ABSTRACT
Since 2007, large-scale blooms have periodically broken out in the Yellow Sea, leading to serious economic and ecological problems in the surrounding coastal environments. Previous studies reported that the blooms originated in the Rudong oceanic area of the southern Yellow Sea and drifted almost 400 km into the Qingdao oceanic area of the northern Yellow Sea. In this study, chlorophyll concentrations, chlorophyll fluorescence, growth rates, and reproductive characteristics of floating Ulva prolifera collected along the Yellow Sea coast were assessed. The results revealed that these four physiological parameters in the Rudong area at the bloom source were considerably higher (2.02 mg/g, 0.68, 28.49% d−1, and 4 × 107, respectively) than in the Qingdao area (0.34 mg/g, 0.31, 5.23% d−1, and 0.67 × 107, respectively). However, the abundance of microscopic propagules of Ulva in the Qingdao area (3980 ind/l) was significantly higher than that in the Rudong area (667 ind/l). These findings help increase our understanding of the formation of the world’s largest Ulva macroalgal blooms.
INTRODUCTION
Species in the green macroalgal genus Ulva are known for their wide global distribution in marine, brackish, and freshwater environments (Canter-Lund & Lund Citation1995; van den Hoek et al. Citation1995). Their abnormal proliferation, termed ‘green tides’ (Fletcher 1996b), which occurred mainly in Europe, America, and Asia during the last three decades (Morand & Briand Citation1996; Ye et al. Citation2011), have caused many serious environmental and ecological problems (Fletcher Citation1996a, Citation1996b; Ye et al. Citation2011). These include alterations to ecosystem structure and reduced biodiversity (Franz & Friedman Citation2002; Hernandez et al. Citation1997; McGlathery Citation2001; Nelson & Lee Citation2001). Due to competition for light and space, green tides may also reduce seagrass populations and their re-establishment capability (Berger et al. Citation2003; Raberg et al. Citation2005). Furthermore, when the algae die and sink to the bottom, the consumption of dissolved O2 may cause a local ‘dead zone’ with hypoxic conditions (Diaz & Rosenberg Citation2008; Hu & He Citation2008). This results in invertebrate, fish, and even marine mammal mortality (Hallegraeff Citation1993). In the last few decades, green tides have increased in severity, frequency, and geographic range and become a growing concern globally (Ye et al. Citation2011).
The world’s largest green tide occurred in the Yellow Sea, China, in 2008 (D. Liu et al. Citation2009; F. Liu et al. Citation2010); the bloom covered an area of approximately 13,000–30,000 km2. More than 16,000 people using over 1000 transportation vehicles and 1600 fishing and transportation vessels volunteered to clean up the massive bloom. Finally, over 700,000 tons of algae were removed from beaches and nearby coastal waters (Hu & He Citation2008). Green tides have subsequently become an annual phenomenon in the Yellow Sea.
Recently, satellite remote sensing and cruise observations were applied to trace the floating path of macroalgal blooms, indicating that scattered floating Ulva macroalgae initially appeared along the coast of Rudong in April and accumulated in the Dafeng oceanic area in May (Z. Wang et al. Citation2015; J. Zhang, Huo, Zhang et al. Citation2013; J. Zhang et al. Citation2014). The patches of free-floating green algae then drifted into the Qingdao oceanic area of the Northern Yellow Sea due to surface currents (Bao et al. Citation2015; Qiao et al. Citation2011; J. Zhang et al. Citation2017; Wu et al. Citation2018). The blooms declined and gradually disappeared in July and August. The dominant species were identified as Ulva prolifera (Müller) J.Agardh (Chlorophyta, Ulvophyceae; Leliaert et al. Citation2009; J. Wang et al. Citation2010), which possesses numerous reproductive modes – including sexual, asexual, and vegetative – to support its massive biomass (Cui et al. Citation2018; Lin et al. Citation2008; Q. Liu et al. Citation2015). Therefore, in this present study, during the bloom drifting process from Rudong to Qingdao oceanic areas, floating U. prolifera was collected to assess physiological characteristics. Ulva microscopic propagules including gametes, meiospores, and zygotes, may play an important role in the rapid development of high-biomass blooms of green algae and were also collected along the Yellow Sea coast to evaluate their role in bloom formation.
