Cortese et al. [Citation1] proposed a link between attention-deficit hyperactivity disorder (ADHD), obesity, and iron deficiency in Postgraduate Medicine. A later meta-analyses conducted by Cortese et al. [Citation2] concluded a positive association between ADHD and obesity and overweight in both children and adults. Importantly, when the authors stratified the analyses by medication status, the association was lost, although this finding was correlational and should not be taken to imply any utility of the medications in the management of obesity and overweight.
The findings of the meta-analyses conducted by Cortese et al. [Citation2] are clarified by the recent investigation of food-eating patterns in Greek preschool children and associated ADHD symptoms. Leventakou et al. [Citation3] report that food responsiveness and emotional overeating were positively associated with symptoms of ADHD. Furthermore, hemoglobin A1C levels were recently shown to be higher in drug-naïve ADHD children compared to control children,[Citation4] suggesting altered glucose homeostasis may accompany abnormal food preferences and obesity risk in ADHD. More research clarifying the neurobiological mechanisms to explain these findings is, therefore, warranted and is this commentary’s offering.
Newman et al. [Citation5] have reported an association between early exposure to traffic-related air pollution and hyperactivity scores at 7 years of age. Forns et al. [Citation6] found that traffic-related air pollution was positively associated with impaired behavioral development in Spanish school children, while noise pollution was significantly associated with ADHD symptomatology. Nitrous oxide (N2O) is an oxide of nitrogen and a recognized environmental air pollutant that is derived from many sources, including agriculture [Citation7] and transportation [Citation8]. Recent studies assessing environmental N2O from indirect agricultural sources (i.e. bodies of water) in southern Minnesota (USA) have reported levels that are significantly higher than established Intergovernmental Panel on Climate Change estimates [Citation7], suggesting that a more comprehensive reevaluation of environmental N2O burden is warranted. Interestingly, obesity rates are climbing significantly in states with large agricultural operations [Citation9], and perhaps more importantly, a recent epidemiological study on ADHD (case discernment derived from inpatient psychiatric evaluation, and therefore representing possible ‘severe’ ADHD) indicated that the highest ADHD prevalence occurred in the states of Nebraska and Minnesota [Citation10,Citation11].
In accord with the literature identifying ADHD symptoms in children exposed to general anesthesia for surgery [Citation12], it has been proposed that increasing exposure to environmental N2O—most especially in light of a marked reduction in other air pollutants [Citation13]—may be the principal exposure contributing to ADHD and other neurodevelopmental pathologies, like autism spectrum disorders (ASD).[Citation10,Citation11,Citation14,Citation15] Through targeting several neural substrates, including those of the opioidergic, cholinergic, dopaminergic, and glutamatergic systems, this exposure may cumulatively induce a chronic parasympathetic state in those exposed [Citation15], as has been shown in healthy humans exposed to inhalational N2O [Citation16]. Given that environmentally relevant concentrations (i.e. trace amounts) of N2O have been found to exert adverse cognitive effects that are represented in ADHD (i.e. working memory) in adult human males [Citation17], the author identifies the empirically documented physiological targets of N2O and how their dysregulation may explain the association between obesity and ADHD, as depicted in .
Figure 1. Inhalational exposure to air pollutants like nitrous oxide (N2O) may spur alterations in opioidergic, glutamatergic, dopaminergic, and cholinergic activity, heightening parasympathetic dominance and reducing sympathetic activity, rendering susceptibility to cognitive deficits characteristic of ADHD and adverse metabolic effects, like obesity. α7nAChR: alpha 7 nicotinic acetylcholine receptor; DaR: dopamine receptor; NmdaR: NMDA receptor; Da: dopamine; DaT: dopamine transporter; KoR: kappa-opioid receptor; nNOS: neuronal nitric oxide synthase; NE: norepinephrine; N2O: nitrous oxide.
