 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The invasive seaweed Rugulopteryx okamurae has recently arrived in Europe from the western Pacific. Its explosive spread on coastal areas of the Gulf of Cádiz (GoC), Strait of Gibraltar and Alboran Sea is spoiling native coastal ecosystems and inflicting heavy losses on ecosystem services. We discovered for the first time large amounts (up to 17 g m–2) of detached R. okamurae thalli on deep-sea bottoms of the GoC that are being dragged from the Strait of Gibraltar shores into the NE Atlantic by the Mediterranean Outflow Water (MOW). Laboratory experiments revealed that collected unattached macroalgae from deep-sea locations were alive and healthy and maintained intact photosynthetic capacity after long dark periods, suggesting a tremendous resilience and invasive potential. Given the rapid transport of healthy thalli by the MOW and massive accumulation of them in the GoC basin, R. okamurae could represent a major threat to NE Atlantic ecosystems, affecting not only coastal but also deep-sea habitats.
INTRODUCTION
The occurrence of a new invasive macroalga, Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & Hy.S. Kim, at the Strait of Gibraltar (SoG) in 2015 marked an unprecedented milestone on the north African-European marine ecosystems (García-Gómez et al. Citation2020; ministerial order TED/1126/2020). This species has very high primary productivity rates, which led to 5,000 tons of biomass removed from the beaches of Ceuta (southern SoG) in 2015 (Ocaña et al. Citation2016). From 2015 to date, R. okamurae has colonized most of the infralittoral rocky bottoms (5–30 m depth) of the SoG and is presently expanding towards Atlantic and Mediterranean coastal areas of the southern Iberian Peninsula, modifying their habitats and associated communities (García-Gómez et al. Citation2018, Citation2020; CAGPDS Citation2019).
This brown macroalga originally comes from subtropical and temperate areas of the western Pacific Ocean (Lee & Kang Citation1986; Silva et al. Citation1987; Yoshida Citation1998). It probably arrived at the SoG through ballast water of large Asian vessels that moored in the Bay of Algeciras or TangerMed ports (Rosas-Guerrero et al. Citation2018; García-Gómez et al. Citation2020). The huge invasive ability of R. okamurae is due to its elevated capacity of vegetative reproduction and survival in adverse environmental conditions (Rosas-Guerrero et al. Citation2018; Altamirano et al. Citation2019). The main pathway of local expansion is through unattached (detached) thalli that are washed away and dispersed by currents from personal observations of Altamirano in (CAGPDS Citation2019). These unattached thalli can finally accumulate on rocks or other hard substrates (natural or artificial) and then start a new invasion far away from their original place. For example, a square centimetre of thallus produces more than 100 asexual monospores and more than 25 vegetative propagules (from personal observations of Altamirano in CAGPDS Citation2019 and MITECO Citation2021). A propagule is a multicellular structure that spontaneously detaches from the parental thallus and gives rise to a new individual (Cecere et al. Citation2011). In R. okamurae, a new clonal individual can be generated from each propagule, regardless of whether the thallus that produces it is fixed to the substrate or free in the water column (from personal observations of Altamirano in CAGPDS Citation2019 and MITECO Citation2021).
On European shores, there are a high number of non-indigenous species of macrophytes (113 species only in the Mediterranean Sea; Zenetos & Galandi Citation2020; Tsiamis et al. Citation2020). Nevertheless, the most successful species are those that share with R. okamurae a series of reproductive features, such as rapid rates of colonization by vegetative growth and long-distance dispersal by fragments or propagules (e.g. Caulerpa cylindracea Sonder, Halimeda incrassata (J. Ellis) J.V. Lamouroux or Gracilaria vermiculophylla (Ohmi) Papenfuss, among other) (Ceccherelli & Cinelli Citation1999; Klein & Verlaque Citation2008; Watanabe et al. Citation2009; Cecere et al. Citation2011; Winter et al. Citation2020). The colonization success of these species might be related to an enhanced capacity for uniparental reproduction (i.e. vegetative and asexual reproduction), which increases the potential for rapid expansion (Krueger-Hadfield et al. Citation2016). Fast spread and further establishment of an invasive species could produce severe negative implications on the indigenous ecosystem (Anton et al. Citation2019; but see Thomsen Citation2020). In the case of R. okamurae invasion, the elevated vegetative reproduction and growth of R. okamurae can lead this macroalga to cover 80%–100% of the seabed, which, unfortunately, results in a regression of many native macroalgae and invertebrate species and in the reduction of the typical benthic heterogeneity of infralittoral rocky shores (CAGPDS Citation2019; García-Gómez et al. Citation2020). An example of this is the recently described change in a Mediterranean coralligenous community structure with a regression of the gorgonian species Paramuricea clavata (Risso) and the calcareous seaweed Mesophyllum expansum (Philippi) Cabioch & M.L. Mendoza, associated with the rapid expansion of R. okamurae (Sempere-Valverde et al. Citation2021). In areas highly colonized by R. okamurae, such as the SoG, the socioeconomic costs of the invasion are huge since fishermen nets are filled with tons of thalli that prevent them from fishing. Indeed, fishing is one of the most affected economic sectors by the R. okamurae invasion, with an economic impact of 0.8 × 106 € in nine months (Altamirano et al. Citation2021).
