ABSTRACT
Surveys of the seaweed flora of the Falkland Islands and of Tierra del Fuego revealed the presence of a new species of brown alga. Dictyota falklandica sp. nov. inhabits the shallow rocky infralittoral in sheltered localities and the lower intertidal in more exposed sites. Dictyota falklandica has a regular to irregular habit of dichotomously branched blades, forming erect thalli composed of a single-layered cortex and medulla, with margins in the apical parts dotted with dormant apical cells. Sporangia occur in irregular groups or longitudinal lines on the thallus surface. Molecular phylogenies based on chloroplast psbA and rbcL and mitochondrial cox1 sequences showed that the species from the Falkland Islands is sister to a clade formed by D. korowai, recently described from New Zealand and D. kunthii known from both the Pacific coast of South America and New Zealand. Temperature tolerance experiments, showing mortality at 25 °C but survival at 20 °C, confirm the cold-temperate affinity of this taxon. Its relationship to other cold-temperate Southern Hemisphere species is discussed, with its closest relatives living in regions with sea surface temperatures of at least 7–10 °C higher.
INTRODUCTION
Dictyota species are common members of the benthic communities in warm-temperate and tropical seas, and the genus is widely considered to have warm-water affinities (Tronholm et al. Citation2010). Among the 97 Dictyota species currently recognized, 87 are described from warm-temperate and tropical seas (Guiry & Guiry Citation2019). Their effective chemical defense against grazing based on diterpenes (Hay et al. Citation1987; Wiesemeier et al. Citation2007) and high constitutive hydrogen peroxide release, likely for the control of bacterial biofilms (Küpper et al. Citation2002), as well as their ability to propagate through fragmentation (Herren et al. Citation2006), enables them to form sizeable populations even in localities under high grazing pressure such as Ascension Island (Tsiamis et al. Citation2017). Significantly, defense against amphipods is inducible in Chilean Dictyota kunthii (C.Agardh) Greville (Macaya & Thiel Citation2008), which may be a more widespread feature in this genus.
In contrast to the ubiquity of Dictyota in warm seas, few reports exist of Dictyota species in cold-temperate seas of the southern Hemisphere (Fig. S1). Asensi & Küpper (Citation2012) reported a Dictyota sp. from Puerto Deseado (Patagonia, Argentina), where it was present year-round, growing on calcareous algae. There is a high chance that this report corresponds to the entity from Argentina identified as D. dichotoma (Hudson) J.V.Lamouroux by Lopes-Filho et al. (Citation2017) using DNA sequence information. Dictyota dichotoma is primarily known from Europe, but is also present in South Africa and the temperate Atlantic coast of Brazil and Argentina (Tronholm et al. Citation2010; O. De Clerck, pers. obs.). Dictyota kunthii occurs in cold to warm-temperate regions along the South American Pacific coast from Peru to Chile, the Juan Fernandez Islands (Ramírez & Osorio Citation2000; Rodríguez-Ruiz et al. Citation2017), and in New Zealand (Hoffmann & Santelices Citation1997; Nelson Citation2013; Ramírez & Santelices Citation1991). The most southern Dictyota described so far is D. decumbens (Ricker) Hörnig, Schnetter et Prud’homme van Reine from Macquarie Island (54 °S, Ricker Citation1987). The species was originally described as Dilophus decumbens Ricker, because the medulla comprised 2–3, irregularly organized layers of cells near the margins. Dilophus decumbens was subsequently transferred to Dictyota (Hörnig et al. Citation1992b, Citation1992a).
Recent work (Mystikou et al. Citation2016) reported a hitherto undescribed Dictyota sp. from the eastern tip of Tierra del Fuego (Argentina) and from two localities in the Falkland Islands. In a psbA phylogeny, this unknown taxon was sister to D. binghamiae J.Agardh from Mexico and California (Mystikou et al. Citation2016). However, more research was needed before it could be formally described as a separate species, especially its relationships with Southern Hemisphere taxa (Chile, Juan Fernandez) such as D. phlyctaenodes Montagne and D. kunthii, which was the rationale for the present study. In this paper, we report on our recent observations and collections of Dictyota from the Falkland Islands, including a formal description as a new species, underpinned by temperature tolerance experiments with cultured isolates.
