ABSTRACT
Objective: This study aims to compare the effectiveness of insulin glargine 300 U/mL (Gla-300) with its accompanying patient support program with that of other basal insulin and available patient support programs in patients with type 2 diabetes (T2D) in a real-world setting in terms of achieving HEDIS (Healthcare Effectiveness Data and Information Set) individualized glycemic targets without documented symptomatic hypoglycemia.
Methods: Achieve Control is a US-based, multicenter, randomized, open-label, active-controlled, parallel group pragmatic Phase IV trial in insulin-naïve patients with T2D uncontrolled on ≥2 oral antidiabetes drugs (OAD) and/or glucagon-like peptide-1 receptor antagonists (GLP-1 RA). Inclusion criteria include a diagnosis of T2D, age ≥18 years, and glycated hemoglobin (HbA1c) between 8.0% and 11.0%. Patients will be assigned to either the Gla-300 or other basal insulin group. The primary end point is the proportion of patients achieving HEDIS HbA1c targets (<8.0% [64 mmol/mol] in patients with comorbidities or aged ≥65 years; <7.0% [58 mmol/mol] in all other patients) without occurrence of symptomatic hypoglycemia (blood glucose ≤70 mg/dL) from baseline to 6 months. Secondary end points include rates of documented symptomatic nocturnal hypoglycemia and severe hypoglycemia; change from baseline in HbA1c, fasting glucose, and body weight; treatment persistence; patient-reported outcomes; and healthcare resource utilization. Planned enrollment is 3270 patients across approximately 400 clinical sites.
Conclusion: Pragmatic clinical trials offer the potential to assess comparative effectiveness in broadly based patient populations receiving care (with or without a corresponding educational support program) in real-world clinical settings. The results of Achieve Control should elucidate the benefits of management of T2D with Gla-300 versus other basal insulins in terms of patient outcomes, experiences, and perceptions, and its impact on healthcare resource utilization and cost.
Clinical trial registration: www.clinicaltrials.gov identifier is NCT02451137.
1. Introduction
Type 2 diabetes (T2D) is a progressive disease characterized by relative insulin deficiency and the eventual need for replacement insulin therapy. It affects millions of people worldwide and is a leading cause of cardiovascular and kidney disease [Citation1–Citation3]. Although the number of patients with T2D who receive basal insulin as part of their therapy is increasing, about 50% of these patients do not achieve adequate glycemic control (generally defined as glycosylated hemoglobin [HbA1c] <7.0% [53 mmol/mol]) [Citation3,Citation4]. Initiation and persistence of basal insulin therapy are also constrained by physician- and patient-perceived barriers to the use of insulin, including patient reluctance, concerns regarding hypoglycemia and/or weight gain, and worries about difficulties associated with managing insulin therapy [Citation5–Citation7].
Basal insulins in clinical development are aimed at providing similar or improved efficacy over currently available insulins, but with an improved safety profile to help more people living with diabetes achieve their treatment goal. In pharmacokinetics and pharmacodynamics studies, insulin glargine 300 U/mL (Gla-300) has demonstrated low variability in exposure over 24 h, a more consistent activity profile, and prolonged maintenance of glucose control compared with insulin glargine 100 U/mL (Gla-100) [Citation8–Citation10]. In the randomized, controlled Phase III EDITION clinical trial program, Gla-300 was shown to be non-inferior to Gla-100 in reducing HbA1c levels, without increasing the risk of hypoglycemia versus Gla-100 in patients with type 1 diabetes (T1D) or T2D at 6 months [Citation11–Citation14] and at 1 year [Citation15,Citation16]. The proportion of patients who experienced ≥1 confirmed (blood glucose [BG] ≤70 mg/dL) or severe nocturnal hypoglycemic event was significantly lower for Gla-300 than for Gla-100 [Citation17]. Data from the EDITION clinical trial program also supports the use of Gla-300 in different patient populations, its ease of use in insulin-naïve patients, and less hypoglycemia when switching from twice-daily insulin [Citation11–Citation13,Citation15,Citation16].
The impact of treatments in the real-world setting is of immense importance to patients, providers, and population-based decision makers within health-care systems. Traditional randomized controlled trials (RCTs) typically focus on carefully selected patients who are treated under tightly controlled and closely monitored conditions to ensure adherence to study protocols and procedures to produce high internal validity. While optimally designed to examine the efficacy of competing interventions, they often provide limited insight on their effectiveness under real-world conditions. Unlike the traditional explanatory RCTs that focus on efficacy and safety in highly selected patients in controlled, experimental settings and under ideal conditions, real-world study designs provide valuable and practical information precisely because they are unconstrained by requirements and design elements of the explanatory RCTs; instead, they investigate a treatment in the patients and settings where it is most likely to be used [Citation18–Citation22].
