ABSTRACT
Objective: Positive-controlled clinical studies have shown a dose dependent effect of buprenorphine transdermal system on QTc interval prolongation. This study provides assessment of the buprenorphine transdermal system and cardiac arrhythmia using US FDA and WHO postmarketing reporting databases.
Methods: Disproportionality analysis of spontaneously reported adverse events to assess whether the reporting rate of cardiac arrhythmia events was disproportionately elevated relative to expected rates of reporting in both FDA and WHO databases. Cardiac arrhythmia events were identified using the standardized Medical Dictionary for Regulatory Activities query for torsade de pointes and/or QT prolongation (TdP/QTP). The threshold for a signal of disproportionate adverse event reporting was defined as the lower 90% confidence limit ≥ 2 of the Empiric Bayes geometric mean in FDA database and as the lower 95% confidence limit of the Informational Component >0 in WHO database.
Results: There were 124 (<1%) and 77 (2%) cardiac arrhythmia event cases associated with buprenorphine transdermal as compared to 3206 (12%) and 2913 (14%) involving methadone in the FDA and WHO databases, respectively. In the FDA database methadone was associated with a signal of disproportionate reporting for TdP/QTP (EB05 3.26); however, buprenorphine transdermal was not (EB05 0.33). In the WHO database methadone was associated with a signal of disproportionate reporting for TdP/QTP (IC025 2.66); however, buprenorphine transdermal was not (IC025 −0.88). Similar trends were observed in sensitivity analyses by age, gender, and specific terms related to ventricular arrhythmia.
Conclusions: The signal identified in the transdermal buprenorphine thorough QTc study, which led to a dose limitation in its US label, does not translate into a signal of increased risk for cardiac arrhythmia in real world use, as assessed by this method of analyzing post-market surveillance data.
1. Introduction
Buprenorphine incorporated into a transdermal matrix patch formulation for 7-day wear was approved in the United States (US) in 2010 for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate (BTDS; BUTRANS®, Purdue Pharma, Stamford, US) and was made available for dispensing in January 2011. Five dosage strengths are available with buprenorphine delivery rates averaging 5, 7.5, 10, 15 and 20 mcg/h over 7 days, which correspond, respectively, to average daily buprenorphine doses of 0.12 mg, 0.18 mg, 0.24 mg, 0.36 mg and 0.48 mg per day. Buprenorphine transdermal systems have been available outside the US as 7-day wear formulations delivering 5–20 mcg/h since 2003 (BTDS-N; NORSPAN®, Napp Pharmaceuticals, Cambridge, England) and as 3-day wear systems delivering 35–70 mcg/h since 2001 (BTDS-T; TRANSTEC®, Grunenthal GMbH, Aachen, Germany). Currently, the 3-day and the 7-day buprenorphine transdermal formulations are marketed in 26 and 44 countries worldwide.
In the US, the full prescribing information for BTDS includes a warning of QTc prolongation at BTDS doses above 20 mcg/h [Citation1]. Two positive-controlled, randomized, double-blind studies of the effects of BTDS on the QTc interval in healthy subjects demonstrated no clinically meaningful effect at a BTDS dose of 10 mcg/h; however, a BTDS dose of 40 mcg/h was observed to prolong the QTc interval [Citation2]. Although the magnitude of the QTc prolongation in these clinical thorough QT studies was modest [Citation3], buprenorphine had also been shown in a preclinical human ether-a-go-go-related gene (hERG) study to block the delayed rectifier potassium ion channel [Citation4], a recognized mechanism for QT-prolongation [Citation5].