This study reports for the first time the massive presence of unattached R. okamurae thalli in deep-sea bottoms transported and dispersed by oceanic undercurrents. It also includes results from a laboratory experiment with unattached R. okamurae collected from bathyal bottoms of the Gulf of Cádiz (GoC) to assess the health condition of the unattached thalli and their survival capacity when transported to distant regions. More specifically, the objectives of the present study are: 1) to describe the massive presence of R. okamurae unattached thalli on the continental slope of the GoC and semi-quantify their abundances; 2) to analyse the role of the Mediterranean Outflow Water (MOW) in unattached thalli dispersion; 3) to study the overall health condition of the unattached thalli collected from bathyal bottoms of the GoC and their survival capacity after a long incubation in continuous darkness by means of their photosynthetic capacity and growth rate. Moreover, some observations on the accumulation of R. okamurae thalli on typical deep-sea benthic invertebrate species were reported. We hypothesize that some tidal cross-shore currents and, finally, the MOW acts as an effective transport of photosynthetically active unattached R. okamurae thalli from shallow coastal areas to deep-sea bottoms, which could potentially reach other distant coastal locations and expand its invasion area.
MATERIAL AND METHODS
Presence of Rugulopteryx okamurae unattached thalli on the continental slope of the Gulf of Cádiz
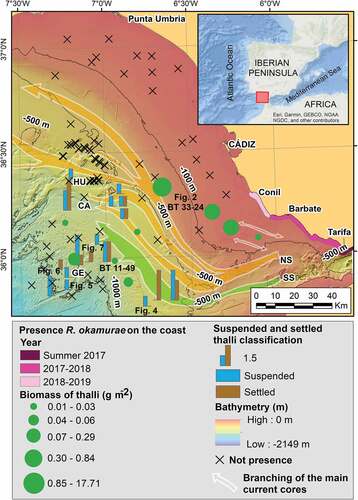
To study the presence, distribution and abundance of R. okamurae thalli on the continental slope of the GoC (South-western Iberian Peninsula), we analysed underwater imagery and samples obtained over three oceanographic expeditions carried out in the GoC in 2019–2021: INPULSE_0719 (9 July–5 August 2019, R/V Ángeles Alvariño), CIRCASUR 2020 (23 October–5 November 2020, R/V Ramón Margalef) and INTEMARES A4 CAD (6 April–20 April 2021, R/V Ramón Margalef). A total of 101 stations (located between 35 and 1141 m deep) were explored, covering a surface of c. 13,216 km2 ().
Fig. 1. Map displaying the main deep currents and seafloor geomorphology of the Gulf of Cádiz, together with the coastal areas where Rugulopteryx okamurae has been detected from 2017 to 2019 according to García-Gómez et al. (Citation2020), and sampling stations where R. okamurae was detected in the oceanographic expeditions of this study (including quantitative and semi-quantitative annotations using a rank system). Suspended thalli classification (1, 1.0–4.9; 2, 4.9–9.9; 3, 9.9–30 thalli min–1); Settled thalli classification (1, 1.0%–14.9%; 2, 15.0%–29.9%; 3, 30.0%–74.9% of bottom coverage). References to figures are indicated on the map. The northern Spartel channel (NS), the southern Spartel channel (SS), the Cádiz channel (CA), the Huelva channel (HU) and the Gil Eanes channel (GE) are shown on the map. Black crosses indicate absence of R. okamurae. The digital bathymetric Model data products have been derived from the EMODnet Bathymetry portal – http://www.emodnet-bathymetry.eu. The main deep currents branches were obtained from Sánchez-Leal et al. (Citation2017).
High-resolution seafloor videos were filmed in 21 of the total 101 stations with acoustically tracked photogrammetric sleds (HORUS and TASIFE). Sleds operated 0.5–2.5 m above the seafloor for 10–20 min, covering track distances of 180–260 m. Records of R. okamurae thalli were quantified using laser pointers for scaling 7.5 cm (HORUS) and 10 cm (TASIFE) separation. Following the methodology recently used in other studies (de la Torriente et al. Citation2018; Urra et al. Citation2021), videos from each station were split into one-minute clips, with a total of 115 clips analysed. These 1-min samples were treated as different samples. The R. okamurae thalli appeared both in suspension and carried away by the bottom currents and/or deposited at the bottom. For each one-minute clip, the number of suspended thalli (thalli min–1) and percentage of bottom covered by settled thalli were recorded. The percentage of bottom cover was estimated by extracting frames from clips in which settled thalli were present. These frames were scaled using the laser points of the photogrammetric sleds and the percentage of bottom cover was calculated by overlaying a grid (Babb et al. Citation2021). This procedure was carried out with the software Image J (Schneider et al. Citation2012). Using the mean values, a semi-quantitative rank was established to indicate the abundance of suspended thalli (1, 1.0–4.9; 2, 4.9–9.9; 3, 9.9–30 thalli min–1) and settled thalli (1, 1.0%–14.9%; 2, 15.0%–29.9%; 3, 30.0%–74.9% of bottom coverage) at each station.
Direct samples of the seabed were also collected during CIRCASUR 2020, with a beam trawl (), which has a 2-m horizontal opening, a 0.6-m vertical opening and a mesh size of 10 mm. The sampling unit consisted of 15-min hauls at a speed of 2–3 knots, covering a trawled distance of generally c. 1,000 m and resulting in a sampling area of c. 2,000 m2 (width of the beam trawl multiplied by the trawled distance). A total of 80 beam trawl samples were taken. In those samples, R. okamurae thalli were weighed and the biomass was corrected to the area sampled by the beam trawl (g m–2). With all this information, we generated a distribution map of unattached R. okamurae thalli in the continental slope of the GoC, in order to know those seafloor areas and geomorphologic structures in which they spread through and/or accumulate, as well as to analyse the potential influence of the MOW on the distribution of the detected unattached thalli ().
Figs 2–7. Information about sample collection, morphology and accumulations of Rugulopteryx okamurae thalli (black arrows) on the deep-sea floor and on benthic fauna.