Explanatory and pragmatic clinical trials address different, complementary questions [Citation23,Citation24]. Guiding principles for pragmatic clinical trials are to identify patients from typical clinical practice sites, minimize inclusion and exclusion criteria to ensure generalizability, minimize requirements for study-related monitoring, limit protocol-mandated visits, limit restrictions on treatment, capture end points that are relevant to decision makers and consonant with usual care practice and data collection, engage with relevant stakeholders, capture patient-reported outcomes (PROs), and limit intervention to randomization if possible [Citation23,Citation24]. Despite their important methodological strengths and relevance to health-care decision makers, there have been few examples of pragmatic clinical trials in assessments of drug therapy because of the challenges associated with executing such studies [Citation25–Citation27].
In diabetes, a pragmatic trial might go beyond the surrogate end point identified for disease (HbA1c) to assess long-term consequences, such as treatment effects on persistence, discontinuation, attainment of quality-of-care metrics such as Healthcare Effectiveness Data and Information Set (HEDIS) glycemic goals or PROs, and effect on health-care resource utilization and cost. Pragmatic trials have been supported through initiatives from various organizations, including, in the United States, the National Institutes of Health [Citation28,Citation29], the Centers for Medicare & Medicaid Services [Citation30], and the Agency for Healthcare Research and Quality [Citation31], as well as the Patient-Centered Outcomes Research Institute, which is investing heavily in these types of comparative effectiveness studies and the creation of research networks to facilitate such trials, as well as studies in priority areas [Citation32].
Given the high prevalence of T2D, it is important to understand clinical and health economic outcomes of interventions aimed at diabetes in real-world conditions; however, there exists a lack of concordance between clinical trial results and real-life experience. A key rationale for this study is to generate data that can translate into the results health-care providers (HCPs) and payers could expect in real life. These data would support the so-called triple aim of improved clinical outcomes and greater patient satisfaction, combined with decreased health-care costs [Citation33]. This publication describes the methodology of Achieve Control, the first real-world trial comparing disease management with Gla-300 versus disease management with other basal insulin as initial therapy for insulin-naïve patients with inadequately controlled T2D.
The primary goal of this study is to ascertain whether Gla-300 compared with Gla-100 and other basal insulins, with its designated patient-support program (PSP), increases the likelihood that insulin-naïve patients with T2D will attain individualized HEDIS HbA1c targets () at 6 months while having a lower risk of hypoglycemia. Comparators include Gla-100 and insulin detemir with their respective available PSPs.
Table 1. HEDIS criteria for individualized HbA1c targets.
2. Methods
2.1. Study design
Achieve Control is a multicenter, randomized, open-label, active-controlled, two-arm parallel group pragmatic trial in patients with T2D in the United States who are uncontrolled on ≥2 OADs and/or a GLP-1 RA and have never been treated with insulin.
In the United States, patients typically initiate basal insulin therapy with the option of participating in a PSP to complement medication therapy, as an integrated disease management approach. Thus, Achieve Control includes this option in both the treatment and comparator arms.
Achieve Control includes two operational phases: a planning phase and a prospective study phase (). During the planning phase of the trial, payer, research organization, and HCP databases will be reviewed to identify appropriate sites and patients eligible for the study, and incidentally to identify any unmet needs in this patient population. We will identify potential study patients within designated practice sites who meet eligibility criteria via database analysis. Two payer research organizations affiliated with large health plans will collaborate and allow access to databases used to identify patients, physicians, and sites potentially eligible for inclusion in the study.
Figure 1. Design of the randomized, open-label, parallel group, real-world, pragmatic Achieve Control study.
1Available Patient Support Program for GLA-300.2Available Patient Support Program for GLA-100 and insulin detemir.ADA: American Diabetes Association; BG: blood glucose; DPP-4: dipeptidyl peptidase-4; FPG: fasting plasma glucose; GLP-1 RA: glucagon-like peptide-1 receptor agonist; HbA1c: glycated hemoglobin; OAD: oral antidiabetes drug; PRO: patient-reported outcome; PSP: patient-support program; Q: quarter; QD: once daily; SGLT-2: sodium-dependent glucose cotransporter-2; SU: sulfonylureas; TZD: thiazolidinedione; T2D: type 2 diabetes.
