ABSTRACT
Approximately 90% of T2D patients in the US are diagnosed and treated in the primary care setting, and the majority of the burden of disease management falls to primary care providers. Here, we discuss the clinical data for once weekly dulaglutide, e.g. the results of seven completed Phase 3 trials, patient preference studies, patient reported outcomes (PRO), and clinical data surrounding the dulaglutide administration device. Dulaglutide 1.5 mg once weekly demonstrated superiority to placebo, metformin, sitagliptin, exenatide BID, and insulin glargine (in 2 trials), and non-inferiority to liraglutide in reduction of HbA1c from baseline, with an acceptable safety profile. Dulaglutide-treated patients achieved the composite endpoint of an HbA1c <7.0% with no hypoglycemia, no severe hypoglycemia, and no weight gain significantly more than metformin, sitagliptin, exenatide BID or insulin glargine treated patients. Dulaglutide consistently showed an early onset of glycemic control, lasting up to 104 weeks. Additionally, PRO and patient preference data support the benefit of once weekly dulaglutide for the treatment of T2D.
Introduction
Type 2 diabetes mellitus (T2D) is an endocrine disorder characterized by hyperglycemia due to inadequate control by the normal glucose regulatory systems (decreased insulin secretion, increased insulin resistance, and others). Abnormalities in these glucoregulatory systems cause chronic high blood sugar due to the lack of an insulin signal for circulating glucose to enter cells [Citation1]. Without timely and appropriate medical intervention, T2D can lead to multiple comorbidities including cardiovascular disease and stroke, kidney failure, blindness, numbness, and tingling in the extremities (leading to amputation), and potential neurological disorders [Citation2]. Alternatively, inappropriately aggressive treatment can lead to low blood sugar (hypoglycemia), which can cause serious and debilitating symptoms or death. With greater than 90% of T2D cases diagnosed and treated in the primary care setting in the US, chronic diseases such as diabetes require long-term provider care, as well as disease self-management, decision support, community support and resources, and health-care organizations committed to a chronic care model [Citation3].
Several standard clinical laboratory measures of diabetes status aid in the diagnosis and treatment of diabetes, including glycated hemoglobin A1c (HbA1c), fasting blood glucose (FBG), oral glucose tolerance test, and finger-stick self-monitored plasma glucose. Supplementary Table 1 provides a detailed description of each measure.
Table 1. Efficacy results at primary end point.
As demonstrated in several large clinical trials investigating different treatment strategies for T2D [Citation2,Citation4–Citation6], a decrease in HbA1c has been shown to decrease microvascular complications of diabetes (nephropathy, neuropathy, and retinopathy) [Citation7]. Available data also indicates that moderate diabetic retinopathy is almost nonexistent in patients maintaining an HbA1c ≤ 6.5% [Citation8]. For this reason, the American Diabetes Association, European Association for the Study of Diabetes, International Diabetes Federation, and others have established treatment goals in terms of HbA1c and other glycemic laboratory measures. Guidance developed by these governing bodies are similar, with HbA1c targets of either <7.0% or ≤6.5% [Citation8–Citation10].
In general, the treatment continuum starts with lifestyle modifications including an improved diet and regular exercise. Metformin is the standard drug for first-line therapy, with second- and third-line oral or injectable options recommended based on treatment individualization and comorbidity/tolerability to various antihyperglycemic agents [Citation8]. However, many patients will eventually need insulin therapy (in one of various insulin regimens), in order to supplement the inability of β-cells to produce insulin [Citation8–Citation10].
Many different classes of antihyperglycemic agents are available to health-care providers (HCPs), each of which may be amenable to multiple pathophysiological abnormalities found in patients with T2D (the ‘ominous octet’: muscle, liver, fat and brain insulin resistance, β-cell failure, incretin dysfunction, hyperglucagonemia, and increased kidney glucose reabsorption), and each with a specific safety and tolerability profile [Citation1]. For example, metformin treatment can cause gastrointestinal side effects, while insulin injections can cause weight gain and potential hypoglycemic events [Citation11]. Additional antihyperglycemic agents, known broadly as incretin-based therapies, have become available globally for the treatment of T2D. This class includes dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) [Citation12].
Briefly, incretins are gut hormones (GLP-1 and gastric inhibitory polypeptide) released by the digestive system in response to ingested nutrients that help regulate glycemia through improved insulin secretion and inhibition of glucagon secretion. Incretin-based drug therapies work either by increasing the concentration of endogenous GLP-1 or by binding the GLP-1 receptor, thus eliciting its antihyperglycemic effects. These drugs act to correct multiple abnormalities found in patients with T2D by both increasing insulin secretion and decreasing glucagon secretion in a glucose-dependent manner, delaying gastric emptying, and may cause weight loss [Citation12–Citation17]. Direct administration of GLP-1 is not an effective treatment because it has a short half-life of 2–3 min because it is quickly degraded by the enzyme DPP-4 [Citation18,Citation19]. Based on this information, two classes of incretin-based therapies have been developed: DPP-4 inhibitors and GLP-1 RAs. The first, DPP-4 inhibitors act by increasing the endogenous concentration of GLP-1 by preventing DPP-4 from degrading GLP-1. The second, GLP-1 RAs are molecules that are able to stimulate the same physiological response as endogenous GLP-1, but exhibit resistance to DPP-4 degradation due to the engineering of the molecule, thereby primarily prolonging the incretin response [Citation12].