Buprenorphine tablet/film products are currently available in the US for treatment of opioid dependence in individual dosage strengths ranging from 1.4 mg to 12 mg with naloxone and 2 mg and 8 mg without naloxone [Citation6–Citation9]. No warning of cardiac arrhythmia or QT prolongation is included in the full prescribing information for these tablets/film formulations. Buprenorphine, primarily used for the treatment of opioid dependence, does not appear to be associated with increased risk for serious cardiac arrhythmia [Citation10–Citation14]. Buprenorphine tablet/film products deliver higher systemic buprenorphine serum levels than BTDS, when administered as directed. A single dose of lowest strength sublingual buprenorphine/naloxone tablet (2 mg/0.5 mg) achieves a buprenorphine maximum plasma concentration (Cmax) of 0.947 ng/ml [Citation7], twice that of the maximum recommended dose of BTDS (20 μg/h) which achieves a buprenorphine Cmax of 0.471 ng/ml [Citation1]. Notably, the recommended target dose of buprenorphine/naloxone for maintenance treatment of opioid dependence is 16 mg/4 mg per day.
The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS database) and the World Health Organization (WHO) Global Individual Case Safety Reports Database System (VigiBase database) can be used to identify disproportionate reporting rates for adverse event and drug combinations which could be indicative of potential risks associated with particular marketed products. Disproportionality methods have been used previously to assess opioid arrhythmic potential using data from the FAERS [Citation14] and the VigiBase [Citation16,Citation17]. Most recently, results of a study evaluating spontaneously reported adverse events reported to the FDA up to June 2011 showed that methadone was associated with disproportionate reporting of cardiac arrhythmia, whereas buprenorphine was not [Citation15]. Since BTDS was made available for dispensing in the US in January 2011, the authors concluded that there was insufficient data to specifically evaluate the transdermal formulation.
The objective of this study was to assess whether there is disproportionate reporting of adverse events consistent with the medical concept of cardiac arrhythmia for BTDS in the US FDA and the WHO pharmacovigilance (spontaneous reporting) databases relative to other adverse events in these databases. Methadone was chosen as a comparator drug in the study since it is an opioid with well-established effects of QT interval prolongation and serious arrhythmia (torsade de pointes, TdP) [Citation18]. Fentanyl was chosen as an additional comparator since it is an opioid formulation delivered via transdermal route and does not have a warning of QT prolongation in its US label [Citation19]. We hypothesized that BTDS would be associated with proportionally lower reporting rates of cardiac arrhythmia as compared to methadone.
2. Methods
2.1. Design
This was a retrospective, observational study of data from two large spontaneous reporting systems that contain information that is primarily generated in the postmarketing setting and is predominantly used by regulatory health authorities.
2.2. Databases
Data were extracted from the FAERS database maintained by the FDA, and from WHO Global Individual Case Safety Reports Database System, VigiBase database, maintained by the Uppsala Monitoring Centre (UMC) in Sweden.
FAERS is a database that contains information on adverse event and medication error reports submitted to the FDA starting 1969 and including those from the Medical Products Reporting Program (MEDWatch) that was introduced in 1993 [Citation20]. MEDWatch is a national passive surveillance system that relies on mandatory reporting by pharmaceutical manufacturers as well as voluntary reporting by healthcare professionals and consumers. In the US, most health care professionals and consumers submit reports of adverse events to the manufacturer rather than directly to the FDA. FAERS is a longitudinal database where subsequent follow-up reports for a specific individual case are compiled into a single case. A report may name a specific disorder/diagnosis (e.g. torsade de pointes) or describe a sign/symptom that might be an element of many disorders (e.g. syncope, loss of consciousness).
VigiBase is a database that contains information on suspected adverse drug reactions submitted to the UMC via the WHO Programme for International Drug Monitoring (PIDM) established in 1968 [Citation21]. PDIM is a global surveillance system that collects cases of suspected adverse drug reactions from over 120 member countries from both voluntary and regulatory sources depending on each national pharmacovigilance system. Cases are received by UMC from health care professionals, hospitals, lawyers, and manufacturers, with a significant proportion of cases from the United States FAERS database.