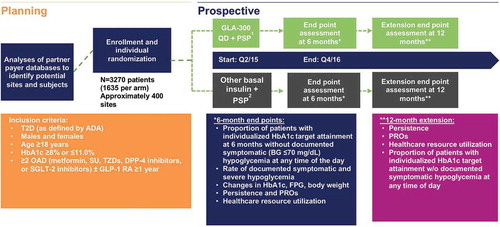
The prospective study phase will consist of a 1-week screening period at study sites during which clinicians will screen patients identified as potential subjects; a 6-month treatment period for the primary efficacy end point and secondary end points; and a 6-month extension period for collecting additional clinical outcomes, resource utilization data, PROs, medication persistence data, and additional safety data. Patients will be assigned to either the Gla-300 or other basal insulin group using patient-level randomization. Given the potential impact of certain variables on the primary end point, randomization will be stratified by HEDIS HbA1c target (<8.0% [64 mmol/mol]/<7.0% [58 mmol/mol]), sulfonylurea (SU) use (yes/no), GLP-1 RA use (yes/no), and baseline HbA1c (≥9/<9%). Individualized HbA1c target attainment will follow HEDIS criteria of <8% for older patients or those with comorbidities, or <7% for other patients () [Citation34]. The maximum study duration will be 53 weeks per patient. For patients who discontinue the trial prematurely, the end-of-treatment visit assessments (Day 180 data) will be performed at the time of discontinuation. In addition, an early end-of-trial case report form (CRF) page will be completed, which includes the reason for stopping the treatment and whether this was the patient’s decision. If possible, patients discontinuing therapy will be assessed using the procedure normally planned for the last dosing day with the investigational medication and will be followed for ≥6 months.
2.2. Sample size
We plan to enroll a total of 3270 patients (1635 patients in each treatment group). Sample size calculations were based on superiority testing of the primary efficacy variable. Treatment-effect assumptions for sample size calculation were determined from the retrospective application of HEDIS targets to the EDITION 3 study, which studied the use of Gla-300 in the treatment of insulin-naïve patients with T2D not adequately controlled with non-insulin OAD therapy [Citation11]. In addition, data from a commercially available database were used to validate the proportion of patients anticipated to be assigned to each HEDIS target, which led to the final assumption of a 70%:30% split for HEDIS targets <7%:<8%. This ratio was then applied to the EDITION 3 results to yield an assumption of an absolute overall (weighted average) difference in the primary end point of 5.5% favoring Gla-300 for all patients combined. Detailed sample size assumptions can be found in the supplementary material.
2.3. Data sources
Planned data sources will include the following: (1) CRFs (along with data from appropriate electronic medical record databases to supplement resource utilization data); (2) administrative claims (captured by both the payer partners and the Smartcard vendor to estimate treatment persistence; health-care utilization and overall treatment costs will be captured by the payer partners); (3) e-diary (to collect self-monitored blood glucose data, capture clinical symptoms of hypoglycemia [residing in a vendor Web-based portal that is not transferred to the database] and report any office visits, emergency department [ED] visits, or hospitalizations); and (4) patient surveys and questionnaires (to capture data on PROs, treatment satisfaction, treatment effectiveness, and hypoglycemia).
2.4. Site and patient recruitment
The study anticipates inclusion of 150 ‘payer sites’ out of the planned 400 study sites. Sanofi, the study sponsor, will provide support from a monitoring and implementation perspective; Sanofi will do the same for sites without prior experience in conducting pragmatic clinical trial research. After recruitment challenges were encountered, the sponsor engaged the payer partners to support the payer sites as well. In addition to the payer sites, around 250 trial-experienced sites with which the study sponsor has worked previously will be included. Study sites have been drawn from 42 states, and therefore are reasonably well-distributed across the United States. Sanofi will ensure that all sites meet eligibility for inclusion and will check for duplicates. At regular intervals during the clinical trial, a monitoring team will contact sites as specified in the monitoring plan. Monitoring visits will be conducted on-site and electronic documentation CRF, interactive Web response system, and CRF Web-based portal [TrialManager™; CRF Health, Plymouth Meeting, PA; http://www.crfhealth.com/platform/] will be appraised to review investigator and patient compliance with clinical trial protocol requirements, study progress, and any emergent problems.
