-powder had no significant impact on the product yield. Under the standard conditions, no reaction was observed in the absence of a catalyst. This shows that the catalyst is essential for the product formation (, Entry 13).
Table 1. Optimization of the reaction conditions in the synthesis of T9.
We used our procedure for the synthesis of substituted 2,3-diarylthiazolidin-4-one derivatives (). This reaction proceeded smoothly, and the desired products were obtained in good yields (mean 72%). In general, aromatic aldehydes and aromatic amines were well tolerated.
Table 2. Synthesis of 2,3-diarylthiazolidin-4-one derivatives using BaSO4 nano-powder as catalyst.
Aromatic aldehydes with electron-donating groups gave higher yields than those with electron-withdrawing groups. When an ortho-substituted aldehyde (, Entry T2) was used, the corresponding product was obtained in good yield. Electron donating groups on the amine appear to facilitate the reactions, giving higher yields of products than the cases using halogen-substituted anilines (). The work-up procedure is very clear-cut; that is, the products were isolated and purified by simple filtration and recrystallization from n-hexane/ethyl acetate.
Next, ethylamine as an aliphatic amine and butyraldehyde and heptanal as aliphatic aldehydes were used to investigate the potential of BaSO4 in promoting the reaction for aliphatic substrates. The desired products were formed in negligible yields, suggesting that BaSO4 is not a suitable catalyst to catalyze this multi-component reaction for aliphatic amines and aliphatic aldehydes.
In order to estimate the efficiency and generality of this methodology, our results on the synthesis of 2,3-diphenylthiazolidin-4-one using BaSO4 nano-powder as catalyst has been compared with those of the previously reported methods ().
Table 3. Comparison results of BaSO4 with other catalysts reported in the literature.
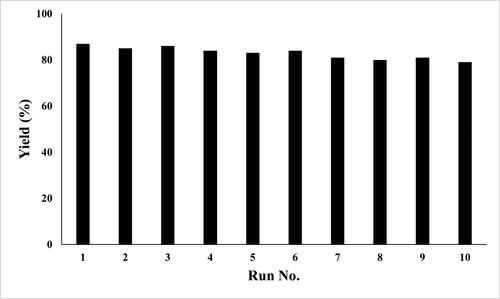
The present method is superior to the reported methods with respect to the reaction time and yield of the product. Our attention was then turned to the possibility of recycling the catalyst from the reaction media since the recovery and reuse of the catalyst are preferable for a green context. At the completion of the reaction, the reaction mixture was poured into chloroform and stirred for 5 min. The separated solid catalyst was filtered, dried and reused for subsequent reactions, with the preparation of T9 as a model. The BaSO4 nanoparticles still had good activity after 10 cycles ().
In conclusion, the nano-powder BaSO4 prepared by a simple precipitation method was used as an efficient catalyst for the preparation of 2,3-diarylthiazolidin-4-one derivatives. Application of 0.18 equivalents of BaSO4 provides good yields of products for the condensation reaction of aromatic aldehydes, aromatic amines, and thioglycolic acid under ambient conditions. We feel that the simplicity of the method system makes it suitable for further research and practical applications.
Experimental section
All reagents were purchased from Merck and Aldrich (Germany), and used without further purification. All yields refer to isolated products after purification. The NMR spectra were recorded on a Bruker Avance DPX 400 MHz instrument (Germany). The spectra were measured in DMSO-d6 relative to TMS (0.00 ppm). Elemental analysis was performed on a Heraeus CHN-O-Rapid analyzer. TLC was performed on silica gel PolyGram SIL G/UV 254 plates to monitor reactions. The powder X-Ray diffraction patterns were measured with a D8 Avance, Bruker, AXS (Germany), diffractometer using Cu-Kα irradiation. FE-SEM was taken on a Hitachi S-4160 (Japan) photograph to examine the shape and size of BaSO4 nanoparticles.
