ABSTRACT
Objectives: To study the effect of transdermal buprenorphine on QTc prolongation at dose levels of 10, 40, and 80 mcg/h, (BTDS 10, BTDS 40, BTDS 80).
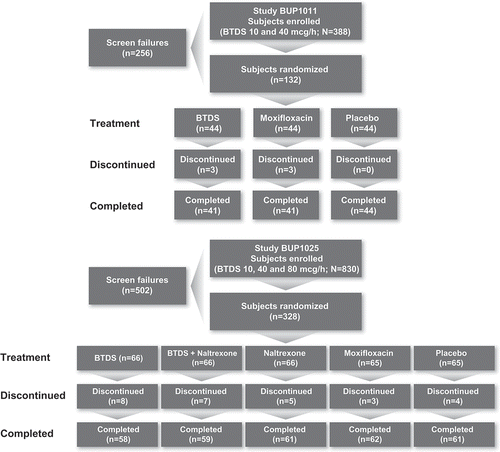
Methods: Two randomized, placebo- and positive-controlled, parallel-group, dose-escalating clinical studies evaluated healthy adult subjects randomized to BTDS, placebo, or moxifloxacin in the first study; and to BTDS only, BTDS plus naltrexone, naltrexone alone at the same dose, placebo, or moxifloxacin in the second study. QT intervals were corrected for heart rate using data from each individual subject (QTcI).
Results: In the first study (n = 44), the maximum upper bounds of the 90% confidence interval (CI) for mean placebo-corrected change from baseline in QTcI across 13 time points over 24 h were: 10.0 msec for BTDS 10 (Day 6) and 13.3 msec for BTDS 40 (Day 13); and 17.0 msec (Day 6) and 15.5 msec (Day 13) for moxifloxacin, respectively.
Similarly, in the second study (n = 66), the upper bound of the 90% CI for mean placebo-corrected change from baseline for QTcI was under 10 msec at all time points for BTDS 10 (maximum upper bound, 5.63 msec), over 10 msec at 5 time points for BTDS 40 (maximum 11.81 msec) and over 10 msec at all 13 time points for BTDS 80 (maximum, 14.14 msec). Naltrexone administered with BTDS eliminated the QTcI prolongation seen with supratherapeutic BTDS doses (BTDS 40, BTDS 80) administered without naltrexone.
Conclusions: At the therapeutic dose of 10 mcg/h, BTDS has no clinically significant effect on QTc. At supratherapeutic doses of 40 and 80 mcg/h, BTDS treatment produces prolongation of QTcI similar in magnitude to that produced by a 400 mg dose of moxifloxacin. Despite the modest, dose-dependent increase in QTcI noted in these studies, transdermal buprenorphine has not been associated with proarrhythmic effects.
1. Introduction
Excessive QTc prolongation increases the risk of potentially fatal ventricular tachyarrhythmias, including torsade de pointes. QTc interval prolongation generally occurs due to blockage of the inward potassium rectifier of the hERG (human ether a go–go-related gene) channel. In vitro studies of opioids such as levomethadyl, methadone, and buprenorphine have demonstrated blockage of the hERG channel activity. Experimental assessment of buprenorphine’s risk of inducing QTc prolongation using a hERG assay has shown that the half maximal inhibitory concentration (IC50) for buprenorphine is in the range of 1–10 μM. As a consequence, buprenorphine has a much larger margin between concentrations obtained following transdermal patch administration versus those required to significantly block the hERG channel.
Buprenorphine is a partial agonist at mu-opioid and at ORL-1 (nociceptin) receptors, an antagonist at kappa opioid receptors, and an agonist at delta opioid receptors [Citation1–Citation3]. Buprenorphine is available in different formulations for example intravenous, sublingual, buccal, and transdermal formulations.
The Buprenorphine Transdermal System (BTDS, 7-day patch; Butrans®) is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. Its efficacy, safety, and tolerability have been established in several randomized trials [Citation4–Citation13]. BTDS systems are approved in the United States in strengths of BTDS 5 (5 mcg/h), BTDS 7.5 (7.5 mcg/h), BTDS 10 (10 mcg/h), BTDS 15 (15 mcg/h), and BTDS 20 (20 mcg/h). Given that the maximum observed plasma concentration (Cmax) for BTDS 20 is approximately 1 nM, the safety margin for potential blockade of hERG by BTDS 20 is at least 1000-fold. Although arrhythmia has been attributed to other opioids such as levacetylmethadol and methadone and other synthetic opioids [Citation14] used to treat heroin addiction, there is little evidence that buprenorphine causes clinically important changes in ECG intervals when BTDS is used at up to the maximum recommended dose of 20 mcg/h [Citation15,Citation16]. Experience with other opioids prompted two clinical studies designed to assess the effects of higher doses of buprenorphine on QTc interval changes.
