Abstract
Prescription opioid analgesics are an important treatment option for patients with chronic pain; however, misuse, abuse and diversion of these medications are a major global public health concern. Prescription opioid analgesics can be abused via intended and non-intended routes of administration, both intact or after manipulation of the original formulation to alter the drug-delivery characteristics. Available data indicate that ingestion (with or without manipulation of the prescribed formulation) is the most prevalent route of abuse, followed by inhalation (snorting, smoking and vaping) and injection. However, reported routes of abuse vary considerably between different formulations. A number of factors have been identified that appear to be associated with non-oral routes of abuse, including a longer duration of abuse, younger age, male sex and a rural or socially deprived location. The development of abuse-deterrent formulations of prescription opioid analgesics is an important step toward reducing abuse of these medications. Available abuse-deterrent formulations aim to hinder extraction of the active ingredient, prevent administration through alternative routes and/or make abuse of the manipulated product less attractive, less rewarding or even aversive. There are currently five opioid analgesics with a Food and Drug Administration abuse-deterrent label, and a number of other products are under review. A growing body of evidence suggests that introduction of abuse-deterrent opioid analgesics in the USA has been associated with decreased rates of abuse of these formulations. The availability of abuse-deterrent formulations therefore appears to represent an important step toward curbing the epidemic of abuse of prescription opioid analgesics, while ensuring the availability of effective pain medications for patients with legitimate medical need.
Introduction
Prescription opioid analgesics are an important treatment option for patients with chronic pain [Citation1]; however, misuse, abuse and diversion of these medications are a global public health concern.[Citation2] Misuse is defined as the intentional therapeutic use of a prescription opioid analgesic in an inappropriate way, excluding events that meet the definition of abuse.[Citation3] Abuse is the intentional, non-therapeutic use of a prescription opioid analgesic to achieve a desirable psychological or physiological effect, for example, euphoric, sedative or anxiolytic effects.[Citation3] Diversion is any intentional act that results in transferring a prescription opioid analgesic from lawful to unlawful distribution or possession.[Citation3]
Results of a structured evidence-based review suggest that abuse develops in <5% of chronic pain patients treated with prescription opioid analgesics, although rates of abuse varied considerably across individual studies.[Citation4] Nevertheless, it is estimated that approximately 33 million people – or 0.7% of the world’s adult population – currently abuse prescription or non-prescription opioids.[Citation2] In 2014, approximately 4.3 million individuals aged 12 years or older (1.6% of the population aged 12 or older) reported non-medical use of prescription opioid analgesics in the USA, making prescription opioid analgesics the second most commonly abused drug class after marijuana.[Citation5] Diversion through family and friends appears to be the most common source of prescription opioid analgesics for abuse.[Citation6] In most cases, these relatives and friends had obtained their prescriptions from a single doctor.[Citation6] Drivers of prescription opioid abuse have been shown to include increased availability of therapeutic opioids,[Citation7–Citation10] a history of illicit substance use and abuse or a substance use disorder [Citation11] and comorbid mental health disorders.[Citation12]
Societal costs of abuse of prescription opioid analgesics in the USA were estimated to be $55.7 billion in 2007.[Citation13] A recent comprehensive review of the clinical and economic burden of abuse of prescription opioid analgesics in the USA found individuals who abused prescription opioid analgesics to be more likely than non-abusers to utilize medical services, such as emergency department visits, physician outpatient visits and inpatient hospital stays.[Citation14] Mean annual excess health-care costs for abusers of prescription opioid analgesics compared with non-abusers ranged from US$14,054 to US$20,546 for those with private insurance and from US$5874 to US$15,183 for those with Medicaid.
Prescription opioid analgesics can be abused in a variety of ways.[Citation15,Citation16] This paper reviews published data on the different routes of abuse of currently available prescription opioid analgesics and assesses the similarities and differences in routes of abuse across different products and populations. The potential impact of emerging abuse-deterrent formulations on routes of abuse will also be assessed. Abuse-deterrent properties are defined as those properties shown to meaningfully deter abuse, even if they do not fully prevent it. The term “tamper-resistant” is sometimes used to describe these formulations, but is in fact more accurately used to describe packaging requirements applicable to certain classes of drugs, devices and cosmetics.
Methods of abuse
Prescription opioid analgesics can be abused simply by taking the medication at or above the recommended dosage via the intended route of administration. Formulations may also be manipulated to alter their drug-delivery characteristics and render them more amenable to abuse, e.g., to overcome extended-release mechanisms, to separate the opioid from excipients and undesirable active ingredients in the formulation, or to permit alternative routes of administration with faster onset of action (e.g., injection). By altering the original formulation, the abuser intends to create a “dose-dumping” effect (i.e., an increased maximum concentration of the opioid in the brain in the shortest possible time); this is associated with the occurrence of a rapid high and other reinforcing effects, which drive further abuse potential.[Citation17]
The primary routes of abuse for prescription opioid analgesics are ingestion (including chewing or taking more than the usual dose), inhalation (e.g., snorting, smoking or inhaling) and injection (intravenous, intramuscular or subcutaneous administration). Common manipulation methods to facilitate abuse include crushing or grinding the product into a powder or small particles, and dissolving in a solvent such as ethanol; extraction of the active ingredient through exposure to hot or cold temperatures is also possible. Abusers may also co-administer prescription opioid analgesics with alcoholic drinks or other substances (e.g., benzodiazepines, barbiturates or antidepressants).[Citation18,Citation19] Furthermore, individual abusers often report utilizing several different routes of abuse.
A considerable amount of information concerning possible methods of manipulation of different prescription opioid analgesics is widely available on the Internet.[Citation20,Citation21] This information includes instructions on how to crush, separate, purify and chemically alter specific formulations to allow changes in dosage, route of administration and time course of drug concentrations and effects. Successful manipulation methods that have widespread appeal evolve into “recipes” and become archived on websites that support recreational drug use.