Preparation of triethylammonium hydrogen sulfate
Triethylammonium hydrogen sulfate was prepared and characterized according to a literature method as follows: Triethylamine (100 mmol) was added to sulfuric acid (100 mmol) drop wise at room temperature. Subsequently, the reaction mixture was stirred at 100 °C for 30 min. Then, to remove the traces of water, the mixture was placed in a vacuum at 80 °C until the weight of the residue remained constant.Citation40
Preparation of BaSO4 nano-powder
Triethylammonium hydrogen sulfate (10 mmol dissolved in 50 mL of EtOH) was added dropwise to an aqueous solution of BaCl2 (10 mmol BaCl2 in 250 mL of water), with vigorous stirring at room temperature. After complete addition, the resulting mixture was left for 30 min. The resulting precipitate was separated from the mother liquor by filtering and then washed with ethanol several times, then dried at 100 °C for 10 min. The obtained BaSO4 nanoparticles were slightly ground for analysis, then characterized using the instrumentation described above. The characterization data were submitted for review and are available in the Supplementary Material or from the corresponding author upon request.
General procedure for synthesis of 2,3-diarylthiazolidin-4-one derivatives
To a mixture of thioglycolic acid (1 mmol), benzaldehyde (1 mmol) and aromatic amine (1 mmol) in ethanol (5 mL), BaSO4 nano-powder (0.18 mmol) was added and the mixture was stirred for the appropriate time at room temperature, with progress of the reaction monitored by TLC. Upon completion, the solvent was evaporated, and the mixture was diluted in dichloromethane. The catalyst was isolated by simple filtration for re-use as described above, and the crude compounds were crystallized from n-hexane/ethyl acetate to afford the pure products (T1-T11) (Scheme 1, ). Known compounds were identified by matching their melting points with values in the literature cited in .
2-(4-Chlorophenyl)-3-p-tolylthiazolidin-4-one (T1)
White powder, m.p. 145-147 °C, 1H NMR (400 MHz, DMSO-d6): δ = 2.24 (s, 3H), 3.68 (d, J = 16.0 Hz, 1H), 3.93 (d, J = 16.0 Hz, 1H), 6.39 (s, 1H), 7.13-7.16 (dd, J = 8.0 Hz, J = 3.2 Hz, 2H), 7.43-7.46 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H), 7.63-7.72 (m, 4H) ppm; 13C NMR (100 MHz, DMSO-d6): 20.8, 33.8, 68.2, 113.6, 129.3, 129.4, 129.7, 132.3, 135.5, 139.2, 144.6, 170.8 ppm.
Anal. Calcd for C16H14ClNOS: C, 63.26; H, 4.65; N, 4.61; S, 10.55. Found: C, 63.33; H, 4.71; N, 4.58; S, 10.46.
2-(2-Chlorophenyl)-3-p-tolylthiazolidin-4-one (T2)
White powder, m.p. 121-123 °C, 1H NMR (400 MHz, DMSO-d6): δ = 2.26 (s, 3H), 3.78 (d, J = 16.0 Hz, 1H), 4.07 (d, J = 16.0 Hz, 1H), 6.13 (s, 1H), 7.08-7.28 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H), 7.29-7.39 (m, 4H), 7.58 (d, J = 8.0 Hz, 1H), 7.70-7.74 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H) ppm; 13C NMR (100 MHz, DMSO-d6): 20.9, 34.0, 68.3, 113.7, 127.5, 127.7, 128.6, 129.7, 130.4, 130.6, 132.4, 133.0, 152.5, 172.4 ppm.
Anal. Calcd for C16H14ClNOS: C, 63.26; H, 4.65; N, 4.61; S, 10.55. Found: C, 63.29; H, 4.68; N, 4.57; S, 10.48.
2-(4-Methoxyphenyl)-3-p-tolylthiazolidin-4-one (T3)
White powder, m.p. 152-154 °C, 1H NMR (400 MHz, DMSO-d6): δ = 2.27 (s, 3H), 3.71 (d, J = 15.6 Hz, 1H), 3.84-3.89 (m, 4H), 5.94 (s, 1H), 6.90-6.93 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H), 7.07-7.30 (m, 6H) ppm.
Anal. Calcd for C17H17NO2S: C, 68.20; H, 5.72; N, 4.68; S, 10.71. Found: C, 68.29; H, 5.78; N, 4.71; S, 10.75.
2-(4-Hydroxyphenyl)-3-p-tolylthiazolidin-4-one (T4)
White powder, m.p. 127-129 °C, 1H-NMR (400 MHz, CDCl3): 2.27 (s, 3H), 3.71 (d, J = 12.4 Hz, 1H), 3.82 (d, J = 12.4 Hz, 1H), 5.14 (s, 1H), 6.70-7.14 (m, 6H), 7.73 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ = 20.6, 34.0, 52.3, 115.7, 120.8,129.7, 130.8, 148.7, 157.2,159.3, 160.7, 170.4 ppm.
