Abstract
Using high-quality longitudinal data on 125,720 singleton live births in Matlab, Bangladesh, we assessed the effects of duration of intervals between pregnancy outcomes on infant and child mortality and how these effects vary over subperiods of infancy and childhood and by the type of outcome that began the interval. Controlling for other correlates of infant and child mortality, we find that shorter intervals are associated with higher mortality. Interval effects are greater if the interval began with a live birth than with another pregnancy outcome. In the first week of the child's life, the effects of short intervals are greater if the sibling born at the beginning of the interval died; after the first month, the effects are greater if that sibling was still alive. Many relationships found are consistent with the maternal depletion hypothesis, and some with sibling competition. Some appear to be due to correlated risks among births to the same mother.
Introduction
There is renewed interest in the effects of pregnancy spacing on health and survival because of continuing high newborn, infant, child, and maternal mortality in a number of less developed countries. This paper is a revised version of one presented in 2005 at a meeting of technical experts convened by the World Health Organization (WHO) to review the evidence on pregnancy spacing and health outcomes (WHO Citation2006).
The relationship between short interbirth intervals and high infant and child mortality has been established in a wide range of populations. (See the systematic literature review and meta-analysis by Rutstein et al. Citation2004.) In addition, several studies—for example, Rutstein (Citation2005) in a cross-country analysis—show that very long intervals (at least 5 years in length) are associated with a slight increase in infant mortality. Most previous studies have considered the intervals between live births and have not taken into account the effects of interpregnancy intervals that begin with pregnancy outcomes other than live births (i.e., miscarriages, induced abortions, and stillbirths, referred to here as non-live births (NLBs)). Nor have they considered whether the effects of these ‘inter-outcome’ intervals vary by the type of pregnancy outcome that began the interval. In the study reported here we investigated the effect on mortality of these inter-outcome intervals by the type of outcomes that began the interval, using a large, high-quality longitudinal dataset from Matlab, Bangladesh, that covers a period of more than 20 years.
In addition, we investigated the following: how the effects of intervals of various lengths vary with the age of the child; the extent to which the ‘effect’ of longer interbirth intervals is a consequence of a NLB between the two births; how short subsequent intervals affect the likelihood of survival of the index child when appropriate attention is given to the reverse causality that can arise because subsequent intervals may be short because the index child died; and whether the effects of short intervals on infant and child mortality are consistent with the maternal depletion hypothesis or with competition (and possibly disease transmission) among closely spaced siblings, or with both these factors.
In the following section we briefly review the reasons why pregnancy spacing might affect infant and child mortality, and what the literature has found about these relationships. We then describe the setting for our study and the data and methods we use in our analyses. The next section presents the results of our analyses of infant and child mortality. The final section discusses their implications and presents our conclusions.
Why pregnancy spacing might affect infant and child mortality
There is limited empirical evidence on the intervening processes through which preceding or subsequent pregnancy intervals influence infant and child mortality. The adverse consequences of a short interval for infant and child survival have been attributed to biological effects related to the ‘maternal depletion syndrome’, or more generally to the woman not having fully recuperated from one pregnancy before supporting the next one. For example, if women become pregnant again before folate restoration is complete, their offspring may be at a higher risk of folate insufficiency throughout the pregnancy, leading to increased risks of neural tube defects, intrauterine growth retardation, and preterm birth (Smits and Essed Citation2001; Zhu Citation2005). Pregnancy (and breastfeeding) can lead to a depletion of protein, energy, and micronutrient reserves, possibly resulting in poorer outcomes for women (e.g., pre-eclampsia and premature rupture of membranes) and their children (e.g., low birth weight, higher rates of mortality) (see Winkvist et al. Citation1994, Citation2000; King Citation2003; Dewey and Cohen Citation2004; Conde-Agudelo et al. Citation2006, Citation2007). The following mechanisms have also been suggested as possible contributors to a detrimental effect of a short preceding interval on infant and child survival: (i) behavioural effects associated with competition between siblings (e.g., competition for parental time or material resources among closely spaced siblings); (ii) the inability (or lack of desire) to give a child adequate attention if his or her birth came sooner than desired; and (iii) disease transmission among closely spaced siblings. Several of these have been discussed extensively in the literature (e.g., DaVanzo et al. Citation1983; National Research Council Citation1989; Miller Citation1991). Some of these mechanisms, for example, maternal depletion, apply to preceding pregnancies regardless of the outcome, though they may depend on the duration of the preceding pregnancy, while others, for example, competition and disease transmission, will only come into play if the preceding child is still alive. Much less attention has been given to why very long intervals might have an adverse effect; see Conde-Agudelo and Belizán Citation2000 for a discussion of this issue.
Relatively little attention has been given to the effects of interpregnancy intervals (the length of time between the end of one pregnancy and the conception of the next pregnancy) and whether they differ by the type of outcome that begins the interval—that is, whether the preceding pregnancy ended in a live birth, stillbirth, miscarriage, or induced abortion. (An exception is Conde-Agudelo et al.'s study (Citation2005) of the effects of post-abortion intervals in Latin America. That study, however, does not compare such effects with those of intervals that began with outcomes other than an abortion, does not distinguish induced from spontaneous abortions, and only considers maternal and perinatal outcomes.) If such distinctions can be made, they can permit further tests of the mechanisms suggested above. For example, because they are shorter, pregnancies that end in a miscarriage or induced abortion should be less depleting than those that end in a full-term birth, while pregnancies that end in a stillbirth may be less depleting than those that end in a live birth because there is no breastfeeding after a stillbirth, and also there is no live child from this previous pregnancy to ‘compete’ for parental resources with the index child. Furthermore, if the effects of intervals differ by the type of previous outcome, such distinctions can enable medical practitioners to tailor to a woman's reproductive history the advice they give her about how long she should wait after one pregnancy before trying to become pregnant again.
There are a number of reasons why there may appear to be a relationship between pregnancy and birth spacing and infant and child mortality when the relationship is not in fact causal. First, holding constant the length of time between a preceding birth and the conception of the index pregnancy, the shorter the duration of the index pregnancy, the shorter will be the interval between births. Since prematurity increases the risk of infant mortality, a shorter gestation of pregnancy could be a reason why a short preceding interbirth interval is related to infant mortality. Miller et al. (Citation1992) control for gestation and find that confounding by prematurity accounts for 20–30 per cent of the excess mortality for infants born after intervals of less than 15 months from the preceding live-birth outcome. Secondly, interbirth intervals may be short when the child born at the beginning of the interval dies because of shorter post-partum amenorrhea as a result of no or shortened breastfeeding or a conscious effort to ‘replace’ the child who died. If certain families have a higher risk of mortality for all of their children, this could lead to a relationship between short intervals and infant mortality. A third possibility is that longer breastfeeding both improves infants’ survival chances and lengthens the intervals following their birth. This could explain a relationship between the length of the subsequent pregnancy interval and the survival of the child born at the beginning of that interval. Fourthly, interbirth intervals may be long because there is an intervening NLB, and the types of women who have NLBs may also be less healthy or give birth to less healthy children. Finally, interbirth intervals may be associated with confounding socio-economic factors associated with both short intervals and high infant and child mortality.