MATERIAL AND METHODS
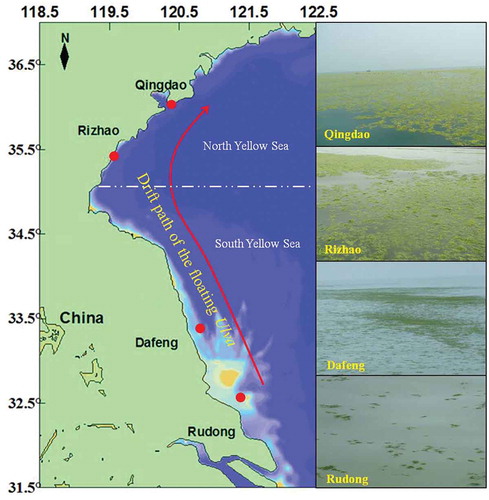
Large-scale green tides occur periodically along the coastline of Jiangsu and Shandong province (30°44′–38°24′N, 119°09′–122°37′E), China. Following the drift path of the green tides, Rudong (32°33′N, 121°26′E), Dafeng (33°18′N, 120°51′E), Rizhao (35°27′N, 119°35′E), and Qingdao (36°03′N, 120°26′E) were selected as the sampling stations (). Floating Ulva samples in the present study were collected in the Rudong, Dafeng, Rizhao, and Qingdao oceanic areas during April to July in 2013. The collected samples (~ 50 g per station in wet weight) were refrigerated at 4 °C and then transported to the laboratory within 1 day. Subsequently, before physiological experimentation, sediments and contaminants were removed from the samples using sterilised seawater and a soft brush. In addition, the samples were identified as Ulva prolifera by molecular methods using internal transcribed spacer and 5S rDNA markers, as described in our previous studies (Huo et al. Citation2016; J. Zhang et al. Citation2014).
Chlorophyll concentration was measured immediately from selected Ulva samples following X. Zhang (Citation1986) and Lin et al. (Citation2009). Chlorophyll concentrations were determined using a spectrophotometer (UV757CRT, Shanghai Precision & Scientific Instrument Co. Ltd., Shanghai, China) after extraction of 0.5 g fresh Ulva fragments in 80% (V/V) acetone:water buffered solution. The total chlorophyll concentrations (CT) were calculated based on the formula: CT (mg/g) = 8.02 A663 + 21.21 A645. A663 and A645 are the absorption wavelength values at 663 nm and 645 nm measured by spectrophotometry.
The in vivo chlorophyll fluorescence (Fv/Fm) of the floating Ulva was measured immediately following Han et al. (Citation2008) and Tang et al. (Citation2009) during the shipboard survey. Chlorophyll fluorescence was determined using a pulse amplitude modulated fluorometer connected to a PC with Phytowin software (Heinz Walz, Effeltrich, Germany). Specimens were dark-adapted for 30 min and chlorophyll fluorescence readings were taken. The maximum fluorescence yield of dark-adapted samples (Fm) and the initial fluorescence yield (F0) were recorded. The maximum quantum yield of photosystem II in the dark-adapted state is expressed as the ratio of variable to maximal chlorophyll fluorescence (Fv/Fm), derived from (Fm − F0)/Fm.
The growth experiment followed Cui et al. (Citation2015). Ulva samples (0.05 g fresh weight) from each station were selected and cultivated in a 500-ml spherical glass flask with Von Stosch Enrichment (VSE) medium (Ott Citation1965) at 20 °C, 100 μmol· m−2· s−1 in a 12 h:12 h light:dark cycle with continuous aeration. The VSE culture medium was refreshed every 3 days. The specific growth rate (SGR) was calculated using the formula SGR (% d−1) = 100 (lnWt − lnW0)/t. The initial wet weight (W0) and final wet weight (Wt) were measured simultaneously after removing surface moisture from the thalli with paper towels; t is the culture period in days.