Health condition and survival experiment
During the CIRCASUR 2020 oceanographic expedition, several unattached R. okamurae thalli were collected from two beam trawl samples located at 258 m (BT 33-24; 36°19.92ʹN, 6°39.90ʹW) and 823 m depth (BT 11-49; 35°59.16ʹN, 6°57.12ʹW) (). The collected thalli, which were visually healthy and free from macroscopic epibiota (), were maintained in tanks under very low irradiance conditions (<5 μmol photons m–2 s–1) and continuous flow of water collected from the ocean surface during the expedition. The origin of these thalli is difficult to know because R. okamurae is established along the coasts from Conil to Algeciras (). However, the healthy appearance of these thalli seems to indicate that they were in the sea bottom for a short time. Taking into account the maximum and minimum velocity of the MOW (1.0 and 0.21 m s–1, respectively) and the tidal cross-shore currents, we estimated that the thalli could have travelled from the coast during 2–9.5 d for those thalli collected at 258 m depth and during 2.3–11 d for those thalli collected at 823 m depth. At the end of the expedition, thalli were transported to the laboratory under similar conditions as during the expedition. In order to study the health condition and further survival capacity of these unattached thalli collected from deep-sea bottoms of the GoC (258 and 823 m), their photosynthetic activity and growth rate was analysed during 74 d of incubation in the laboratory under either coastal light conditions or continuous darkness. For this, several independent thalli of R. okamurae, selected from the stock obtained during the CIRCASUR 2020 expedition, were incubated in five-litre tanks (weighing about 5 g in total per tank) in a controlled-temperature chamber (EG701 Equitec) at 19°C, either at an irradiance of 70 μmol photons m–2 s–1 provided by white LED-lights with a photoperiod of 12:12 h light:dark (coastal light conditions), or in continuous darkness (0:24 h light:dark photoperiod). Three tanks were used for each condition, and all of them possessed continuous aeration of the medium. Filtered natural seawater was used for these incubations, which was renewed every week. This temperature was chosen based on the average temperature of the surface seawater used to maintain the thalli onboard during the two-week expedition, because they could only be acclimated to this temperature during the expedition and we did not want to further stress the thalli. The photoperiod used for the light treatment corresponded to the photoperiod of our latitude in October, and the irradiance used for the light treatment represented the saturating irradiance for photosynthesis based on preliminary rapid light curves carried out with a pulse amplitude modulated (PAM) fluorometer in some of the collected thalli, as explained below.
The photosynthetic activity of the thalli after 41 and 74 d of incubation at each condition (coastal light or continuous dark) was estimated by chlorophyll fluorescence measurements using a PAM fluorometer (Junior-PAM, Walz, Effeltrich, Germany).
Three different parameters were calculated for estimating the photosynthetic activity of the thalli: maximum electron transport rate (ETRmax), the light requirement for saturating ETR (Ek) and the optimal quantum yield for photosystem II (PSII) fluorescence (Fv/Fm). Fv/Fm was measured after 15 min of incubation in darkness, as described by Schreiber et al. (Citation1986). Immediately afterwards, rapid light curves were measured using the same device, where the effective quantum yield of PSII (ФPSII = ΔF/Fm´) was estimated for 8 different actinic (blue) light irradiances. The relative electron transport rate between PSII and PSI (ETR) at each irradiance was calculated as:
where 0.5 stands for the assumption of equal contribution of excitons from PSI and PSII. ETR versus irradiance curves were fitted to the nonlinear least squares regression model by Eilers & Peeters (Citation1988) using the Solver function of Excel (Microsoft, Redmond, USA), to obtain the photosynthetic parameters analysed (ETRmax and Ek).
Relative growth rate (RGR; % d–1) of the thalli after 74 d of incubation at each condition (coastal light or continuous dark) was calculated according to Lüning (Citation1990), using the equation:
where FW1 and FW2 represent the fresh weight in grams at initial (T1) and final (T2) times.
Differences between photosynthetic parameters and growth rate under coastal light and continuous dark conditions were analysed by using a one-way ANOVA. Normality of the data and equality of variances across comparison groups were checked a priori. When these assumptions were not realized, data were square-root transformed. The analyses were done using R v4.2.1 (R Core Team Citation2020).
RESULTS
Presence of unattached thalli of Rugulopteryx okamurae on the continental slope of the Gulf of Cádiz
Using the combined information gathered from the underwater videos and beam trawl samples, it was possible to estimate the magnitude of the transport and accumulation rates of R. okamurae thalli on the seafloor. Unattached thalli were detected between 35 and 1141 m depth on the shelf (circalittoral level) and continental slope (bathyal level) of the GoC and up to 120 km offshore. These thalli appeared both suspended and/or deposited on the seafloor in different areas, including turbiditic channels extending along the continental slope, contouritic channels (i.e. northern and southern Spartel channels at the SoG; Huelva, Cádiz and Gil Eanes channels further away) and other adjacent areas (). The observed distribution of R. okamurae followed the MOW pathway (). The contouritic channels convey a huge freight of seaweed debris into the deeper GoC (between 1.7 ± 2.2 and 27.7 ± 27.2 thalli min–1), being the deeper and southern channels the most active ones with maximum rates of transported thalli of 42.6 ± 33.6 thalli min–1 (; supple-mentary video). Regarding R. okamurae thalli collected with beam trawl, the highest biomass accumulations were found in some shelf areas close to the SoG coastline as well as on the head of a turbiditic channel at 258 m depth (up to 17.7 g m–2; ). The occurrence of R. okamurae accumulations on the continental slope seems to be related to currents and contouritic channels, so that, outside the main flows, no R. okamurae thalli were detected (). The highest accumulations were located on the banks of the most active contouritic channels, with values of R. okamurae bottom coverage ranging between 65% and 90% (; supplementary video). In these areas, R. okamurae thalli probably settled due to a reduction of the bottom current speed or due to the presence of obstacles. In some cases, a large number of R. okamurae thalli hooked to common bathyal invertebrates of the GoC, such as the sea-urchin Cidaris cidaris (Linnaeus) (), the sea anemone Actinauge richardi (Marion), different types of cerianthiids (supplementary video), the soft bottom fragile octocorals Isidella elongata (Esper) () and Radicipes gracilis (Verrill) (supplementary video), the hexactinellid sponge Pheronema carpenteri (Thomson) (supplementary video), as well as other hard bottom gorgonians (Acanthogorgia spp) and sponges (Phakelia spp) ().