Study setting, data, and methods
Study setting and data
This study used data from Matlab, a typical rural subdistrict of Bangladesh. Our data on pregnancies and their outcomes were collected through the Demographic Surveillance System (DSS) of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). The DSS covers a large population (220,000 people in 2002) and has collected data on pregnancy outcomes in two areas—the ‘Treatment’ (or MCH–FP) Area and the ‘Comparison’ Area. The Comparison Area is typical of much of Bangladesh in contraceptive practice (ICDDR,B Centre for Health and Population Research Citation2000) and fertility (Mitra et al. Citation1994). Since October 1977, the Treatment Area has been exposed to the Maternal Child Health–Family Planning (MCH–FP) intervention of the ICDDR,B, which provides better family planning and health services than the standard government services available to people in the Comparison Area (Bhatia Citation1983; Van Ginneken et al. Citation1998). This has resulted in greater contraceptive use and lower fertility and infant and child mortality in the former area than in the latter (Greenspan Citation1993; LeGrand and Phillips Citation1996). The two areas are otherwise similar.
The DSS data we used to study infant and child mortality contain information on a large number of pregnancies (145,816), births (125,720 live singleton births), and infant and child deaths (12,372 before age 5) that occurred in both areas between 1982 and 2002, a period of considerable demographic change in Bangladesh. Between 1980 and 2000, the total fertility rate in Bangladesh declined from 6.1 children per woman to 3.1, the infant mortality rate decreased from 129 infant deaths per 1,000 live births to 54, and under-5 mortality from 205 under-5 deaths per 1,000 live births to 82 (World Bank Citation2005).
The DSS data on the timing of pregnancy outcomes and of deaths are thought to be of very high quality because they have been collected during regular household visits (every 2 weeks until the late 1990s and every month since then) by well-trained, trusted female community health workers (CHWs) (D'Souza Citation1981; Van Ginneken et al. Citation1998). However, there has been no systematic evaluation of the quality of the data, and, given the sensitive nature of reporting of adverse pregnancy outcomes, it is possible that there was some underreporting of induced abortions and foetal losses within 28 weeks of conception. Our estimates of induced abortions, miscarriages, and stillbirths are slightly higher than those found in a population-based study in India (Pallikadavath and Stones Citation2005). Compared with clinical studies, however, the rates of induced abortion, miscarriage, and stillbirths appear to be underreported in our data compared to those reported by Leridon (Citation1976), though that study is now quite dated.
Our dataset contains information on a number of variables that may affect pregnancy spacing or infant and child mortality, or both, for example, duration of the pregnancy, breastfeeding, age and education of the mother, sex of child, household space (a proxy for the household's economic status), and religion. If not controlled, these could contribute to associations between spacing and mortality.
Dependent variables and the samples used for analyses of them
Our analyses of infant and child mortality considered all singleton live births that occurred between 1982 and 2002 that are documented in the DSS. (Because multiple births have a considerably higher risk of mortality and the influences on their survival may differ from those on singletons, we excluded multiple births (3,043 children in all) from our analyses.) We used the following dependent variables and samples:
| • | Early neonatal mortality: whether a live-born child died in the first week of life. We consider 125,720 live singleton births. Of these, 3,631 (2.9 per cent) died during the first week of life. | ||||
| • | Late neonatal mortality: whether infants who survived the first week of life (n=121,936) died in the subsequent 3 weeks; 1,734 (1.4 per cent) died in this 3-week period. | ||||
| • | Post-neonatal mortality: whether infants who survived the first 4 weeks of life (n=119,718) died before their first birthday; 3,684 (3.1 per cent) died between the end of the fourth week of life and their first birthday. | ||||
| • | Mortality at ages 1–4: whether children who survived until their first birthday (n=110,191) died before their fifth birthday; 3,323 (3.0 per cent) died between their first and fifth birthdays. | ||||
The samples for subperiods after the first are decremented by deaths, migration out of the DSS area, and by censoring by the end of our study period (end of 2002) during the previous subperiod.
Interbirth, inter-outcome, and interpregnancy intervals
As noted above, previous studies have generally considered the preceding interval between births—the interbirth interval—as their measure of spacing. This is probably the appropriate measure to use when considering the effect of competition or disease transmission from another young child in the family. However, the interbirth interval includes the duration of the index pregnancy, which may have its own effect on infant and child mortality. Furthermore, sometimes there is a pregnancy that resulted in an outcome other than a live birth (NLB) between two live births, in which case the interbirth interval will include two (or more) interpregnancy intervals. These concepts are illustrated in the Appendix. Some of the hypotheses about why reproductive spacing may affect infant and child health and survival are related to the interpregnancy interval. For example, it is during the interval between pregnancies (and, for preceding live births, perhaps after the end of intensive breastfeeding) that the woman ‘recuperates’ from the preceding pregnancy. Furthermore, an intervening NLB may reflect something about the mother's health that may affect the health of her children.
We would have liked to include both the duration of gestation of the index pregnancy (because babies born prematurely are more likely to die) and the duration of the interpregnancy interval that precedes it in our analyses. Unfortunately, we did not have gestation data for our full sample, and there were systematic differences between the cases with gestation data and those without them. We had data on pregnancy duration for 64,944 singleton live births, nearly all in the MCH–FP Area. Furthermore, within the MCH–FP Area, the children of the women for whom we did not know the duration of gestation were more likely to die than those for whom we did know duration. Rather than focus only on the selected sample for which we knew the duration of gestation and lose many cases in the process, we used the entire sample. We included the duration of gestation for those for whom we knew it and a missing dummy variable identifying those for whom we did not, and we focused on the inter-outcome interval (IOI)—the number of months between the preceding outcome and the birth of the index child—as our measure of spacing. This approach yielded the same estimate of the effect of gestation duration as if we had considered only the sample for which we knew it, but it enabled us to consider the entire sample for estimating the effects of other explanatory variables. If we had included IOIs and gestation in the models, the estimated effects of IOIs would be the same as the effects of interpregnancy intervals. See the Appendix.
We investigated the effects of seven categories of IOI: less than 15, 15–17, 18–23, 24–35, 36–59, 60–83, and 84 or more months. These categories correspond to those used in previous studies and those considered in the policy debate (e.g., whether to change the recommendation that births should be spaced at least 2 years apart to a recommendation that the optimal interbirth interval (IBI) is 3–5 years) and are groups across which we find differences.
Our large number of observations allowed us to look at narrower distinctions and shorter intervals than those studied by many previous researchers. For example, Cleland and Sathar (Citation1984), Koenig et al. (Citation1990), and Rutstein (Citation2003) used interval groupings of <2 years, 2–3 years, 3–4 years, and 4+ years. Miller et al. (Citation1992) considered shorter intervals, but investigated only a dichotomous distinction of <15 months vs. 15 or more months. Furthermore, through the use of interaction terms, we allowed the effects of intervals to vary by the type of pregnancy outcome that began the interval and, for those that began with a live birth, we distinguished whether the child born at the beginning of the interval was alive at the beginning of the at-risk period under consideration (e.g., at the time of the child's first birthday when we analyse mortality at ages 1–4).