The reproductive experiment followed Chen et al. (Citation2011) and J. Zhang, Huo, Yu et al. (Citation2013). The release of Ulva germ cells was evaluated using a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with a DXM-1200 digital camera system (Nikon). Six full microscope fields of view were observed randomly on each selected thallus. During maturation, the numbers of germ cell cysts and released single germ cells were photographed. The VSE medium was refreshed every 3 days. The number of released zoids (Rs) was calculated using the formula Rs = As (An × G1/S1), where Rs represents the release amount of germ cells per unit thalli area, As represents the area of thalli released germ cells (μm2), An represents the number of germ cell cysts under a full microscope field, G1 represents the number of germ cells released from a single germ cell cyst, and S1 represents the area of a full microscope field (μm2).
Surface seawater samples were also collected from stations at Rudong, Dafeng, Rizhao, and Qingdao. Three parallel samples at each station were selected. The collected surface seawater samples were filtered through a 150-μm mesh sieve to remove zooplankton, refrigerated at 4 °C, and then transported to the laboratory within 1 day for microscopic propagule cultivation. Microscopic propagules were cultured following Huo et al. (Citation2014). The surface seawater samples were transferred and cultivated in a 1-liter glass beaker with continuous aeration at 15 °C, 80–100 μmol · m−2· s−1 in a 12 h:12 h light:dark cycle. Meanwhile, nutrient concentrations were adjusted to 500 μmol l−1 NO3-N and 30 μmol l−1 PO4-P, followed by the addition of 500 ml saturated GeO2 to control diatoms. One week later, all cultured surface seawater samples were filtered through a CuSO4 solution and then aerated. During the subsequent culture period, the VSE medium was refreshed each week until the green algal germlings were 1-5cm diameter and were counted. These germlings were derived from microscopic spores or gametes or from those that had been attached to particles that were invisible to the naked eye at time of sampling.
All data were expressed as mean ± standard deviation. The difference among means was analysed by one-way ANOVA. For post hoc analysis, Tukey’s test was used with a significance level of P < 0.05. The tests were performed using SPSS v13.0 (Everitt & Landau Citation2003; SPSS Inc., Stanford, California USA).
RESULTS
Morphological variation in floating Ulva from in different areas
The morphological characteristics of floating Ulva prolifera thalli are shown in –. In the Rudong oceanic area, the floating algae were a fresh green, with new branches developing from the old thalli. In the Dafeng oceanic region, thalli exhibited a profusion of fine long branches, with smaller and multiple slender branchlets on the main axis. Compared with those in the southern Yellow Sea, thalli were light green, hollow, and tubular in the Rizhao oceanic area; and the blooming algae in the Qingdao oceanic region were light green or yellow, markedly tubular, had folded blade thalli morphology, and had a mature partial thallus.
Physiological parameters of floating Ulva prolifera in different oceanic areas
Results (–) revealed that floating Ulva prolifera in Rudong presented high chlorophyll concentrations, Fv/Fm, SGR, and Rs of 2.02 mg/g, 0.68, 28.49% d−1, and 4 × 107, respectively. However, these physiological parameters decreased gradually northward. Statistical analysis revealed that these physiological parameters were not significantly different between floating Ulva in Rudong and Dafeng areas (P > 0.05) but were significantly higher than those for floating Ulva in the Qingdao oceanic region (P < 0.05).
Quantitative characteristics of microscopic propagules
The density of Ulva developing from microscopic propagules was calculated from the seawater samples. Density of microscopic propagules gradually increased from 667 to 3980 ind l-1 along the drift path of floating Ulva (). The highest density of microscopic propagules was observed in the Qingdao area (3980 ± 1340 ind/l), which was significantly higher than in the Rudong (667 ± 376 ind/l) and Dafeng (913 ± 556 ind/l) regions.