Frequency of different routes of abuse
Interpretation of data concerning routes of abuse of prescription opioid analgesics is limited by a number of factors, most notably differences in study populations and study design, particularly whether or not respondents are able to indicate more than one route of abuse, since abusers may utilize multiple routes of abuse.[Citation15] The vast majority of published data concerning routes of abuse of prescription opioid analgesics have been obtained in the USA (). Available data suggest that ingestion (with or without manipulation of the prescribed formulation) is the most prevalent route of abuse of prescription opioid analgesics, followed by inhalation and injection.[Citation6,Citation11,Citation22–Citation31]
Table 1. Key US data on routes of abuse of prescription opioid analgesics.
Comprehensive data on methods of manipulation and routes of abuse of prescription opioid analgesics have been generated through the US National Health and Wellness Survey.[Citation31] The prevalence of prescription opioid analgesic abuse in the 3 months before the survey was estimated at 1.3% of the adult population in the USA. Most (91%) of the 225 abusers of prescription opioid analgesics who completed the survey reported taking the abused formulation via the intended route of administration. None of the manipulation methods or routes of abuse identified was reported by substantially more individuals than any other (); these included snorting (38%), chewing (38%), administration with alcoholic drinks (37%), dissolving in water (36%), injection (both intravenous [32%] and by other routes [34%]), smoking (34%) and rectal administration (29%).
Figure 1. Proportions of respondents reporting different manipulation methods and routes of abuse for prescription opioid analgesics in the USA (unweighted findings, across all drugs). Data from [Citation31]. i.v.: intravenous.
![Figure 1. Proportions of respondents reporting different manipulation methods and routes of abuse for prescription opioid analgesics in the USA (unweighted findings, across all drugs). Data from [Citation31]. i.v.: intravenous.](/cms/asset/1da4508d-eb2e-46d3-b4cf-500bbae38208/ipgm_a_1120642_f0001_c.jpg)
The relative frequencies of different routes of abuse generally appear similar in other parts of the world, although data are sparse. An analysis of discussions of prescription opioid analgesic abuse on German-language Internet forums found oral intake to be the most frequently mentioned route of abuse (45%), followed by injection (32%) and inhalation (18%).[Citation32] In another recent study assessing patterns of opioid abuse among 200 patients presenting to a treatment center in a tertiary-care hospital in Kashmir, oral administration was found to be the most frequently reported route of abuse (70%), followed by inhalation (33%) and intravenous administration (26%).[Citation33] Of note, 41% of patients in this study reported more than one route of abuse. In contrast, recent data from the National Opioid Medication Abuse Deterrence (NOMAD) study in Australia for 606 individuals with a history of regular prescription opioid analgesic abuse found injection to be the most common route of abuse across all prescription opioid analgesic formulations.[Citation34] For example, injection of oxycodone was reported by 95% of respondents, with other routes of abuse (snorting, smoking, chewing, or dissolving) reported by only 26%.
Differences in routes of abuse between formulations
There are notable differences in reported routes of abuse between different prescription opioid analgesic formulations. The preferred route of abuse most likely reflects what the individual abuser finds attractive and/or unattractive about a specific formulation.[Citation16] In a study of experienced abusers, ease of extraction, rapid onset of effects and duration of effect were all positively associated with the attractiveness of a formulation for abuse.[Citation35]
Immediate-release formulations generally have a lower barrier to abuse than extended-release formulations. However, extended-release formulations are generally more attractive to abusers than immediate-release formulations due to the greater amount of opioid contained in the product.[Citation16] Available data suggest that immediate-release formulations are more often abused intact (e.g., by over-ingestion), whereas extended-release formulations are more likely to be manipulated and then swallowed, inhaled or injected.[Citation15,Citation29] Eighty-three percent of individuals with a history of prescription opioid abuse reported oral abuse for an immediate-release formulation of oxycodone/acetaminophen (swallowing intact tablets and chewing combined), with inhalation reported by 44% and injection by 0.5%.[Citation36] Similarly, 89% of individuals abusing immediate-release hydrocodone/acetaminophen used the oral route, with inhalation reported by 40% and injection by 1%.[Citation36] In contrast, inhalation (snorting and smoking) has been reported by 57–92% of individuals abusing extended-release oxycodone and injection by 23–59%.[Citation15,Citation23,Citation27,Citation36] The proportion of individuals reporting the oral route of abuse for extended-release oxycodone ranged from 27 to 89%.[Citation15,Citation23,Citation27,Citation36] A study in opioid-dependent persons enrolling in methadone-maintenance treatment programs found the proportion of individuals reporting abuse by injection to be 8% for hydrocodone, 15% for immediate-release oxycodone, 38% for controlled-release oxycodone, 57% for fentanyl, 85% for morphine and 90% for hydromorphone.[Citation37]
The most recent data from the US National Addictions Vigilance Intervention and Prevention Program for individuals entering substance-abuse treatment facilities clearly highlight the differences in route of abuse between different prescription opioid analgesic formulations ().[Citation29] Immediate-release formulations of hydrocodone and oxycodone were most likely to be abused via their intended route of administration (89 and 81%, respectively, compared with 62% for extended-release oxycodone, 47% for immediate-release oxymorphone, 42% for extended-release morphine, 36% for immediate-release morphine, 33% for hydromorphone, 32% for extended-release oxymorphone, 13% for sublingual fentanyl and 9% for transdermal fentanyl). However, rates of abuse via chewing were relatively consistent across the different opioid formulations (8–20%).[Citation29]
Figure 2. Route of administration for different prescription opioid analgesic formulations reported by individuals entering substance-abuse treatment facilities. Data from [Citation29]. Individuals may have reported multiple routes of abuse.