Minimal study inclusion/exclusion criteria will be considered and applied to relevant databases to include appropriate patients representing the broadest patient profile to optimize generalizability of the results. Patients will have actively sought care at the corresponding site in the last 12 months. Review of these databases and the resultant attrition chart based on application of the eligibility criteria may also identify unmet medical needs of the patient population currently on non-insulin treatment regimens. Patients who were health-plan members or appropriate provider database members with evidence of a T2D diagnosis for ≥1 year at the time of the screening visit are eligible for the planning phase. After identification, individual patients will be screened at the practice site to determine eligibility for the prospective phase of the study.
Eligible patients are insulin-naïve adults and must have had inadequate glycemic control after ≥1 year of treatment with ≥2 of the following: oral agents (metformin, SU, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, or sodium-dependent glucose cotransporters-2 (SGLT-2) inhibitors) or GLP-1 RA approved for use with basal insulin. Each patient will be required to have ≥12 months of baseline medical data available prior to randomization. All patients will be required to sign an informed consent form (including informed consent to share claims data) and a Health Insurance Portability and Accountability Act (HIPAA) authorization form. A list of patient exclusion criteria can be found in the supplementary material.
2.5. Study treatments
Patients will be randomly assigned to treatment with Gla-300 and its designated PSP, or commercially available insulin detemir or Gla-100 and their respective available diabetes management support programs. The choice of comparator basal insulin will be based on usual practice based on the investigator’s choice at the clinical site.
The Gla-300 PSP is an integrated diabetes management program available to patients receiving Gla-300. The program tailors support based on a patient’s needs, including their stage of disease, willingness to engage (Patient Activation Measure), and communication preferences (live tailored support, digital/mobile support, text messaging). It provides a clinical educator (usually a Certified Diabetes Educator) to assist with product initiation training (the specific insulin dose titration algorithm will be left to the discretion of the provider), a Patient Support Navigator who will assess the patient’s specific support needs and suggest a tailored program based on those needs, and tailored programs ranging from high touch to medium touch, including peer mentoring.
Insulin glargine, given as either commercially available Gla-300 or Gla-100, will be self-administered using disposable pen devices by subcutaneous injection once daily in the morning or evening according to the local label. Commercially available insulin detemir will be self-administered by subcutaneous injection using a disposable pen device once or twice daily in the morning and/or evening according to the local label.
Patients will be initiated on basal insulin therapy according to prescribing instructions and titrated to a target (FPG) consistent with each patient’s individualized HbA1c target. Insulin use will follow the standard of care used at each site defined by American Diabetes Association (ADA) guidelines, local practice guidelines, and investigator discretion. There is no titration algorithm and no insulin oversight review implemented.
Patients in both treatment groups will continue their other antidiabetes drugs as background therapy at the discretion of the investigator and consistent with the local labeling guidelines for use with insulin. All patients in the trial will receive diet and lifestyle counseling provided at the clinical practice site according to their standard practice. Patients being treated with Gla-100 or insulin detemir are encouraged to enroll in diabetes management programs or PSPs available through their payer, provider system, or a local professional organization.
The sponsor will provide financial support for the cost of commercially available basal insulins and Gla-300.
2.6. Outcomes of interest
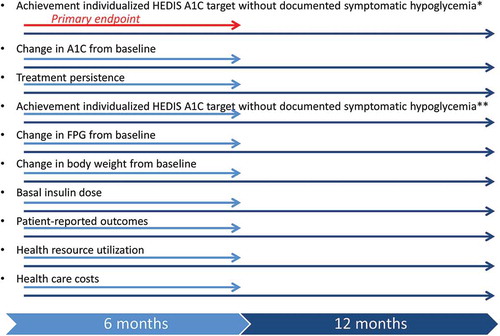
The primary end point of the study is the proportion of patients treated with Gla-300 or another basal insulin who achieve individualized HbA1c targets according to HEDIS criteria after 6 months of treatment without a documented symptomatic hypoglycemic event (blood glucose [BG] ≤70 mg/dL) at any time of day from baseline to 6 months. HbA1c targets are <8.0% (64 mmol/mol) for patients aged ≥65 years or with defined comorbidities or <7.0% (58 mmol/mol) for all other patients. HEDIS measures are used by >90% of US health plans to document the plan’s performance in health care and provide benchmarks for quality and value. In light of the growing interest in establishing individualized treatment goals, recent changes to the 2015 HEDIS Comprehensive Diabetes Care measures require patient age and health status to determine the HbA1c goal to be attained [Citation34].