DISCUSSION
Growth and photosynthetic performance in different oceanic areas along the Yellow Sea coast
Chlorophyll content and fluorescence are useful parameters for assessing the physiological state of macroalgae (Abreu et al. Citation2009; Gao et al. Citation2010). Using data from five successive field monitoring years from 2009-2013, We found that the colour of floating Ulva thalli gradually changed from dark green to yellow during the drifting process from Rudong to Qingdao (–). It has been reported that darker Ulva thalli have significantly higher chlorophyll concentrations than lighter ones (Lin et al. Citation2011). Chlorophyll concentrations and photosynthetic activity of floating Ulva in the southern Yellow Sea were considerably higher than for thalli in the northern Yellow Sea (J. Zhang, Huo, Zhang et al. Citation2013). These studies support research in which chlorophyll concentrations, Fv/Fm, and SGR of floating Ulva in the Rudong area were significantly higher than those in the Rizhao and Qingdao areas (–). Lin et al. (Citation2009) reported that the maximal photosystem II quantum yield of floating thalli in the Qingdao area was significantly lower than the normal level. Therefore, the present physiological data together with the results described by Lin et al. (Citation2009) demonstrate that thalli in the Qingdao area were under stress; that is, the natural conditions of the Qingdao area were not suitable for growth of U. prolifera. Consequently, the blooms gradually declined. Photosynthetic performance and growth of green tide algae can be regulated by environmental parameters, especially sea surface temperature (SST) and irradiance (Hurd et al. Citation2014; Lüning Citation1990). This has been demonstrated in the laboratory (Cui et al. Citation2015; Tang et al. Citation2009) where the optimal temperature for photosynthesis and growth of floating Ulva thalli in the Yellow Sea was 15–20 °C, and higher or lower temperatures inhibited photosynthesis and growth rate. In the present study, according to the environmental parameters measured in the field, SST in Rudong and Dafeng oceanic areas was relatively low (10–20 °C) and irradiance was approximately 2000 μmol·m−2·s−1, which is suitable for the growth of Ulva green algae. Compared with Rizhao and Qingdao areas, SST and irradiance were much higher, 20–25 °C and 2300 μmol·m−2· s−1, respectively, becoming inhibitory for Ulva growth. These results were consistent with those of previous reports (Keesing et al. Citation2011; D. Liu et al. Citation2013). Thus, in the source areas of Rudong and Dafeng, Ulva blooms grew rapidly, reaching up to c. 400 km2 in only 1 month; when the blooms drifted into the Rizhao and Qingdao oceanic areas of the northern Yellow Sea, the blooms declined and quickly disappeared.
Significant Ulva biomass and eutrophication in the Yellow Sea
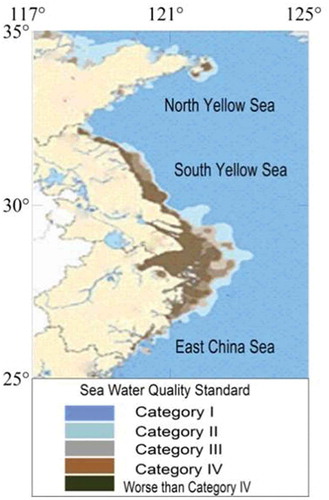
Ulva spp., with their tubular morphology and a high surface area to volume ratio, have high rates of nutrient uptake (Xu et al. Citation2012). In addition, because of their high growth rate, Ulvaspp. require higher N levels than slow-growing species (de Paula Silva et al. Citation2008). Therefore, they are dependent on constant high levels of N in the medium to survive and bloom. The putative cause of the blooms has been attributed to eutrophication (Nelson et al. Citation2008; Schramm Citation1999), a common problem for all coastal areas (Leliaert et al. Citation2009). Official monitoring of nutrients in the Yellow Sea by the State Oceanic Administration of China (SOA Citation2011) indicated that nutrient levels in the Rudong and Dafeng oceanic areas were much higher than those in the Rizhao and Qingdao areas (). Severe eutrophication in the southern Yellow Sea supported the initial and rapid formation of the blooms. When the blooms drifted into the northern Yellow Sea, the coverage reached more than 400 km2; however, the relatively low nutrient levels were insufficient for continuous growth.
Production potential of floating Ulva in different areas
Previous research revealed that morphological thalli including filament-like, tubular, cystic, and folded blades were present during the Ulva bloom (J. Zhang, Huo, Yu et al. Citation2013). The filament-like thalli grew much faster than the other three morphological thalli (J. Zhang, Huo, Yu et al. Citation2013). In the Rudong and Dafeng oceanic areas, filamentous thalli were the dominant morphology (, ) and had the highest growth potential. However, in the Rizhao and Qingdao areas, the Ulva had a cyst and folded morphology (, ) that no longer had growth potential, resulting in the rapid decline of blooms in the northern Yellow Sea. In addition, the density of microscopic propagules in the Qingdao area was significantly higher than in Rudong and Dafeng (). This is because the higher SSTs and irradiance in the Qingdao area induced maturation and zoid production. Abundant microscopic propagules, as the ‘seed’ bank, tolerated the hot summer; they could grow into new Ulva thalli and create the initial Ulva biomass for the subsequent year. This explains the annual re-occurrence of the blooms.