![Figure 2. Route of administration for different prescription opioid analgesic formulations reported by individuals entering substance-abuse treatment facilities. Data from [Citation29]. Individuals may have reported multiple routes of abuse.](/cms/asset/ade9a955-d915-4244-9d23-31909be50568/ipgm_a_1120642_f0002_c.jpg)
Compared with other drugs, hydromorphone and both immediate- and extended-release morphine were more likely to be abused via injection (58, 57 and 48%, respectively, vs 25% for extended-release oxycodone, 22% for transdermal fentanyl, 20% for immediate-release oxymorphone, 10% for sublingual fentanyl, 8% for extended-release oxymorphone, 6% for immediate-release oxycodone, and only 1% for immediate-release hydrocodone).[Citation29] Oxymorphone was more likely to be abused via inhalation than other opioid formulations (77% for extended-release oxymorphone and 60% for immediate-release oxymorphone vs 46% for extended-release oxycodone, 28% for immediate-release oxycodone, 28% for extended-release morphine, 20% for immediate-release morphine, 24% for immediate-release hydromorphone, 19% for immediate-release hydrocodone, 18% for sublingual fentanyl, and 2% for transdermal fentanyl).[Citation29]
In the same study, fentanyl was found to be more likely to be associated with alternative routes of abuse than other prescription opioid analgesics (i.e., abuse via routes other than that intended or via inhalation, injection or chewing); 70% of individuals abusing transdermal fentanyl and 69% of those abusing sublingual fentanyl reported other routes of abuse compared with 3–13% for other drugs.[Citation29] Other routes of abuse that have been reported for transdermal fentanyl formulations include application of multiple patches,[Citation38] placing the patch in the mouth,[Citation39–Citation41] chewing or sucking,[Citation42–Citation44] swallowing whole,[Citation45,Citation46] injection of extracted patch contents,[Citation47–Citation49] smoking [Citation50,Citation51] and rectal insertion.[Citation52] Patches may also be boiled to make a “tea”, which can be ingested or injected.[Citation53–Citation55]
Available data suggest that injection is the most common route of abuse for buprenorphine,[Citation56–Citation61] although abuse via inhalation has also been reported.[Citation60,Citation62–Citation64] However, differences in routes of abuse have been reported between different buprenorphine formulations.[Citation65,Citation66] Both overall rates of abuse and rates of abuse by non-oral routes of administration appear lower with buprenorphine/naloxone film than with buprenorphine or buprenorphine/naloxone tablets.[Citation65,Citation66]
Differences in routes of abuse between populations
Available data indicate that the route of abuse may differ according to duration of abuse, age, sex and geographic location. In general, less experienced abusers appear to prefer the oral route of abuse,[Citation11,Citation22,Citation24] with frequency of non-oral routes of abuse increasing with duration of abuse.[Citation22,Citation23,Citation28] Results of a survey of opioid-dependent subjects showed that the most common initial route of abuse was ingestion (83%), followed by inhalation (16%) and injection (1%); however, at the time of admission to a treatment center, the most common route of abuse was inhalation (58%), followed by ingestion and injection (both 21%).[Citation22] Among undergraduate students with a history of prescription opioid analgesic abuse, the main route of abuse was oral administration (97% vs 18% for inhalation [including snorting and smoking] and only 0.5% for injection).[Citation11] In contrast, 80% of prescription drug abusers entering a treatment facility reported manipulating the intended delivery system of the abused product.[Citation23] For extended-release oxycodone, 92% reported manipulating the formulation for intranasal administration, 49% reported chewing before swallowing and 23% reported manipulating for intravenous injection. High rates of abuse via injection were also seen in a study of patients in methadone-maintenance treatment for heroin addiction, although rates varied according to the product abused (90% for hydromorphone, 85% for morphine, 57% for fentanyl, 35% for oxycodone, 12% for methadone and 8% for hydrocodone).[Citation37] Similarly, recent data from the NOMAD study in Australia revealed high rates of abuse via injection among individuals with a history of prescription opioid analgesic abuse (95% for oxycodone).[Citation34] Although this would appear to suggest that use of non-oral routes of abuse might increase with age, increasing age has in fact been shown to be negatively correlated with non-oral routes of abuse (e.g., snorting, smoking and injecting) ().[Citation15,Citation28,Citation30]
Figure 3. Route of abuse of prescription opioid analgesics according to age. Data from a custom analysis of the 2006 Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Treatment Episode Data Set (TEDS) database. From Katz N, et al. [Citation15] (Taylor & Francis Ltd, www.tandfonline.com reprinted with permission from the publisher).
![Figure 3. Route of abuse of prescription opioid analgesics according to age. Data from a custom analysis of the 2006 Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Treatment Episode Data Set (TEDS) database. From Katz N, et al. [Citation15] (Taylor & Francis Ltd, www.tandfonline.com reprinted with permission from the publisher).](/cms/asset/0f7a1acb-cd0f-452a-861d-cbacd6e7f9cc/ipgm_a_1120642_f0003_c.jpg)
Available data suggest that men are more likely than women to abuse prescription opioid analgesics.[Citation31,Citation67–Citation69] Men also appear to be more likely than women to abuse prescription opioid analgesics via non-oral routes (e.g., snorting or injection) ().[Citation28,Citation67,Citation70] Other clinically relevant gender differences in patterns of abuse have also been noted. In particular, women appear to be older than men when they start to abuse prescription opioid analgesics, but advance more quickly to regular abuse (i.e., weekly) than male subjects.[Citation70]
Figure 4. Gender differences in routes of abuse of prescription opioid analgesics. Data from [Citation70].