MATERIALS & METHODS
Specimens of Dictyota were collected in the shallow subtidal zone at two localities in East Falkland (; ): Blue Beach, San Carlos (51° 34.2676ʹ S, 59° 2.1236ʹ W, on 24 January 2017, at 1 m depth, and the south of North Arm (52° 8.4014ʹ S, 59º 22.174ʹ W) on 26 January 2017, at 2 m depth (–). Herbarium specimens were prepared in parallel to subsamples which were fixed in 4% formalin-seawater (for microscopy), CTAB buffer and silica gel (both for DNA extraction). Samples in Chile were collected in the Juan Fernandez Archipelago (Robinson Crusoe Island), in the low intertidal (D. kunthii) and the shallow subtidal at 1 m depth (D. phlyctaenodes) from El Palillo (33°38.3177ʹ S, 78°49.2879ʹ W) on 12–13 February 2014, respectively. Additional samples of D. kunthii were collected from northern Chile in the low intertidal at Caleta Errazuriz (23°26.689’S, 70°35.3039ʹ W) on 12 November 2012 and from central Chile in the low intertidal at Cocholgüe (36°36.3011ʹ S, 72°58.4962ʹ W) from August to November 2015.
Table 1. Comparison of key features of Dictyota decumbens and Dictyota falklandica sp. nov.
Fig. 1. Localities in the Falkland Islands where Dictyota falklandica sp. nov. was found: San Carlos and North Arm.
Figs 2–5. Dictyota falklandica sp. nov. in its natural habitat at North Arm on 26 January 2017, with epiphytes on its thallus and surrounding vegetation. Scale bar = 5 mm.

Underwater photographs were taken with an Olympus Tg4 camera, while micrographs were taken with an Olympus DP50 digital camera (Melville, NY, USA) mounted on a Leitz Diaplan compound microscope or Leica Wild M10 (Wetzlar, Germany) stereo microscope.
Total genomic DNA was extracted from silica gel-dried samples using a modified CTAB-extraction method (Steen et al. Citation2017). Sequences were generated for the plastid-encoded PSII reaction centre protein D1 (psbA) and the mitochondrial-encoded cytochrome oxidase subunit 1 (cox1) gene. PCR primers and conditions are described in Tronholm et al. (Citation2010). Sequences were aligned by eye using MEGA 7 (Kumar et al. Citation2016) and added to a reference species-level alignment of Dictyota and related genera (Canistrocarpus, Dictyotopsis, Dilophus, Scoresbyella and Rugulopteryx). The matrix, a multi-locus alignment of mitochondrial cox1 and cox3 genes, and chloroplast psaA, psbA and rbcL genes, consisted of 64 taxa and 5446 positions. The matrix was 81% filled at the gene level (Table S1).
A maximum-likelihood (ML) tree was generated from the concatenated alignment, partitioned by organelle and codon position. The partition scheme and substitution models were estimated using PartitionFinder 2 (Lanfear et al. Citation2017). ML analyses were conducted using RAxML v8.1 under a GTR + GAMMA model (Stamatakis Citation2014). The robustness of the resulting phylogenies was tested using 1000 replicates of a rapid bootstrap heuristic (Stamatakis Citation2006). In addition, a Bayesian tree was estimated using MrBayes 3.2. (Ronquist et al. Citation2012), applying a GTR+GAMMA model applied to every partition. Two runs, consisting of four chains each, were run for 10 million generations. Stationarity and convergence of runs was assessed visually using Tracer v1.6 (Rambaut et al. Citation2018) and a majority rule consensus tree was calculated after removal of a burn-in of 15%.
For all specimens, we collected mean sea surface temperature (SST mean) based on locality information and environmental layers present in Bio-Oracle v2.0 (Assis et al. Citation2018). Averaged mean surface temperatures correlate equally well or better with marine species distribution ranges compared to average minimum or maximum temperatures as demonstrated by Bosch et al. (Citation2018). Ancestral states of SST affinities were reconstructed and visualised on the phylogeny using the fastAnc and contMap functions of the R package phytools (Revell Citation2012).