Key secondary clinical end points will be assessed at baseline and at 6 and 12 months, and include change in HbA1c, HbA1c target attainment without documented hypoglycemia (BG ≤ 70 mg/dL at 12 months and <54 mg/dL at 6 months and at 12 months), proportion of patients who remain on assigned basal insulin therapy; changes in FPG, change in body weight, and basal insulin dose ().
Figure 2. Major outcomes measures and timing of their ascertainment.
*Defined as a symptomatic event with documented BG ≤70 mg/dL at any time of day.**Defined as a symptomatic event with documented BG <54 mg/dL at any time of day.HbA1c: glycated hemoglobin; BG: blood glucose; FPG: fasting plasma glucose; HEDIS: Health Effectiveness Data and Information Set.
Medication possession ratio (MPR) will be assessed by estimating the number of days that patients had possession of the study drug based on the prescription date and days of supply [Citation35]. An MPR of 80% will define patients as being persistent on study medication. Pharmacy claims data or third-party vendor databases will be used to evaluate discontinuation at 6 and 12 months for patients enrolled in the study. A gap of 45 days or more between the last day of the previous prescription fill and the day of the next prescription fill will define treatment discontinuation.
Patients’ experience with their treatment will be assessed using specific, validated PRO instruments, including the Global Evaluation of Treatment Effectiveness measure, the Diabetes Treatment Satisfaction Questionnaire Status version [Citation36], and the Hypoglycemia Patient Questionnaire () [Citation37].
Table 2. Patient-reported outcomes instruments.
Health resource utilization will be assessed through physician office visits, ED visits and hospitalizations, and health-care costs over 6 and 12 months. Data for these end points will be obtained from the health claims database of the payers involved in the study. The study will also collect pharmacy-related utilization and costs through reimbursed pharmacy claims obtained from the health plans, using similar to those employed to estimate medical care costs. The mean number of office visits, ED visits, and hospitalizations per patient will be compared between cohorts. For patients identified through payer sites, the study will assess diabetes-specific health resource utilization and costs where such claims data are available. Health-care costs for medical services will be summarized as cumulative costs over time for comparison at 6 and 12 months using payer claims data. Health-care costs will be estimated based on payer reimbursed costs and any out-of-pocket patient costs for services that would include office visits, ED visits, hospitalizations, and diabetes supply costs. Mean overall and diabetes-related costs will be compared at each time point. In case of non-payer sites and/or non-payer patients, where a health claims database is unavailable, health resource utilization information will be collected through a supplemental CRF at each site. Patients are required to report any office visits, ED visits, and hospitalizations in their e-diary, which will then be transcribed into supplemental CRFs at regular time intervals. Health-care costs will not be collected from non-payer sites and patients.
The study design, and broad population being studied, will also allow for the evaluation of responses, especially in patient subgroups, as well as an assessment of the impact of support programs in an exploratory manner.
2.7. Safety analysis
Planned safety assessments are hypoglycemia, adverse events, serious adverse events, injection site reactions, hypersensitivity reactions, laboratory data, and vital signs. The ADA defines hypoglycemia in diabetes as BG ≤ 70 mg/dL (see for details) [Citation37]. Adverse events will be examined using information from the clinical database and claims data, respectively; if any discrepancies exist between the two data sources, they will not be reconciliated.
Table 3. ADA definitions of hypoglycemia in diabetes [Citation37].
The following end points for hypoglycemia will be assessed in this study: (1) incidence of documented symptomatic nocturnal (midnight to 6 am) hypoglycemia from baseline to 6 and 12 months; (2) incidence of documented symptomatic 24-h hypoglycemia from baseline to 12 months; (3) rates of documented 24-h and nocturnal symptomatic hypoglycemia from baseline to 6 and 12 months; (4) incidence and rates of documented 24-h and nocturnal symptomatic (BG < 54 mg/dL) hypoglycemia from baseline to 6 and 12 months; and (5) incidence and rate of severe 24-h hypoglycemia from baseline to 6 and 12 months.
3. Discussion
The EDITION studies provided efficacy and safety data to support the use of Gla-300 in the treatment of T1D and T2D. While information from so-called explanatory RCTs is essential for evaluating the efficacy and safety of new medications, they may provide insufficient information on the effectiveness of such interventions in clinical practice. Thus, additional data are required to establish the value of Gla-300 in a real-world clinical setting. Achieve Control is a comparative effectiveness research study designed to address the question of which basal insulin is the most effective and safe for initiating insulin therapy in insulin-naïve patients with T2D in a real-world setting. As a primary composite end point, the study aims to demonstrate that Gla-300, with or without its available diabetes management program, will be superior to insulin detemir or Gla-100 with their respective available diabetes management support programs in achieving individualized HbA1c targets at 6 months without episodes of documented symptomatic hypoglycemia.