![Figure 4. Gender differences in routes of abuse of prescription opioid analgesics. Data from [Citation70].](/cms/asset/27e56641-d703-4b5e-b702-4b1f27317e00/ipgm_a_1120642_f0004_c.jpg)
The preferred route of abuse has also been shown to vary according to geographic location.[Citation34,Citation71] In one study of 212 recreational prescription drug abusers in the USA, rural abusers were found to be more likely to utilize non-oral forms of abuse (particularly injection) than urban abusers.[Citation71] Recent data from Australia also show geographic differences in routes of abuse among individuals who regularly manipulate prescription opioid analgesics; socially disadvantaged subjects were most likely to inject prescription opioid analgesics, including oxycodone, fentanyl and methadone.[Citation34]
Medical outcomes associated with different routes of abuse
Abuse and misuse of prescription opioid analgesics have serious consequences, including physical dependence, addiction, overdose, drug-related road traffic accidents, spreading of blood-borne infectious diseases via shared drug paraphernalia (e.g., human immunodeficiency virus (HIV) and/or hepatitis C virus infection) and death.[Citation9,Citation14] Individuals who abuse prescription opioid analgesics have higher rates of both HIV and hepatitis infection than non-abusers (1.4% vs 0.3% for HIV infection and 6.5% vs 0.2% for hepatitis A, B, and C).[Citation72]
Abuse routes associated with the highest morbidity are injection and inhalation.[Citation15] Abuse of prescription opioid analgesics has been independently related to risky injection behaviors, such as sharing syringes.[Citation73] Individuals who abuse prescription opioid analgesics via injection have been shown to be at greater risk of serious infectious diseases, including hepatitis C virus and HIV infection.[Citation30,Citation74–Citation78] In the recent HIV outbreak in Indiana in a population historically at low risk of infection, the majority of cases were found to be linked to intravenous abuse of oxymorphone.[Citation78] Serious cutaneous complications have also been reported in individuals who crush and inject buprenorphine tablets; such complications appear to be at least in part due to the presence of silica in generic formulations.[Citation79,Citation80] Intravenous injection of opioid formulations not intended for parenteral use has also been associated with pulmonary complications [Citation81,Citation82] and endocarditis.[Citation83,Citation84] Costs associated with acute bacterial infections such as endocarditis, cellulitis, osteomyelitis and sepsis in individuals who abuse via injection have been shown to be substantial.[Citation85] Intranasal abuse of prescription opioid analgesics, particularly of formulations containing acetaminophen, has been linked to nasal pain, tissue necrosis with potential septal and palatal perforation, and serious fungal infections.[Citation86–Citation88]
Of the 22,134 drug overdose deaths involving pharmaceuticals (not including overdose of illegal drugs) that occurred in 2010 in the USA, 75% involved opioids.[Citation89] Indeed, it is estimated that non-medical use of prescription opioid analgesics contributes to over 16,500 deaths annually (i.e., 46 deaths/day) in the USA.[Citation90] The Annual Report of the American Association of Poison Control Centers’ National Poison Data System for 2013 showed that 690 of the 2113 reported fatalities (33%) were attributable to analgesics.[Citation91] Of these 690 deaths, 110 were attributed to hydrocodone/acetaminophen, 109 to methadone, 98 to oxycodone, 58 to morphine, 26 to fentanyl, 23 to tramadol and 20 to unspecified opioids. In addition, the Centers for Disease Control and Prevention estimates that for every prescription painkiller overdose death in the USA, there are 10 treatment admissions for abuse, 32 emergency department visits for abuse or misuse, 130 people who abuse or who are dependent and 824 non-medical users.[Citation92] Overall, the medical and prescription costs associated with misuse, abuse and diversion of prescription opioid analgesics in the USA have been estimated at US$72.5 billion annually.[Citation93]
Potential impact of abuse-deterrent formulations
The magnitude of the problem of prescription opioid analgesic abuse has spurred the development of numerous strategies to reduce its impact, including the development of novel formulations that are more difficult to manipulate for purposes of abuse.[Citation94–Citation100] Available abuse-deterrent formulations aim to hinder extraction of the active ingredient, limit its bioavailability, prevent administration through alternative routes and/or make abuse of the manipulated product much less attractive, less rewarding or even aversive. Possible approaches to abuse deterrence include physical and chemical barriers to prevent manipulation of the formulation and extraction of the active ingredient, combination with an antagonist, aversion technologies, use of new molecular entities or prodrugs, and novel delivery systems.[Citation100] These approaches can be combined to further reduce the potential for abuse by different routes.
There are currently five extended-release opioid analgesic formulations available in the USA with Food and Drug Administration (FDA) abuse-deterrent labeling. OxyContin® (Purdue Pharma LP), an abuse-deterrent reformulation of controlled-release oxycodone, has been available in the USA since 2010. This product has physical and chemical properties intended to deter manipulation for the purposes of oral and intranasal abuse by making tablet crushing both more difficult and less effective. The formulation is also resilient to ethanol dose-dumping and other chemical extraction techniques. If attempts are made to dissolve the tablet, it gradually forms a viscous gel that resists passage through a hypodermic needle, thus deterring intravenous abuse. Studies have shown that it is less attractive to recreational drug abusers than the original extended-release formulation.[Citation101,Citation102]
Targiniq™ ER (Purdue Pharma LP) was approved in the USA in July 2014. This abuse-deterrent formulation contains oxycodone combined with naloxone – an antagonist that blocks the euphoric effects of oxycodone if the tablet is crushed for snorting or manipulated for injection.[Citation103] Embeda® (King Pharmaceuticals, Inc.), which consists of pellets of morphine sulfate each containing a core of sequestered naltrexone that is intended to remain sequestered when the product is taken as prescribed,[Citation104] was approved with abuse-deterrent labeling in the USA in October 2014. This formulation was first approved in 2009, but was voluntarily withdrawn from the market in March 2011 due to testing that found stability concerns in the manufacturing process; it was relaunched in the USA in 2015. Studies have indicated that the quantity of naltrexone released upon manipulation is sufficient to attenuate the desired subjective effects of morphine following oral, intranasal or intravenous administration.[Citation105–Citation107] Hysingla® ER (Purdue Pharma LP) is an abuse-deterrent formulation of hydrocodone that was approved in the USA in November 2014. This formulation has physical and chemical properties that render it difficult to crush, break or dissolve; these abuse-deterrent properties are expected to deter abuse via chewing, snorting and injection.[Citation108] Approved by the FDA in October 2015, MorphaBond™ ER (Inspirion Delivery Technologies) is the first abuse-deterrent extended-release morphine product without an antagonist to be marketed in the USA.[Citation109] Laboratory test data show that, relative to morphine sulfate tablets, MorphaBond has increased resistance to cutting, crushing or breaking using a variety of tools. Furthermore, when subjected to a liquid environment, the manipulated formulation forms a viscous material that resists passage through a needle.