Three unialgal isolates (coded FI 17–182 and FI 17–186 from San Carlos, and FI 17–203 from North Arm) were obtained from thallus apices. The culture medium was autoclaved, half-strength Provasoli-enriched sea water (Coelho et al. Citation2012), which for the first weeks contained 4 mg l−1 GeO2 to inhibit diatom growth. During isolation, the algae received natural daylight near a north-facing window, and the temperature ranged between 12–14 ºC.
In a laboratory-based experiment, the gross temperature tolerance was determined with cultures pre-cultivated at 15 °C. On day 1, a dish (10 ml) of each of the three isolates (FI 17–182, FI 17–186, FI 17–203) was placed at 15 °C, 20 °C and 25 °C under white light of 30 μmol m−1 sec−1. On day 11, the medium was changed in all cultures, and those which had been incubated at 20 and 25 °C were transferred to 15 °C (where all cultures were kept). On days 24 and 38, the medium was changed in all dishes, and all cultures were continued at 15 °C until final examination two months after start of the experiment.
RESULTS
In situ observations
At both San Carlos and North Arm, Dictyota falklandica grew on rocky substrata at 1–3 m beneath low tide level, typically under a canopy of Macrocystis pyrifera (Linnaeus) C.Agardh (–). Synchronous with our subtidal collections on the Falkland Islands we examined the rocky intertidal zone close to the two sites. We did not find the species on the rocks or in shallow tide pools.
Phylogeny
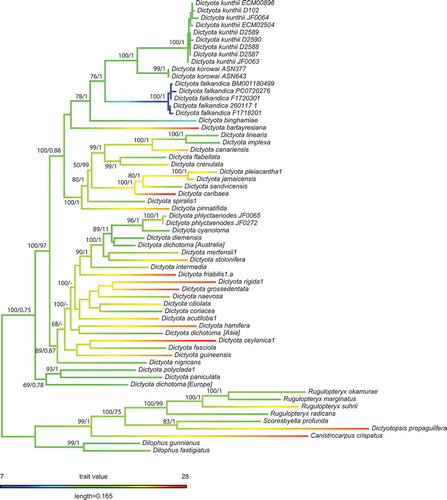
The maximum-likelihood and Bayesian phylogenies () were in good agreement, differing only in parts of the tree that received low support. Dictyota falklandica sp. nov. was resolved sister to a clade formed by D. korowai, a species recently described from northern New Zealand (Nelson et al. Citation2019) and D. kunthii from the Pacific coast of South America and New Zealand. Another closely related species is D. binghamiae from the northeastern Pacific. D. phlyctaenodes, described from, and possibly endemic to, the Juan Fernandez Islands, appears only distantly related to D. binghamiae, D. falklandica, D. korowai and D. kunthii. D. phlyctaenodes is resolved in a clade with predominantly Australian species. Likewise, D. coriacea, occurring on both sides of the northern Pacific Ocean, and eastern Pacific species with tropical affinities (D. crenulata, D. flabellata) are all distantly related to D. falklandica.
Fig. 6. Maximum-likelihood tree of the concatenated alignment (-Ln = 45270.45) with rapid bootstrap (left) and posterior probabilities (right) values shown on branches. Branch colours are the maximum-likelihood estimates of the ancestral states of mean sea surface temperature.
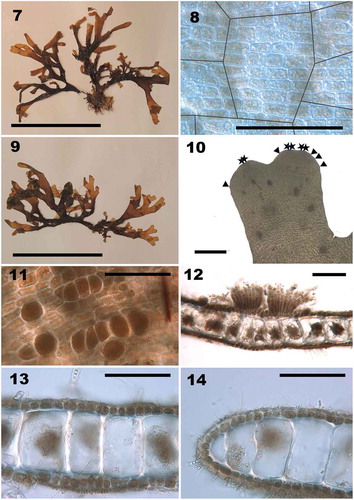
Figs 7–14. Herbarium specimens and micrographs of Dictyota falklandica sp. nov.