Our sample includes 29,741 first births. First births have been found in previous research (e.g., National Research Council Citation1989) to have poorer outcomes than higher-order births. We compared the risks associated with first births with those associated with short intervals. Over 8 per cent (n=2,706) of the first births in our sample had an associated preceding inter-outcome interval because the birth was preceded by a pregnancy that resulted in a NLB, and many of these were short intervals. Our analyses distinguished cases where the first birth was also the first pregnancy and those where it was not.
For 20,303 of the non-first pregnancies we considered, we do not know the date of the preceding pregnancy outcome (because it occurred before our study period or before the woman migrated into the study area), and hence do not know the length of the preceding IOI. In our multivariate analysis, this group was identified by a ‘missing information’ dichotomous indicator. All remaining births are associated with an IOI, and when a live birth preceded the index child, with an IBI.
The distributions of IOIs by the type of pregnancy outcome that began them and of IBIs are shown in . Looking at the two right-most columns of the table, we see that of IOIs of known duration, 9.8 per cent are less than 15 months long. Because IBIs sometimes contain more than one IOI, the percentage of IBIs of known duration that are less than 15 months in duration is considerably smaller—4.3 per cent. In all, 56.0 per cent of all IOIs of known duration and 49.2 per cent of all IBIs of known duration are less than 36 months in length. Very long intervals (84 months or longer) account for 4.1 per cent of IOIs of known duration and for 5.1 per cent of IBIs of known duration.
Table 1 Frequency distribution (per cent) of durations of inter-outcome intervals (by type of outcome that began the interval) and interbirth intervals. Matlab, Bangladesh, 1982–2002 (n = 125,720).1 (Numbers in parentheses are the percentages of the row totals)
The rest of presents the frequency of IOI duration/outcome-of-previous-pregnancy combinations, and shows that the two are not independent. Short intervals are much more likely to begin with a NLB than are longer ones. The majority (59 per cent) of IOIs of less than 15 months began with a NLB, as did 40 per cent of IOIs of 15–17 months and 18 per cent of those of 18–23 months, but only 5 per cent or less of those of 24 months or longer did. The longer the IOI, the more likely it is to begin with a live birth, particularly if the child survived. IOIs of less than 15 months and of 15–17 months in duration were more likely to begin with the live birth of a child who subsequently died than of one who survived. By contrast, the vast majority of all IOIs of at least 18 months duration began with the live birth of a child who survived.
Methods of estimation
For each of the dependent variables for infant and child mortality, we estimated a Cox proportional hazards model explaining whether the child died during the subperiod under consideration. This technique enabled us to include censored observations in our analyses (e.g., children who were less than 5 years old at the end of our study period or those who migrated out of the study area before the end of the subperiod under consideration). The 125,720 births in our sample occurred to 54,366 women. Using the cluster command in Stata 9.0, all standard errors were adjusted to account for family effects, that is, for the non-independence of births to the same mother.
We conducted sensitivity analyses to assess whether the results change depending on the measure of intervals used and on which other explanatory variables were controlled. For each of our dependent variables, we first estimated an equation that included only interbirth interval duration (IBI) and first parity. Next we considered inter-outcome intervals (IOIs) instead of IBIs. Third, we added a control for pregnancy gestation to see if that changed the IOI effects. Fourth, we allowed the effects of IOIs to differ by the type of pregnancy outcome that began the interval (miscarriage, induced abortion, stillbirth, live birth of a child still alive at the beginning of the at-risk period, live birth of a child who died before the beginning of the at-risk period). Finally, we also controlled for other parities and other explanatory variables. (The additional explanatory variables were mother's age, parity, residence in the MCH–FP [Treatment] Area, mother's education, father's education, religion, household space, month of birth, and calendar year. These were all treated as categorical variables. Some analyses, for the MCH–FP Area only, also controlled for breastfeeding and immunizations; these are described below.) For mortality at ages 1–4 we also assessed the effects of subsequent intervals by including variables indicating whether the woman became pregnant again or had another birth before the index child's first birthday. The hazard model coefficients for the interval and first-parity variables are presented in . Results for all other explanatory variables are available on request.
Table 2(a) Results of Cox proportional hazards models (relative risks (RR) and standard errors (SE)) of first-week mortality: interbirth vs. inter-outcome intervals, without and with controls for duration of gestation, and effects of inter-outcome intervals by type of preceding pregnancy outcome, without and with controls for other explanatory variables. Matlab, Bangladesh, 1982–2002 (n = 125,720)
Table 2(b) Results of Cox proportional hazards models (relative risks (RR) and standard errors (SE)) of late neonatal mortality: interbirth vs. inter-outcome intervals, without and with controls for duration of gestation, and effects of inter-outcome intervals by type of preceding pregnancy outcome, without and with controls for other explanatory variables. Matlab, Bangladesh, 1982–2002 (n=121,936)
Table 2(c) Results of Cox proportional hazards models (relative risks (RR) and standard errors (SE)) of post-neonatal mortality: interbirth vs. inter-outcome intervals, without and with controls for duration of gestation, and effects of inter-outcome intervals by type of preceding pregnancy outcome, without and with controls for other explanatory variables. Matlab, Bangladesh, 1982–2002 (n=119,718)
Table 2(d) Results of Cox proportional hazards models (relative risks (RR) and standard errors (SE)) of child mortality: interbirth vs. inter-outcome intervals, without and with controls for gestation, and effects of inter-outcome intervals by type of preceding pregnancy outcome, without and with controls for other explanatory variables (n=110,191). Matlab, Bangladesh, 1982–2002
Effects of birth and pregnancy spacing on infant and child mortality
Effects of interbirth and inter-outcome intervals and of controlling for gestation of pregnancy on infant and child mortality
In we show the effects of interbirth intervals (IBIs) vs. inter-outcome intervals (IOIs) and assess the effects of controlling for gestation on mortality during four subperiods of infancy and childhood. The results indicated by squares show the mortality risks associated with IBIs of different lengths relative to those 3–5 years long, while those indicated by triangles show IOI effects. These models do not control for other explanatory variables except for first parity. The results indicated by diamonds show IOI effects when duration of pregnancy is also controlled.