A number of other potential opioid abuse-deterrent formulations are under review by the FDA. Some other currently available opioid analgesics have also been reported to have potential abuse-deterrent properties; however, these have not yet met the necessary regulatory requirements for an ADF label.[Citation100] Examples include Exalgo® (Mallinckrodt Pharmaceuticals), Nucynta® ER (Depomed Inc.), Opana® ER (Endo Pharmaceuticals Inc.), Oxaydo™ (Egalet Corporation), Xartemis™ XR (Mallinckrodt Pharmaceuticals) and Zohydro® ER (Zogenix Inc).[Citation94–Citation99]
A growing body of evidence suggests that introduction of the abuse-deterrent formulation of oxycodone has been associated with decreased rates of abuse and diversion of this opioid analgesic in the USA.[Citation101,Citation110–Citation119]. Cost savings have also been reported to be associated with reductions in abuse following the introduction of the abuse-deterrent reformulation of oxycodone in the USA.[Citation119–Citation121] It has been estimated that reformulated oxycodone is associated with annual medical cost savings at the US population level of US$430 million (including savings of $86 million from reductions in diagnosed abuse and $344 million from reductions in undiagnosed abuse) and indirect cost savings of US$605 million, for total annual societal cost savings of approximately US$1.0 billion.[Citation120,Citation121] The societal cost savings associated with reductions in abuse may be expected to increase over time as more abuse-deterrent opioid analgesic formulations are introduced. However, these benefits have been accompanied by a significant increase in abuse of other opioids, most notably heroin, in some studies.[Citation112,Citation116–Citation118,Citation122] Epidemiological data showing the potential impact of other abuse-deterrent opioid analgesic formulations on rates of prescription opioid abuse and diversion are not yet available.
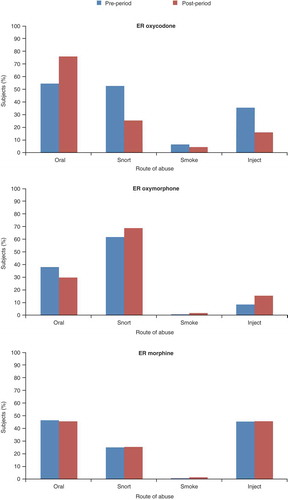
The impact of the abuse-deterrent reformulation of oxycodone on routes of abuse has been assessed in 189 subjects with a history of oxycodone abuse.[Citation114] The past 30-day prevalence of abuse was found to be lower for the reformulated oxycodone compared with that of both the previous extended-release and immediate-release oxycodone formulations through any route (33% vs 74% and 96%, respectively), snorting (5% vs 39% and 70%) and injection (0.5% vs 41% and 51%). The past 30-day prevalence of oral abuse was also lower for the reformulation than for the immediate-release formulation (21% vs 29%), but not compared with the previous extended-release formulation (7%). In a larger sample of 140,496 individuals assessed for substance-abuse treatment at 357 centers in the USA between June 2009 and March 2012, the overall rate of abuse of the abuse-deterrent reformulation of oxycodone decreased by 41% for any route and by 66% for non-oral routes compared with historical rates for the original extended-release formulation; oral abuse decreased by 17%.[Citation111] Abuse patterns of extended-release oxymorphone and extended-release morphine were not found to change over the same period ().
Figure 5. Percentages of individuals abusing extended-release oxycodone, oxymorphone and morphine via specific routes of administration* before and after introduction of the abuse-deterrent reformulation of oxycodone.
An Australian study also assessed attempted manipulation methods and routes of abuse for the abuse-deterrent reformulation of oxycodone in a prospective cohort of 522 people who regularly abused pharmaceutical opioids.[Citation123] Despite the majority (88%) reporting recent abuse of the original oxycodone formulation, low levels of abuse of reformulated oxycodone were evident, with only 12% reporting successful manipulation of the reformulation. In addition, most (66%) of those who had abused reformulated oxycodone reported doing so only once in the preceding month, suggesting low levels of pervasive use. The primary route of abuse for reformulated oxycodone was swallowing (15%), with low levels of recent successful injection (6%), chewing (2%), drinking/dissolving (1%) and smoking (<1%).
Analysis of Internet forums to evaluate reactions to the abuse-deterrent reformulation of oxycodone revealed a reduction in discussion levels and endorsing content following its introduction.[Citation124] Despite discussion of recipes (32 of which were deemed feasible, i.e., able to abuse), the frequency of posts reporting abuse via these recipes was low and decreased over time. Of the 5677 posts mentioning the abuse-deterrent reformulation, 498 reported abuse (41 reported injecting and 128 reported snorting).
Conclusions
Prescription opioid analgesics can be abused via intended and non-intended routes of administration, both intact or after manipulation of the original formulation to change the drug-delivery characteristics. Ingestion is the most common route of abuse, followed by inhalation (snorting, smoking and vaping) and injection. However, routes of abuse vary widely between opioid analgesics and formulations, and may also change when new formulations become available.
Abuse-deterrent formulations aim to hinder extraction of the active ingredient, prevent administration through alternative routes and/or make abuse of the manipulated product less attractive, less rewarding or even aversive. There are currently five extended-release opioid analgesic formulations with an FDA abuse-deterrent label, and a number of other products are under review. A growing body of evidence suggests that introduction of abuse-deterrent formulations has been associated with decreased rates of abuse and diversion of these prescription opioid analgesics in the USA. The availability of abuse-deterrent formulations therefore appears to represent an important step toward curbing the epidemic of prescription opioid analgesic abuse and diversion, while ensuring the availability of effective pain medications for patients with legitimate medical need.
At present, it is not mandatory that clinicians prescribe abuse-deterrent formulations of opioid analgesics. Thus, it is up to prescribers to choose the most appropriate formulation for individual patients. This decision can be difficult to make and, for various reasons, both prescribers and patients may hesitate to accept it. For example, if abuse-deterrent formulations of opioid analgesics are not universally prescribed, some physicians may be reluctant to prescribe them to avoid false labeling of their patients as “addicts”; similarly, some patients may feel they are being identified as potential abusers if prescribed an abuse-deterrent formulation. As abuse-deterrent formulations are more expensive than those without abuse-deterrent properties, insurers may also resist paying for them. A number of factors have been identified that appear to increase the likelihood of riskier non-oral routes of abuse of prescription opioid analgesics, including a longer duration of abuse, younger age, male sex and a rural or socially deprived setting. Clinicians should take these factors into consideration when assessing the risks and benefits of treating pain with a given formulation in an individual patient.
Increased use of abuse-deterrent opioid analgesic formulations may be expected to confer broader public health benefits similar to the societal impact of vaccination or use of seat belts in motor vehicles. Abuse-deterrent formulations may be used alongside Risk Evaluation and Mitigation Strategies, Prescription Drug Monitoring Programs and interventions such as risk assessment tools, opioid therapy agreements and management plans and urine drug screening in routine clinical practice to further combat the risk of prescription opioid analgesic abuse. Future research should focus on evaluating the comparative risks and benefits of different abuse-deterrent formulations and technologies once these become available for prescription. Genome research may also lead to the identification of genetic biomarkers associated with increased risk of opioid abuse.