Fig. 7. Isotype (PC accession no. 0776066). Scale bar = 5 cm. Fig. 8. Surface view, with walls of larger medullar cells indicated by black lines. Scale bar = 50 µm. Fig. 9. Holotype specimen (BM accession no. BM013828094). Scale bar = 5 cm. Fig. 10. Apical region of branching thallus, with actively growing apical cells (asterisks) as well as marginal dormant apical cells (arrowheads). Scale bar = 1 mm. Fig. 11. Spores on thallus surface in irregular longitudinal groups. Scale bar = 50 µm. Fig. 12. Cross section of thallus with two hair tufts. Scale bar = 100 µm. Fig. 13. Cross section of central blade area. Scale bar = 100 µm. Fig. 14. Cross section of blade margin. Scale bar = 100 µm.
Taken together, this suggests that this is a species new to science, which is formally described below:
Dictyota falklandica F.C.Küpper, A.F.Peters, A.O.Asensi & O.DeClerck sp. nov.–, –
DIAGNOSIS
Species characterised by a regular to irregular dichotomously branching habit of erect blades up to 65 mm long and 5 mm wide, each branch growing by division of a meristematic apical cell. Margins of the whole thallus dotted with dormant apical cells. In situ colour medium to pale brown, retaining the same colour when dry.
HOLOTYPE
Herbarium specimen from San Carlos (F39) was deposited in the Natural History Museum (BM), London, UK as No. BM013828094, accompanied by vials with CTAB samples (Vial F17-186 BM013792001; Vial F18-203 BM013792002; Vial F17-182 BM013792003).
ISOTYPE
An isotype from North Arm was deposited in the Muséum National d’Histoire Naturelle – Paris, as no. PC 0776066. Material from the 3 live cultures fixed in CTAB buffer was deposited in the Muséum National d’Histoire Naturelle – Paris, accompanying the isotype specimen, as no. PC 0776066.
TYPE LOCALITY
Blue Beach, San Carlos, East Falkland, Falkland Islands, 51º 34.2676ʹ S, 59º 2.1236ʹ W. The type locality of this species at San Carlos is historically significant as the landing site of the forces who liberated the Falkland Islands in 1982.
ETYMOLOGY
The organism is named after the Falkland Islands, where the type material was collected.
REPRESENTATIVE SEQUENCES
MK516759 – MK516759 (cox1) and MK516799- MK516800 (psbA), and MK516815 (rbcL).
ISOTYPE CULTURE
A unialgal culture isolated on 31 January 2017 from the type material collected at San Carlos was deposited in the Culture Collection of Algae and Protozoa (no. CCAP 1335/1).
Vegetative and reproductive morphology
Dictyota falklandica grew upright, but lacked a conspicuous base. The species was attached by patches of rhizoids, present near basal parts of thalli (). Apices were obtuse, with protruding lens-shaped apical cells. Irregular branching in the apical parts of thalli might be related to regained meristematic activity of additional apical cells which dot the margins of the apical parts of the thallus (, ). Dichotomies were evenly spaced every 13–15 mm. The branching angle was approximately 45–50°. Surface and margins were smooth, and lacked teeth or proliferations ().
The thallus was composed of a single-layered cortex and medulla (–15). Tangential divisions of cortical and medulla cells, resulting in a multi-layered cortex or medulla, were not observed, not even in the most basal regions. Cells contained multiple discoid plastids devoid of pyrenoids. Cortical cells were rectangular to nearly isodiametric in surface view (), (22–) 30 (–38) μm long, (12–) 17 (–22) μm wide and 10–12 μm high (, ). Medullary cells were (77–) 101 (–129) μm long, (46–) 57 (–77) μm wide () and 95–120 μm high ().
Male and female gametophytes were not observed. Immature sporangia occurred in irregular groups or longitudinal lines on the thallus surface (). Sporangia were not surrounded by an involucrum and were borne on a single stalk cell. Thalli had tufts of hairs randomly scattered on both surfaces ().