Health condition and survival experiment
Chlorophyll a fluorescence measurements revealed maximum quantum yield (Fv/Fm) values of 0.72–0.76 () for R. okamurae thalli after 74 d of cultivation under either dark or light conditions, which are within the optimum range for brown seaweeds (0.7–0.79; see Celis-Plá et al. Citation2015; Iñiguez et al. Citation2016a, Citationb), indicating that thalli from both conditions were overall healthy. In addition, rapid light curves indicated similar values of maximum electron transport rate (ETRmax) and saturating irradiance (Ek) for thalli incubated under light (70 µmol photons m–2 s–1; 12:12 h photoperiod) or continuous dark conditions, either after 41 or 74 d of incubation ().
Table 1. Photosynthetic parameters calculated from Chlorophyll a fluorescence measurements (mean ± standard deviation, n = 6) of Rugulopteryx okamurae after 41 and 74 d of culture under either light (70 µmol photons m–2 s–1; 12:12 h photoperiod) or continuous dark conditions. No significant differences were found between light and dark conditions (p < 0.05). R > 0.99 was obtained for all curve fittings.
Relative growth rates between thalli incubated under light (70 µmol photons m–2 s–1; 12:12 h photoperiod) or continuous dark conditions were significantly different, with a positive growth rate of 1.45% ± 0.59% d–1 for those thalli cultured under light conditions and a slightly negative growth rate of –0.34% ± 0.29% d–1 for those thalli maintained under complete darkness.
DISCUSSION
This study constitutes the first direct evidence of a massive presence of R. okamurae on deep-sea bottoms. The Mediterranean Outflow Water (MOW) seems to be the main spread vector of these unattached thalli in the bathyal areas of the Gulf of Cádiz (GoC) basin. Moreover, laboratory experiments of the present study demonstrated that these unattached thalli flowing through the bathyal seafloor were alive and in an overall healthy condition, as demonstrated by the high Fv/Fm values after long periods of incubation in continuous darkness (74 d at 19°C), thus keeping their invasive capacity. In fact, these Fv/Fm values were even significantly higher than those reported by Figueroa et al. (Citation2020) for coastal free-floating thalli (2 m depth; Fv/Fm of 0.65) and attached thalli (0.5 m depth; Fv/Fm of 0.5) of R. okamurae collected in Tarifa (Cádiz), which might be related to the photochemical relaxation experienced by the unattached thalli during their transport to deep-sea bottoms under complete darkness. In contrast, ETRmax and Ek values are much lower than those reported by Figueroa et al. (Citation2020) for intertidal free-floating and attached thalli of R. okamurae due to acclimation to a significantly lower dose of radiation in the laboratory than those experienced in intertidal and shallow coastal areas. The north-eastern Gulf of Cádiz has large river inputs, high primary production and low water transparencies, with a photic depth (with at least 1% of surficial irradiance) between 30 and 60 m in areas located near the coast and 80 to 100 m in offshore areas (Navarro et al. Citation2006; García La Fuente & Ruiz Citation2007). Therefore, R. okamurae unattached thalli have been exposed to almost zero irradiance during their transport by currents from the infralittoral to the bathyal zone of the Gulf of Cádiz.
Although the seawater temperature of the MOW branches ranges between 12.9 and 14.0°C (Bellanco & Sánchez-Leal Citation2016; Sánchez-Leal et al. Citation2017), we decided to culture the thalli during the laboratory experiments at the surface seawater temperature (19°C), because they were already acclimated to this temperature during the expedition and we did not want to further stress the thalli. In addition, this is also the average temperature for coastal waters at this latitude and time of the year, and we wanted to know if the thalli would be able to survive and grow after arriving to coastal waters, which has been demonstrated by our results. Moreover, if the thalli were able to survive during 74 d under complete darkness at 19°C, it is expected that they would be able to survive even longer at 12–14°C (range of MOW water temperature), given that lower temperatures generally reduced the algal metabolic requirements under complete darkness (i.e. reduction in respiratory rates; see Dunton & Schell Citation1986; Peters & Thomas Citation1996; Borum et al. Citation2002; Li et al. Citation2020). In addition, other temperate Dictyotales like Dictyota dichotoma (Hudson) J.V. Lamouroux have shown maximal survival rates close to 90% after more than 6 months at 8°C (Bogaert et al. Citation2016), indicating that low temperatures allow their long-term cultivation due to a reduced metabolic activity.
The deepest unattached R. okamurae thalli occur along the main GoC contourite channels (). These channels are the main conduits that steer the MOW once flowing westwards the Strait of Gibraltar (SoG) (Sánchez-Leal et al. Citation2017). The spatial coincidence () suggests that the MOW undercurrent is steering the westward spread of R. okamurae. The suggested mechanism is through thalli resuspension and dragging by the energetic bottom currents. These would include tidal cross-shore currents from the shallower grounds near the SoG and along-slope gravity currents below (Sánchez-Leal et al. Citation2017). Macroalgal fragments would drift towards less energetic areas, usually flat areas, where they might form large accumulations (; supplementary video).