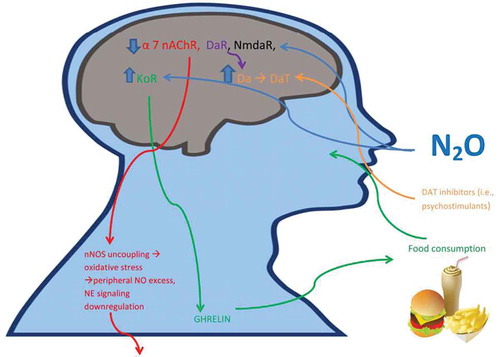
Exposure to N2O has been shown to reversibly inhibit human alpha 7 nicotinic acetylcholine receptors (α7 nAChR).[Citation18] As the nicotinic receptor enhances glutamatergic firing that potentiates working memory and attention-related circuitry in prefrontal cortex [Citation19], inhibition of this particular receptor subtype from chronic exposure to N2O may play a role in ADHD etiopathology, as well. The consequence to cognitive processes may be further compounded by N2O’s antagonism of N-methyl-D-aspartate receptors [Citation20].
Central stimulation of α7 nAChR has also been implicated in eating reward behavior and satiety processes via its action on glutamatergic firing and consequent neuronal release of dopamine [Citation21]. Peripherally, α7 nAChR acts as anti-inflammatory and activation has been demonstrated to improve obesity-induced inflammation [Citation22], suggesting its perturbation from increasing air pollution exposures, like N2O, could augur both adverse neurologic and metabolic effects in humans.
Since α7 nAChR has been shown to be coupled with neuronal nitric oxide synthase (nNOS) [Citation23], inhibition of α7 nAChR may be expected to promote a nNOS uncoupling [Citation24], leading to compensatory peripheral formation of oxidative stress markers, like hydrogen peroxide (H2O2), to reestablish central nitric oxide (NO) levels.[Citation25] A meta-analysis investigating oxidative stress in ADHD has found elevated levels of peripheral NO.[Citation26] As NO promotes parasympathetic tone [Citation27], excess levels of NO could initiate a parasympathetic dominant state in ADHD, as has been shown [Citation15]. This is further corroborated since activation of α7 nAChR on sympathetic nerves stimulated norepinephrine (NE) release, facilitating neuronal vasodilation [Citation28], and therefore, N2O-mediated inhibition of α7 nAChR may directly negatively affect sympathetic activity, although the analgesic action of N2O includes NE release in spinal cord [Citation29]. This action may overcome the cholinergic inhibition of central sympathetic activity, and therefore institute a compensatory downregulation in NE synthesis downstream. While increased peripheral oxidative stress biomarkers may be necessary to restore central NO metabolism, it is also possible that elevated peripheral inflammation may be linked to independent endogenous mechanisms (i.e. hypertension) to restore autonomic balance in humans [Citation30]. This may explain the usefulness of the spontaneously hypertensive rat model in studying ADHD prior to induction of a hypertensive state [Citation31]. This dysregulation of central sympathetic activity may also be accompanied by direct N2O-mediated enhancements in parasympathetic control to further aggravate obesogenic risk in ADHD subjects.
N2O targets the dynorphin/kappa opioid receptor system (KOR) [Citation32]. Activation of the KOR system has been implicated in parasympathetic control, as atropine methyl nitrate treatment (vagal blocker) prevented the bradycardia elicited by a highly selective KOR agonist, U50488, injection in dorsal motor nucleus of the vagus [Citation33]. Rao, Conley, and Dunbar confirm the reduction in mean arterial pressure from central administration of the endogenous KOR ligand, dynorphin [Citation34]. Hypothalamic KOR activation is thought to induce gastrointestinal ghrelin secretion to spur food intake [Citation35], and administration of KOR antagonists in hypothalamic paraventricular nucleus has been shown to reduce deprivation, glucoprivic, and sucrose intake in rats [Citation36]. Expectedly then, central KOR responsiveness on cardiovascular activity is decreased in dietary-induced obesity [Citation37], suggesting initiation of sympathetic resuscitation, including the mesolimbic dopaminergic system, in obesity as part of an endogenous feedback loop.