Figs 6–9. Physiological parameters of floating Ulva prolifera in the four areas. Error bars are standard deviation (n = 3). Different letters (a–c) on the error bars indicate statistically significant differences (P < 0.05).
Fig. 6. Chlorophyll concentration in the four areas.
Fig. 7. Chlorophyll fluorescence Fv/Fm in the four areas.
Fig. 8. Specific growth rate in the four areas.
Fig. 9. Number of released zoids in the four areas.
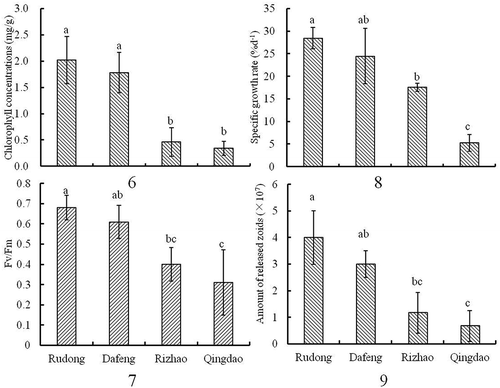
Fig. 10. Density of filamentous Ulva species developed from microscopic propagules in seawater samples collected from four investigated areas of the Yellow Sea. Error bars represent standard deviation (n = 3). *Surface seawater samples in the Rizhao area were provided by Dr Liang Hua from the Marine Scientific Research Institute of Shanghai Ocean University.
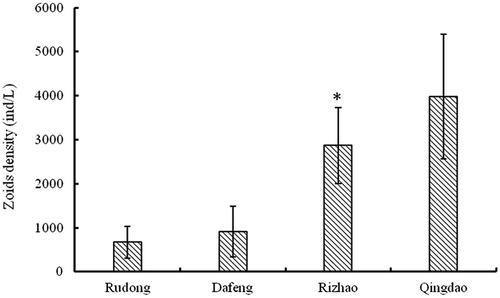
Fig. 11. Seawater quality standards in oceanic areas of China (Category I: clean marine areas, suitable for marine fish culture, marine nature reserves, and marine endangering life protection zones, DIN < 0.20 mg l-1, PO43 --P < 0.015 mg l-1. Category II: less clean marine areas, suitable for aquiculture, marine bathing, marine sports in which people have direct contact with water or recreation; water suitable for the human food industry, DIN < 0.30 mg -1, PO43 --P < 0.030 mg -1. Category III: lightly polluted marine areas; suitable for ordinary industrial park water usage, DIN < 0.40 mg l-1, PO43 --P < 0.030 mg l-1. Category IV: medium-polluted marine areas, suitable only for marine ports and marine development areas, DIN < 0.50 mg l-1, PO43 --P < 0.045 mg l-1. Worse than Category IV: Seriously polluted marine areas.
ACKNOWLEDGEMENTS
The authors thank Dr Liang Hua for support in collecting surface seawater samples. We thank Charles Yarish and anonymous reviewers for their helpful comments and corrections.
Additional information
Funding
REFERENCES
- Abreu M.H., Varela D.A., Henríquez L., Villarroel A., Yarish C., Sousa-Pinto I. & Buschmann A.H. 2009. Traditional vs integrated multi-trophic aquaculture of Gracilaria chilensis C.J. Bird, J.McLachlan & E.C.Oliveira: productivity and physiological performance. Aquaculture (Amsterdam, Netherlands) 293: 211–220. DOI: 10.1016/j.aquaculture.2009.03.043.
- Bao M., Guan W., Yang Y., Cao Z. & Chen Q. 2015. Drifting trajectories of green algae in the western Yellow Sea during the spring and summer of 2012. Estuarine, Coastal and Shelf Science 163: 9–16. DOI: 10.1016/j.ecss.2015.02.009.
- Berger R., Henriksson E., Kautsky L. & Malm T. 2003. Effects of filamentous algae and deposited matter on the survival of Fucus vesiculosus L. germlings in the Baltic Sea. Aquatic Ecology 37: 1–11. DOI: 10.1023/A:1022136900630.