The rapid spread of R. okamurae along the southern coast of Spain is unprecedented in Europe. Its success seems to be related with their main strategy for local expansion, through floating unattached thalli which are swept away by currents (CAGPDS Citation2019; Altamirano et al. Citation2019). Other invasive species belonging to the genera Caulerpa, Asparagopsis or Gracilaria also have this high propagation ability with the help of detached mats or fragments (Ceccherelli & Cinelli Citation1999; Klein & Verlaque Citation2008; Cecere et al. Citation2011). Nevertheless, only Gracilaria vermiculophylla in the North Atlantic American coasts probably had a comparable invasion success to R. okamurae (Krueger-Hadfield et al. Citation2016), and, in the case of G. vermiculophylla, it was also related to its free-living lifestyle. In addition, Gracilaria species are also able to reproduce vegetatively through thallus fragmentation (Kain & Destombe Citation1995). These free-floating thalli can grow indefinitely and propagate naturally without holdfasts (Krueger-Hadfield et al. Citation2016). This is somewhat coincident with the laboratory experiments of the present study using unattached R. okamurae thalli collected at bathyal depths (more than 800 m) which were alive and healthy, maintaining an active growth and production of propagules under light conditions. Although the thalli biomass exposed to continuous dark conditions slowly decreased in time at a rate of 0.34% ± 0.29% d–1, chlorophyll a fluorescence data indicate that their photosynthetic capacity was unaltered after 74 d of incubation, since ETRmax and Ek values were not significantly different between thalli incubated at coastal light conditions and those incubated under complete darkness. In a recent study, even a slight biomass increase was detected in R. okamurae during a dark period of 20 d (Rosas-Guerrero et al. Citation2018). In addition, Rosas-Guerrero et al. (Citation2018) reported a survival rate of 80%–100% when those thalli exposed to 20 d of continuous dark conditions were transferred to light conditions, independently of the temperature and time elapsed since the abandonment of the dark conditions. Therefore, it appears that the invasion success of R. okamurae is related to the ability of their unattached thalli to maintain 1) a high reproductive potential through vegetative (propagules) and asexual (mitotic spores) strategies (CAGPDS Citation2019; Altamirano et al. Citation2019; Figueroa et al. Citation2020), and 2) an intact photosynthetic capacity even after long periods of darkness (this study).
The observations of the present study reveal an active transport of huge quantities of detached R. okamurae thalli driven by the MOW towards bathyal areas of the GoC. In addition, the survival capacity shown by the thalli collected at bathyal depths of the GoC indicates that these thalli retain their huge invasive potential and that the MOW may provide a source of its dispersion. The MOW can spread R. okamurae thalli throughout the south-western Iberian margin at a high rate. Considering a current velocity range between 1 and 0.21 m s–1 (Sánchez-Leal et al. Citation2017), thalli detached near the SoG may travel about 200 km (corresponding to the deepest point at which R. okamurae thalli were collected for the survival experiment) in 2.3–11 d. Therefore, the possibilities of R. okamurae expansion to other regions by deep dragged thalli are potentially high and raise concern. A recent study based on species distribution modelling showed that all the Mediterranean countries are very favourable areas for R. okamurae proliferation, but also the Atlantic coast of Morocco, Western Sahara, Portugal and France, including some areas of Ireland or Norway (Muñóz et al. Citation2019). However, R. okamurae has been recently reported not only close to its expansion area in Morocco, but also far away from it, in the Azores Islands (NE Atlantic) (El Aamri et al. Citation2018; Faria et al. Citation2022). Faria et al. (Citation2022) attributed the R. okamurae introduction in Azores Islands to human-assisted transport, via ballast waters and/or fouling (inlays in boat hulls). However, the drag transport of unattached thalli driven by the MOW linked to commercial transport and to recreational and professional fishing (thalli harvested with trawls or hooked to longlines or trammel nets) could also facilitate the arrival of R. okamurae to some of these areas through the different MOW branches, contouring the European Margin or flowing southwards to North Africa (Llave et al. Citation2019).
Macroalgae are the top primary producers in coastal systems and macroalgal carbon may be widespread, extending from shallow to deep-sea sediments, across a broad range of depths into the sediment, from surface and subsurface layers down to a hundred meters into the sediment (reviewed in Krause-Jensen & Duarte Citation2016). The preservation potential of macroalgal carbon in sediments depends on the liability of the organic carbon, which varies between species (Trevathan-Tackett et al. Citation2015), but it can represent a significant contribution to deep-sea carbon sequestration (Krause-Jensen & Duarte Citation2016). Some studies have shown that macroalga and seagrass debris may play an important role in fuelling deep-sea benthic communities and, potentially, enhance secondary production of commercial species (Wolff Citation1976; Suchanek et al. Citation1985; Ramirez-Llodra et al. Citation2016). The present study has provided evidence of a significant transport of R. okamurae thalli from the shelf to the slope but could not provide estimates of the carbon input and further sequestration. Further studies should quantify this carbon input, its potential sequestration in marine sediments and its consumption by secondary producers in order to know the role of this new organic carbon source for the deep-sea ecosystems of the Gulf of Cádiz.