However, exposure to N2O may target dopaminergic activity through possible impairment of dopamine receptor 4 (DR4) via N2O-mediated inhibition of methionine synthase [Citation10,Citation11]. DR4 is a receptor that has been implicated in ADHD [Citation38]. Activation of dopamine 2 receptor, a receptor previously implicated in both parasympathetic tone in humans [Citation39] and compulsive eating behavior in obese rats [Citation40], has been shown to mediate the antinociceptive properties of N2O [Citation41]. Striatal expression of this receptor subtype is downregulated in obesity, suggesting a compensatory neural mechanism to counter chronic N2O exposure [Citation40]. These dopamine receptor-specific, parasympathetic-promoting consequences from chronic N2O exposure could explain the significant increase in neural levels of dopamine [Citation42] (which has also been shown in rats exposed to environmentally relevant concentrations of N2O [Citation43]) and attendant increase in dopamine transporters in ADHD as shown in the neuroimaging literature on ADHD [Citation44]. This dopaminergic dysregulation is perhaps why psychostimulants that rescue dopaminergic activity are effective in ADHD management.
Psychostimulants used in the management of ADHD exert their effects via enhancement of catecholaminergic activity in certain neural regions [Citation45]. The documented effects of these widely used psychostimulants may explain why Cortese [Citation2] did not find any association between ADHD and obesity when controlling for medication status. In the nonmedicated ADHD subject, however, central catecholaminergic activity may remain dysregulated as has been described here, potentiating disruption of the critical neuro-adipose cross talk on a myriad of levels and facilitating the development of an obesity phenotype [Citation46]. Obesity and overweight have been previously associated with sympathetic activity in humans [Citation47], likely serving as a feedback mechanism to reverse the complex and pathological parasympathetic dominant state attributed to certain environmental exposures, such as N2O. Elevations in obesity-induced sympathetic tone may also be positively associated with leptin expression [Citation48], and leptin has been directly implicated in hepatic hepcidin expression in vitro, highlighting a mechanism by which obesity may further promote a dysfunctional regulation of iron homeostasis in the body.[Citation49]
Collectively, these studies suggest that chronic exposure to environmental N2O may impart a parasympathetic dominant state through central cholinergic inhibition of α7 AChR, targeting of KOR, and dysregulation of dopaminergic functioning. These mechanisms may stimulate uncontrolled eating behaviors and impair the critical neuro-adipose crosstalk, leading to the development of obesity. Paradoxically, an obesity-mediated phenotype may serve a homeostatic purpose to restore sympathetic tone, which is also accompanied by other obesity-mediated physiological changes, like aberrations in iron homeostasis and perhaps other vitamin deficiencies [Citation15], as has been emphasized by Cortese et al.[Citation1,Citation2]. Additional research is needed to better quantify air pollution exposures and their relevance or not to metabolic dysfunction [Citation50], with particular focus on conditions where obesity and overweight are disproportionately represented, like ADHD and ASD.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Cortese S, Angriman M. Attention-deficit/hyperactivity disorder, iron deficiency, and obesity: is there a link? Postgrad Med. 2014;126:155–170. doi:10.3810/pgm.2014.07.2793.
- Cortese S, Moreira-Maia CR, St Fleur D, et al. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry. 2015. doi:10.1176/appiajp.201515020266.
- Leventakou V, Micali N, Georgiou V, et al. Is there an association between eating behaviour and attention-deficit/hyperactivity disorder symptoms in preschool children? J Child Psychol Psychiatry. 2015. doi:10.1111/jcpp.12504.
- Lindblad F, Eickhoff M, Forslund AH, et al. Fasting blood glucose and HbA1c in children with ADHD. Psychiatry Res. 2015;226(2–3):515–516. doi:10.1016/j.psychres.2015.01.028.