Although individual reports may be duplicated in the FAERS and WHO databases, FAERS contains adverse event reports for brand and generic buprenorphine/naloxone tablets/film products indicated for opioid maintenance therapy at average daily doses of 16 mg/4 mg prescribed in the US, while VigiBase provides adverse event data for transdermal buprenorphine mostly used outside of the US, in countries where the 7-day transdermal buprenorphine is approved for management of pain at dosage strengths up to 40 mcg/hour and 3-day transdermal buprenorphine product have been used for over a decade at dosage strengths up to 70 mcg/hour.
2.3. Events of interest
Adverse events are categorized in the FAERS and VigiBase using the Medical Dictionary for Regulatory Activities (MedDRA), a standard hierarchically organized medical terminology developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
Standardized MedDRA queries (SMQs) have been developed jointly by the Council for International Organizations of Medical Sciences (CIOMS) and the MedDRA Maintenance and Support Services Organization (MSSO) as a tool to assist in retrieval of cases of interest in MedDRA coded databases [Citation22]. SMQs contain terms related to diagnoses and laboratory test data as well as signs, symptoms, syndromes, and physical findings connected with the medical condition of interest. Currently over 40 SMQs have been developed by the MSSO. During development they undergo testing to assure that they are able to retrieve cases of interest within the defined scope of the SMQ. Based on event definition, SMQs may be of narrow scope, consisting of highly specific terms likely to represent the condition of interest, and broad scope, containing additional more general search terms which may assist in identifying more cases increasing the sensitivity of the search.
Adverse events (AEs) of interest consistent with the medical concept of cardiac arrhythmia were identified using the SMQ for TdP and/or QT prolongation. The SMQ consist of 20 individual adverse event preferred terms (PTs) of which six are specific PTs (electrocardiogram QT interval abnormal, electrocardiogram QT prolonged, long QT syndrome, long QT syndrome congenital, torsade de pointes, and ventricular tachycardia) and 14 are broad PTs (cardiac arrest, cardiac death, cardiac fibrillation, cardio-respiratory arrest, electrocardiogram repolarization abnormality, electrocardiogram U-wave abnormality, loss of consciousness, sudden cardiac death, sudden death, syncope, ventricular arrhythmia, ventricular fibrillation, ventricular flutter, and ventricular tachyarrhythmia). Narrow scope refers to a search that identifies AEs of interest in the database coded to any of the six specific terms, while broad scope search identifies AEs coded to any of the 20 terms.
2.4. Drugs of interest
In the FAERS analysis, drugs evaluated included all formulations of buprenorphine, fentanyl and methadone that were categorized as a suspect, interacting, or concomitant medication. Buprenorphine subgroups included all transdermal, BTDS (Butrans® patch 5–20 mcg/hour), and sublingual buprenorphine/naloxone tablet/film. Fentanyl subgroups included transdermal formulation.
In the VigiBase analyses, drugs evaluated included buprenorphine and methadone that were categorized as suspect, interacting, or concomitant medication. Buprenorphine subgroups included all transdermal, BTDS-N (Norspan® patch 5–20 mcg/h), and BTDS-T (Transtec® patch 35–70 mcg/h). Methadone subgroup included all formulations.
2.5. Statistical analysis
Disproportionality analyses were used as the method to identify any potential safety signals for adverse events suggestive of cardiac arrhythmia in the FAERS and the VigiBase. Disproportionality analyses assess the extent of reporting of adverse event(s) for a product relative to this same reported adverse event(s) for all other products in the database. Unexpectedly high relative reporting rates may signal a potential association between the particular adverse event and the product.
Two frequently used methods for data mining of pharmacovigilance databases are the multi-item Gama-Poisson shrinker (MGPS), and the Bayesian Confidence Propagation Neural Network (BCPNN) [Citation23]. These methods are widely used by regulatory agencies to identify potential safety concerns for marketed products.