2. Methods
2.1 Study BUP1011
BUP1011 (NCT01148537) was a single-center, randomized, placebo- and positive-controlled, parallel group, dose-escalating study conducted in healthy subjects to evaluate the effect of BTDS 10 and BTDS 40 on QTc interval. The primary objective of this study was to evaluate the effect of BTDS 40 (2 × BTDS 20; a supratherapeutic dose) on QTcI intervals. The secondary objectives were to evaluate the effect of BTDS 10 (a therapeutic dose) on QTcI intervals and to characterize the safety of dose escalation up to BTDS 40.
Healthy subjects 18–55 years with a BMI < 30 kg/m2, normal cardiac conduction, and resting heart rate (HR) 50–85 bpm were enrolled, while those with a personal or family history of prolonged QT interval or disorders of cardiac rhythm were excluded from the trial. The study protocol was approved by an institutional review board and all subjects provided written informed consent.
ECG intervals were evaluated in three treatment groups: BTDS, matching placebo transdermal systems (placebo TDS), and moxifloxacin (positive control). BTDS and placebo TDS were administered in double-blind fashion. Moxifloxacin treatment was open label to subjects, the investigator, and staff members at the study site. Personnel involved in the assessment of digital ECGs were blinded to all three treatments.
The dose-escalation sequence was BTDS 5 on Days 1–3, BTDS 10 on Days 4–6, BTDS 20 on Days 7–9, and BTDS 20 × 2 on Days 10–13. The ECG evaluation was performed on Days 6 and 13. A 400-mg moxifloxacin tablet was administered to subjects in the positive control group on Days 6 and 13 only. The placebo group received matching placebo TDS throughout the study. A taper period (for subjects assigned to BTDS or placebo TDS) began after the dose escalation had been completed. During the taper, BTDS-treated subjects received BTDS 10 for 7 days, followed by BTDS 5 for 7 days.
A continuous 12-lead digital ECG recording system was used for 24-h ECG collections (Mortara H+ System, Mortara Instruments, Milwaukee, WI USA). Subjects were admitted to the study unit during the baseline period and underwent a 24-h digital 12-lead ECG and simultaneous 24-h telemetry. Beginning at approximately 8 AM on Day 2, a second baseline 24-h digital ECG recording was obtained. Subjects were randomized on Day 1 to receive either BTDS, placebo TDS, or moxifloxacin. The ECG recordings were sent to a central ECG laboratory (ERT, Gladwyne, PA, USA) for blinded ECG evaluation. All subjects were confined to the facility from baseline until Day 14 (moxifloxacin group) or Day 16 (BTDS and placebo TDS).
ECG data were determined for each of 4 ECGs extracted over an approximate 10-min interval at each of the 13 nominal time points over a 23.5-h period. The average ECG interval data from the replicate ECGs at each nominal time point were calculated and morphology changes in the wave form assessed. The average ECG interval data over the 2 pretreatment days were used as the baseline values. QTcI corrections were calculated using the pretreatment baseline ECG data for each subject. Pharmacokinetics (PK) blood samples were obtained during the 24-h Holter ECG recording periods on Day 6 at 49, 61, and 72 h after BTDS 10 application and on Day 13 at 73, 85, and 96 h after BTDS 40 application. Plasma from these samples was assayed to determine concentrations of buprenorphine and its metabolite norbuprenorphine.
The primary variable for the comparison of moxifloxacin to placebo, and for BTDS to placebo, was the average difference from baseline (across all nominal time points) using QT corrected from within subject data (QTcI) on Day 13. An ANCOVA was used, with average baseline as a covariate, and with treatment as effect, to calculate 90% confidence intervals (CIs) for these differences. For each subject, the mean time-matched QTc from the 2 pretreatment days was subtracted from the time-matched QTc on Day 13. The full analysis population (FAP) for ECGs was defined as subjects with at least one postdose digital QT/QTc evaluation.
2.2 Study BUP1025
The primary objective of the BUP1025 study (NCT01999114) was to evaluate ECG effects of BTDS (10, 40, and 80 mcg/h) alone or BTDS with naltrexone, relative to placebo.
Studies BUP1025 and BUP1011 were generally similar with several notable differences (): In Study BUP1025, continuous 24-h, digital, 12-lead Holter ECG collections for ECG interval evaluation were performed and blood samples collected at baseline on Days 2 and 1, and on treatment Days 6, 13, and 17 to characterize the digital ECG and PK/PD effects of the 5 treatments. The study evaluated 10, 40, and 80 mcg/h doses of BTDS, administered both alone (BTDS-only) and in combination with naltrexone (BTDS/naltrexone), and the time-matched effects of placebo, moxifloxacin, and naltrexone.