- Newman NC, Ryan P, Lemasters G, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 2013;121:731–736. doi:10.1289/ehp.1205555.
- Forns J, Dadvand P, Foraster M, et al. Traffic-related air pollution, noise at school, and behavioral problems in Barcelona schoolchildren: a cross-sectional study. Environ Health Perspect. 2015;124. doi:10.1289/ehp.1409449.
- Turner PA, Griffis TJ, Lee X, et al. Indirect nitrous oxide emissions from streams within the US Corn Belt scale with stream order. Proc Natl Acad Sci U S A. 2015;112:9839–9843. doi:10.1073/pnas.1503598112.
- Lipman TE, Delucchi MA. Emissions of nitrous oxide and methane from conventional and alternative fuel motor vehicles. Climatic Change. 2002;53:477–516.
- Perry S Minnesota’s obesity rate rose to 27.6% in 2014. MinnPost. 2015 Aug 22 [cited 2016 Mar 18]. Available from: https://www.minnpost.com/second-opinion/2015/09/minnesotas-obesity-rate-rose-276-2014
- Fluegge KR, Fluegge KR. Retracted due to publication error, but passed peer review: glyphosate predicts ADHD in Healthcare Cost and Utilization Project net (HCUPNET). Plos One. 2015;10(8):e0133525. doi:10.1371/journal.pone.0133525.
- Fluegge K, Fluegge K. Glyphosate use predicts healthcare utilization for ADHD in the Healthcare Cost and Utilization Project net (HCUPnet): a two-way fixed-effects analysis. Pol J Env Studies. 2016;25(4):1–15. doi:10.15244/pjoes/61742
- Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87(2):120–129. doi:10.1016/j.mayocp.2011.11.008.
- Environmental Protection Agency. Air quality trends. Research Triangle Park (NC); 2015 [cited 2016 Jan 2]. Available from: http://www3.epa.gov/airtrends/aqtrends.html
- Fluegge K. Do toxic synergies of underlying etiologies predispose the positive association between traumatic brain injury and ADHD? J Atten Disord. 2016. doi:10.1177/1087054716633858.
- Fluegge K. A reply to Wang T, Shan L, Du L, Feng J, Xu Z, Staal WG, Jia F. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2015; doi: 10.1007/s00787-015-0786-1. Eur Child Adolesc Psychiatry. 2015. doi:10.1007/s00787-015-0803-4.
- Okushima K, Kohjitani A, Asano Y, et al. Inhalational conscious sedation with nitrous oxide enhances the cardiac parasympathetic component of heart rate variability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):e1–e5. doi:10.1016/j.tripleo.2008.08.020.
- Bruce DL, Bach MJ, Arbit J. Trace anesthetic effects on perceptual, cognitive, and motor skills. Anesthesiology. 1974;40:453–458.
- Suzuki T, Ueta K, Sugimoto M, et al. Nitrous oxide and xenon inhibit the human (alpha 7)5 nicotinic acetylcholine receptor expressed in Xenopus oocyte. Anesth Analg. 2003;96(2):443–448.
- Yang Y, Paspalas CD, Jin LE, et al. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2013;110(29):12078–12083. doi:10.1073/pnas.1307849110.
- Jevtović-Todorović V, Todorović SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4(4):460–463.
- McFadden KL, Cornier M-A, Tregellas JR. The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol. 2014;5:553. doi:10.3389/fpsyg.2014.00553.
- Wang X, Yang Z, Xue B, et al. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152(3):836–846. doi:10.1210/en.2010-0855.
- Haberberger RV, Henrich M, Lips KS, et al. Nicotinic receptor alpha 7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem Cell Biol. 2003 Sep;120(3):173–181.
- Fluegge K. A reply to ‘metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study’. Transl Psychiatry. 2016;6:e793. doi:10.1038/tp.2016.24.
- Shimizu S, Shiota K, Yamamoto S, et al. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med. 2003;34(10):1343–1352.