The suspended thalli transported by the MOW through the GoC contourite channels may get entangled around obstacles such as rocks and marine litter, but also sessile and mobile benthic invertebrates. Several deep-sea habitats and associated species displayed large quantities of entangled R. okamurae thalli in the underwater images of the present study. Some of these species are very sensitive to physical impacts (e.g. friction, abrasion) such as the delicate and rigid soft bottom octocorals Isidella elongata and Radicipes gracilis, followed by other species that may display a stronger resilience to physical impacts such as the flexible hard bottom gorgonians Swiftia dubia (Thomson) and Acanthogorgia hirsuta (Gray), and the sponges P. carpenteri or Phakelia spp (; supplementary video). Moreover, some of them are included in conservation lists because of the decline that they have experienced in the last decades due to the increasing human impacts in the deep sea, especially from bottom-trawling. In fact, the bamboo coral I. elongata was included in 1995 in Annex II of the List of endangered or threatened species of the Barcelona Convention, and it is catalogued as Critically Endangered with a decreasing population by the IUCN Red List. The high amount of R. okamurae thalli that was detected hooked to several I. elongata colonies may harm them because of the slow growth and fragility of this species, especially its apical parts where polyps generally occur. The implications of this finding are yet to be discovered, but in the recent INTEMARES A4 CAD expedition (April 2021), many I. elongata colonies filmed in deep slope bottoms of the GoC with no bottom trawling activity had their apical parts damaged or were totally missing, with a large number of colonies mutilated and pulled out of the substrate and lying in the direction of the bottom current. This could probably be related to the weight and current resistance generated by the accumulation of R. okamurae thalli on them, as detected in previous expeditions (; supplementary video). In addition to this physical damage, the decomposition of thalli can release molecules that are toxic to the invertebrates on which they attach or those that might feed on them. There are several bioactive metabolites of the terpenoid class in R. okamurae with toxic effects against predation, ranging from deterrent activity, inhibition of larval settlement to even lethal effects (Kurata et al. Citation1989; Suzuki et al. Citation2002; Casal-Porras et al. Citation2021). The present study provides evidence of R. okamurae attached to sea urchins, sea anemones, sponges and gorgonians from the deep-sea bottoms and, taking into account the toxicity of their secondary metabolites, presumable negative effects could be produced on these animals. Therefore, future research should be focused on R. okamurae impacts not only on neritic but also on bathyal ecosystems.
AUTHOR CONTRIBUTIONS
AMR, CI, LMFS, RFSL and JLR contributed to conceptualization and design of the article. AMR, CI, LMFS, RFSL, CF, MJB, JG and JLR contributed to acquisition of the data. AMR, CI, LMFS, RFSL and JLR contributed to analysis and interpretation of the data. All authors contributed to the writing of the article and approved the final version.
Supplemental Material
Download MS Word (17.8 KB)Supplemental Material
Download MP4 Video (88.7 MB)ACKNOWLEDGEMENTS
We would like to thank the captains and all the crews of RV Ramón Margalef and RV Ángeles Alvariño, as well as other colleagues during the INPULSE 0719, CIRCASUR 2020 and INTEMARES A4 CAD expeditions.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00318884.2023.2177057
DISCLOSURE STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
REFERENCES
- Altamirano M., De la Rosa J., Carmona R., Zanolla M. & Muñoz A.-R. 2019. Macroalgas invasoras en las costas andaluzas. Algas 55e: 10–13. ISSN:1695-8160.
- Altamirano M., De la Rosa J., Kawai H., Hanyuda T., Carmona R., Zanolla M. & Muñoz A.-R. 2021. Cryptic introduction and invasion of Rugulopteryx okamurae (Dictyotales, Ochrophyta) at the Strait of Gibraltar: a real threat to the Mediterranean ecosystems. 12th International Phycological Congress (IPC2021), Chile 22–26 March 2021, abstract, p. 17.
- Anton A., Geraldi N., Lovelock C., Apostololaki E.T., Bennett S., Cebrian J., Krause-Jensen D., Marbà N., Martinetto P., Pandolfi M.J. et al. 2019. Global ecological impacts of marine exotic species. Nature Ecology & Evoution 3: 787–800. DOI: 10.1038/s41559-019-0851-0.
- Babb I., Conroy C., Govert N., Schneeberger C., Walton O., Auster P. & Zajac T. 2021. Preliminary map products from an ecological characterization of Eastern Long Island sound and Fishers Island sound regions: Long Island sound cable fund phase II survey area. Technical Report. 42 pp. DOI: 10.13140/RG.2.2.16488.60166.
- Bellanco M.J. & Sánchez-Leal R. 2016. Spatial distribution and intra-annual variability of water masses on the Eastern Gulf of Cadiz seabed. Continental Shelf Research 128: 26–35. DOI: 10.1016/j.csr.2016.09.001.
- Bogaert K., Beeckman T. & De Clerck O. 2016. Abiotic regulation of growth and fertility in the sporophyte of Dictyota dichotoma (Hudson) J.V. Lamouroux (Dictyotales, Phaeophyceae). Journal of Applied Phycology 28: 2915–2924. DOI: 10.1007/s10811-016-0801-z.
- Borum J., Pedersen M., Krause-Jensen D., Christensen P.B. & Nielsen K. 2002. Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord, NE Greenland. Marine Biology 141: 11–19−.
- CAGPDS 2019. Programa de gestión sostenible del medio marino. Consejeria de Agricultura, Ganaderia, Pesca y Desarrollo Sostenible, Informe Regional 2019, Sevilla, Spain. 151 pp. https://www.juntadeandalucia.es/medioambiente/portal/documents/20151/3227240/informe_anual_medio_marino_2019.pdf/eed625c9-1488-292b-c607-bb282a2ee83e?t=1606215965664
- Casal-Porras I., Zubía E. & Brun F.G. 2021. Dilkamural: a novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuarine, Coastal & Shelf Science 257: Article 107398. DOI: 10.1016/j.ecss.2021.107398.
- Ceccherelli G. & Cinelli F. 1999. The role of vegetative fragmentation in dispersal of the invasive alga Caulerpa taxifolia in the Mediterranean. Marine Ecology Progress Series 182: 299–303. DOI: 10.3354/meps182299.
- Cecere E., Petrocelli A. & Verlaque M. 2011. Vegetative reproduction by multicellular propagules in Rhodophyta: an overview. Marine Ecology 32: 419–437. DOI: 10.1111/j.1439-0485.2011.00448.x.
- Celis-Plá P.S.M., Hall-Spencer J.M., Horta P.A., Milazzo M., Korbee N., Cornwall C.E. & Figueroa F.L. 2015. Macroalgal responses to ocean acidification depend on nutrient and light levels. Frontiers in Marine Science 2: Article 26.