Dulaglutide is a once weekly human GLP-1 RA approved for the treatment of adult patients with T2D. The dulaglutide molecule is composed of two identical peptide chains, with each chain comprised of a chain of human GLP-1 analog and a linker, which binds to an immunoglobulin (Ig) G4 heavy chain (). The GLP-1 analog portion of dulaglutide is approximately 90% homologous to native human GLP-1. Three alterations to the GLP-1 portion of the molecule were incorporated to optimize its clinical profile, including protection from DPP-4 inactivation, increased solubility, and reduction of immunogenicity [Citation20]. The addition of the IgG4 fragment crystallizable (Fc) portion helped to decrease the rate of renal clearance of dulaglutide. Additionally, the Fc fragment of IgG4 was also modified to prevent antibody formation and to reduce the potential for immunologic cytotoxicity. As a result of this engineering, dulaglutide has a half-life of approximately 5 days, with a peak concentration at a median of 48 h, making it suitable for once weekly subcutaneous administration. The molecule is absorbed after subcutaneous injection, and reaches steady state between 2 and 4 weeks after treatment initiation [Citation21]. The pharmacokinetic data suggest that dose adjustment of dulaglutide is not necessary on the basis of age, sex, race, ethnicity, body weight, or injection site. In patients with varying degrees of renal or hepatic impairment, no relevant change in dulaglutide exposure was observed relative to the degree of renal or hepatic impairment. Dulaglutide is presumed to be degraded into component peptides and amino acids by general protein catabolism mechanisms [Citation22].
Figure 1. The dulaglutide molecule.
This figure has been reprinted with permission from Kuritzky et. al 2014 [Citation23].
Color code in B is representative of region color in A.
GLP-1: glucagon-like peptide-1; IgG: immunoglobulin gamma; Fab: fragment antigen binding; Fc: fragment crystallizable
![Figure 1. The dulaglutide molecule.This figure has been reprinted with permission from Kuritzky et. al 2014 [Citation23].Color code in B is representative of region color in A.GLP-1: glucagon-like peptide-1; IgG: immunoglobulin gamma; Fab: fragment antigen binding; Fc: fragment crystallizable](/cms/asset/6d9bfd91-98a6-4301-ae58-4c7d95c27581/ipgm_a_1218260_f0001_c.jpg)
Dulaglutide exhibits several antihyperglycemic actions of GLP-1. In the presence of elevated glucose concentrations, dulaglutide acts on pancreatic β-cells leading to insulin release and also suppresses glucagon secretion. It suppresses glucagon secretion which is known to be inappropriately elevated in patients with T2D. Lower glucagon concentrations lead to decreased hepatic glucose output.
Dulaglutide is available commercially in the US and EU as 0.75 mg/0.5 mL or 1.5 mg/0.5 mL solution in a disposable, ready-to-use pen, with no need for reconstitution based on its solubility profile. Each ready-to-use pen contains only one weekly dose of dulaglutide (0.75 mg or 1.5 mg).
A recent study highlighted the widespread use of GLP-1 RAs for treatment of T2D in the UK [Citation24]. Physicians’ reports of prescription patterns and reasons for prescribing these medications suggested that they generally have a good understanding of the potential benefits of this class of drug for specific types of patients. However, almost a 25% of general practice physicians reported that they still do not have enough information to prescribe within the GLP-1 RA class. This finding suggests that increased dissemination of information to targeted groups of physicians may be useful for ensuring that all appropriate treatment options are considered. Hence, although there have already been published primary manuscripts and reviews [Citation25], this review article is intended to provide practicing physicians with an updated overview of dulaglutide efficacy data, safety profile, and key patient-reported outcomes (PROs) from the clinical program.
Overview of completed dulaglutide AWARD clinical trials
The Assessment of Weekly AdministRation of LY2189265 (dulaglutide) in Diabetes (AWARD) clinical trial program reported in this review consisted of seven completed Phase 3 clinical trials (AWARD-1 to AWARD-6 and AWARD-8) comparing dulaglutide 1.5 mg and/or dulaglutide 0.75 mg to a variety of common antihyperglycemic medications. These Phase 3 studies were designed to assess efficacy, safety, and PROs in patients across different stages of the T2D treatment continuum from monotherapy (AWARD-3), in patients taking concomitant sulfonylurea (SU) (AWARD-8), metformin (AWARD-5 and AWARD-6), metformin and thiazolidinedione (TZD) (AWARD-1), metformin and SU (AWARD-2), or in combination with prandial insulin with or without metformin (AWARD-4) with a study duration of 24–104 weeks () [Citation26–Citation33]. A diverse patient population representing the continuum of T2D progression was examined across the seven trials, with a mean baseline age of 54.1–59.4 years, a mean duration of diabetes of 2.6–12.7 years, mean HbA1c of 7.6–8.5%, mean weight of 85.5–96.0 kg, FBG of 154–177 mg/dL, and previous treatment ranging from antihyperglycemic medication naive to third-line therapy.
Figure 2. Completed and published AWARD clinical trials across the diabetes continuum.
Abbreviations: BID: twice-daily; OAM: oral antihyperglycaemic medication; SU: sulfonylurea; TZD: Thiazolidinedione
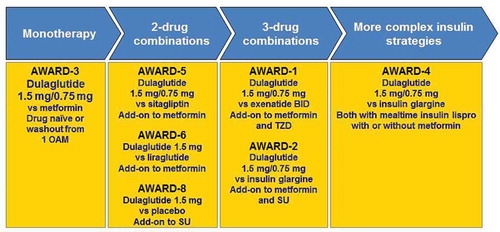
The seven completed Phase 3 controlled studies presented in this report were designed to establish the superiority of dulaglutide for HbA1c change from baseline to placebo and/or noninferiority/superiority of dulaglutide to active comparator. Comparators were chosen amongst a variety of antihyperglycemic therapeutic classes, such as biguanides (metformin), DPP-4 inhibitors (sitagliptin), GLP-1 RAs (liraglutide, exenatide twice-daily [BID]), and insulin (basal insulin glargine). The primary outcome measure in all seven studies was HbA1c change from baseline to the primary end point (6–12 months depending on the study); secondary efficacy measures, safety, and PRO measures were also evaluated. Long-term, comparator-controlled efficacy, safety, and PRO data were collected through the final end points (6 months through 24 months).