- Joseph N, Zhang-James Y, Perl A, et al. Oxidative stress and ADHD: a meta-analysis. J Atten Disord. 2015;19(11):915–924. doi:10.1177/1087054713510354.
- Chowdhary S, Vaile JC, Fletcher J, et al. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36(2):264–269.
- Si M-L, Lee TJF Alpha7-nicotinic acetylcholine receptors on cerebral perivascular sympathetic nerves mediate choline-induced nitrergic neurogenic vasodilation. Circ Res. 2002 12;91(1):62–69.
- Zhang C, Davies MF, Guo TZ, et al. The analgesic action of nitrous oxide is dependent on the release of norepinephrine in the dorsal horn of the spinal cord. Anesthesiology. 1999;91(5):1401–1407.
- Chae CU, Lee RT, Rifai N, et al. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38(3):399–403.
- Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi:10.1186/1744-9081-1-9.
- Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9–18. doi:10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2.
- Hassen AH, Broudy EP. Selective autonomic modulation by mu- and kappa-opioid receptors in the hindbrain. Peptides. 1988;9(Suppl 1):63–67.
- Rao SP, Conley A, Dunbar JC. Cardiovascular responses to central administration of mu and kappa opioid receptor agonist and antagonist in normal rats. Peptides. 2003;24(5):745–754.
- Romero-Picó A, Vázquez MJ, González-Touceda D, et al. Hypothalamic κ-opioid receptor modulates the orexigenic effect of ghrelin. Neuropsychopharmacology. 2013;38(7):1296–1307. doi:10.1038/npp.2013.28.
- Koch JE, Glass MJ, Cooper ML, et al. Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neuroscience. 1995;66(4):951–957.
- Barnes MJ, Jen K-LC, Dunbar JC. The effect of CNS opioid on autonomic nervous and cardiovascular responses in diet-induced obese rats. Peptides. 2004;25(1):71–79.
- Yuen EY, Yan Z. Cellular mechanisms for dopamine D4 receptor-induced homeostatic regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:24957–24965. doi:10.1074/jbc.M111.221416.
- Kaya D, Ellidokuz E, Onrat E, et al. The effect of dopamine type-2 receptor blockade on autonomic modulation. Clin Auton Res. 2003;13(4):275–280.
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi:10.1038/nn.2519.
- Koyanagi S, Himukashi S, Mukaida K, et al. Dopamine D2-like receptor in the nucleus accumbens is involved in the antinociceptive effect of nitrous oxide. Anesth Analg. 2008;106(6):1904–1909. doi:10.1213/ane.0b013e318172b15b.
- Sakamoto S, Nakao S, Masuzawa M, et al. The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study. Anesth Analg. 2006;103(6):1459–1463.
- Abdul-Kareem HS, Sharma RP, Drown DB. Effects of repeated intermittent exposures to nitrous oxide on central neurotransmitters and hepatic methionine synthetase activity in CD-1 mice. Toxicol Ind Health. 1991;7:89–108.
- Spencer TJ, Biederman J, Madras BK, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57(11):1293–1300.
- Spencer RC, Devilbiss DM, Berridge CW. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol Psychiatry. 2015;77:940–950. doi:10.1016/j.biopsych.2014.09.013.
- Zeng W, Pirzgalska RM, Pereira MMA, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94. doi:10.1016/j.cell.2015.08.055.
- Lambert E, Sari CI, Dawood T, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi:10.1161/HYPERTENSIONAHA.110.155663.
- Pontiroli AE, Pizzocri P, Paroni R, et al. Sympathetic overactivity, endothelial dysfunction, inflammation, and metabolic abnormalities cluster in grade III (World Health Organization) obesity: reversal through sustained weight loss obtained with laparoscopic adjustable gastric banding. Diabetes Care. 2006;29(12):2735–2738.
- Chung B, Matak P, McKie AT, et al. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137(11):2366–2370.
- McConnell R, Shen E, Gilliland FD, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect. 2015;123(4):360–366. doi:10.1289/ehp.1307031.