- de la Torriente A., Serrano A., Fernández-Salas L.M., García M. & Aguilar R. 2018. Identifying epibenthic habitats on the Seco de los Olivos Seamount: species assemblages and environmental characteristics. Deep-Sea Research I 135: 9–22. DOI: 10.1016/j.dsr.2018.03.01.
- Dunton K.H. & Schell D.M. 1986. Seasonal carbon budget and growth of Laminaria solidungula in the Alaskan High Arctic. Marine Ecology Progress Series 31: 57–66−.
- Eilers P.H.C. & Peeters J.C.H. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Model 42: 199–215. DOI:10.1016/0304-3800(88)90057-9.
- El Aamri F., Idhalla M. & Tamsouri M.N. 2018. Occurrence of the invasive brown seaweed Rugulopteryx okamurae (E.Y.Dawson) I.K.Hwang, W.J.Lee & H.S.Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). Mediterranean Fisheries and Aquaculture Research 1: 92–96.
- Faria J., Prestes A.C.L., Moreu I., Martins G.M., Neto A.I. & Cacabelos E. 2022. Arrival and proliferation of the invasive seaweed Rugulopteryx okamurae in NE Atlantic islands. Botanica Marina 65: 45–50. DOI: 10.1515/bot-2021-0060.
- Figueroa F., Vega J., Gómez-Valderrama M., Korbee N., Mercado J.M., Bañares E. & Flores A. 2020. Invasión de la especie exótica Rugulopteryx okamurae en Andalucía I: estudios preliminares de la actividad fotosintética. Algas 56: 35–46. ISSN: 1695-8160.
- García Lafuente J. & Ruiz J. 2007. The Gulf of Cádiz pelagic ecosystem: a review. Progress in Oceanography 74: 228–251. .
- García-Gómez J.C., Sempere-Valverde J., Ostalé-Valriberas E., Martínez M., Olaya-Ponzone L., Roi A., Espinosa F., Sánchez-Moyano E., Megina C. & Parada J.A. 2018. Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim (Dictyotales, Ochrophyta), alga exótica “explosiva” en el estrecho de Gibraltar. Observaciones preliminares de su distribución e impacto. Almoraima 49: 97–113.
- García-Gómez J.C., Sempere-Valverde J., Roi A., Martínez-Chacón M., Oloya-Ponzone L., Sánchez-Moyano E., Ostalé-Valriberas E. & Megina C. 2020. From exotic to invasive in record time: the extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Science of the Total Environment 704: Article 135408. DOI: 10.1016/j.scitotenv.2019.135408.
- Iñiguez C., Carmona R., Lorenzo M.R., Niell F.X., Wiencke C. & Gordillo F.J.L. 2016a. Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta. Polar Biology 39: 1979–1991. DOI: 10.1007/s00300-015-1724-x.
- Iñiguez C., Carmona R., Lorenzo M.R., Niell F.X., Wiencke C. & Gordillo F.J.L. 2016b. Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula. Marine Biology 163: Article 248. DOI: 10.1007/s00227-016-3024-6.
- Kain J.M. & Destombe C. 1995. A review of the life history, reproduction and phenology of Gracilaria. Journal of Applied Phycology 7: 269–281.
- Klein J. & Verlaque M. 2008. The Caulerpa racemosa invasion: a critical review. Marine Pollution Bulletin 56: 205–225. DOI: 10.1016/j.marpolbul.2007.09.043.
- Krause-Jemsen D. & Duarte M.C. 2016. Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience 9: 737–742. DOI: 10.1038/NGEO2790.
- Krueger-Hadfield S.A., Kollars N.M., Byers J.E., Greig T.W., Hammann M., Murray D.C., Murren C.J., Strand A.E., Terada R., Weinberger F. et al. 2016. Invasion of novel habitats uncouples haplo-diplontic life cycles. Molecular Ecology 25: 3801–3816. DOI: 10.1111/mec.13718.
- Kurata K., Taniguchi K., Shiraishi K. & Suzuki M. 1989. Structures of secospatane-type diterpenes with feeding-deterrent activity from the brown alga Dilophus okamurai. Tetrahedron Letters 30: 1567–1570. DOI: 10.1016/S0040-4039(00)99521.
- Lee I.K. & Kang J.A. 1986. A check list of marine algae in Korea. Korean Journal of Phycology 1: 311–325.
- Li H., Scheschonk L., Heinrich S., Valentin K., Harms L., Glöckner G., Corre E. & Bischof K. 2020. Transcriptomic responses to darkness and the survival strategy of the kelp Saccharina latissima in the early polar night. Frontiers in Marine Sciences 7: Article 592033.
- Llave E., Hernández-Molina F.J., García M., Ercilla G., Roque C., Juan C., Mena A., Preu B., Van Rooij D., Rebesco M. et al. 2019. Contourites along the Iberian continental margins: conceptual and economic implications. Geological Society Special Publication 476: 403–436. DOI: 10.1144/SP476-2017-46.
- Lüning K. 1990. Seaweeds: their environment, biogeography, and ecophysiology. Wiley. WileyInterscience, New York, USA. xiii + 527 pp.
- MITECO 2021. EU non-native organism risk assessment scheme: Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim 2009. Ministerio para transición ecológica y reto demográfico, Spain, and CIRCABC, European Union. https://circabc.europa.eu/sd/a/3ea80df2-56d7-4c1f-80f7-ee8d3e91bb8a/Rugulopteryx_okamurae_final_20210413.pdf
- Muñoz A.R., Martin-Taboada A., De la Rosa J., Carmona R., Zanolla M. & Altamirano M. 2019. La modelación de la distribución de especies como herramienta en la gestión de invasiones biológicas en el medio marino: el caso de Rugulopteryx okamurae (Dictyotaceae, Ochrophyta) en el Mediterráneo. Algas 55: 37–40.