Early and sustained glycemic control
Weekly GLP-1 RAs are relatively new to the T2D treatment pharmacopoeia and, in general, demonstrate significant glucose lowering ability with an acceptable safety profile. Treatment with dulaglutide resulted in significant reductions from baseline in FBG. In studies where FBG was measured early in the course of treatment, the near-maximal effect on FBG concentrations occurred by 2 weeks. The improvement in FBG was sustained through the longest study duration of 104 weeks in AWARD-5 [Citation31]. In AWARD-3 study (monotherapy vs. metformin), treatment with dulaglutide also resulted in significant reductions in postprandial glucose (PPG) from baseline after 2 weeks and this reduction lasted through the study [Citation28]. Consistent with the early reduction in FBG and PPG, dulaglutide was shown to reduce HbA1c in patients with T2D as early as 4 weeks [Citation28,Citation30,Citation31,Citation33] after the start of treatment, and the reduction was shown to be sustained through 104 weeks [Citation31].
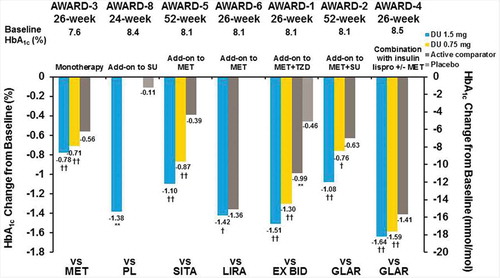
The range of HbA1c reduction from baseline to the primary end point with dulaglutide 1.5 mg was −0.78% to −1.64% and −0.71% to −1.59% for dulaglutide 0.75 mg, and −0.39% to −1.41% for active comparators ( and ) in the seven studies included in this report [Citation26–Citation33]. Across studies, dulaglutide 1.5 mg was superior, based on HbA1c change from baseline, to placebo in AWARD-1, AWARD-5, and AWARD-8 (; placebo data will not be discussed further in the context of this manuscript), and superior to the active comparators evaluated: metformin, sitagliptin, exenatide BID, and insulin glargine (the comparator in two separate studies), and noninferior to liraglutide at the primary end point. This effect was sustained in all studies through the final end point () [Citation26–Citation30,Citation32]. In four of the five Phase 3 studies that examined dulaglutide 0.75 mg-treated patients, at the primary end point, dulaglutide 0.75 mg was superior based on HbA1c change from baseline to placebo and the active comparators of metformin, sitagliptin, exenatide BID, and insulin glargine (AWARD-4) [Citation26,Citation28–Citation30,Citation33]. Dulaglutide 0.75 mg was noninferior to insulin glargine in a fifth study (AWARD-2) at the primary end point (, and ) [Citation27].
Table 2. Efficacy results at final end point.
Figure 3. AWARD trial efficacy outcomes at the primary endpoint, HbA1c change from baseline.
††multiplicity adjusted 1-sided p-value <0.025 for superiority, and †Multiplicity adjusted 1-sided p < 0.025 for non-inferiority (no adjustment for AWARD-6), versus active comparator **p < 0.001 for dulaglutide or active comparator versus placebo. Data presented are LS means, ITT, LOCF ANCOVA analysis except AWARD-6 (MMRM analysis).
Abbreviations: DU: dulaglutide; EX BID: exenatide twice-daily; GLAR: insulin glargine; HbA1c: glycated haemoglobin A1c; lispro: insulin lispro; LIRA: liraglutide; MET: metformin; PL: placebo; SITA: sitagliptin; SU: sulfonylurea; TZD: thiazolidinedione
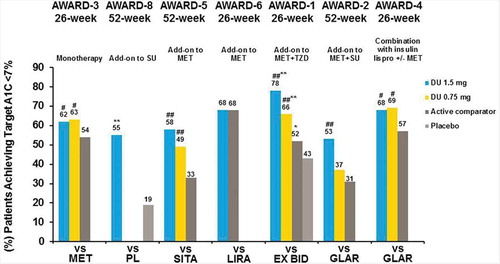
As previously indicated, specific HbA1c targets are associated with clinical outcomes and risks for diabetes-related comorbidities. In AWARD clinical trials, the ranges for percentages of patients reaching a target HbA1c of <7.0% were as follows: 53–78% for dulaglutide 1.5 mg, 37–69% for dulaglutide 0.75 mg, and 31–68% for patients treated with active comparators at the primary end point [Citation26,Citation27,Citation29,Citation32,Citation33]. Significantly more patients achieved an HbA1c target of <7.0% with dulaglutide 1.5 mg versus metformin, sitagliptin, exenatide BID, and insulin glargine (p < 0.05), with no difference observed in AWARD-6 versus liraglutide ( and ). Similar results were observed at the final end point for the percentages of patients achieving HbA1c goal of <7.0% () [Citation26–Citation30].
Figure 4. AWARD trial efficacy outcomes at the primary endpoint, percentage of patients achieving HbA1c targets.
*p < 0.05, **p < 0.001 for dulaglutide or active comparator versus placebo and #p < 0.05, ##p < 0.001 for dulaglutide versus active comparator. Data presented are LS means, ITT, LOCF ANCOVA analysis except AWARD-6 (MMRM analysis).