Figure 1 Interbirth intervals vs. inter-outcome intervals: how length of preceding interval affects mortality, without and with controls for duration of gestation. Matlab, Bangladesh, 1982–2002
Note: Open symbols indicate that the relative risk is not different from 1.0 at a significance level of p<0.05
Source: Table 2(a)–(d), Columns 1–3
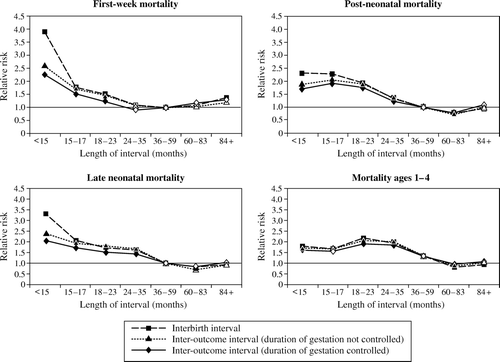
For all four subperiods of infancy and childhood, the relative mortality risks of short intervals (less than 3 years) decrease when the definition of interval changes from IBI to IOI. For example, compared with intervals of 36–59 months, the relative risk of first-week mortality is 3.90 (p<0.001) for IBIs of less than 15 months but 2.62 (p<0.001) for IOIs of less than 15 months. The difference between the effect of short IBIs and short IOIs is largest for neonatal mortality and is still substantial for the post-neonatal period, but it is negligible for mortality at ages 1–4.
Adding a control for duration of pregnancy reduces the size of the effect of short IOIs, but to a relatively small extent, and the effects of short IOIs remain quite large and statistically significant. For example, for babies born after an IOI of less than 15 months, the relative risk of first-week mortality is reduced from 2.62 (p<0.001) to 2.26 (p<0.001) with the addition of the variables measuring the duration of pregnancy. The extent of this reduction is smaller than that found by Miller et al. (Citation1992).
The highest-risk IOI changes as the subperiod of life studied progresses. Considering the model that controls for pregnancy duration, during the neonatal periods the highest risk of mortality is associated with the shortest IOI (<15 months) (RR = 2.26, p<0.001, for early neonatal mortality and RR = 2.05, p<0.001, for late neonatal). During the post-neonatal period, the highest risk is associated with IOIs of 15–17 months (RR = 1.90, p<0.001), while for children aged 1–4, the highest risk occurs for IOIs of 18–23 months (RR = 1.52, p<0.001).
We find a significant detrimental effect of very long IBIs (84+ months) on first-week mortality (RR =1.34, p<0.05). Because nearly 20 per cent of interbirth intervals longer than 7 years in our data include an intervening NLB (DaVanzo et al. Citation2004), it is possible that this may reflect something about the types of women who have NLB outcomes. However, this does not appear to be the case, because we also see significant effects (p<0.10) of IOIs of this length on first-week mortality, even when gestation duration is controlled (RR = 1.27). Very long intervals are not significantly associated with late neonatal or post-neonatal mortality. Very long IBIs are associated with lower child mortality (RR = 0.72, p<0.05), but this effect does not persist when IOIs are considered.
How do effects of inter-outcome intervals vary by the type of outcome of the preceding pregnancy, by interval length, and by subperiod of infancy and childhood?
As noted above, we used interactions to allow the effects of IOIs to differ depending on whether the interval began with the birth of a child who was still alive at the beginning of the at-risk period, the birth of a child who died before the beginning of the at-risk period, a stillbirth, miscarriage, or an induced abortion. We will refer to these as IOIlba, IOIlbd, IOIsb, IOIm, and IOIia, respectively. We consider all of these categories for IOIs of less than 15 months. Because of small cell sizes (see ), we combine the three NLB categories for IOIs of 15–59 months in duration; and for IOIs of 60 months or longer we consider only the distinction between intervals that began with a birth of a child who survived and all other IOIs (‘Not lba’).
In , we show how relative risks for IOIlba and IOIlbd (and Not lba for intervals of 60 months or longer) vary by IOI duration (all relative to IOIlba = 36–59 months) when all of the explanatory variables listed above are controlled. The hazard models underlying these figures and results for intervals that began with NLBs are shown in Column 5 of . (The results for the full models are available on request.) In general, the interval effects change little when the additional variables (beyond first parity and pregnancy duration) are controlled (compare Columns 4 and 5 of ). In the discussion below we note the main effects of the other variables considered in the hazard models and mention the few instances where controlling other explanatory variables substantially changes the interval effects.
Figure 2 Relative risks associated with inter-outcome intervals of various lengths (compared to intervals of 3–5 years following births of children who survived), by type of outcome of preceding pregnancy. Matlab, Bangladesh, 1982–2002
Note: Solid symbols indicate that relative risks are significantly different at p<0.05
Source: Table 2(a)–(d), Column 5
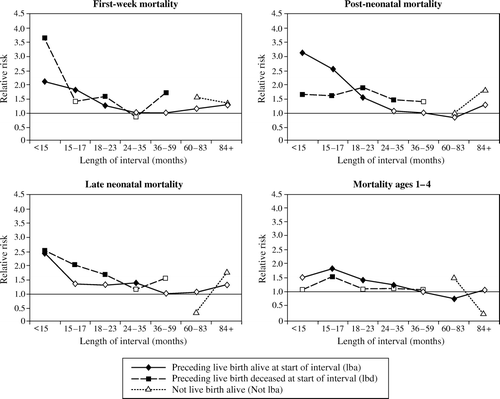
Before proceeding we note that intervals of unknown duration are typically associated with higher mortality (see ), though beyond the first week of life their effects are reduced substantially when other explanatory variables are controlled. This suggests that there is something different (both observed and unobserved) about the cases for which interval durations cannot be calculated.
First-week mortality
The highest risk of first-week mortality is observed for births following the shortest IOIs (<15 months) after live births of infants who then died (RR = 3.64, p<0.001, compared with IOIlba = 36–59 months). Infants born after an IOI of <15 months following the birth of a child who survived (lba) also have a high risk of first-week mortality (RR = 2.11, p<0.001). During this subperiod, IOI effects are generally greater if the child born at the beginning of the interval died than if that child survived, but the difference is statistically significant only for IOI < 15 months. An IOIlba of 15–17 months is also associated with an increased risk of early neonatal mortality compared with one of 36–59 months (RR = 1.83, p<0.01). The risks of first-week mortality for IOIlba fall as the intervals become longer, reaching a minimum at 24–59 months.
Very short intervals (<15 months) following stillbirths and miscarriages are also associated with significantly increased risks of early neonatal mortality (RR = 1.87, p<0.001, and RR = 1.48, p<0.01, respectively) compared with an IOIlba of 36–59 months. IOIs of 15–17, 24–35, and 36–59 months that began with pregnancies that did not result in a live birth are also associated with significantly higher risks of mortality (RR = 1.49, 1.47, and 1.55, respectively, all p<0.05) compared with IOIlba of 36–59 months. The effects of having a preceding pregnancy that did not result in a live birth do not vary much with interval length.
First births that are also first pregnancies also have a higher risk of mortality (RR = 2.07, p<0.001)—one that is virtually the same as that associated with IOIlba < 15 months (RR = 2.11), but lower than that for IOIlbd < 15 months (RR = 3.64). First births that are not first pregnancies also have an elevated, but smaller, risk (RR = 1.54, p<0.001). The difference between the two first-birth coefficients is statistically significant (p<0.01).
Although the results for the other variables are not the focus of this paper, we note that high education of the mother and being born in the spring are associated with lower first-week mortality. Characteristics associated with significantly higher first-week mortality include short gestation period (pregnancy durations of less than 35 weeks compared with those of 36–37 weeks), young maternal age (<20 years), parity ≥ 8, not being Muslim, and being a male child.