Temperature tolerance
Two months after the start of the experiment, cultures of the three isolates exposed to 25 °C for 11 days showed clear signs of stress – two were dead, and one culture was mostly bleached with only small tissue parts remaining pigmented. In contrast, the cultures at 20 or 15 °C for the same duration had healthy (brown – dark brown) pigmentation and growth.
DISCUSSION
The order Dictyotales is frequent in temperate to tropical seas, and particularly diverse in Australia and New Zealand (Guiry & Guiry Citation2019; Nelson et al. Citation2019; Phillips Citation2001). However, the nearby subantarctic region is largely devoid of this order, and it is missing in Antarctica (Papenfuss Citation1964; Wiencke & Clayton Citation2002; Wiencke et al. Citation2014). This study highlights that the overall diverse seaweed flora of the Falkland Islands still has significant potential for new discoveries, warranting further surveys and taxonomic studies (Küpper & Kamenos Citation2018). Dictyota falklandica sp. nov. from the Falkland Islands and southernmost South America is one of the few species able to grow in cold-temperate waters, and it appears to be geographically confined to this cold-temperate region. Its closest relatives, D. korowai, D. binghamiae and D. kunthii, live in regions with sea surface temperatures at least 7–10 ºC higher. In the Falkland Islands, surface water temperatures oscillate between 5 and 9 °C (Arkhipkin et al. Citation2013), similar to temperatures present in eastern Tierra del Fuego (Rivas & Pisoni Citation2010). One of the collecting sites, North Arm, lies in the SE part of the Falkland Islands, which is strongly influenced by cold subantarctic waters. In high-temperature tolerance experiments, our cultures of D. falklandica survived 20 °C but not 25 ºC for an 11-day duration. It resembles other subantarctic species from South America, which have upper survival temperatures between 19.9 and 24.5 but not 25 °C (Peters & Breeman Citation1993). Such species, albeit adapted to the cold-temperate subantarctic environment, have a large ‘safety margin’ which may allow occurrence in milder habitats such as shallow pools or the intertidal. At Bahía Thetis (Tierra del Fuego; Mystikou et al. Citation2016), D. falklandica had been collected in the mid-intertidal zone of a more exposed site, as judged from the presence of Durvillaea antarctica (Chamisso) Hariot.
Mystikou et al. (Citation2016) concluded that D. falklandica sp. nov. (as Dictyota sp.) is morphologically and genetically different from other Dictyota species. In the present study, Dictyota falklandica was resolved as sister to D. kunthii from the Pacific coast of South America, the Juan Fernandez Islands, New Zealand and Australia (Guiry & Guiry Citation2019) and D. korowai from northern New Zealand (Nelson et al. Citation2019). However, the phylogenetic relationship of D. falklandica to D. decumbens endemic to subantarctic Macquarie Island is unknown, as no sequences are available of D. decumbens. The hydrographic conditions at Macquarie Island just north of the Antarctic Convergence resemble those at the Falkland Islands, with even slightly lower sea surface temperatures of 3.0–7.3 °C (Ricker Citation1987). However, although one can hypothesize that D. falklandica sp. nov. may be related to D. decumbens, the geographic distance and morphological differences (the latter was initially described as Dilophus decumbens due to a double-layered medulla; Ricker Citation1987) suggests they are not conspecific. A number of recent studies on macroalgae with a circum-Antarctic distribution show that only large species or taxa that are good drifters [Macrocystis pyrifera, Durvillaea antarctica, Adenocystis utricularis (Bory) Skottsberg] and possibly their symbionts and pathogens such as Maullinia braseltonii P.Murúa, P.Goecke & S.Neuhauser (Blake et al. Citation2017; Murua et al. Citation2017), Herpodiscus durvilleae (Lindauer) G.R.South (Fraser & Waters Citation2013) and Laminariocolax aecidioides (Rosenvinge) Peters (Bernard et al. Citation2018) are genetically similar in the different land masses and islands, whereas less good drifters and smaller algae [e.g. Lessonia spp., the cryptic species included in Bostrychia intricata (Bory) Montagne] rather form different species in the distant localities (Fraser & Waters Citation2013; Fraser et al. Citation2013; Martin & Zuccarello Citation2012; Muangmai et al. Citation2014). Like D. decumbens, D. falklandica sp. nov. was found in the subtidal zone. However, D. decumbens has a prostrate habit. It forms several cm thick mats on boulders or other macroalgae (Ricker Citation1987), whereas the alga described here was erect and always epilithic. Available herbarium specimens of D. decumbens had been fixed in formalin before mounting (Ricker Citation1987) and are therefore unsuitable for the extraction of DNA. We prefer to describe the Falkland Dictyota as a separate species based on morphological differences and geographic separation.