Abbreviations: DU: dulaglutide; EX BID: exenatide twice-daily; GLAR: insulin glargine; HbA1c: glycated haemoglobin A1c; lispro: insulin lispro; LIRA: liraglutide; MET: metformin; PL: placebo; SITA: sitagliptin; SU: sulfonylurea; TZD: thiazolidinedione
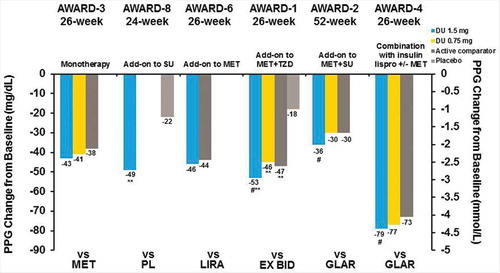
At the primary end point, treatment with dulaglutide 1.5 mg alone or in combination with oral antihyperglycemic medications or prandial insulin resulted in significant reductions from baseline in FBG, as measured by the central laboratory. Patients demonstrated FBG changes of −4.9 to −42.8 mg/dL with dulaglutide 1.5 mg, 4.0 to −34.2 mg/dL with dulaglutide 0.75 mg, and −16.2 to −34.3 mg/dL for active comparators ( and ). FBG reduction was similar at the final end point for both dulaglutide doses () [Citation26,Citation29–Citation32]. PPG decreased, depending on the study, from a minimum of −36.3 mg/dL to a maximum of −78.5 mg/dL with dulaglutide 1.5 mg, −29.7 to −76.5 mg/dL with dulaglutide 0.75 mg, and −29.7 to −72.5 mg/dL for active comparators at the primary end point ( and ) [Citation26,Citation27]. This effect was similar at the final end point ().
Figure 5. AWARD trial efficacy outcomes at the primary endpoint, FBG change from baseline.
**p < 0.001 for dulaglutide or active comparator versus placebo and #p < 0.05, ##p < 0.001 for dulaglutide versus active comparator. Data presented are LS means, ITT, LOCF ANCOVA analysis except AWARD-6 (MMRM analysis).
Abbreviations: DU: dulaglutide; EX BID: exenatide twice-daily; GLAR: insulin glargine; HbA1c: glycated haemoglobin A1c; lispro: insulin lispro; LIRA: liraglutide; MET: metformin; PL: placebo; SITA: sitagliptin; SU: sulfonylurea; TZD: thiazolidinedione
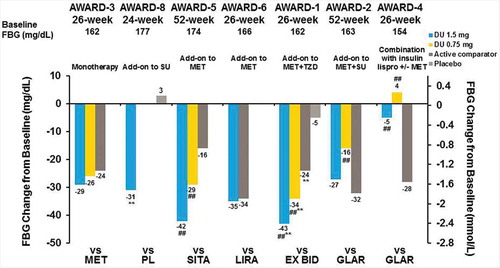
Figure 6. AWARD trial efficacy outcomes at the primary endpoint, PPG change from baseline.
**p < 0.001 for dulaglutide or active comparator versus placebo and #p < 0.05, ##p < 0.001 for dulaglutide versus active comparator. Data presented are LS means, ITT, LOCF ANCOVA analysis except AWARD-6 (MMRM analysis).
Abbreviations: DU: dulaglutide; EX BID: exenatide twice-daily; GLAR: insulin glargine; HbA1c: glycated haemoglobin A1c; lispro: insulin lispro; LIRA: liraglutide; MET: metformin; PL: placebo; SITA: sitagliptin; SU: sulfonylurea; TZD: thiazolidinedione
Weight change
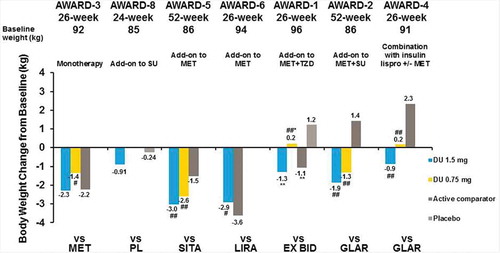
Excess weight and obesity are important factors in T2D, as excess body weight leads to glucose intolerance through insulin resistance, and can ultimately lead to the development of T2D. Additionally, body weight is linked to cardiovascular health and other key T2D comorbidities [Citation2]. One concern patients experience during T2D treatment is that some T2D therapies, including insulins, SUs, glinides, and TZDs, are associated with weight gain [Citation34]. Since weight and T2D disease processes are strongly linked, weight gain can exacerbate T2D as well as discourage patients from initiating therapy with a potential for weight gain. In the AWARD clinical trials reported in this article, patients experienced a weight change of −0.9 to −3.0 kg with dulaglutide 1.5 mg, 0.2 to −2.6 kg with dulaglutide 0.75 mg, and 2.3 to −3.6 kg with active comparators at the primary end point ( and ) [Citation26,Citation29,Citation30,Citation32,Citation33]. The weight changes were similar at the final end point (). Patients treated with liraglutide in AWARD-6 experienced statistically significantly greater weight loss than dulaglutide 1.5 mg (mean difference: 0.71 kg [0.17–1.26]; p < 0.05) [Citation32]. Of interest, patients treated with dulaglutide and insulin concomitantly experienced either attenuation of the weight gain generally experienced with insulin treatment or weight loss [Citation27,Citation29].
Figure 7. AWARD trial efficacy outcomes at the primary endpoint, weight change from baseline.
*p < 0.05, **p < 0.001 for dulaglutide or active comparator versus placebo and #p < 0.05, ##p < 0.001 for dulaglutide versus active comparator. Data presented are LS means, ITT, LOCF ANCOVA analysis except AWARD-6 (MMRM analysis).
Abbreviations: DU: dulaglutide; EX BID: exenatide twice-daily; GLAR: insulin glargine; HbA1c: glycated haemoglobin A1c; lispro: insulin lispro; LIRA: liraglutide; MET: metformin; PL: placebo; SITA: sitagliptin; SU: sulfonylurea; TZD: thiazolidinedione
Device and delivery benefits
In addition to the clinical benefits of glycemic control and potential for weight loss, the dulaglutide molecule and the device in which it is delivered have several novel characteristics. The large molecular size and protection from degradation by DPP-4 allow dulaglutide to be administered once weekly by subcutaneous injection. Weekly injections have the potential to improve patient perceptions. In fact, a study of injection-naive patients with T2D demonstrated that 83% of patients’ significantly preferred a once weekly dulaglutide medication profile compared to a once daily liraglutide medication profile (p ≤ 0.0001) [Citation35].