- Navarro G., Ruiz J., Huertas I.E., García C.M., Criado Aldeanueva F. & Echevarría F. 2006. Basin scale structures governing the position of the deep fluorescence maximum in the Gulf of Cádiz. Deep Sea Research II 53: 1261–1281. DOI: 10.1016/j.dsr2.2006.04.013
- Ocaña Ó., Afonso-Carrillo J. & Ballesteros E. 2016. Massive proliferation of a dictyotalean species (Phaeophyceae, Ochrophyta) through the Strait of Gibraltar (research note). Revista de la Academia Canaria de Ciencias 28: 165–170.
- Peters E. & Thomas D.N. 1996. Prolonged darkness and diatom mortality I: marine Antarctic species. Journal of Experimental Marine Biology and Ecology 207: 25–41.
- Ramirez-Llodra E., Rinde E., Hege Gundersen H., Christie H., Fagerli C.W., Fredriksen S., Gitmark J.K., Norling K., Walday M.G. & Norderhaug K.M. 2016. A snap shot of the short-term response of crustaceans to macrophyte detritus in the deep Oslofjord. Scientific Reports 6: Article 23800. DOI: 10.1038/srep23800.
- R Core Team 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Rosas-Guerrero J., Meco Y.E. & Altamirano M. 2018. Could Rugulopteryx okamurae (Dictyotales, Ochrophyta) have been introduced by ballast waters? Algas 54: 52.
- Sánchez-Leal R., Bellanco M.J., Fernández-Salas L.M., García-Lafuente J., Gasser-Rubinat M., González-Pola C., Hernández-Molina F.J., Pelegrí J.L., Peliz A., Relvas P. et al. 2017. The Mediterranean overflow in the Gulf of Cadiz: a rugged journey. Science Advances 3: Article eaao0609. DOI: 10.1126/sciadv.aao0609.
- Schneider C.A., Rasband W.S. & Eliceiri K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675.
- Schreiber U., Schliwa U. & Bilger W. 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research 10: 51–62. DOI: 10.1007/BF00024185.
- Sempere-Valverde J., Ostalé-Valriberas E., Maestre M., González-Aranda R., Bazairi H. & Espinosa F. 2021. Impacts of the non-indigenous seaweed Rugulopteryx okamurae on a Mediterranean coralligenous community (Strait of Gibraltar): the role of long-term monitoring. Ecological Indicators 121: Article 107135. DOI: 10.1016/j.ecolind.2020.107135.
- Silva P.C., Meñez E.G. & Moe R.L. 1987. Catalogue of the benthic marine algae of the Philippines. Smithsonian Contributions to Marine Sciences 27: 1–179. DOI: 10.5479/si.1943667X.27.1.
- Suchanek T.H., Williams S.L., Ogden J.O., Hubbard D.K. & Gill P. 1985. Utilization of shallow-water seagrass detritus by Caribbean deep-sea macrofauna: δ13C evidence. Deep-Sea Research Part A 35: 201–214 . DOI:10.1016/0198-0149(85)90028-7.
- Suzuki M., Yamada H. & Kurata K. 2002. Dictyterpenoids A and B, two novel diterpenoids with feeding-deterrent activity from the brown alga Dilophus okamurae. Journal of Natural Products 65: 121–125. DOI: 10.1021/np010234b.
- Thomsen M.S. 2020. Indiscriminate data aggregation in ecological meta-analysis underestimates impacts of invasive species. Nature Ecology & Evolution 4: 312–314. DOI: 10.1038/s41559-020-1117-6.
- Trevathan-Tackett S., Kelleway J., Macreadie P.I., Beardall J., Ralph P. & Bellgrove A. 2015. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 96: 3043–3057. DOI: 10.1890/15-0149.1.
- Tsiamis K., Azzurro E., Bariche M., Çinar M.E., Crocetta F., De Clerck O., Galil B., Gómez F., Hoffman R., Jensen K.R. et al. 2020. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 794–845. DOI: 10.1002/aqc.3267.
- Urra J., Palomino D., Lozano P., González-García E., Farias C., Mateo-Ramírez Á., Fernández-Salas L.M., López-González N., Vila Y., Orejas C. et al. 2021. Deep-sea habitat characterization using acoustic data and underwater imagery in Gazul mud volcano (Gulf of Cádiz, NE Atlantic). Deep Sea Research Part I 169: Article 103458. DOI: 10.1016/j.dsr.2020.103458.
- Watanabe S., Metaxas A. & Scheibling R.E. 2009. Dispersal potential of the invasive green alga Codium fragile ssp. fragile. Journal of Experimental Marine Biology and Ecology 381: 114–125. DOI:10.1016/j.jembe.2009.09.012.
- Winters G., Beer S., Willette D.A., Viana I.G., Chiquillo K.L., Beca-Carretero P., Villamayor B., Azcárate-García T., Shem-Tov R., Mwabvu B. et al. 2020. The tropical seagrass Halophila stipulacea: reviewing what we know from its native and invasive habitats, alongside identifying knowledge gaps. Frontiers in Marine Science 7: Article 300. DOI: 10.3389/fmars.2020.00300.
- Wolff T. 1976. Utilization of seagrass in the deep sea. Aquatic Botany 2: 161–174. DOI:10.1016/0304-3770(76)90017-6.
- Yoshida T. 1998. Marine algae of Japan. Uchida Rokakuho, Tokyo, Japan. 1222 pp.
- Zenetos A. & Galandi M. 2020. Mediterranean non-indigenous species at the start of the 2020s: recent changes. Marine Biodiversity Records 13: Article 10. DOI:10.1186/s41200-020-00191-4.