Supplemental Material
Download Zip (22.6 KB)Acknowledgements
Special thanks to Paul Brickle (South Atlantic Environmental Research Institute) for hosting our expedition.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Arkhipkin A., Brickle P. & Laptikhovsky V. 2013. Links between marine fauna and oceanic fronts on the Patagonian shelf and slope. Arquipelago - Life and Marine Sciences 30: 19–37.
- Asensi A.O. & Küpper F.C. 2012. Seasonal periodicity and reproduction of brown algae (Phaeophyta) at Puerto Deseado (Patagonia). Botanica Marina 55: 217–228. DOI: 10.1515/bot-2012-0002.
- Assis J., Tyberghein L., Bosch S., Verbruggen H., Serrao E.A. & De Clerck O. 2018. Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Global Ecology and Biogeography 27: 277–284. DOI: 10.1111/geb.12693.
- Bernard M., Strittmatter M., Murúa P., Heesch S., Cho G.Y., Leblanc C. & Peters A.F. 2018. Diversity, biogeography and host specificity of kelp endophytes with a focus on the genera Laminarionema and Laminariocolax (Ectocarpales, Phaeophyceae). European Journal of Phycology 54: 39–51. DOI: 10.1080/09670262.2018.1502816.
- Blake C., Thiel M., Lopez B.A. & Fraser C.I. 2017. Gall-forming protistan parasites infect southern bull kelp across the Southern Ocean, with prevalence increasing to the south. Marine Ecology Progress Series 583: 95–106. DOI: 10.3354/meps12346.
- Bosch S., Tyberghein L., Deneudt K., Hernandez F. & De Clerck O. 2018. In search of relevant predictors for marine species distribution modelling using the MarineSPEED benchmark dataset. Diversity and Distributions 24: 144–157. DOI: 10.1111/ddi.12668.
- Coelho S.M., Scornet D., Rousvoal S., Peters N., Dartevelle L., Peters A.F. & Cock J.M. 2012. How to cultivate Ectocarpus. Cold Spring Harbor Protocols 2012: 258–261.
- Fraser C.I. & Waters J.M. 2013. Algal parasite Herpodiscus durvillaeae (Phaeophyceae, Sphacelariales) inferred to have traversed the Pacific Ocean with its buoyant host. Journal of Phycology 49: 202–206. DOI: 10.1111/jpy.12017.
- Fraser C.I., Zuccarello G.C., Spencer H.G., Salvatore L.C., Garcia G.R. & Waters J.M. 2013. Genetic affinities between trans-oceanic populations of non-buoyant macroalgae in the high latitudes of the Southern Hemisphere. PLoS ONE 8: e69138. DOI: 10.1371/journal.pone.0069138.
- Guiry M.D. & Guiry G.M. 2019. AlgaeBase. National University of Ireland, Galway. World-wide electronic publication. http://www.algaebase.org.
- Hay M.E., Duffy J.E. & Pfister C.A. 1987. Chemical defense against different marine herbivores - are amphipods insect equivalents? Ecology 68: 1567–1580. DOI: 10.2307/1939849.
- Herren L.W., Walters L.J. & Beach K.S. 2006. Fragment generation, survival, and attachment of Dictyota spp. at Conch Reef in the Florida Keys, USA. Coral Reefs 25: 287–295. DOI: 10.1007/s00338-006-0096-7.
- Hoffmann A. & Santelices B. 1997. Flora marina de Chile central. Marine flora of central Chile. Ediciones Universidad Católica de Chile, Santiago. 434 pp.