Additionally, another molecular modification increases solubility and allows dulaglutide to be delivered as a solution, with no reconstitution necessary prior to dosing. The molecular characteristics of dulaglutide allow it to be supplied in a ready-to-use pen that is discarded after use. The needle, with a 5 mm injection depth, is incorporated and hidden into the pen. The dose is premeasured, so the patient neither needs to handle or attach the needle, nor to measure the weekly dose.
To inject, the device is first uncapped, correctly positioned, and unlocked. Pressing the button on the top of the pen automatically initiates subcutaneous injection, and is followed by automatic needle retraction after the dose has been delivered. Associated with the injection steps, an audible ‘click’ can be heard when the injection is initiated, and another ‘click’ is heard when the needle retracts [Citation36].
A single-arm, placebo-controlled, open-label, out-patient study was conducted to understand patients’ perceptions regarding the dulaglutide device [Citation37]. Specifically, the dulaglutide ready-to-use pen filled with placebo was used to determine if injection-naive patients with T2D could safely and effectively use the ready-to-use pen and to assess patients’ perceptions of the device. The key study results showed that 99.1% of patients achieved the primary outcome of final self-injection successfully. Additionally, 99.0% reported that overall, the ready-to-use pen was ‘easy’ or ‘very easy’ to use. Thus, the clinical study demonstrated that the dulaglutide ready-to-use pen could be used safely and effectively by patients with T2D.
PROs in dulaglutide clinical trials
PRO measures are used in clinical trials to aid in the understanding of patients’ perceptions of their conditions and treatment. In T2D treatment, PRO end points are often used in concert with clinical end points to capture important elements of treatment regimens. Thus, in clinical trials, PRO measures can complement clinical outcomes by providing information beyond traditional efficacy and safety measures.
For example, treatment satisfaction is important largely because it is thought to provide an indication of treatment adherence [Citation38–Citation40]. In general, patients who are satisfied with their treatment can be expected to adhere to prescribed treatment regimens more than patients who are unsatisfied. Therefore, patient satisfaction is necessary in order to maximize treatment effectiveness and collecting such data in a clinical trial setting helps provide patients’ perspectives regarding their treatment.
PRO measures were administered in Phase 3 AWARD clinical trials of patients with T2D treated with dulaglutide. Significant improvements from baseline at several end points across several studies were observed for both dulaglutide doses with regard to weight-related PRO measures, treatment satisfaction, and overall quality of life measures [Citation41,Citation42]. Dulaglutide-treated patients also demonstrated significantly greater improvement than comparator therapies for these PRO measures at several end points. In addition, PROs are included in the Summary of Product Characteristics (SmPCs) where it specifically states the following: Dulaglutide significantly improved total treatment satisfaction compared to exenatide BID. In addition, there was significantly lower perceived frequency of hyperglycemia and hypoglycemia compared to exenatide BID [Citation43].
Additional PRO measurements related to dulaglutide have been conducted outside the AWARD clinical studies. Two such studies involved obtaining stated preferences from patients with T2D. In the first study, using discrete choice experiment (DCE) methodology, in-person interviews were conducted in the UK with injection-naive patients with T2D [Citation35]. The DCE examined six attributes of T2D treatment associated with dulaglutide and liraglutide: ‘dosing frequency,’ ‘HbA1c change,’ ‘weight change,’ ‘type of delivery system,’ ‘frequency of nausea,’ and ‘frequency of hypoglycemia.’ The study results showed the relative importance for the attributes in rank order as follows: ‘dosing frequency’ (41.6%), ‘type of delivery system’ (35.5%), ‘frequency of nausea’ (10.4%), ‘weight change’ (5.9%), ‘HbA1c change’ (3.6%), and ‘frequency of hypoglycemia’ (3.0%). Moreover, significantly more participants preferred the dulaglutide profile (83.1%) compared to the liraglutide profile (16.9%; p < 0.0001).
The second PRO-focused study investigated weekly GLP-1 RA injection device characteristics [Citation44]. Specifically, this study focused on the patient preferences that were associated with three treatment-related attributes that distinguish among the weekly GLP-1 therapies: (1) requirements for reconstitution (i.e. mixing the medication) prior to injection, (2) required waiting time prior to the injection, and (3) and the need to handle the injection needle. The study found that even small differences in the GLP-1 RA weekly devices can have an impact on patient preferences. In fact, each individual injection administration inconvenience (i.e. reconstitution, waiting, and needle handling) had a decreased patient preference while simultaneous multiple inconveniences had an even more substantial negative impact on patient preference (e.g., mixing plus needle handling). However, a dulaglutide-like weekly device profile did not demonstrate any negative stated patient preference attributes when compared to the other GLP-1 RA device profiles.
Safety profile of dulaglutide
Similar to other GLP-1 RAs, the most frequently reported adverse events (AEs) associated with dulaglutide treatment were gastrointestinal in nature and included nausea, vomiting, and diarrhea. With either dulaglutide 1.5 or 0.75 mg, 8–29% of patients reported nausea (2–28% for comparators), 4–17% reported vomiting (1–12% for active comparators), and 8–17% reported diarrhea (6–14% for active comparators) [Citation26–Citation29,Citation31]. These events were mild to moderate in severity, peaked at 2 weeks, and rapidly declined over the next 4 weeks, after which the rate remained relatively constant [Citation43]. In clinical pharmacology studies conducted up to 6 weeks in patients with T2D, the majority of gastrointestinal events were reported during the first 2–3 days after the initial dose and declined with subsequent doses [Citation43].