Anal. Calcd for C16H15NO2S: C, 67.34; H, 5.30; N, 4.91; S, 11.23. Found: C, 67.39; H, 5.36; N, 4.94; S, 11.17.
2,3-bis(4-Methoxyphenyl)thiazolidin-4-one (T5)
White powder, m.p. 121-123 °C, 1H NMR (400 MHz, DMSO-d6): δ = 3.72-3.79 (m, 7H), 3.98 (d, J = 16.0 Hz, 1H), 6.15 (s, 1H), 6.77-6.80 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H), 6.98 (d, J = 7.2 Hz, 2H), 7.08-7.10 (dd, J = 8.0 Hz, J = 2.4 Hz, 2H), 7.37 (d, J = 7.2 Hz, 2H) ppm; 13C NMR (100 MHz, DMSO-d6): 33.7, 55.0, 57.6, 68.2, 114.3, 123.4, 127.6, 139.1, 144.6, 156.9, 160.4, 170.9 ppm.
Anal. Calcd for C17H17NO3S: C, 64.74; H, 5.43; N, 4.44; S, 10.17. Found: C, 64.78; H, 5.47; N, 4.39; S, 10.14.
2-(4-Chlorophenyl)-3-(4-methoxyphenyl)thiazolidin-4-one (T6)
White powder, m.p. 155-157 °C, 1H NMR (400 MHz, DMSO-d6): δ = 3.78-3.81 (m, 4H), 4.01 (d, J = 16.0 Hz, 1H), 5.98 (s, 1H), 6.87-6.90 (dd, J = 7.6 Hz, J = 2.8 Hz, 2H), 7.12-7.15 (dd, J = 7.6 Hz, J = 2.4 Hz, 2H), 7.32-7.34 (dd, J = 7.6 Hz, J = 2.8 Hz, 2H), 7.46-7.48 (dd, J = 8.0 Hz, J = 2.8 Hz, 2H) ppm; 13C NMR (100 MHz, DMSO-d6): 33.9, 55.1, 68.2, 114.4, 123.5, 129.4, 129.5, 135.6, 139.2, 144.7, 156.9, 170.9 ppm.
Anal. Calcd for C16H14ClNO2S: C, 60.09; H, 4.41; N, 4.38; S, 10.02. Found: C, 60.16; H, 4.48; N, 4.32; S, 9.96.
2-(4-Nitrophenyl)-3-(4-methoxyphenyl)thiazolidin-4-one (T7)
White powder, m.p. 149-151 °C, 1H NMR (400 MHz, DMSO-d6): δ = 3.77-3.85 (m, 4H), 3.99 (d, J = 16.0 Hz, 1H), 6.39 (s, 1H), 6.79-6.82 (dd, J = 8.0 Hz, J = 2.4 Hz, 2H), 7.13-7.16 (dd, J = 8.4 Hz, J = 2.8 Hz, 2H), 7.50-7.52 (dd, J = 7.6 Hz, J = 2.4 Hz, 2H), 8.14-8.17 (dd, J = 7.6 Hz, J = 2.4 Hz, 2H) ppm; 13C NMR (100 MHz, DMSO-d6): 33.8, 55.1, 68.2, 114.3, 123.4 (2C), 127.6, 139.1, 144.6, 147.9, 156.9, 170.9 ppm.
Anal. Calcd for C16H14N2O4S: C, 58.17; H, 4.27; N, 8.48; S, 9.70. Found: C, 58.21; H, 4.30; N, 8.46; S, 9.64.
2-Phenyl-3-(4-chlorophenyl)thiazolidin-4-one (T8)
White powder, m.p. 166-168 °C, 1H NMR (400 MHz, DMSO-d6): δ = 3.85 (d, J = 15.6 Hz, 1H), 4.11 (d, J = 15.6 Hz, 1H), 6.31 (s, 1H), 7.30-7.59 (m, 7H), 7.97 (t, J = 5.2 Hz, 2H) ppm.
Anal. Calcd for C15H12ClNOS: C, 62.17; H, 4.17; N, 4.83; S, 11.06. Found: C, 62.25; H, 4.23; N, 4.86; S, 11.09.