Declaration of interest
Jennifer Coward of Anthemis Consulting provided medical writing support, funded by TEVA Pharmaceuticals, Frazer, PA. M Gasior, M Bond and R Malamut are employees of TEVA Pharmaceuticals. Other TEVA personnel provided a single medical accuracy review of the final draft. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130.
- United Nations Office on Drugs and Crime. World Drug Report 2015. [cited 2015 Oct 20]. Available from: http://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf
- Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:2287–2296.
- Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459.
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. HHS Publication No. SMA 15-4927, NSDUH Series H-50. 2015 [cited 2015 Oct 20]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.htm
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2014. Treatment Episode Data Set (TEDS): 2002-2012. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. Rockville (MD): Substance Abuse and Mental Health Services Administration. [cited 2015 Oct 20]. Available from: http://www.samhsa.gov/data/sites/default/files/TEDS2012N_Web.pdf
- Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–251.
- Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur Addict Res. 2012;18:228–245.
- Manchikanti L, Helm S 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(Suppl.):ES9–E38.
- Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol. 2014;78:1159–1166.
- McCabe SE, Cranford JA, Boyd CJ, et al. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict Behav. 2007;32:562–575.
- Sullivan MD, Edlund MJ, Zhang L, et al. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093.
- Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667.
- Meyer R, Patel AM, Rattana SK, et al. Prescription opioid abuse: a literature review of the clinical and economic burden in the United States. Popul Health Manag. 2014;17:372–387.
- Katz N, Dart RC, Bailey E, et al. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217.
- Kirsh K, Peppin J, Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother. 2012;26:348–361.
- Farré M, Camí J. Pharmacokinetic considerations in abuse liability evaluation. Br J Addict. 1991;86:1601–1606.
- Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125:8–18.
- Gudin JA, Mogali S, Jones JD, et al. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125:115–130.
- Cone EJ. Ephemeral profiles of prescription drug and formulation tampering: evolving pseudoscience on the Internet. Drug Alcohol Depend. 2006;83(Suppl. 1):S31–S39.
- Schifano F, Deluca P, Baldacchino A, et al. Drugs on the web; the Psychonaut 2002 EU project. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:640–646.
- Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23:1–9.
- Passik SD, Hays L, Eisner N, et al. Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. J Pain Palliat Care Pharmacother. 2006;20:5–13.
- Carise D, Dugosh KL, McLellan AT, et al. Prescription OxyContin abuse among patients entering addiction treatment. Am J Psychiatry. 2007;164:1750–1756.
- Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO): a real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf. 2008;17:1142–1154.
- Davis WR, Johnson BD. Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug Alcohol Depend. 2008;92:267–276.
- Katz N, Fernandez K, Chang A, et al. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535.
- Butler SF, Black RA, Serrano JM, et al. Characteristics of prescription opioid abusers in treatment: prescription opioid use history, age, use patterns, and functional severity. J Opioid Manag. 2010;6:239–252.
- Butler SF, Black RA, Cassidy TA, et al. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29.
- Surratt H, Kurtz SP, Cicero TJ. Alternate routes of administration and risk for HIV among prescription opioid abusers. J Addict Dis. 2011;30:334–341.
- Vietri J, Joshi AV, Barsdorf AI, et al. Prescription opioid abuse and tampering in the United States: results of a self-report survey. Pain Med. 2014;15:2064–2074.
- Krüger R, Meißner W, Zimmer A. [Misuse of opioid analgesics. An Internet analysis]. Schmerz. 2014;28:473–482. German.
- Farhat S, Hussain SS, Rather YH, et al. Sociodemographic profile and pattern of opioid abuse among patients presenting to a de-addiction centre in tertiary care Hospital of Kashmir. J Basic Clin Pharm. 2015;6:94–97.
- Peacock A, Bruno R, Cama E, et al. Jurisdictional differences in opioid use, other licit and illicit drug use, and harms associated with substance use among people who tamper with pharmaceutical opioids. Drug Alcohol Rev. 2015;34:611–622.
- Butler SF, Fernandez KC, Chang A, et al. Measuring attractiveness for abuse of prescription opioids. Pain Med. 2010;11:67–80.
- Villapiano A, Butler SF, Budman SH, et al. Demonstration of the feasibility of real-time, product-specific, prescription opioid abuse surveillance: the NAVIPPRO system. Poster presented at: Annual Meeting of the College on Problems of Drug Dependence; 2006 Jun 20; Scottsdale, AZ. Poster 173.
- Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71.
- Arvanitis ML, Satonik RC. Transdermal fentanyl abuse and misuse. Am J Emerg Med. 2002;20:58–59.
- Liappas IA, Dimopoulos NP, Mellos E, et al. Oral transmucosal abuse of transdermal fentanyl. J Psychopharmacol. 2004;18:277–280.
- Woodall KL, Martin TL, McLellan BA. Oral abuse of fentanyl patches (Duragesic®): seven case reports. J Forensic Sci. 2008;53:222–225.
- Bektas F, Eken C, Sayrac V. Opioid toxicity as a result of oral/transmucosal administration of transdermal fentanyl patch. Eur J Emerg Med. 2009;16:344–345.
- Van Rijswijk R, Van Guldener C. A delirious patient with opioid intoxication after chewing a fentanyl patch. J Am Geriatr Soc. 2006;54:1298–1299.
- Carson HJ, Knight LD, Dudley MH, et al. A fatality involving an unusual route of fentanyl delivery: chewing and aspirating the transdermal patch. Leg Med (Tokyo). 2010;12:157–159.
- Faust AC, Terpolilli R, Hughes DW. Management of an oral ingestion of transdermal fentanyl patches: a case report and literature review. Case Rep Med. 2011;2011:495938.
- Thomas S, Winecker R, Pestaner JP. Unusual fentanyl patch administration. Am J Forensic Med Pathol. 2008;29:162–163.
- Mrvos R, Feuchter AC, Katz KD, et al. Whole fentanyl patch ingestion: a multi-center case series. J Emerg Med. 2012;42:549–552.