Late neonatal mortality
From the start of the second to the end of the fourth week of life, the relative risks of mortality are highest for the shortest intervals (<15 months) that began with a live birth. For these (i.e., IOIlba < 15 months) the risks are larger for late neonatal mortality than for first-week mortality. The effects on late neonatal mortality of intervals that began with live births are somewhat smaller (especially for lbd) when other variables are controlled. IOI effects on late neonatal mortality are generally greater if the child born at the beginning of the interval died than if that child survived, but the differences are not statistically significant.
Late neonatal mortality is higher (p<0.10) following very short intervals that began with a miscarriage (RR = 1.41) or induced abortion (RR = 1.78) than an IOIlba of 36–59 months.
Babies born after an IOIlba of 24–35 months have a 37 per cent increased risk of mortality (p<0.01) during the late neonatal period relative to births preceded by an IOIlba of 36–59 months. Risks of late neonatal mortality for IOIlba are lowest for intervals of 36–83 months.
IOIs of 18–23 and 36–59 months that began with pregnancies that did not result in a live birth are also associated with a higher risk of mortality than an IOIlba of 36–59 months (RR = 1.59 and 1.82, respectively, both p<0.10).
First births that are first pregnancies have high risks during this period (RR = 2.05, p<0.001), but the effect of first births that are not first pregnancies is not statistically significant (though the effects of the IOIs that precede these also need to be considered).
The effects of the other explanatory variables are similar to those observed for first-week mortality. Higher levels of mother's and father's education are protective against late-neonatal mortality, while a short gestation period (pregnancies shorter than 35 weeks compared with those 36–37 weeks long), young age of mother (<18 years), and not being Muslim are associated with significantly higher risks of mortality during this period. In addition, a December birth and being born between 1982 and 1991 (rather than between 1992 and 2002) are also associated with higher late neonatal mortality.
Post-neonatal mortality
During the post-neonatal period (between the 5th and 52nd week of life), mortality is highest after IOIlba < 15 months (RR = 3.14, p<0.001, a relative risk that is higher than for neonatal mortality) and declines nearly monotonically as the duration of IOIlba increases, reaching a minimum at 24–83 months. (The risks for 24–35, 36–59, and 60–83 months do not differ significantly from each other.) Very long intervals (84+ months) that began with the birth of a child who survived are associated with an increase in mortality (RR = 1.28, p<0.10, compared with IOIlba = 36–59 months).
Intervals that began with the birth of a child who then died are also associated with increased risks of post-neonatal mortality. The effects of IOIlbd are smaller than those of IOIlba for intervals of less than 18 months in duration, but the opposite is true for longer intervals. The difference is statistically significant (p<0.05) for IOI = 24–35 months.
Very short IOIs that began with a stillbirth also have a higher risk of post-neonatal mortality (RR = 1.53, p<0.05) compared with IOIlba = 35–59 months, and IOInlb = 15–17 months is associated with significantly higher post-neonatal mortality (RR = 1.41, p<0.05).
During the post-neonatal subperiod, many of the explanatory variables mentioned above still have significant effects on mortality (first births that are first pregnancies, mother's age, gestation duration, and mother's education). In addition, household space (an indicator of socio-economic status) begins to make a difference, with the lowest quartile associated with increased mortality risk. Being born in April, May, or June is also associated with a lower risk of mortality during the post-neonatal subperiod than being born in December. Religion does not have a significant effect on mortality during this subperiod. Controlling for these additional explanatory variables does not change the IOI effects much.
Mortality at ages 1–4
Between ages 1 and 4, the magnitudes of IOI effects on mortality are generally smaller than they were in the previous subperiods. The risk of mortality at ages 1–4 is highest for IOIlba = 15–17 months (RR = 1.83, p<0.01). Risks are also significantly higher (compared with IOIlba = 36–59 months) for IOIlba durations of less than 15 months (RR = 1.50, p<0.10), 18–23 months (RR = 1.41, p<0.001), and 24–35 months (RR = 1.23, p<0.01). For births following the births of children who survived, mortality risks are lowest for intervals of 60–83 months. The relative risk associated with IOIlba = 60–83 months is significantly lower than that for IOIlba = 36–59 months (RR = 0.75, p<0.05) (and the difference between IOIlba < 15 months and IOIlba = 60–83 months is significant at p<0.01). During this subperiod, for nearly all IOI durations, mortality risk is higher for IOIlba than for the other previous outcome categories. The difference between the effect of IOIlba and IOIlbd is statistically significant (p<0.01) for IOI = 15–17 months.
In this subperiod we also assess the effect of short subsequent intervals. We see that the mother being pregnant again by the beginning of the subperiod (i.e., by the time of the index child's first birthday) substantially increases the index child's risk of mortality at ages 1–4 (RR = 2.31, p<0.001). The relative risk of mortality for those who had a subsequent birth before the index child's first birthday is also increased (RR = 1.34), but this effect is not statistically significant. There were very few women who had already given birth within 1 year of giving birth to the index child. Including variables for a subsequent pregnancy and subsequent birth does not affect the size of the effects of short preceding intervals on mortality.
The magnitudes of the effects of parity, mother's age, and pregnancy duration on child mortality are generally smaller than they are in the previous subperiods, and, of these, only high parity (four or more children) has a statistically significant effect on child mortality. This is probably because these are primarily biological variables, whose effects are greatest shortly after birth. By contrast, the effects of socio-economic factors such as household space and mother's education increase in size compared with previous subperiods. While female newborns have a lower risk of first-week mortality than male newborns (RR = 0.84, p<0.001), female children have a higher risk of mortality at ages 1–4 than male children (RR = 1.49, p<0.001), a finding similar to those of other studies in rural Bangladesh (D'Souza and Chen Citation1980; Chen et al. Citation1981; Fauveau et al. Citation1991). Month of birth is unrelated to mortality at ages 1–4.
Does controlling for breastfeeding and immunizations alter the estimates of the effects of inter-outcome intervals?
In the analyses presented above, we were unable to control for breastfeeding and immunizations because these variables were not available for the full DSS sample. To the extent that these variables are correlated with lengths of intervals, their exclusion may bias the effects of the intervals. We have conducted analyses only for the MCH–FP Area, for which these variables are available, that include these variables and allow for the fact that they are time-varying characteristics; we did so in such a way that the estimates would not be biased by the possibility of reverse causation (by measuring these variables at a time before the exposure interval began; see DaVanzo et al. Citation2004 for details). We found that breastfeeding (but not immunizations) during the first year of life did indeed significantly reduce infant and child mortality, but that the sizes of the effects of short intervals on infant and child mortality barely changed when these additional variables were controlled. These results can be seen in DaVanzo et al. (Citation2004).
Discussion and conclusions
Why are short intervals associated with higher mortality?