FAERS data signal scores were computed using MGPS, a signal detection algorithm used by FDA [Citation24]. MPGS is a disproportionality method that uses an empirical Bayesian model to detect the magnitude of drug–event associations in a database. MPGS calculates adjusted reporting ratios for pairs of drug event combinations. The adjusted reporting ratio value, called the empiric Bayes geometric mean (EBGM), indicates the strength of the reporting relationship between a particular drug and event pair. To reduce confounding, the MPGS program systematically stratifies expected rates by variables collected in the database (e.g. demographics, year, number reports per quarter). Depending on how much information exists about a drug–event combination, MPGS adjusts for multiplicity and false positive errors by systematically ‘shrinking’ observed–expected toward 1 (no association). Drug–event combinations with few reports for the event are shrunk by a large amount, while drug–event combinations with many reports for an event are shrunk by a small amount. Data from the FAERS from 1969 to December 2015 were extracted and analyzed using Oracle Health Sciences quantitative signal detection tool, Empirica Signal™ (Version 7.3). The MGPS algorithm was applied to extracted FAERS data to generate EBGM values with corresponding confidence intervals (CIs). The lower limit of the 90% EBGM confidence interval (EB05) of ≥2 was used as standard measure of an increase in disproportionate reporting [Citation25].
VigiBase disproportionality signal scores were computed at UMC using BCPNN to screen the WHO Adverse drug reaction database as part of routine signal detection processes. To assess disproportionality, an informational component (IC) and credibility interval is calculated for each drug and adverse drug reaction in the dataset [Citation23]. IC measures the disproportionality between the observed and the expected reporting of a drug-adverse drug reaction (drug-ADR) pair. A positive IC value indicates that a particular drug-ADR pair is reported more often than expected based on all the reports in the database, while a negative IC value indicates that a drug-ADR pair is reported less frequently than expected. The IC value is based on the total number of reports in the database on the ADR term, the drug, and the specific drug-ADR pair.
Data from 1978 to June 2014 were extracted by staff at UMC and IC values and credibility intervals calculated. The lower limit of the 95% informational component credibility interval (IC025) of > 0 was used as a standard measure of an increase in disproportionality [Citation26,Citation27].
Disproportionality analyses were further stratified by gender, age, and specific adverse event term.
3. Results
3.1. FDA Adverse Event Reporting System (FAERS)
A total of 8,270,285 cases in FAERS were evaluated of which 32,067 involved buprenorphine (22,454 any transdermal; 21,610 BTDS; and 11,206 buprenorphine/naloxone tablets/film), 88,335 involved fentanyl (39,976 any transdermal), and 26,519 involved methadone. The EBGM and Upper (EB95) and Lower (EB05) 90% confidence intervals for the cardiac arrhythmia adverse events (narrow and broad term search) by opioid product grouping are presented in . No signal of disproportionate reporting for cardiac arrhythmia was identified for any buprenorphine product grouping or fentanyl product grouping (all EB05 values <2); however, methadone was associated with disproportionate reporting of cardiac arrhythmia.
Figure 1. Disproportionality analysis of cardiac arrythmia events reported to FDA between 1969 and December 2015. The figure displays Empirical Bayesian Geometric Mean (EBGM) Values and Upper (EB95) and Lower (EB05) limits of the 90% Confidence Intervals for cardiac arrhythmia events by opioid product grouping. A standard measure of an increase in disproportionality is an EB05 of ≥ 2.
Broad: includes 1 or more of 20 adverse event terms: electrocardiogram QT interval abnormal, electrocardiogram QT prolonged, long QT syndrome, long QT syndrome congenital, torsade de pointes, and ventricular tachycardia cardiac arrest, cardiac death, cardiac fibrillation, cardio-respiratory arrest, electrocardiogram repolarization abnormality, electrocardiogram U-wave abnormality, loss of consciousness, sudden cardiac death, sudden death, syncope, ventricular arrhythmia, ventricular fibrillation, ventricular flutter, ventricular tachyarrhythmia.
Narrow: includes 1 or more of 6 adverse event terms: electrocardiogram QT interval abnormal, electrocardiogram QT prolonged, long QT syndrome, long QT syndrome congenital, torsade de pointes, and ventricular tachycardia.