- Hörnig I., Schnetter R. & Vanreine W.F.P. 1992a. The genus Dictyota (Phaeophyceae) in the North Atlantic. 2. Key to the species. Nova Hedwigia 54: 397–402.
- Hörnig I., Schnetter R., Vanreine W.F.P., Coppejans E., Achenbachwege K. & Over J.M. 1992b. The genus Dictyota (Phaeophyceae) in the North Atlantic. 1. A new generic concept and species. Nova Hedwigia 54: 45–62.
- Kumar S., Stecher G. & Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. DOI: 10.1093/molbev/msw054.
- Küpper F.C. & Kamenos N.A. 2018. The future of marine biodiversity and marine ecosystem functioning in UK coastal and territorial waters (including UK overseas territories) – with an emphasis on marine macrophyte communities. Botanica Marina 61: 521–535. DOI: 10.1515/bot-2018-0076.
- Küpper F.C., Müller D.G., Peters A.F., Kloareg B. & Potin P. 2002. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of the sporophytes of laminariales. Journal of Chemical Ecology 28: 2057–2081.
- Lanfear R., Frandsen P.B., Wright A.M., Senfeld T. & Calcott B. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. DOI: 10.1093/molbev/msw260.
- Lopes-Filho E.A.P., Salgueiro F., Nascimento S.M., Gauna M.C., Parodi E.R. & Campos De Paula J. 2017. Molecular evidence of the presence of Dictyota dichotoma (Dictyotales: Phaeophyceae) in Argentina based on sequences from mtDNA and cpDNA and a discussion of its possible origin. New Zealand Journal of Botany 55: 293–305. DOI: 10.1080/0028825X.2017.1326387.
- Macaya E.C. & Thiel M. 2008. In situ tests on inducible defenses in Dictyota kunthii and Macrocystis integrifolia (Phaeophyceae) from the Chilean coast. Journal of Experimental Marine Biology and Ecology 354: 28–38. DOI: 10.1016/j.jembe.2007.10.005.
- Martin P. & Zuccarello G.C. 2012. Molecular phylogeny and timing of radiation in Lessonia (Phaeophyceae, Laminariales). Phycological Research 60: 276–287. DOI: 10.1111/j.1440-1835.2012.00658.x.
- Muangmai N., West J.A. & Zuccarello G.C. 2014. Evolution of four Southern Hemisphere Bostrychia (Rhodomelaceae, Rhodophyta) species: phylogeny, species delimitation and divergence times. Phycologia 53: 593–601. DOI: 10.2216/14-044.1.
- Murua P., Goecke F., Westermeier R., van West P., Küpper F.C. & Neuhauser S. 2017. Maullinia braseltonii sp. nov. (Rhizaria, Phytomyxea, Phagomyxida): a cyst-forming parasite of the bull kelp Durvillaea spp. (Stramenopila, Phaeophyceae, Fucales). Protist 168: 468–480. DOI: 10.1016/j.protis.2017.07.001.
- Mystikou A., Asensi A.O., De Clerck O., Müller D.G., Peters A.F., Tsiamis K., Shewring D.M., Fletcher K.I., Westermeier R., Brickle P., et al. 2016. New records and reassessment of macroalgae and associated pathogens from the Falkland Islands, Patagonia and Tierra del Fuego. Botanica Marina 59: 105–121. DOI: 10.1515/bot-2015-0071.
- Nelson W.A. 2013. New Zealand seaweeds. An illustrated guide. Te Papa Press, Wellington. 328 pp.
- Nelson W.A., Sutherland J.E., Ringham S. & Murupaenga H. 2019. Dictyota korowai sp. nov. (Dictyotales, Phaeophyceae) from Manawatawhi/Three Kings Islands, northern New Zealand, previously confused with Dictyota intermedia. Phycologia. 58: 433–442. DOI: 10.1080/00318884.2019.1625256.
- Papenfuss G.F. 1964. Catalogue and bibliography of Antarctic and Sub-Antarctic benthic marine algae. American Geophysical Union, Washington, DC. 76 pp.