Our results shed some light on the reasons why short intervals are associated with higher infant and child mortality. Some, but very little, of the effect is explained by the fact that shorter interbirth and inter-outcome intervals are associated with shorter gestations of pregnancy. Also, as the child ages, a very small portion of some of the effects of short inter-outcome intervals seen in bivariate analyses is explained by socio-economic factors associated with both short intervals and higher risks of mortality, but this rarely affects the statistical significance of the relationships. Some of our analyses have also controlled for breastfeeding and immunizations—variables that have been suggested as possible reasons for the effects of short intervals—but the sizes of the effects of short intervals barely change when these additional variables are controlled. The effects of short intervals typically continue to be sizable and statistically significant when all of these other variables are controlled.
Our results give some credence to the maternal depletion hypothesis. We see that effects of intervals are generally greatest for the shortest intervals (which allow the smallest amount of time for recuperation from the previous pregnancy), and they are greater during infancy, when physiological factors should matter more, than during childhood. We see that very short inter-outcome intervals are generally more detrimental when they follow a live birth or stillbirth than when they follow a preceding miscarriage or induced abortion. Because of their longer gestation, live births and stillbirths should be more depleting than miscarriages or induced abortions. The breastfeeding that follows a live birth leads to further maternal depletion. Indeed we find that the effects of short inter-outcome intervals are greatest when the preceding outcome was a live birth.
Another possible reflection of a physiological mechanism is the somewhat elevated mortality risk we see for very long intervals (7 years or longer). This is not a consequence of intervening non-live-birth outcomes because we see it for inter-outcome intervals as well as for interbirth intervals. Our companion analyses of maternal morbidity (Razzaque et al. Citation2005), pregnancy outcomes (DaVanzo et al. Citation2007), and maternal mortality (DaVanzo et al. Citation2004) in Matlab and analyses of Latin American data (Conde-Agudelo and Belizán Citation2000) show that long intervals are often associated with adverse outcomes for women, and these are similar in magnitude to those for first pregnancies. One possibility is that the physiology of a mother who becomes pregnant after a long interval is similar to that of a woman who is pregnant for the first time (Conde-Agudelo and Belizán Citation2000). In addition, some women may have health problems that both make it difficult for them to become pregnant (and hence they have long intervals) and adversely affect the health of the children they bear, raising some question about whether the relationship between long IOIs and poorer outcomes is causal.
We also find some support for the sibling competition hypothesis. During the post-neonatal period and during childhood the effects of short intervals are stronger if the child born at the beginning of the interval is still alive at the time of the index child's birth (and hence can compete with the index child for the family's resources) than if the child born at the beginning of the interval has died, a finding that is similar to those of other studies (e.g., Whitworth and Stephenson Citation2002). The effects of very short IOIs that began with the births of children who survived become stronger as the child ages during the first year of life. We also find significant negative effects of subsequent short interpregnancy intervals on child survival (and have investigated this in a way that avoids reverse causality): a child is much more likely to die between ages 1 and 5 if the mother became pregnant again before this index child's first birthday (RR = 2.31, p<0.001). (Once this is controlled, we do not find, however, that actually having the subsequent birth before the index child's first birthday significantly increases the risk that the index child will die, but there are very few women who give birth again so quickly.) These results for short IOIlba and short subsequent intervals could also reflect disease transmission between children of similar ages. We do not have a way to distinguish between the effects of sibling competition and disease transmission in our data.
However, in some cases, particularly during the neonatal period, we find that interval effects are greater if the preceding live-born child died than if it survived. This is not consistent with the competition hypothesis, but instead appears to reflect a higher family-level risk for all children in a family, as was found by Curtis et al. (Citation1993). The effects of very short IOIs that began with the births of children who died are strongest early in the child's life, when biological (rather than behavioural) factors tend to be most important, and become weaker as the child ages. Similar relationships have been found in other studies (e.g., Alam Citation1995; Whitworth and Stephenson Citation2002).
Implications for future research
We have shown that short preceding interbirth intervals have stronger effects on infant mortality than short inter-outcome intervals, and that this occurs because intervals that began with live births have stronger effects than those that began with other pregnancy outcomes. Because the effects of short inter-outcome intervals vary by whether the intervals began with a live birth, stillbirth, miscarriage, or induced abortion, we recommend, where data permit, that future studies consider inter-outcome intervals, and allow their effects to vary by the type of outcome that began the interval, rather than only considering interbirth intervals.
Our finding that, early in a child's life, interval effects are greater if the preceding live-born child died than if it survived appears to reflect a higher family-level risk for all children in a family. Future research should attempt to take this unobserved family-level heterogeneity into account. Furthermore, the fact that over 90 per cent of the very short IOIs (<15 months) in our data occur among women who recently had a NLB outcome or a live-born child who died suggests that such outcomes (i.e., the woman becoming pregnant again so soon) affect pregnancy spacing. In addition, the fact that the effects of intervals that began with NLBs sometimes do not vary much with interval length suggests that there may be something different about women who have NLBs that leads to health problems in their live-born children. The approach of Bhalotra and van Soest (Citation2008), which jointly models influences on interval lengths and mortality, allowing for correlated risks among different births to the same mother, is promising and should be tried with the Matlab data (which are richer than the DHS data that Bhalotra and van Soest use), taking into account NLBs as well as child deaths.
In addition, future research should try to explain the role that breastfeeding (and its intensity) plays in the relationship between birth spacing and infant and child mortality. For example, to what extent does the breastfeeding of one child lead to maternal depletion that has detrimental effects on the next child?
Policy implications
Although there are some reasons to question the extent to which the relationships shown here reflect causal mechanisms—and future research should try to address this issue—the results nonetheless identify the types of pregnancies that lead to births that have a higher risk of mortality. These are particularly first births that are the outcomes of first pregnancies and births following short intervals that began with a live birth, but also very short intervals that began with stillbirths or miscarriages and births that were preceded by the birth of child who died or by a pregnancy that did not result in a live birth. Such higher-risk pregnancies and births merit special attention in antenatal and postnatal care.
To the extent that the relationships shown here reflect cause and effect, they have implications for the advice that should be given to women about pregnancy spacing. Although we generally find the effect of inter-outcome intervals to be largest when the interval began with a live birth, we also find an increased risk of early neonatal mortality after inter-outcome intervals of less than 15 months that began with a stillbirth or miscarriage, suggesting that women who experience one of these types of pregnancy outcomes should be advised to wait at least 6 months before becoming pregnant again.
In the past, health professionals have advocated birth intervals of at least 2 years in length. Our results are consistent with the findings of recent studies (e.g., Rutstein Citation2003, Citation2005), from both developed and less developed countries, that show an association between even longer interbirth intervals and lower infant and child mortality. In our data, inter-outcome intervals of 24–35 months that began with the birth of a child who survived are associated with a 37 per cent increased risk of late neonatal mortality and a 23 per cent increased risk of mortality at ages 1–4 relative to intervals of 36–59 months (both p<0.01). After the first week of life, the lowest mortality rates are generally found for intervals of 60–83 months. Thus, the previously recommended birth interval of at least 2 years could arguably be increased to at least 3 years. Considering that 30 per cent of inter-outcome intervals of known duration in our data are 24–35 months in length, a change in pregnancy spacing consistent with this recommendation could help reduce infant and child mortality rates.