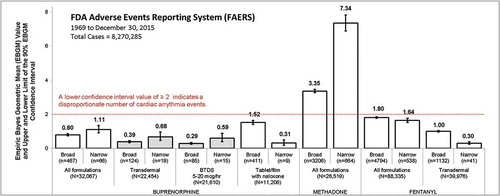
Case characteristics and disproportionality analysis results further stratified by gender, age, and specific term are summarized in . Cardiac arrhythmia adverse events, identified using the broad scope PT search, were reported in <1% of cases involving any buprenorphine transdermal, <1% involving BTDS 5–20 mcg/h patch, 4% for buprenorphine/naloxone tablets/film, and 12% for methadone. Age range and gender distributions of reports containing an arrhythmia adverse event were generally similar between groups. However, age was not reported in >50% of cases involving BTDS.
Table 1. Cardiac arrhythmia disproportionality results, FAERS database, 1969-30 December 2015 (8,270,285 total cases).
Disproportionate reporting of cardiac arrhythmia for methadone (EB05 3.26) was observed in broad scope term analysis. No disproportionality was observed for buprenorphine including: all buprenorphine formulations (EB05 0.74), all buprenorphine transdermal (EB05 0.33), BTDS 5–20 mcg/h patch (EB05 0.24) and buprenorphine tablets/film (EB05 1.40). Similar trends were observed for each individual preferred terms within the broad scope grouping with the exception of ‘loss of consciousness’ which was disproportionally reported for buprenorphine/naloxone tablets/film but not for other buprenorphine products. Loss of consciousness is a known adverse reaction secondary to opioid induced CNS depression.
Stratifying the broad scope cardiac arrhythmia term analysis by females, males, or individuals aged <65 years and ≥65 years, did not identify a disproportionate reporting signal associated with any buprenorphine product (all EB05 < 2). However, for methadone, a signal was observed in female individuals (EB05 3.57), male individuals (EB05 3.21), and individuals age <65 years (EB05 3.40; ).
When analysis was limited to specific, narrow scope, cardiac arrhythmia terms, no disproportionate reporting signal for any buprenorphine product was identified (all EB05 < 2); however, a signal for methadone (EB05 6.89) was observed. Similar trends were presented for nearly all of the individual preferred terms within the narrow scope grouping including TdP (EB05 22.16), electrocardiogram QT prolonged (EB05 8.67) and ventricular tachycardia (EB05 3.38). Sensitivity analyses that further limited the data by gender and age also showed similar trends.
3.2. WHO drug reporting system (Vigibase)
A total of 9,350,995 VigiBase cases were evaluated, of which 16,266 involved buprenorphine, (5095 any transdermal, 2118 BTDS-N patch 5–20 mcg/h, and 1638 BTDS-T patch 35–70 mcg/h) and 20,167 involved methadone. Case characteristics and disproportionality analysis results further stratified by gender, age, and specific term are summarized in .
Table 2. Cardiac arrhythmia disproportionality results, VigiBase database, 1978–June 2014 (9,350,995 total cases).
Cardiac arrhythmia events, identified using the broad scope PTs, were reported in 2% of cases involving any buprenorphine formulation, 2% involving all transdermal buprenorphine, 1% involving BTDS-N patch 5–20 mcg/h, 2% involving BTDS-T patch 35–70 mcg/h, and 14% involving methadone. Age range and gender distributions of reports containing an arrhythmia event were generally similar between groups. However, cases involving the buprenorphine patch more frequently involved females and individuals aged >65 years.
No signal of disproportionate reporting of cardiac arrhythmia was identified for any of the buprenorphine product groupings: all formulations (IC025 –0.04), all transdermal (IC025 –0.88), BTDS-N patch 5–20 mcg/hour (IC025 –1.90) and BTDS-T patch 35–70 mcg/hour (IC025 –1.07). However, the broad scope term analysis showed a signal of disproportionate reporting for methadone (IC025 2.66; ).