Supplemental Material
Download MS Word (1.9 MB)Acknowledgments
We thank the Research Council of Najafabad Branch, Islamic Azad University, for partial support of this research. Financial assistance from the Shiraz University of Medical Sciences by way of grant number 23670 is also gratefully acknowledged.
References
- C. Tieh-Chi and W. Jeng-long, J. Nucl. Radiochem. Sci., 1, 5 (2000). doi:https://doi.org/10.14494/jnrs2000.1.5
- C. A. Menzie, B. Southworth, G. Stephenson, and N. Feisthauer, Hum. Ecol. Risk Assess., 14, 974 (2008). doi:https://doi.org/10.1080/10807030802387622
- F. Ye, X. Guo, J. Hou, X. Liu, L. Wang, Z. Wang, H. Jin, CIESC Journal, 69, (2018).
- K. Prutviraj, T. Narayan Ramesh, Inorg. Nano-Met. Chem., 49, (2019). doi:https://doi.org/10.1080/24701556.2019.1603162.
- H. Bala, W. Fu, Y. Guo, J. Zhao, Y. Jiang, X. Ding, K. Yu, M. Li, and Z. Wang, Colloids Surf. A Physicochem. Eng. Asp., 274, 71 (2006). doi:https://doi.org/10.1016/j.colsurfa.2005.08.050
- G. Wu, H. Zhou, and S. Zhu. Mater. Lett., 61, 168 (2007). doi:https://doi.org/10.1016/j.matlet.2006.04.096
- Q. A. Wang, J. X. Wang, M. Li, L. Shao, J. F. Chen, L. Gu, and Y. T. An, Chem. Eng. J., 149, 473 (2009). doi:https://doi.org/10.1016/j.cej.2009.02.018
- G. Chen, G. Luo, J. Xu, and J. Wang, Powder Technol., 153, 90 (2005). doi:https://doi.org/10.1016/j.powtec.2005.03.002
- C. M. Patel, Z. V. P. Murthy, and M. Chakraborty, J. Ind. Eng. Chem., 18, 1450 (2012). doi:https://doi.org/10.1016/j.jiec.2012.02.005
- N. Nandakumar and P. Kurian, Powder Technol., 224, 51 (2012). doi:https://doi.org/10.1016/j.powtec.2012.02.022
- H. Zhou, S. Wu, S. Zhai, A. Liu, Y. Sun, R. Li, Y. Zhang, S. Ekins, P. W. Swaan, B. Fang, B. Zhang and B. Yan, J. Med. Chem., 51, 1242 (2008). doi:https://doi.org/10.1021/jm7012024
- M. L. Barreca, J. Balzarini, A. Chimirri, E. De Clercq, L. De Luca, H. Dieter Höltje, M. Höltje, A. M. Monforte, P. Monforte, C. Pannecouque, A. Rao, and M. Zappalà, J. Med. Chem., 45, 5410 (2002). doi:https://doi.org/10.1021/JM020977
- V. Raja Solomon, W. Haq, K. Srivastava, S. K. Puri, and S. B. Katti, J. Med. Chem., 50, 394 (2007). doi:https://doi.org/10.1021/jm061002i
- G. Küçükgüzel, A. Kocatepe, E. De Clercq, F. Şahin, and M. Güllüce, Eur. J. Med. Chem., 41, 353 (2006). doi:https://doi.org/10.1016/j.ejmech.2005.11.005
- M. V. Diurno, O. Mazzoni, E. Piscopo, A. Calignano, F. Giordano, and A. Bolognese. J. Med. Chem., 35, 2910 (1992). doi:https://doi.org/10.1021/jm00093a025
- Archana, V. K. Srivastava, and A. Kumar, Eur. J. Med. Chem., 37, 873 (2003). doi:https://doi.org/10.1016/S0223-5234(02)01389-2
- K. G. Desai, and K. R. Desai, J. Sulfur Chem., 27, 315 (2006). doi:https://doi.org/10.1080/17415990600786409
- C. M. Jackson, B. Blass, K. Coburn, L. Djandjighian, G. Fadayel, A. J. Fluxe, S. J. Hodson, J. M. Janusz, M. Murawsky, J. M. Ridgeway, R. E.White, and S. Wu, Bioorganic Med. Chem. Lett., 17, 282 (2007). doi:https://doi.org/10.1016/j.bmcl.2006.07.007