After reviewing recent studies, including an earlier version of this paper, experts at the 2005 WHO technical consultation recommended that, after a live birth, the interval should be at least 24 months before a subsequent pregnancy is attempted (a 33 month birth-to-birth interval) (WHO Citation2006). They also recommended a 6-month pregnancy interval after an induced abortion or miscarriage. In response, a number of countries have launched communication and outreach campaigns that reflect these messages. The US Agency for International Development has adopted ‘Healthy Timing and Spacing of Pregnancy’ as a slogan that reflects the priority that should be given to efforts to educate policy-makers, health providers, youth, and families on the importance of delaying the first pregnancy until at least age 18 and spacing subsequent pregnancies for improved health and quality of life.
Notes
1. Julie DaVanzo is at RAND, 1776 Main Street, PO Box 2138, Santa Monica, CA 90407-2138, USA. E-mail: [email protected]. Lauren Hale is at the State University of New York, Stony Brook; Abdur Razzaque is at the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B); Mizanur Rahman is at Pathfinder International.
2. Support provided by the Office of Population and Reproductive Health, Bureau for Global Health, US Agency for International Development under the terms of Cooperative Agreement No. HRN-A-00-00-00003-00 awarded to CATALYST Consortium. The consortium, which ended in 2005, was a partnership between Pathfinder International and the Academy for Educational Development, the Centre for Development and Population Activities, Meridian Group International, Inc., and PROFAMILIA/Colombia. The views expressed are those of the authors and do not reflect the opinions of the staff of the US Agency for International Development, RAND, ICDDR,B, or Pathfinder International. An earlier version of this paper was presented at USAID in March 2004; at the annual meeting of the Population Association of America, in Boston, in April 2004; at a meeting of the Systematic Literature Review Expert Panel at the CATALYST Consortium, in April 2004, in Washington, DC; and at a Technical Consultation and Scientific Review of Birth Spacing at the World Health Organization in Geneva in June 2005. Some of DaVanzo's work on this paper was undertaken while she was a visiting fellow at the Australian Demographic and Social Science Research Institute at the Australian National University. Some of Hale's work was done while she was an NICHD-funded postdoctoral fellow at the RAND Corporation. Open Access to this paper was made possible through the Extending Service Delivery (ESD) project funded by USAID. The authors thank Stan Becker for his helpful comments, Suzanne Knecht for her guidance, Maureen Norton for her useful comments and her encouragement, and Greg Ridgeway and Lionel Galway for their statistical advice. ICDDR,B acknowledges with gratitude the commitment of USAID and CATALYST Consortium to the Centre's research efforts.
References
- Alam , N. 1995 . Birth spacing and infant and early childhood mortality in a high fertility area of Bangladesh: age dependant and interactive effects . Journal of Biosocial Science , 27 ( 4 ) : 393 – 404 .
- Bhalotra , S. and van Soest , A. 2008 . Birth spacing, fertility and neonatal mortality in India: dynamics, frailty and fecundity . Journal of Econometrics , 143 ( 2 ) : 274 – 290 .
- Bhatia , S. 1983 . Contraceptive users in rural Bangladesh—a time trend analysis . Studies in Family Planning , 14 ( 1 ) : 20 – 28 .
- Chen , L. C. , Huq , E. and D'Souza , S. 1981 . Sex bias in the allocation of food and health care in rural Bangladesh . Population and Development Review , 7 ( 1 ) : 55 – 70 .
- Cleland , J. G. and Sathar , Z. A. 1984 . The effect of birth spacing on childhood mortality in Pakistan . Population Studies , 38 ( 3 ) : 401 – 418 .
- Conde-Agudelo , A. and Belizán , J. 2000 . Maternal morbidity and mortality associated with interpregnancy interval . British Medical Journal , 321 ( 7271 ) : 1255 – 1259 .
- Conde-Agudelo , A. , Belizán , J. M. , Breman , R. , Brockman , S. C. and Rosas-Bermudez , A. 2005 . Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America . International Journal of Gynecology and Obstetrics , 89 ( S1 ) : S34 – S40 .
- Conde-Agudelo , A. , Rosas-Bermúdez , A. and Kafury-Goeta , A. C. 2006 . Birth spacing and risk of adverse perinatal outcomes: a meta-analysis . Journal of the American Medical Association , 295 ( 15 ) : 1809 – 1823 .
- Conde-Agudelo , A. , Rosas-Bermúdez , A. and Kafury-Goeta , A. C. 2007 . Effect of birth spacing on maternal health: a systematic review . American Journal of Obstetrics and Gynecology , 196 ( 4 ) : 297 – 308 .
- Curtis , S. L. , Diamond , I. and McDonald , J. W. 1993 . Birth interval and family effects on postneonatal mortality in Brazil . Demography , 30 ( 1 ) : 33 – 43 .
- DaVanzo , J. , Butz , W. P. and Habicht , J.-P. 1983 . How biological and behavioural influences on mortality in Malaysia vary during first year of life . Population Studies , 37 ( 3 ) : 381 – 402 .
- DaVanzo , J. , A. Razzaque , M. Rahman , and L. Hale with K. Ahmed , M. A. Khan , G. Mustafa , and K. Gausia . 2004 . The effects of birth spacing on infant and child mortality, pregnancy outcomes, and maternal morbidity and mortality in Matlab, Bangladesh . Working Paper 198, RAND Corporation, Santa Monica, CA .
- DaVanzo , J. , Hale , L. , Razzaque , A. and Rahman , M. 2007 . Effects of interpregnancy interval and outcome of the preceding pregnancy on pregnancy outcomes in Matlab, Bangladesh . BJOG: An International Journal of Obstetrics and Gynaecology , 114 ( 9 ) : 1079 – 1087 .
- Dewey , K. G. and R. J. Cohen . 2004 . Birth spacing literature review: maternal and child nutrition outcomes . Draft Report, CATALYST Consortium .
- D'Souza , S. and Chen , L. C. 1980 . Sex differentials in mortality in rural Bangladesh . Population and Development Review , 6 ( 2 ) : 257 – 270 .
- D'Souza , S. 1981 . A population laboratory for studying disease processes and mortality—the Demographic Surveillance System , Matlab, Bangladesh, Special Publication No. 13 .
- Fauveau , V. , Koenig , M. A. and Wojtyniak , B. 1991 . Excess female deaths among rural Bangladeshi children—an examination of cause-specific mortality and morbidity . International Journal of Epidemiology , 20 ( 3 ) : 729 – 735 .
- Greenspan , A. 1993 . Family planning's benefits include improved child health and nutrition: new data from Bangladesh . Asia-Pacific Population & Policy , 26 : 1 – 4 .
- ICDDR,B Centre for Health and Population Research, Dhaka, Bangladesh . 2000 . Health and Demographic Surveillance System—Matlab: registration of demographic and contraceptive use 1998 , Scientific Report 87 .
- King , J. C. 2003 . The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies . Journal of Nutrition , 133 : 1732S – 1736S .