Stratifying the broad scope PT search data by females, males, or individual’s age ≥65 years, did not identify a signal of disproportionate reporting associated with any buprenorphine product (all IC025 < 0). However, a signal was observed for methadone in female individuals (IC025 2.46), male individuals (IC025 2.35), and individuals age ≥65 years (IC025 0.69).
When data was limited to narrow scope PTs, reflective of a more specific diagnosis of TdP/QT-prolongation, no signal of disproportionately reporting was identified for any buprenorphine product (all IC025 < 0). However, a signal was observed for methadone (IC025 3.36). Additionally, for methadone, signals were also identified when analyzing the majority of individual PTs, including TdP (IC025 4.62), electrocardiogram QT prolonged (IC025 3.86), and ventricular tachycardia (IC025 1.95). Data further stratified by gender and age showed similar trends.
4. Discussion
This analysis of spontaneously reported adverse events in the FDA and WHO databases did not identify any potential proarrhythmic signal for transdermal buprenorphine formulations available in the US at unit strengths from 5 to 20 mcg/h and outside the US at unit strengths from 35 to 70 mcg/h. In addition, no proarrhythmic signal was identified for buprenorphine/naloxone tablets/films which provide higher daily systemic exposures to buprenorphine than the transdermal formulation. In contrast, a signal was observed for methadone, an opioid with a clinically well-established association with cardiac arrhythmia events, indicating that the method of Bayesian disproportionality analyses of the FDA and WHO databases were sensitive to increases in reporting of events consistent with a proarrhythmic potential of a drug.
There are limitations to this analysis. The voluntary nature of adverse event reporting to the FDA and WHO pharmacovigilance systems may limit this analysis to a subset of adverse events occurring in the general population. Reports of an association between a product and an event do not necessarily indicate causality. Similarly, a signal identified by a disproportionality analysis may be indicative of an association that may not be causal. Comparisons of adverse event rates using measures of disproportionate reporting can be influenced by many factors including absolute report numbers, presence of other adverse events associated with the same drug, and reporting bias such as underreporting, overreporting, poor data quality and diagnostic uncertainty. The number of cases reported for some of the sensitivity analysis in some cases may be too small to support robust assessment of data.
Other factors that can influence an association between the frequency of adverse event reporting and a product include the length of time that product has been marketed, or the extent of publicity about a new safety concern. One such example is the FDA cardiac warning letter for methadone-associated serious cardiac arrhythmia issued to healthcare providers in November 2006, which could have prompted increasing reporting of arrhythmic events for the drug; however, we conducted additional analysis of FAERS data limited to 1969 to November 2006 which confirmed the presence of a signal of disproportionate reporting prior to issuance of the warning letter.
Spontaneous reports of adverse events do not always contain information on dosing, plasma concentration levels, concomitant medications, and other confounding factors to allow for a complete medical assessment. Such information may have provided insights into the lack of correlation between the proarrhythmic risk, as suggested by two QT clinical studies, and the findings of this postmarketing data analysis. Interestingly, increases in the QTc interval have been characterized to be highly sensitive but not very specific for predicting the risk of ventricular proarrhythmia. Currently efforts are ongoing to develop new assays to measure the proarrythmic risks of drugs in development [Citation28,Citation29].
5. Conclusion
The signal identified in the transdermal buprenorphine thorough QTc study, which led to a dose limitation in its US label, does not translate into a signal of increased risk for cardiac arrhythmia in real world use, as assessed by this method of analyzing postmarket surveillance data. In contrast to methadone, no disproportionality for cardiac arrhythmias was observed for any of the buprenorphine formulations.