Discontinuation due to AEs was generally low with 1–11% of the study population treated with dulaglutide discontinuing due to AEs (2–6% of comparators in AWARD-1, AWARD-2, AWARD-3, AWARD-4, and AWARD-6. In AWARD-5, after 104 weeks of treatment, 21% of patients from each dulaglutide 1.5 mg, 0.75 mg, and sitagliptin discontinued due to an AE. In AWARD-5, patients who developed persistent or worsening hyperglycemia based on prespecified thresholds were discontinued, and this was recorded as an AE of hyperglycemia [Citation31]. AE data for all trials can be found in [Citation27,Citation29,Citation31–Citation33].
Table 3. Summary of serious AEs, treatment-emergent AEs, most frequent GI events, and discontinuations due to AEs.
Hypoglycemia is a significant consideration when prescribing an antihyperglycemic medication, as hypoglycemia can be debilitating and potentially life-threatening. Clinical trials of dulaglutide monitored multiple hypoglycemia parameters and defined hypoglycemia as an event where blood glucose was ≤70 mg/dL. Due to the unique mechanism of action of GLP-1 RAs (glucose-dependent, i.e. increased insulin secretion in response to hyperglycemia, but not during normoglycemia or hypoglycemia, and the inhibition of glucagon only during hyperglycemia), previous clinical trials have not commonly reported significant hypoglycemic events with a GLP-1 RA as monotherapy or as combination therapy with antihyperglycemic medications other than secretagogues and insulins. The incidence and rates of hypoglycemia in the AWARD clinical trial program can be found in .
Table 4. Incidence and rates of hypoglycemia.
Briefly, no severe hypoglycemia was reported in patients treated with dulaglutide as monotherapy (AWARD-3) [Citation28], with background non-secretagogues, like metformin (AWARD-5 and AWARD-6) [Citation31,Citation32], or background metformin and pioglitazone (AWARD-1) [Citation27]. The incidence and rate of hypoglycemia were similar for dulaglutide and non-secretagogue active comparators (metformin, sitagliptin, exenatide BID, and liraglutide; incidence ranged from 9% to 12.8% for dulaglutide 1.5 mg, 8.6% to 11.1% for dulaglutide 0.75 mg, and 6% to 15.9% for active comparators) [Citation26,Citation28,Citation31,Citation32]. See for detailed information on the incidence, rates, and total hypoglycemia in AWARD-3, AWARD-5, AWARD-6, and AWARD-1.
Incidence and rates of hypoglycemia in dulaglutide arms (AWARD-8, AWARD-2, and AWARD-4) and comparator arms were higher with the concomitant use of high-dose glimepiride (AWARD-8 and AWARD-2) or insulin lispro (AWARD-4). The findings are consistent with the data for the incretin therapy class. In AWARD-8 with SU as background therapy, total hypoglycemia occurred in 20.9% dulaglutide patients and 3.3% placebo patients, with a mean (standard deviation) rate of 2.37 (7.22) events/patient/year for dulaglutide compared with 0.07 (0.39) for placebo. There were no cases of severe hypoglycemia [Citation33]. Notably, in the setting of concomitant metformin plus glimepiride in AWARD-2, the rate of total hypoglycemia was significantly lower for both dulaglutide 1.5 mg and 0.75 mg through week 78 compared to insulin glargine. A total of four patients experienced severe hypoglycemia: two patients in the insulin glargine group while on concomitant glimepiride and two in the dulaglutide 1.5 mg group with one patient on concomitant glimepiride and another who had previously discontinued glimepiride [Citation27]. In AWARD-4, where dulaglutide or glargine was used in combination with titrated prandial insulin lispro (with or without metformin), the rate of total hypoglycemia for dulaglutide 1.5 mg was significantly lower than insulin glargine. Reports of severe hypoglycemia were numerically higher in the glargine group [Citation29].
The administration of any biologic agent has the potential to elicit an immune response. During the development of dulaglutide, potential immunogenicity was addressed by an amino acid substitution in the GLP-1 analog portion of the molecule, to remove a potential T-cell epitope, i.e. an area of the antigen to which antibodies bind and may result in an immune response, as well as the fusion of the GLP-1 domain to a modified IgG4 Fc fragment, to reduce complement-dependent and antibody-dependent cell-mediated cytotoxicity, and reduce interaction with high-affinity Fc receptors. Treatment-emergent dulaglutide antidrug antibodies were detected in 1–2.8% of patients treated with dulaglutide, with none reporting systemic hypersensitivity reactions and limited number of patients reporting injection site reactions. A small fraction of these patients developed native GLP-1 cross-reactive antibodies, and due to the low incidence, analyses of the effect on glycemic response on these patients were not considered appropriate [Citation22].
Thyroid safety was carefully assessed in the AWARD clinical trial program, and suspected medullary thyroid carcinoma (MTC) cases were independently adjudicated. One case of MTC was reported in a dulaglutide-treated patient in AWARD-5, and this case was judged to be preexisting [Citation45]. Mean calcitonin laboratory values did not change during any of the AWARD trials.
Pancreatic safety was also carefully assessed through laboratory and clinical signs/symptoms. Seven events of adjudicated pancreatitis occurred across all treatment groups in the AWARD trials described in this report. In AWARD-5, events from two sitagliptin-treated patients and one placebo/sitagliptin-treated patient were adjudicated [Citation31]. In AWARD-2, two cases were reported in patients treated with dulaglutide 1.5 mg and one in a dulaglutide 0.75 mg-treated patient [Citation27]. In AWARD-1, one event from dulaglutide 1.5 mg-treated patient was adjudicated [Citation28]. Laboratory assessments of pancreatic amylase, total amylase, and lipase indicated 14–20% mean increases in lipase and/or pancreatic amylase for dulaglutide-treated patients, with placebo-treated patients experiencing mean increases of up to 3% [Citation22]. In the absence of other signs and symptoms of acute pancreatitis, elevations in pancreatic enzymes alone are not predictive of acute pancreatitis. Mean increases were also observed in patients treated with metformin, sitagliptin, exenatide, and liraglutide [Citation26,Citation28,Citation31,Citation32].