- Canter-Lund H. & Lund J.W.G. 1995. Freshwater algae: their microscopic world explored. BioPress, Bristol, UK. 360 pp.
- Chen Q., He P., Feng Z., Tang W., Li X., Zhang T., Wang Y., Cai C., Huo Y. & Ma J. 2011. [Reproduction of spores/gametes of floating green tide algae Ulva prolifera]. Journal of Fishery Sciences of China 18: 1069–1076. DOI: 10.3724/SP.J.1118.2011.01069.
- Cui J., Shi J., Zhang J., Wang L., Fan S., Xu Z., Huo Y., Zhou Q., Lu Y. & He P. 2018. Rapid expansion of Ulva blooms in the Yellow Sea, China through sexual reproduction and vegetative growth. Marine Pollution Bulletin 130: 223–228. DOI: 10.1016/j.marpolbul.2018.03.036.
- Cui J., Zhang J., Huo Y., Zhou L., Wu Q., Chen L., Yu K. & He P. 2015. Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Marine Pollution Bulletin 101: 660–666. DOI: 10.1016/j.marpolbul.2015.10.033.
- de Paula Silva P.H., McBride S., de Nys R. & Paul N.A. 2008. Integrating filamentous green tide algae into tropical pond-based aquaculture. Aquaculture (Amsterdam, Netherlands) 284: 74–80. DOI: 10.1016/j.aquaculture.2008.07.035.
- Diaz R.J. & Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929. DOI: 10.1126/science.1156401.
- Everitt B.S. & Landau S. 2003. A Handbook of Statistical Analyses Using SPSS. CRC Press.
- Fletcher R.L. 1996a. The British Isles. In: Marine benthic vegetation: recent changes, the effects of eutrophication (Ed. by W. Schramm & P.H. Nienhuis), pp. 150–223. Springer, Berlin, Germany.
- Fletcher R.L. 1996b. The occurrence of “green tides” – a review. In: Marine benthic vegetation, recent changes and the effects of eutrophication, Ecological studies 123 (Ed. by W. Schramm & P.H. Nienhuis), pp. 7–43. Springer, Berlin.
- Franz D.R. & Friedman I. 2002. Effects of a macroalgal mat (Ulva lactuca) on estuarine sand flat copepods: an experimental study. Journal Experimental Marine Biology and Ecology 271: 209–226. DOI: 10.1016/S0022-0981(02)00045-X.
- Gao S., Chen X., Yi Q., Wang G., Pan G., Lin A. & Peng G. 2010. A strategy for the proliferation of Ulva prolifera, main causative species of green tides, with formation of sporangia by fragmentation. PLoS ONE 5: e8571. DOI: 10.1371/journal.pone.0008571.
- Hallegraeff G.M. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia 32: 79–99. DOI: 10.2216/i0031-8884-32-2-79.1.
- Han T., Kang S.H., Park J.S., Lee H.K. & Brown M.T. 2008. Physiological responses of Ulva pertusa and U. armoricana to copper exposure. Aquatic Toxicology 86: 176–184. DOI: 10.1016/j.aquatox.2007.10.016.
- Hernandez I., Peralta G., Perez-Llorens J.L. & Vergara J.J. 1997. Biomass and dynamics of growth of Ulva species in Palmones River estuary. Journal of Phycology 33: 764–772. DOI: 10.1111/j.0022-3646.1997.00764.x.
- Hu C. & He M. 2008. Origin and offshore extent of floating algae in Olympic sailing area. Eos, Transactions American Geophysical Union 89: 302–303. DOI: 10.1029/2008EO330002.
- Huo Y., Han H., Hua L., Wei Z., Yu K., Shi H., Kim J.K., Yarish C. & He P. 2016. Tracing the origin of green macroalgal blooms based on the large scale spatio-temporal distribution of Ulva microscopic propagules and settled mature Ulva vegetative thalli in coastal regions of the Yellow Sea, China. Harmful Algae 59: 91–99. DOI: 10.1016/j.hal.2016.09.005.