Pragmatic trials are useful across countries and health-care system environments, but need to be tailored closely to the needs and characteristics of particular settings. Similar to its two ongoing European counterparts, the Reach Control and Regain Control studies, which include patients who are insulin-naïve or currently uncontrolled on basal insulins glargine, detemir, or degludec [Citation38], Achieve Control uses a novel, pragmatic design to shed light on outcomes in real-world clinical practice and to minimize problems of generalizability with traditional RCTs. In contrast to typical RCTs, which seek to determine the effects of an intervention under idealized conditions, the Achieve/Reach/Regain Control trials aim to explore whether the intervention works under normal conditions in the management of T2D.
Pragmatic clinical trials focus on outcomes that can affect multiple aspects of health-care delivery, such as differences in treatment effectiveness rather than efficacy or safety. Data from pragmatic RCTs, in contrast to explanatory RCTs, can potentially provide a much more generalizable picture of how interventions work in the real world and can be of greater interest to patients, providers, and payers. This type of study design has been used successfully by others to compare the effectiveness and costs of two alternative strategies for treating patients with elevated cholesterol levels [Citation25], to compare the effect of interventions on decreasing craving and posttraumatic stress in patients with co-occurring substance abuse and psychiatric disorders [Citation39], to assess interventions to improve adherence to pediatric asthma controller medication [Citation40], and to examine whether a targeted pharmacist-led intervention might improve blood-pressure control among diabetes patients with persistent hypertension and poor refill adherence or insufficient medication intensification [Citation41].
Achieve Control has been designed rigorously to minimize inclusion and exclusion criteria to ensure generalizability and requirements for monitoring, to limit protocol-mandated visits and restrictions on treatment, and to capture end points that are relevant to decision makers and consonant with usual care practice and data collection. The study protocol allows for nearly all OADs and GLP-1 RAs currently labeled for use with basal insulin as background medication, mirroring real-world clinical practice. Additionally, long-term outcomes, including treatment persistence, discontinuation, and health-care utilization are evaluated to provide further insight into the impact of Gla-300 and its PSP in clinical practice.
The pragmatic nature of the Achieve Control trial led to a number of initial and ongoing operational challenges. Achieve Control was the first pragmatic trial that the study sponsor conducted in diabetes mellitus. Operational challenges included (1) study scope and design, (2) identification of payer partners, (3) complying with both sponsor and payer standard operating procedures (SOPs), (4) site identification, (5) patient consent, (6) site research experience, and (7) identification of patients with availability of claims data. Unlike traditional RCTs, the involvement of payer partners required first the identification of partners and then the alignment on the scope and execution of the study. Instead of working with only a single payer partner, the sponsor chose to include two payer partners to engage in this trial to ensure a higher number of patients whose claims data could be analyzed. The management of two distinct payer partners in the same study proved to be especially challenging, as the need to engage and align three legal and procurement teams added a significant amount of time to the study start-up process. Alignment between sponsor and partner SOPs, including differing HIPAA requirements for each partner, consumed considerable effort. Furthermore, the majority of sponsor SOPs needed refinement to adapt to the pragmatic nature of the study. Sites were identified by both the sponsor’s clinical study unit and the payer partners. Payer partner sites were identified based on a review of the claims data which identified potential candidates for the study. Managing the overlap of the sites identified by the study sponsor and the payer partners required an unanticipated encryption process, as the site lists identified were not able to be freely shared among the payer partners. The study has begun randomizing patients and is slated to conclude enrollment by the third quarter of 2016.
Patients provided consent for collection of both clinical and claims-based data. To maintain the real-world nature of the study, a combination of sites with research experience and sites with less experience were selected. The sites with less experience required more support and oversight, resulting in an extension of the site initiation period. During the recruitment period, we noticed the highest enrolling sites were those that were supported by both the study sponsor and a payer partner.