Dulaglutide cardiovascular safety has been assessed across the clinical development program, as well as in an ambulatory blood pressure monitoring study. In 2008, the Food and Drug Administration (FDA) required comprehensive cardiovascular safety analyses for all marketed T2D therapies. Ambulatory blood pressure monitoring study comparing dulaglutide to placebo, demonstrated a mean decrease in systolic blood pressure of 2.8 mm Hg compared to placebo, no change in diastolic blood pressure, and a 2–4 beat-per-minute increase in heart rate [Citation46]. These findings were generally consistent with the AWARD clinical trials. Dulaglutide had minor effects on laboratory lipid values, with minor potentially favorable effects in AWARD-1, AWARD-3, and AWARD-4 [Citation26,Citation28,Citation29]. Following the FDA guidance, a meta-analysis to assess cardiovascular safety was performed, and a long-term cardiovascular outcomes trial is ongoing (REWIND, NCT01394952), which has enrolled more than 9000 patients. The meta-analysis of Phase 2 and Phase 3 studies (not including AWARD-6 and AWARD-8) suggested that dulaglutide does not increase the risk of major cardiovascular events in T2D patients [Citation47]. The results from the long-term cardiovascular outcomes study are predicted to be available in April 2019.
Benefit/risk of dulaglutide as determined by composite end point analysis
A composite end point combines individual outcomes of a clinical trial into a single measure. Composite end points are of particular interest because they allow for a combined assessment of both efficacy and safety. A composite end point was performed for AWARD-1, AWARD-2, AWARD-3, AWARD-5, and AWARD-6 to identify the proportion of patients who achieved an HbA1c of <7.0% with no hypoglycemia (glucose < 3.0 mmol/L or severe hypoglycemia) and no weight gain at week 26. The end point analysis compared dulaglutide 1.5 mg and 0.75 mg to active comparators in the aforementioned AWARD trials [Citation48]. Composite end point results for AWARD-4 were previously reported in Blonde et al. (2015) [Citation29].
At the week 26 end point, 37–58% of dulaglutide 1.5 mg patients, 27–49% of dulaglutide 0.75 mg patients, and 9–61% of active comparator patients achieved the composite end point. Significantly more dulaglutide 1.5 mg patients achieved the composite end point than metformin, sitagliptin, exenatide BID, or insulin glargine-treated patients (p < 0.001 for all except metformin where p < 0.05). Significantly more dulaglutide 0.75 mg-treated patients achieved the composite end point than sitagliptin or insulin glargine-treated patients (p < 0.001, both) [Citation48]. In AWARD-4, significantly more patients achieved the composite end point than insulin glargine at both weeks 26 and 52 (p < 0.05, all) [Citation29].
Conclusions
GLP-1 RAs are an important addition to the available options for HCPs specifically in the primary care setting for the treatment of T2D due to their efficacy and safety profile, potential for weight loss, and less risk for hypoglycemia when used without secretagogues or insulin. However, education and experience with GLP-1 RAs is lacking, as demonstrated by the UK physician survey [Citation24]. In this review, we have strived to increase knowledge regarding the benefit–risk profile of GLP-1 RAs, and specifically, the clinical trials surrounding dulaglutide which has been approved in the US, EU, Japan, and numerous other geographies.
In the AWARD clinical trials presented here, dulaglutide demonstrated early and sustained glucose reduction in T2D patients as measured by FBG, PPG, and HbA1c leading to a high percentage of patients achieving an HbA1c goal of <7.0%. Similar to other GLP-1 RAs, dulaglutide treatment also resulted in weight loss or attenuation in weight gain. Dulaglutide also demonstrated significantly more patients achieving a composite end point of HbA1c < 7.0% without hypoglycemia or weight gain than with several active comparators from across antihyperglycemic classes. In studies where FBG was measured early in the course of treatment, the near-maximal effect on FBG concentrations occurred by 2 weeks. The improvement in fasting glucose was sustained through the longest study duration of 104 weeks in AWARD-5 [Citation26,Citation31]. Additionally, dulaglutide was associated with improvements in PRO measures in patients with T2D. The PRO data support the benefit of dulaglutide for the treatment of T2D and highlight the potential importance of nonclinical factors such as dosing frequency and type of delivery system as important drivers of patient preference. The safety profile of dulaglutide was similar to the GLP-1 RA class, with gastrointestinal side effects being most common (nausea, vomiting, and diarrhea), mild to moderate in severity, and generally improved over time. The established early onset and sustained glycemic control, effect on weight, an acceptable safety profile similar to other GLP-1 RAs, and the ready-to-use pen (single use, no reconstitution needed, and no need for needle attachment) makes dulaglutide an attractive treatment option available for the management of T2D in a primary care setting. Dulaglutide is approved in the US and EU as an adjunct to diet and exercise to improve glycemic control in adults with T2D [Citation22,Citation43].
Declaration of interest
The studies of dulaglutide within this manuscript were sponsored and funded by Eli Lilly and Company, Indianapolis, Indiana, USA. JE Anderson has been on the advisory panel and speaker’s bureau for Eli Lilly, Boehringer Ingelheim, AstraZeneca, Janssen and Sanofi and the advisory panel alone for Abbot. V Thieu, K Boye, R Hietpas and LE Garcia-Perez, are employees and shareholders of Eli Lilly. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplementary_Table_1.docx
Download MS Word (15.2 KB)Acknowledgments
The authors would like to thank Chrisanthi Karanikas, MS (Eli Lilly and Company) for writing and editorial assistance of this manuscript.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Defronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi:10.2337/db09-9028.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412.
- Siminerio LM, Siminerio LM, Drab SR, et al. Diabetes educators: implementing the chronic care model. Diabetes Educ. 2008;34:451–456. doi:10.1177/0145721708316627.