- Huo Y., Hu L., Wu H., Zhang J., Cui J., Huang X., Yu K., Shi H., He P. & Ding D. 2014. Abundance and distribution of Ulva microscopic propagules associated with a green tide in the southern coast of the Yellow Sea. Harmful Algae 39: 357–364. DOI: 10.1016/j.hal.2014.09.008.
- Hurd C.L., Harrison P.J., Bischof K. & Lobban C.S. 2014. Seaweed ecology and ecophysiology, ed. 2. Cambridge University Press, New York, USA. 549 pp.
- Keesing J.K., Liu D., Fearns P. & Garcia R. 2011. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Marine Pollution Bulletin 62: 1169–1182. DOI: 10.1016/j.marpolbul.2011.03.040.
- Leliaert F., Zhang X., Ye N., Malta E., Engelen A.H., Mineur F., Verbruggen H. & De Clerck O. 2009. Identity of the Qingdao algal bloom. Phycological Research 57: 147–151. DOI: 10.1111/j.1440-1835.2009.00532.x.
- Lin A., Shen S., Wang G., Yi Q., Qiao H., Niu J. & Pan G. 2011. Comparison of chlorophyll and photosynthesis parameters of floating and attached Ulva prolifera. Journal of Integrative Plant Biology 53: 25–34. DOI: 10.1111/j.1744-7909.2010.01002.x.
- Lin A., Shen S., Wang J. & Yan B. 2008. Reproduction diversity of Enteromorpha prolifera. Journal of Integrative Plant Biology 50: 622–629. DOI: 10.1111/j.1744-7909.2008.00647.x.
- Lin A., Wang C., Qiao H., Pan G., Wang G., Song L., Wang Z., Sun S. & Zhou B. 2009. Study on the photosynthetic performances of Enteromorpha prolifera collected from the surface and bottom of the sea of Qingdao sea area. Chinese Science Bulletin 54: 399–404.
- Liu D., Keesing J.K., He P., Wang Z., Shi Y. & Wang Y. 2013. The world’s largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuarine, Coastal and Shelf Science 129: 2–10. DOI: 10.1016/j.ecss.2013.05.021.
- Liu D., Keesing J.K., Xing Q. & Shi P. 2009. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin 58: 888–895. DOI: 10.1016/j.marpolbul.2009.01.013.
- Liu F., Pang S., Chopin T., Xu N., Shan T., Gao S. & Sun S. 2010. The dominant Ulva strain of the 2008 green algal bloom in the Yellow Sea was not detected in the coastal waters of Qingdao in the following winter. Journal of Applied Phycology l22: 531–540. DOI: 10.1007/s10811-009-9489-7.
- Liu Q., Yu R., Yan T., Zhang Q. & Zhou M. 2015. Laboratory study on the life history of bloom-forming Ulva prolifera in the Yellow Sea. Estuarine, Coastal and Shelf Science 163: 82–88. DOI: 10.1016/j.ecss.2014.08.011.
- Lüning K. 1990. Seaweeds: their environment, biogeography and ecophysiology. Wiley-Interscience, New York, USA. 527 pp.
- McGlathery K.J. 2001. Macroalgal blooms contribute to the decline of seagrass in nutrient-enriched coastal waters. Journal of Phycology 37: 453–456. DOI: 10.1046/j.1529-8817.2001.037004453.x.
- Morand P. & Briand X. 1996. Excessive growth of macroalgae: a symptom of environmental disturbance. Botanica Marina 39: 491–516. DOI: 10.1515/botm.1996.39.1-6.491.
- Nelson T.A., Haberlin K., Nelson A.V., Ribarich H., Hotchkiss R., Alstyne K.L.V., Buckingham L., Simunds D.J. & Fredrickson K. 2008. Ecological and physiological controls of species composition in green macroalgal blooms. Ecology 89: 1287–1298.
- Nelson T.A. & Lee A. 2001. A manipulative experiment demonstrates that blooms of the macroalga Ulvaria obscura can reduce eelgrass shoot density. Aquatic Botany 71: 149–154. DOI: 10.1016/S0304-3770(01)00183-8.
- Ott F.D. 1965. Synthetic media and techniques for the xenic cultivation of marine algae and Flagellata. Virginia Journal of Science 16: 205–218.