Enrollment of insulin-naïve patients, in particular patients meeting the defined HbA1c criteria, has resulted in higher screening failure rates than initially anticipated which has impacted study timelines. Finally, we realized that the payers may also have access to claims data for patients enrolled in non-payer sites. We therefore needed to develop a process to assist in the identification of these patients and arranged a third-party vendor to manage data integration. Achieve Control was one of the first pragmatic trials that the study sponsor conducted with some centers selected in partnership with payers to ensure better investigation and extrapolation of resource and cost utilization. The sponsor learned from the ongoing Achieve Control study about the importance of engaging research partners in the study design very early in the process to foster true partnerships. Process evaluation and implementation of operational insights early in the design procedure are crucial to ensure that the study design remains feasible. If regulatory concerns are anticipated, the US FDA could be engaged to review the study design. We will continue to monitor for unforeseeable challenges encountered in the trial so that future pragmatic clinical studies can benefit from the lessons.
The hypothesis driving Achieve Control is that treatment with Gla-300 versus usual care, by providing glycemic control with fewer episodes of symptomatic hypoglycemia as demonstrated in EDITION, with an improved diabetes management program, will contribute to improvements in achieving individualized HEDIS HbA1c targets across the spectrum of insulin therapy in T2D care. Appropriate insulin titration by the site, which did not involve any involvement of the sponsor, and assistance from the PSP, should improve safety and may enhance patients’ engagement in their diabetes management. It is thought that enhanced patient engagement will improve glycemic outcomes, including achieving individualized HbA1c target, which in turn may increase patient and provide ratings of treatment effectiveness, enhance persistence and adherence, and potentially provide cost offsets in the long term.
As a further potential benefit of this study, analysis of the databases used, and the resultant attrition chart based on the application of the inclusion/exclusion criteria, may identify unmet medical needs of the patient population currently on non-insulin treatment regimens. This provides a platform for identifying the quality of and gaps in T2D care as it is currently practiced.
Thus, the outcomes of the pragmatic Achieve Control study will be highly relevant in real-world clinical practice. Indeed, the focus on differences in treatment effectiveness, rather than addressing efficacy or safety separately, has clinical relevance and is also useful for clinical decision-making. Results from Achieve Control should allow a better understanding of the impact of interventions on patient outcomes and experiences with treatment, as well as on health resource utilization and cost of care.
4. Conclusions
Achieve Control is a US-based, multicenter, randomized, active-controlled, open-label, two-arm parallel group, comparative real-world pragmatic clinical trial comparing disease management strategies in patients with T2D who are insulin-naïve and uncontrolled on ≥2 OADs and/or GLP-1 RA. The study seeks to determine whether a disease management strategy using Gla-300 with its available PSP is superior to disease management strategies with other basal insulins in terms of the likelihood of patients achieving individualized HbA1c targets at 6 months without experiencing documented symptomatic hypoglycemia. This pragmatic study aims to model real-world clinical practice using a rigorous design that may address some limitations of explanatory RCTs and allow patients, HCPs, and payers to better understand the real-world value of interventions in T2D, while trying to address the ‘triple aim’ in the management of populations with diabetes.
Declaration of interest
Achieve Control is supported by Sanofi US, Inc. The authors received writing and editorial support from Tessa Hartog, PhD, of Excerpta Medica, funded by Sanofi US, Inc. G Oster reports serving as a consultant for Amgen, Bristol-Myers Squibb, Celgene, CSL Behring, Flexion, Gilead, GlaxoSmithKline, Hollister, Mitsubishi Tanabe, InVivo, Novartis, Pfizer, Roche, Sanofi, Seattle Genetics, Shire, Vertex, and ZS Pharma. S Sullivan reports serving as a consultant for Sanofi US, Inc. M Rojeski, J Sung, B Johnstone, L Traylor, and J Van Vleet are employees of Sanofi US, Inc. M Dalal, M Kazemi, H Anhalt, and M Hull report that they were employees of Sanofi US, Inc. at the time the study was conducted. C Wysham reports serving as a consultant for Astra Zeneca, Boerhinger Ingelheim, Eli Lilly, Janssen, and Sanofi, and serving as a speaker for Astra Zeneca, Boerhinger Ingelheim, Eli Lilly, Novo Nordisk, Janssen, and Sanofi. A Cali is an employee of Sanofi, France. L Wei is a professor of biostatistics at Harvard and reports serving as a consultant for Sanofi, Merck, Pfizer, J&J, and Novartis. L Meneghini reports serving as a consultant for Novo Nordisk and Sanofi Aventis.
Supplementary_Material.docx
Download MS Word (37.3 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- UK Prospective Diabetes Study Group. U.K. Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258.
- Turner RC, Cull CA, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012.