- Reeves MD, Ginifer CJ. Fatal intravenous misuse of transdermal fentanyl. Med J Aust. 2002;177:552–553.
- Lilleng PK, Mehlum LI, Bachs L, et al. Deaths after intravenous misuse of transdermal fentanyl. J Forensic Sci. 2004;49:1364–1366.
- Tharp AM, Winecker RE, Winston DC. Fatal intravenous fentanyl abuse: four cases involving extraction of fentanyl from transdermal patches. Am J Forensic Med Pathol. 2004;25:178–181.
- Marquardt KA, Tharratt RS. Inhalation abuse of fentanyl patch. Clin Toxicol. 1994;32:75–78.
- Chapman E, Leipsic J, Satkunam N, et al. Pulmonary alveolar proteinosis as a reaction to fentanyl patch smoke. Chest. 2012;141:1321–1323.
- Coon TP, Miller M, Kaylor D, et al. Rectal insertion of fentanyl patches: a new route of toxicity. Ann Emerg Med. 2005;46:473.
- Barrueto F Jr, Howland MA, Hoffman RS, et al. The fentanyl tea bag. Vet Hum Toxicol. 2004;46:30–31.
- Darling SS, Mærkedahl R. [Fentanyl tea]. Ugeskr Laeger. 2014;17:176(8A). Danish.
- Schauer CK, Shand JA, Reynolds TM. The fentanyl patch boil-up – a novel method of opioid abuse. Basic Clin Pharmacol Toxicol. 2015;117:358–359.
- Vidal-Trecan G, Varescon I, Nabet N, et al. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend. 2003;69:175–181.
- Auriacombe M, Fatséas M, Dubernet J, et al. French field experience with buprenorphine. Am J Addict. 2004;13(Suppl. 1):S17–S28.
- Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78.
- Aitken CK, Higgs PG, Hellard ME. Buprenorphine injection in Melbourne, Australia: an update. Drug Alcohol Rev. 2008;27:197–199.
- Yokell MA, Zaller ND, Green TC, et al. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev. 2011;4:28–41.
- Häkkinen M, Heikman P, Ojanperä I. Parenteral buprenorphine-naloxone abuse is a major cause of fatal buprenorphine-related poisoning. Forensic Sci Int. 2013;232:11–15.
- Strang J. Abuse of buprenorphine (Temgesic) by snorting. BMJ. 1991;302:969.
- Roux P, Villes V, Bry D, et al. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addict Behav. 2008;33:1625–1629.
- Horyniak D, Dietze P, Larance B, et al. The prevalence and correlates of buprenorphine inhalation amongst opioid substitution treatment (OST) clients in Australia. Int J Drug Policy. 2011;22:167–171.
- Larance B, Lintzeris N, Ali R, et al. The diversion and injection of a buprenorphine-naloxone soluble film formulation. Drug Alcohol Depend. 2014;136:21–27.
- Lavonas EJ, Severtson SG, Martinez EM, et al. Abuse and diversion of buprenorphine sublingual tablets and film. J Subst Abuse Treat. 2014;47:27–34.
- Back SE, Payne RA, Waldrop AE, et al. Prescription opioid aberrant behaviors: a pilot study of sex differences. Clin J Pain. 2009;25:477–484.
- Back SE, Payne RL, Simpson AN, et al. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35:100–107.
- Kerridge BT, Saha TD, Chou SP, et al. Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions – III. Drug Alcohol Depend. 2015;156:47–56.
- Back SE, Payne RL, Wahlquist AH, et al. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse. 2011;37:313–323.
- Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct J. 2010;7:24.
- White AG, Birnbaum HG, Mareva MN, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11:469–479.
- Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23:638–645.
- Bruneau J, Roy E, Arruda N, et al. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107:1318–1327.
- Valdiserri R, Khalsa J, Dan C, et al. Confronting the emerging epidemic of HCV infection among young injection drug users. Am J Public Health. 2014;104:816–821.
- Zibbell JE, Hart-Malloy R, Barry J, et al. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104:2226–2232.
- Lankenau SE, Kecojevic A, Silva K. Associations between prescription opioid injection and hepatitis C virus among young injection drug users. Drugs (Abingdon Engl). 2015;22:35–42.
- Conrad C, Bradley HM, Broz D, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone–Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:443–444.
- Bouquié R, Wainstein L, Pilet P, et al. Crushed and injected buprenorphine tablets: characteristics of princeps and generic solutions. PLoS One. 2014;9:e113991.
- Wainstein L, Bernier C, Gérardin M, et al. Livedo-like dermatitis and necrotic lesions after high-dose buprenorphine injections: a national French survey. Br J Dermatol. 2015;172:1412–1414.
- Marchiori E, Lourenço S, Gasparetto TD, et al. Pulmonary talcosis: imaging findings. Lung. 2010;188:165–171.
- Nguyen VT, Chan ES, Chou SH, et al. Pulmonary effects of i.v. injection of crushed oral tablets: “excipient lung disease”. AJR Am J Roentgenol. 2014;203:W506–W515.
- Chong E, Poh KK, Shen L, et al. Infective endocarditis secondary to intravenous Subutex abuse. Singapore Med J. 2009;50:34–42.
- Ho RC, Ho EC, Tan CH, et al. Pulmonary hypertension in first episode infective endocarditis among intravenous buprenorphine users: case report. Am J Drug Alcohol Abuse. 2009;35:199–202.
- Tookes H, Diaz C, Li H, et al. A cost analysis of hospitalizations for infections related to injection drug use at a county safety-net hospital in Miami, Florida. PLoS One. 2015;10:e0129360.
- Alexander D, Alexander K, Valentino J. Intranasal hydrocodone-acetaminophen abuse induced necrosis of the nasal cavity and pharynx. Laryngoscope. 2012;122:2378–2381.
- Houlton JJ, Donaldson AM, Zimmer L, et al. Intranasal drug-induced fungal rhinopharyngitis. Int Forum Allergy Rhinol. 2012;2:130–134.
- Vosler PS, Ferguson BJ, Contreras JI, et al. Clinical and pathologic characteristics of intranasal abuse of combined opioid-acetaminophen medications. Int Forum Allergy Rhinol. 2014;4:839–844.
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2010;309:657–659.