- Peters A.F. & Breeman A.M. 1993. Temperature tolerance and latitudinal range of brown algae from temperate Pacific South America. Marine Biology 115: 143–150. DOI: 10.1007/BF00349396.
- Phillips J.A. 2001. Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora? Biodiversity and Conservation 10: 1555–1577. DOI: 10.1023/A:1011813627613.
- Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. 2018. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol 10.
- Ramírez M.E. & Osorio C. 2000. Patrones de distribución de macroalgas y macroinvertebrados intermareales de la isla Robinson Crusoe, archipiélago de Juan Fernández, Chile. Investigaciones Marinas 28: 1–13. DOI: 10.4067/S0717-71782000002800002.
- Ramírez M.E. & Santelices B. 1991. Catalogo de las algas marinas bentonicas de la costa temperada del Pacifico de Sudamerica. Monografías Biológicas. Ediciones Pontifica Universidad Católica de Chile, Santiago, Chile. 437 pp.
- Revell L.J. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. DOI: 10.1111/j.2041-210X.2011.00169.x.
- Ricker R.W. 1987. Taxonomy and biogeography of Macquarie Island seaweeds. British Museum Natural History, London. 344 pp.
- Rivas A.L. & Pisoni J.P. 2010. Identification, characteristics and seasonal evolution of surface thermal fronts in the Argentinean continental shelf. Journal of Marine Systems 79: 134–143. DOI: 10.1016/j.jmarsys.2009.07.008.
- Rodríguez-Ruiz M.C., Andreu-Cazenave M., Ruz C.S., Ruano-Chamorro C., Ramírez F., González C., Carrasco S., Pérez-Matus A. & Fernández M. 2017. Initial assessment of coastal benthic communities in the Marine Parks at Robinson Crusoe Island. Latin American Journal of Aquatic Research 42: 918–936. DOI: 10.3856/vol42-issue4-fulltext-16.
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Hohna S., Larget B., Liu L., Suchard M.A. & Huelsenbeck J.P. 2012. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. DOI: 10.1093/sysbio/sys029.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. DOI: 10.1093/bioinformatics/btl446.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. DOI: 10.1093/bioinformatics/btu033.
- Steen F., Aragay J., Zuljevic A., Verbruggen H., Mancuso F.P., Bunker F., Vitales D., Garreta A.G. & De Clerck O. 2017. Tracing the introduction history of the brown seaweed Dictyota cyanoloma (Phaeophyceae, Dictyotales) in Europe. European Journal of Phycology 52: 31–42. DOI: 10.1080/09670262.2016.1212998.
- Tronholm A., Steen F., Tyberghein L., Leliaert F., Verbruggen H., Ribera Siguan M.A. & de Clerck O. 2010. Species delimitation, taxonomy, and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). Journal of Phycology 46: 1301–1321. DOI: 10.1111/j.1529-8817.2010.00908.x.
- Tsiamis K., Peters A.F., Shewring D.M., Asensi A.O., Van West P. & Küpper F.C. 2017. Marine benthic algal flora of Ascension Island, South Atlantic. Journal of the Marine Biological Association of the United Kingdom 97: 681–688. DOI: 10.1017/S0025315414000952.
- Wiencke C., Amsler C.D. & Clayton M.N. 2014. Macroalgae. In: Biogeographic atlas of the Southern Ocean (Ed. by C. De Broyer, P. Koubbi, H.J. Griffiths, B. Raymond, C. Udekem, A.P. d’Acoz, B. Van de Putte, B. Danis, S. David, J. Grant, et al.), pp. 66–73. Scientific Committee on Antarctic Research, Cambridge.
- Wiencke C. & Clayton M.N. 2002. Antarctic seaweeds. A.R.G. Gantner, Ruggell, Liechtenstein, 239 pp.
- Wiesemeier T., Hay M.E. & Pohnert G. 2007. The potential role of wound-activated volatile release in the chemical defence of the brown alga Dictyota dichotoma: blend recognition by marine herbivores. Aquatic Sciences 69: 403–412. DOI: 10.1007/s00027-007-0889-y.