Declaration of interest
All authors are employees of Purdue Pharma L.P. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Butrans [Full Prescribing Information]. Stamford (CT): Purdue Pharma L.P.; 2014 [cited 2016 Sep]. Available from: https://www.butrans.com/hcpportal/f?p=BUTRANSRX:ISI
- Harris SC, Morganroth J, Ripa SR, et al. Effects of buprenorphine on QT intervals in healthy subjects: results of 2 randomized positive- and placebo-controlled trials. Postgrad Med. 2017;129(1):69–80.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs: ICH E14. 2005 May 12. [cited 2016 Dec 7]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf
- Katchman AN, McGroary KA, Kilborn MJ, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther. 2002;303:688–694.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals: S7B. 2005 May 12 [cited 2016 Dec 7]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf
- Subutex (buprenorphine and naloxone) sublingual tablets [Full Prescribing Information]. Richmond (VA): Reckitt Benckiser Pharmaceuticals, Inc. 2016 [cited 2016 Mar 10]. Available from: http://www.accessdata.fda gov/drugsatfda_docs/label/2014/020733Orig1s014lbl.pdf
- Suboxone (buprenorphine and naloxone) sublingual tablets. Richmond (VA): Reckitt Benckiser Pharmaceuticals, Inc. 2014 12. [cited 2016 Dec 8]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020733Orig1s014lbl.pdf
- Zubsolv Sublingual Tablets [Full Prescribing Information]. New York (NY): Orexo US, Inc. 2015 8 [cited 2016 Dec 8]. Available from: https://www.zubsolv.com/wp-content/uploads/2015/01/Zubsolv FullPrescribingInformation.pdf
- Bunavail Buccal Film [Full Prescribing Information]. Raleigh (NC): BioDelivery Sciences International, Inc. [cited 2016 Dec 8]. Available from: http://www.bunavail.com/assets/pdf/ BUNAVAIL_Full_Prescribing_Information.pdf
- Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469–2475.
- Poole SA, Pecoraro A, Subramaniam G, et al. Presence or absence of QTc prolongation in buprenorphine-naloxone among youth with opioid dependence. J Addict Med. 2016;10:26–33.
- Stallvik M, Nordstrand B, Kristensen O, et al. Corrected QT interval during treatment with methadone and buprenorphine–relation to doses and serum concentrations. Drug Alcohol Depend. 2013;129:88–93.
- Fareed A, Patil D, Scheinberg K, et al. Comparison of QTc interval prolongation for patients in methadone versus buprenorphine maintenance treatment: a 5-year follow-up. J Addict Dis. 2013;32:244–251.
- Kao D, Bartelson BB, Khatri V, et al. Trends in reporting methadone-associated cardiac arrhythmia 1997–2011. Ann Intern Med. 2013;158:735–741.
- Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the food and drug administration. Addiction. 2015;110:1468–1475.
- Meyer-Massetti C, Vaerini S, Ratz Bravo AE, et al. Comparative safety of antipsychotics in the WHO pharmacovigilance database: the haloperidol case. Int J Clin Pharmacol. 2011;33:806–814.
- Freedman SB, Uleryk E, Rumantir M, et al. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Am Coll Emerg Phys. 2014;64:19–25.
- Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in Collaboration with the Heart Rhythm Society. J Pain. 2014;15:321–337.
- Duragesic [Full Prescribing Information]. Titusville (NJ): Janssen Pharmaceuticals, Inc; 2014 [cited 2016 Oct 31]. Available from: http://www.duragesic.com/assets/pdf/duragesic_0.pdf
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. Jama. 1993;269:2765–2768.
- Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–419.
- Mozzicato P. Standardized MedDRA queries. Their role in signal detection. Drug Saf. 2007;30:617–619.
- Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov Today. 2009;14:343–357.
- DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat. 1999;53:177–196.
- Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25:381–392.
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–436.
- Van Puijenbroek EP, Bate A, Leufkens HGM, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10.
- Sager PT, Gintant G, Turner JR, et al. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am Heart J. 2014;167(3):292–300.
- Colatsky T, Fermini B, Gintant G, et al. Stockbridge. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative - Update on progress. J Pharmacol Toxicol Methods. 2016;81:15–20.