- Koenig , M. A. , Phillips , J. F. , Campbell , O. M. and D'Souza , S. 1990 . Birth intervals and childhood mortality in rural Bangladesh . Demography , 27 ( 2 ) : 251 – 265 .
- LeGrand , T. K. and Phillips , J. F. 1996 . The effect of fertility reductions on infant and child mortality: evidence from Matlab in rural Bangladesh . Population Studies , 50 ( 1 ) : 51 – 68 .
- Leridon , H. 1976 . Facts and artifacts in study of intrauterine mortality—reconsideration from pregnancy histories . Population Studies , 30 ( 2 ) : 319 – 335 .
- Miller , J. E. 1991 . Birth intervals and perinatal health: an investigation of three hypotheses . Family Planning Perspectives , 23 ( 2 ) : 62 – 70 .
- Miller , J. E. , Trussell , J. , Pebley , A. R. and Vaughan , B. 1992 . Birth spacing and child mortality in Bangladesh and the Philippines . Demography , 29 ( 2 ) : 305 – 318 .
- Mitra , S. , M. Ali , S. Islam , A. Cross , and T. Saha . 1994 . Bangladesh Demographic and Health Survey, 1993–1994 . Dhaka, Bangladesh: Mitra Associates and Calverton, MD: Macro International, Inc .
- National Research Council . 1989 . Contraception and Reproduction: Health Consequences for Women and Children in the Developing World . Washington, DC : National Academy Press .
- Pallikadavath , S. and Stones , R. W. 2005 . Miscarriage in India: a population-based study . Fertility and Sterility , 84 ( 2 ) : 516 – 518 .
- Razzaque , A. , DaVanzo , J. , Rahman , M. , Gausia , K. , Hale , L. , Khan , M. A. and Mustafa , A. H. M. G. 2005 . Pregnancy spacing and maternal morbidity in Matlab, Bangladesh . International Journal of Gynecology and Obstetrics , 89 ( S1 ) : S41 – S49 .
- Rutstein , S. 2003 . Effect of birth intervals on mortality and health: multivariate cross country analyses . Presentation to USAID-sponsored Conference on Optimal Birthspacing for Central America, Antigua, Guatemala .
- Rutstein , S. , K. Johnson , and A. Conde-Agudelo . 2004 . Systematic literature review and meta-analysis of the relationship between interpregnancy or interbirth intervals and infant and child mortality . Paper prepared for the CATALYST Consortium .
- Rutstein , S. 2005 . Effects of preceding birth intervals on neonatal, infant and under-five years mortality and nutritional status in developing countries: evidence from the Demographic and Health Surveys . International Journal of Gynecology and Obstetrics , 89 ( S1 ) : S7 – S24 .
- Smits , L. J. and Essed , G. G. 2001 . Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion . Lancet , 358 ( 9298 ) : 2074 – 2077 .
- Van Ginneken , J. , R. Bairagi , A. De Francisco , A. M. Sarder , and P. Vaughan . 1998 . Health and demographic surveillance in Matlab: past, present, and future , Scientific Publication No. 72 .
- Whitworth , A. and Stephenson , R. 2002 . Birth spacing, sibling rivalry, and child mortality in India . Social Science and Medicine , 55 ( 12 ) : 2107 – 2119 .
- Winkvist , A. , Jalil , F. , Habicht , J.-P. and Rasmussen , K. M. 1994 . Maternal energy depletion is buffered among women in Punjab, Pakistan . Journal of Nutrition , 124 ( 12 ) : 2376 – 2385 .
- Winkvist , A. , Habicht , J.-P. , Rasmussen , K. M. and Frongillo , E. A. J. 2000 . Malnourished mothers maintain their weight throughout pregnancy and lactation: results from Guatemala . Advances in Experimental Medicine and Biology , 478 : 415 – 416 .
- World Bank . 2005 . 2005 World Development Indicators .
- World Health Organization (WHO) . 2006 . Report of a WHO Technical Consultation on Birth Spacing , Geneva, Switzerland, 13–15 June 2005 . Geneva : WHO .
- Zhu , B.-P. 2005 . Effect of interpregnancy interval on birth outcomes: findings from three recent US studies . International Journal of Gynecology , 89 ( S1 ) : S25 – S33 .
Appendix: The effects of interbirth vs. inter-outcome intervals
The diagrams below illustrate the following concepts:
| 1. | Interbirth interval duration (IBI) = Birth date of index child − Birth date of preceding live birth. | ||||
| 2. | Inter-outcome interval duration (IOI) = Date of outcome of index pregnancy − Date of outcome of preceding pregnancy (even if one or both of these pregnancies had a non-live-birth outcome). | ||||
| 3. | Interpregnancy interval duration (IPI) = Date of conception of index pregnancy − Date of outcome of preceding pregnancy = Inter-outcome interval − Duration of gestation of index pregnancy. For studies that focus only on live births, this has also been called the birth-to-conception interval (e.g., Miller et al. Citation1992). | ||||
If there is no intervening NLB, the preceding interbirth interval (IBI) is the same as the inter-outcome interval (IOI), and they are the duration of the preceding interpregnancy interval (IPI) plus the gestation of the index pregnancy (G); that is, IBI = IPI + G, or IPI = IBI – G.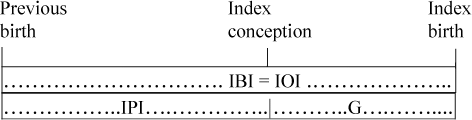
If there is an intervening NLB, the IBI will include two (or more) IOIs, each of which consists of an IPI and the duration of the pregnancy that follows it.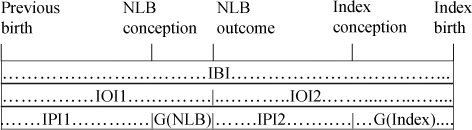
We show below that if we include inter-outcome intervals and gestation in the models, the estimated effect of inter-outcome intervals would be the same as the effect of interpregnancy intervals (when we assume the effects to be linear).
If the effects of intervals and gestation are linear and IPI does not affect G (which it does not significantly in our data), the coefficient (b) for the inter-outcome interval variable will be the same as it would be for the interpregnancy interval.
IOI = the duration of the inter-outcome interval
IPI = the duration of the interpregnancy interval
G = the duration of gestation of the index pregnancy
M = mortality of the index birth.
Even though IPI refers to time between pregnancies and IOI refers to time between outcomes, their coefficients (b) are identical. Hence, for a linear specification, it is sufficient to use IOI (which we know for the vast majority of our sample) instead of IPI (which we can calculate accurately only for a selected sample) as long as we also control for G. In our empirical analyses of infant and child mortality in this paper, we allow the effect of both IOI and gestation to be nonlinear (by using dummy indicators for categories of durations). Nonetheless, the effects we estimate for our indicators of IOI should give us essentially the same ones we would get if we had used indicators of IPI as long as we also control for G. Granted, we don't know G for many cases (primarily in the Comparison Area), but we deal with this by including the additional control for Gestation Unknown (and also an interaction between Gestation Unknown and residence in the MCH–FP Area).