- American Diabetes Association. Standards of medical care in diabetes—2016. Glycemic Targets Diabetes Care. 2016;39(Suppl 1):S39–S46.
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271–2279.
- Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62:860–868.
- Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–2545.
- Polinski JM, Smith BF, Curtis BH, et al. Barriers to insulin progression among patients with type 2 diabetes: a systematic review. Diabetes Educ. 2013;39:53–65.
- Becker RH, Nowotny I, Teichert L, et al. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17:261–267.
- Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 units·mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care. 2015;38:637–643.
- Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17:254–260.
- Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–394.
- Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–2762.
- Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–3243.
- Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015;38:2217–2225.
- Riddle MC, Yki-Järvinen H, Bolli GB, et al. One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17:835–842.
- Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–1149.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859–867.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply? Lancet. 2005;365:82–93.
- Gandhi GY, Murad MH, Fujiyoshi A, et al. Patient-important outcomes in registered diabetes trials. JAMA. 2008;299:2543–2549.
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892.
- Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13:217–224.
- Lakey WC, Barnard K, Batch BC, et al. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56:1226–1235.
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chron Dis. 1967;20:637–648.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475.
- Oster G, Borok GM, Menzin J, et al. A randomized trial to assess effectiveness and cost in clinical practice: rationale and design of the Cholesterol Reduction Intervention study (CRIS). Control Clin Trials. 1995;16:3–16.
- Levin PA, Zhang Q, Mersey JH, et al. Glycemic control with insulin glargine plus insulin glulisine versus premixed insulin analogues in real-world practices: a cost-effectiveness study with a randomized pragmatic trial design. Clin Ther. 2011;33:841–850.
- Kauf TL, McKinnon P, Corey GR, et al. An open-label, pragmatic, randomized controlled clinical trial to evaluate the comparative effectiveness of daptomycin versus vancomycin for the treatment of complicated skin and skin structure infection. BMC Infect Dis. 2015;15:503.
- NIH Health Care Systems (HCS) Research Collaboratory [Internet]. Bethesda (MD): National Institutes of Health; [ cited 2012 Jan 24]. Available from: http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-12-002.html
- Lieu TA, Au D, Krishnan JA, et al. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the National Heart, Lung, and Blood Institute workshop. Am J Resp Crit Care Med. 2011;184:848–856.
- Shrank W. The Center For Medicare And Medicaid Innovation’s blueprint for rapid-cycle evaluation of new care and payment models. Health Aff. 2013;32:807–812.
- AHRQ: comparative effectiveness research portfolio [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; [ reviewed 2012 Oct]. Available from: http://www.ahrq.gov/cpi/portfolios/comparative-effectiveness/index.html
- PCORI: research we support [Internet]. Washington (DC): Patient-Centered Outcomes Research Institute; [updated 2015 Oct 20; cited 2014 Sep 10]. Available from: http://www.pcori.org/research-results/research-we-support
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27:759–769.
- NCQA: HEDIS summary table of measures, product lines and changes [Internet]. Washington (DC): National Committee for Quality Assurance; 2015. [cited 2015 July]. Available from: http://www.ncqa.org/Portals/0/HEDISQM/Hedis2015/List_of_HEDIS_2015_Measures.pdf
- Baser O, Bouchard J, DeLuzio T, et al. Assessment of adherence and healthcare costs of insulin device (FlexPen®) versus conventional vial/syringe. Adv Ther. 2010;27:94–104.
- Bradley C. Diabetes treatment satisfaction questionnaire (DTSQ). In: Bradley C, editor. Handbook of psychology and diabetes. Chur: Harwood Academic Publishers; 1994. p. 111–132.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395.
- Sanofi. Sanofi Announces Study Program Evaluating Toujeo® in a Real-Life Setting [Internet]. [ cited 2015 Jun 4]. Available from: http://www.prnewswire.com/news-releases/sanofi-announces-study-program-evaluating-toujeo-in-a-real-life-setting-300094110.html
- Garland EL, Roberts-Lewis A, Tronnier CD, et al. Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: proximal outcomes from a pragmatic randomized trial. Behav Res Ther. 2016;77:7–16.
- Bender BG, Cvietusa PJ, Goodrich GK, et al. Pragmatic trial of health care technologies to improve adherence to pediatric asthma treatment: a randomized clinical trial. JAMA Pediatr. 2015;169:317–323.
- Heisler M, Hofer TP, Schmittdiel JA, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation. 2012;125:2863–2872.