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi:10.1056/NEJMoa0802743. Action to Control Cardiovascular Risk in Diabetes Study Group.
- ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi:10.1056/NEJMoa0802987.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi:10.1056/NEJMoa0808431. VADT Investigators.
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi:10.2337/dc09-9033.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi:10.2337/dc14-2441.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Aace/Ace comprehensive diabetes management algorithm 2015. Endocr Practice: Official Journal Am Coll Endocrinol Am Assoc Clin Endocrinologists. 2015;21:438–447. doi:10.4158/EP15693.CS.
- International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1–52. doi:10.1016/j.diabres.2012.10.001.
- GLUCOPHAGE and GLUCOPHAGE XR [Prescribing Information]. Princeton (NY): Bristol-Myers Squibb Company; 2009.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi:10.1016/S0140-6736(06)69705-5.
- Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304.
- Ritzel R, Orskov C, Holst JJ, et al. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP-1 [7-36 amide] after subcutaneous injection in healthy volunteers. Dose-response-relationships. Diabetologia. 1995;38:720–725.
- Naslund E, Gutniak M, Skogar S, et al. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530.
- Wettergren A, Schjoldager B, Mortensen PE, et al. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665–673.
- Näslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1999;23:304–311.
- Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131.
- Deacon CF. Circulation and degradation of GIP and GLP-1. Hormone and Metabolic Research. 2004;36:761–765. doi:10.1055/s-2004-826160.
- Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287–296. doi:10.1002/dmrr.1080.
- Geiser, JS, Heathman MA, Cui X, et al. Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analyses of data from clinical trials. Clin Pharmacokinet. 2016;55(5):625–634. doi:10.1007/s40262-015-0338-3.
- Trulicity [Prescribing Information]. Indianapolis (IN): Lilly USA, LLC; 2014.
- Kuritzky L, Umpierrez G, Ekoé JM, et al. Safety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetes. Postgrad Med. 2014;126:60–72. doi:10.3810/pgm.2014.10.2821.
- Matza, L. S., Curtis, S. E., Jordan, J. B., et al. Physician perceptions of GLP-1 receptor agonists in the UK. Curr Med Res Opin. 2016;1–8. doi:10.1185/03007995.2016.1147025.
- Jendle J, Grunberger G, Blevins T, et al., Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016. doi:10.1002/dmrr.2810. [Epub ahead of print].
- Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37:2159–2167. doi:10.2337/dc13-2760.
- Giorgino F, Benroubi M, Sun J-H, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38:2241–2249. doi:10.2337/dc14-1625.
- Umpierrez G, Tofe Povedano S, Perez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37:2168–2176. doi:10.2337/dc13-2759.
- Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–2066. doi:10.1016/S0140-6736(15)60936-9.
- Nauck M, Weinstock RS, Umpierrez GE, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37:2149–2158. doi:10.2337/dc13-2761.
- Weinstock RS, Guerci B, Umpierrez G, et al. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–858. doi:10.1111/dom.12479.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi:10.1016/S0140-6736(14)60976-4.
- Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24-week study to evaluate the efficacy and safety of once weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8). Diabetes Obes Metab. 2016;18:475–482. doi:10.1111/dom.12634.
- Hollander P. Anti-diabetes and anti-obesity medications: effects on weight in people with diabetes. Diabetes Spectr. 2007;20:159–165.
- Gelhorn HL, Poon JL, Davies EW, et al. Evaluating diabetes patients’ preferences for profiles of Glp-1 treatments in the United Kingdom: a discrete choice experiment. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18:A614. doi:10.1016/j.jval.2015.09.2133.
- Trulicity [Instruction for Use]. Indianapolis (IN): Lilly USA, LCC; 2014.
- Matfin G, Van Brunt K, Zimmermann AG, et al. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naive patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9:1071–1079. doi:10.1177/1932296815583059.
- Revicki DA. Patient assessment of treatment satisfaction: methods and practical issues. Gut. 2004;53(Suppl 4):iv40–44.
- Shikiar R, Flood E, Siddique R, et al. Development and validation of the gastroesophageal reflux disease treatment satisfaction questionnaire. Dig Dis Sci. 2005;50:2025–2033. doi:10.1007/s10620-005-3002-1.
- Speight J. Assessing patient satisfaction: concepts, applications, and measurement. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2005;8(Suppl 1):S6–8. doi:10.1111/j.1524-4733.2005.00071.x.
- Reaney M, Yu M, Lakshmanan M, et al. Treatment satisfaction in people with type 2 diabetes mellitus treated with once-weekly dulaglutide: data from the AWARD-1 and AWARD-3 clinical trials. Diabetes Obes Metab. 2015;17:896–903. doi:10.1111/dom.12527.
- Yu M, van Brunt K, Varnado OJ, et al. Patient-reported outcome results in patients with type 2 diabetes treated with once weekly dulaglutide: data from the AWARD phase 3 clinical trial programme. Diabetes Obes Metab. 2015. doi:10.1111/dom.12624.
- Trulicity [Summary of Product Characteristics]. Houten (The Netherlands): Eli Lilly and Company; 2014.
- Matza LS, Stewart KD, Davies EW, et al. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. The 18th Annual European ISPOR Congress; 2015 Nov 7–11; Milan, Italy; 2015.
- Skrivanek Z, Gaydos BL, Chien JY, et al. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5). Diabetes Obes Metab. 2014;16(8):748–756. doi:10.1111/dom.12305.
- Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. doi:10.1161/HYPERTENSIONAHA.114.03062.
- Ferdinand KC, Botros FT, Atisso CM, et al. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol. 2016;15:38. doi:10.1186/s12933-016-0355-z.
- Dungan KM, Raz I, Skrivanek Z, et al. Achieving the composite endpoint of glycated haemoglobin <7.0%, no weight gain and no hypoglycaemia in the once-weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18:49–55. doi:10.1111/dom.12575.