- C. Nitsche, V. N. Schreier, M. A. M. Behnam, A. Kumar, R. Bartenschlager, and C. D. Klein, J. Med. Chem., 56, 8389 (2013). doi:https://doi.org/10.1021/jm400828u
- Y. Liu, F. Jing, Y. Xu, Y. Xie, F. Shi, H. Fang, M. Li, and W. Xu, Bioorg. Med. Chem., 19, 2342 (2011). doi:https://doi.org/10.1016/j.bmc.2011.02.019
- S. Sucheta Tahlan, and P. K. Verma, Chem. Cent. J., 11, 130 (2017). doi:https://doi.org/10.1186/s13065-017-0357-2
- T. Srivastava, W. Haq, and S. B. Katti, Tetrahedron, 58, 7619 (2002). doi:https://doi.org/10.1016/S0040-4020(02)00866-9
- U. R. Pratap, D. V. Jawale, M. R. Bhosle, and R. A. Mane, Tetrahedron Lett., 52, 1689 (2011). doi:https://doi.org/10.1016/j.tetlet.2011.01.143
- N. Azgomi, and M. Mokhtary, J. Mol. Catal. A Chem., 398, 58 (2015). doi:https://doi.org/10.1016/j.molcata.2014.11.018
- M. Ghashang, H. Taghrir, M. N. Biregan, N. Heydari, and F. Azimi, J. Sulfur Chem., 37, 61 (2016). doi:https://doi.org/10.1080/17415993.2015.1089440
- M. Lashkari, and M. Ghashang, Res. Chem. Intermed., 47, 589 (2021). doi:https://doi.org/10.1007/s11164-020-04287-5
- A. K. Yadav, M. Kumar, T. Yadav, and R. Jain, Tetrahedron Lett., 50, 5031 (2009). doi:https://doi.org/10.1016/j.tetlet.2009.06.091
- X. Zhang, X. Li, D. Li, G. Qu, J. Wang, P. M. Loiseau, and X. Fan, Bioorganic Med. Chem. Lett., 19, 6280 (2009). doi:https://doi.org/10.1016/j.bmcl.2009.09.101
- V. Kanagarajan, J. Thanusu, and M. Gopalakrishnan, Green Chem. Lett. Rev., 2, 161 (2009). doi:https://doi.org/10.1080/17518250903251767
- N. Foroughifar and S. Ebrahimi, Chinese Chem. Lett., 24, 389 (2013). doi:https://doi.org/10.1016/j.cclet.2013.03.019
- D. Kumar, M. Sonawane, B. Pujala, V.K. Jain, S. Bhagat, and A.K. Chakraborti, Green Chem., 15, 2872 (2013). doi:https://doi.org/10.1039/c3gc41218k
- D. Prasad, A. Preetam, M. Nath, RSC Adv., 2, 3133 (2012). doi:https://doi.org/10.1039/c2ra20171b
- N. Sheikhan-Shamsabadi, and M. Ghashang, Main Group Met. Chem., 40, 19 (2017). https://doi.org/10.1515/mgmc-2016-0034
- M. Ghashang, Curr. Org. Synth., 9, 727 (2012). doi:https://doi.org/10.2174/157017912803251800
- Z. Golestaneh, and M. Ghashang, Tetrahedron Lett. 60, 151194 (2019). doi:https://doi.org/10.1016/j.tetlet.2019.151194
- M. Ghashang. Res. Chem. Intermed., 39, 3753 (2013). doi:https://doi.org/10.1007/s11164-012-0802-8
- M. Yousefi, and M. Ghashang, J. Heterocycl. Chem., 56, 3152 (2019). doi:https://doi.org/10.1002/jhet.3715
- M. Abaszadeh, S.J. Roudbaraki, and M. Ghashang, Org. Prep. Proced. Int., 51, 255 (2019). doi:https://doi.org/10.1080/00304948.2019.1600124
- H. Taghrir, and M. Ghashang, Synth. React. Inorganic, Met. Nano-Metal Chem., 46, 246 (2016). doi:https://doi.org/10.1080/15533174.2014.988227
- Z. Karimi-Jaberi, B. Masoudi, A. Rahmani, and K. Alborzi, Polycycl. Aromat. Comp., 40, 99 (2020). doi:https://doi.org/10.1080/10406638.2017.1363061