- Centers for Disease Control and Prevention. CDC Vitalsigns™: opioid painkiller prescribing. 2014 Jul [cited 2015 Oct 20]. Available from: http://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf
- Mowry JB, Spyker DA, Cantilena LR Jr, et al. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032–1283.
- Centers for Disease Control and Prevention. Policy impact: prescription painkiller overdoses. 2011 Nov [cited 2015 Oct 20]. Available from: http://www.cdc.gov/HomeandRecreationalSafety/pdf/PolicyImpact-PrescriptionPainkillerOD.pdf
- Katz NP, Birnbaum H, Brennan MJ, et al. Prescription opioid abuse: challenges and opportunities for payers. Am J Manag Care. 2013;19:295–302.
- Schneider JP, Matthews M, Jamison RN. Abuse-deterrent and tamper-resistant opioid formulations: what is their role in addressing prescription opioid abuse? CNS Drugs. 2010;24:805–810.
- Lourenço LM, Matthews M, Jamison RN. Abuse-deterrent and tamper-resistant opioids: how valuable are novel formulations in thwarting non-medical use? Expert Opin Drug Deliv. 2013;10:229–240.
- Schaeffer T. Abuse-deterrent formulations, an evolving technology against the abuse and misuse of opioid analgesics. J Med Toxicol. 2012;8:400–407.
- Alexander L, Mannion RO, Weingarten B, et al. Development and impact of prescription opioid abuse deterrent formulation technologies. Drug Alcohol Depend. 2014;138:1–6.
- Mastropietro DJ, Omidian H. Abuse-deterrent formulations: part 1 - development of a formulation-based classification system. Expert Opin Drug Metab Toxicol. 2015;11:193–204.
- Mastropietro DJ, Omidian H. Abuse-deterrent formulations: part 2: commercial products and proprietary technologies. Expert Opin Drug Metab Toxicol. 2015;16:305–323.
- Food and Drug Administration. Abuse-deterrent opioids: evaluation and labeling guidance for industry. 2015 Apr [cited 2015 Oct 20]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf
- Sellers EM, Perrino PJ, Colucci SV, et al. Attractiveness of reformulated OxyContin® tablets: assessing comparative preferences and tampering potential. J Psychopharmacol. 2013;27:808–816.
- Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol. 2014;54:468–477.
- Colucci SV, Perrino PJ, Shram M, et al. Abuse potential of intravenous oxycodone/naloxone solution in nondependent recreational drug users. Clin Drug Investig. 2014;34:421–429.
- Katz N, Sun S, Johnson F, et al. ALO-01 (morphine sulfate and naltrexone hydrochloride) extended release capsules in the treatment of chronic pain of osteoarthritis of the hip or knee: pharmacokinetics, efficacy, and safety. J Pain. 2010;11:303–311.
- Stauffer J, Setnik B, Sokolowska M, et al. Subjective effects and safety of whole and tampered morphine sulfate and naltrexone hydrochloride (ALO-01) extended-release capsules versus morphine solution and placebo in experienced non-dependent opioid users: a randomized, double-blind, placebo-controlled, crossover study. Clin Drug Investig. 2009;29:777–790.
- Setnik B, Roland CL, Goli V, et al. A clinical trial to determine if corelease of morphine and naltrexone from crushed extended-release capsules induces withdrawal in opioid-dependent patients: a descriptive analysis of six patients. J Opioid Manag. 2013;9:139–150.
- Webster LR, Johnson FK, Stauffer J, et al. Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users. Drugs R D. 2011;11:259–275.
- Extended-release hydrocodone (Hysingla ER) for pain. Med Lett Drugs Ther. 2015;57:71–72.
- Inspiron Delivery Technologies LLC. Inspirion Delivery Technologies receives FDA approval for MorphaBond™ (morphine sulfate) extended-release tablets CII, an opioid analgesic formulated with abuse-deterrent properties. 2015 Oct 5 [cited 2015 Oct 20]. Available from: http://www.prnewswire.com/news-releases/inspirion-delivery-technologies-receives-fda-approval-for-morphabond-morphine-sulfate-extended-release-tablets-cii-an-opioid-analgesic-formulated-with-abuse-deterrent-properties-300153910.html?$G1Ref
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–189.
- Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14:351–358.
- Coplan PM, Kale H, Sandstrom L, et al. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22:1274–1282.
- Severtson SG, Bartelson BB, Davis JM, et al. Reduced abuse, therapeutic errors, and diversion following reformulation of extended-release oxycodone in 2010. J Pain. 2013;14:1122–1130.
- Havens JR, Leukefeld CG, DeVeaugh-Geiss AM, et al. The impact of a reformulation of extended-release oxycodone designed to deter abuse in a sample of prescription opioid abusers. Drug Alcohol Depend. 2014;139:9–17.
- Sessler NE, Downing JM, Kale H, et al. Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf. 2014;23:1238–1246.
- Cicero TJ, Ellis MS. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from OxyContin. JAMA Psychiatry. 2015;72:424–430.
- Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248.
- Larochelle MR, Zhang F, Ross-Degnan D, et al. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med. 2015;175:978–987.
- White AG, LeCates J, Birnbaum HG, et al. Positive subjective measures in abuse liability studies and real-world nonmedical use: potential impact of abuse-deterrent opioids on rates of nonmedical use and associated healthcare costs. J Opioid Manag. 2015;11:199–210.
- Kirson NY, Shei A, White AG, et al. Societal economic benefits associated with an extended-release opioid with abuse-deterrent technology in the United States. Pain Med. 2014;15:1450–1454.
- Rossiter LF, Kirson NY, Shei A, et al. Medical cost savings associated with an extended-release opioid with abuse-deterrent technology in the US. J Med Econ. 2014;17:279–287.
- Cassidy TA, DasMahapatra P, Black RA, et al. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 2014;15:440–451.
- Peacock A, Degenhardt L, Hordern A, et al. Methods and predictors of tampering with a tamper-resistant controlled-release oxycodone formulation. Int J Drug Policy. 2015 June 7. [Epub ahead of print].
- McNaughton EC, Coplan PM, Black RA, et al. Monitoring of internet forums to evaluate reactions to the introduction of reformulated OxyContin to deter abuse. J Med Internet Res. 2014;16:e119.