- Qiao F., Wang G., Lu X. & Dai D. 2011. Drift characteristics of green macroalgae in the Yellow Sea in 2008 and 2010. Chinese Science Bulletin 56: 2236–2242. DOI: 10.1007/s11434-011-4551-7.
- Raberg S., Berger-Jonsson R., Bjorn A., Graneli E. & Kautsky L. 2005. Effects of Pilayella littoralis on Fucus vesiculosus recruitment: implications for community composition. Marine Ecology Progress Series 289: 131–139. DOI: 10.3354/meps289131.
- Schramm W. 1999. Factors influencing seaweed responses to eutrophication: some results from EU-project EUMAC. Journal of Applied Phycology 11: 69–78. DOI: 10.1023/A:1008076026792.
- State Oceanic Administration People’s Republic of China (SOA). 2011. Marine Environment Quality Status of the National SeaWaters in 2011. Available from: http://www.soa.gov.cn/soa/hygbml/hjgb/hjgb/webinfo/2012/06/1340488547097174.htm.
- Tang W., Li X., Huang H., Cai C., Huo Y. & He P. 2009. [Effects of different light intensity and temperature treatment on photosynthesis and chlorophyll fluorescence in Ulva linza]. Journal of Fisheries of China 33: 762–769.
- van den Hoek C., Mann D.G. & Jahns H.M. 1995. Algae. An introduction to phycology. Cambridge University Press, Cambridge. xiv + 623 pp.
- Wang J., Jiang P., Cui Y., Li N., Wang M., Lin H., He P. & Qin S. 2010. Molecular analysis of green-tide-forming macroalgae in the Yellow Sea. Aquatic Botany 93: 25–31. DOI: 10.1016/j.aquabot.2010.03.001.
- Wang Z., Xiao J., Fan S., Li Y., Liu X. & Liu D. 2015. Who made the world’s largest green tide in China? – an integrated study on the initiation and early development of the green tide in Yellow Sea. Limnology Oceanography 60: 1105–1117. DOI: 10.1002/lno.10083.
- Wu H. L., Zhang J. H., Yarish C., He P., & Kim J. K. 2018. Bioremediation and nutrient migration during blooms of Ulva in the Yellow Sea, China. Phycologia 57: 223–231. DOI: 10.2216/17-32.1.
- Xu D., Gao Z., Zhang X., Fan X., Wang Y., Li D., Wang W., Zhuang Z. & Ye N. 2012. Allelopathic interactions between the opportunistic species Ulva prolifera and the native macroalga Gracilaria Lichenoides. PLoS ONE 7: e33648. DOI: 10.1371/journal.pone.0033648.
- Ye N., Zhang X., Mao Y., Liang C., Xu D., Zou J., Zhuang Z. & Wang Q. 2011. ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecological Research 26: 477–485. DOI: 10.1007/s11284-011-0821-8.
- Zhang J., Huo Y., Yu K., Chen Q., He Q., Han W., Chen L., Cao J., Shi D. & He P. 2013. Growth characteristics and reproductive capability of green tide algae in Rudong coast, China. Journal of Applied Phycology 25: 795–803. DOI: 10.1007/s10811-012-9972-4.
- Zhang J., Huo Y., Yu K., Kim J.K., Yarish C., Qin Y., Liu C., Xu R. & He P. 2014. The origin of the Ulva macroalgae blooms in the Yellow Sea. Marine Pollution Bulletin 89: 276–283. DOI: 10.1016/j.marpolbul.2014.09.049.
- Zhang J., Huo Y., Zhang Z., Yu K., He Q., Zhang L., Yang L., Xu R. & He P. 2013. Variations of morphology and photosynthetic performances of Ulva prolifera during the whole green tide blooming process in the Yellow Sea. Marine Environmental Research 92: 35–42. DOI: 10.1016/j.marenvres.2013.08.009.
- Zhang J., Zhao P., Huo Y., Yu K. & He P. 2017. The fast expansion of Pyropia aquaculture in “Sansha” regions should be mainly responsible for the Ulva blooms in Yellow Sea. Estuarine, Coastal and Shelf Science 189: 58–65. DOI: 10.1016/j.ecss.2017.03.011.
- Zhang X. 1986. [The method of extracting chlorophyll concentrations of plant using the mixture of acetone and alcohol]. Liaoning Agriculture Science 3: 26–28.