In BUP1025, subjects in the BTDS-only treatment group received BTDS 5 (Days 1–3), BTDS 10 (Days 4–6), BTDS 20 (Days 7–9), BTDS 40 (i.e. BTDS 20 × 2; Days 10–13), and BTDS 80 (i.e. BTDS 20 × 4; Days 14–17). This dose-escalation sequence is consistent with BTDS dosing instructions. After completion of final ECG and plasma sample collections, active doses of BTDS were tapered to BTDS 40 (Days 18–20), BTDS 10 (Days 21–23), and BTDS 5 (Days 24–26). Subjects randomized to placebo, BTDS/naltrexone, or moxifloxacin received matching placebo TDS during the taper period.
The same dosing schedule was used for subjects in the BTDS/naltrexone treatment group. No taper in BTDS was required for this group due to naltrexone dosing (50 mg every 12 h on Days 4–20 following the removal of active BTDS at the end of Day 17). Placebo TDS systems were used on Days 1–3 and Days 18–26.
Subjects in the naltrexone, placebo, and moxifloxacin treatment groups received only placebo TDS on Days 1–26. The size, number, and timing of applications of placebo TDS matched those in the BTDS and BTDS/naltrexone groups.
All subjects in naltrexone/matching placebo group received active naltrexone (50 mg q12 h or matching placebo) beginning on Day 4 and continuing through Day 20. Active naltrexone was administered to subjects randomized to BTDS/naltrexone or naltrexone alone beginning on Day 4 and continuing through Day 17, followed by matching placebo dosing on Days 18–20. Subjects in the BTDS, placebo, and moxifloxacin treatment groups received matching placebo on Days 4–20.
All subjects received single 400 mg doses of moxifloxacin (moxifloxacin treatment group) or matching placebo (all other treatment groups) on the morning of Days 6, 13, and 17 only.
PK blood samples were collected after treatment on Days 6, 13, and 17. Plasma from these samples for the BTDS-only and the BTDS/naltrexone treatment groups was analyzed for buprenorphine and norbuprenorphine. Samples from the moxifloxacin treatment group were analyzed for moxifloxacin, and samples from the BTDS/naltrexone and the naltrexone alone treatment groups were analyzed for naltrexone.
Subjects in all treatment groups were confined to the study unit for the entire period.
The effects of BTDS on cardiac repolarization were assessed based on the corrected QT intervals (QTc) since QT duration varies inversely with HR. The primary end point was the time-matched change from baseline in QT data corrected for HR, and placebo corrected, based on an individual correction (QTcI) method (ΔΔQTcI), as recommended by ICH E14 [Citation17]. Mean (90% CI) time-matched double-corrected QTc, and ECG-derived values for HR, PR, QRS, and QT were determined. The effect of BTDS 10, BTDS 40, and BTDS 80 on QT intervals was compared with the moxifloxacin-positive control after placebo and baseline correction.
Two-sided 90% CIs were calculated using a mixed-effect general linear model with the following covariates: time (categorical), treatment, and time-by-treatment interaction. The estimates of the ΔΔ and its CIs were performed using a least squares method.
The upper limit of the two-sided 90% CI on treatment was compared to the 10-msec bound only for the BTDS treatments versus placebo. If the upper limit of the two-sided 90% CI for the supratherapeutic treatment versus placebo fell below 10 msec, it was concluded that the supratherapeutic dose did not prolong the QTc interval to a clinically significant degree. The procedure was repeated for the clinical doses. If the upper limit of the two-sided 90% CI for a treatment versus placebo also fell below 10 msec, it was concluded that this dose did not prolong the QTc interval to a clinically significant degree.
The FAP for ECG included subjects who were randomized, received at least one dose of study drug, and had at least one time-matched baseline and one on-treatment ECG. The FAP for PK/PD included subjects who received study drug and had at least one quantifiable PK metric. The PK/PD analysis required a time-matched plasma concentration.
The statistical approaches were similar between the two studies.
3. Results
3.1 Study BUP1011
Forty-four subjects were randomized to each treatment (BTDS, placebo, and moxifloxacin). Six subjects discontinued: three receiving BTDS (two due to adverse events [AEs]) and three receiving moxifloxacin (one due to AE). In the BTDS, placebo, and moxifloxacin treatment groups, the proportions of females were 50%, 52% and 52%, the proportions of white subjects were 50%, 59%, and 41%, and the mean age for each group was 31, 34, and 29 years, respectively. The mean BMI for each group was 26, 25, and 25 kg/m2 respectively.
Change from baseline in QTcI results is shown in – and and . The difference in least square means (90% CI) compared with placebo was 0.39 msec (−1.8, 2.6) for BTDS 10 (on Day 6) and 5.91 msec (3.4, 8.4) for BTDS 40 (on Day 13). The QTcI prolongation seen with BTDS 40 was comparable in magnitude to that seen in the moxifloxacin treatment group on the same day (5.86 msec [3.3, 8.4]).
Table 1. Time-matched, placebo-adjusted QTcI change from baseline (msec) for BTDS 10 and moxifloxacin on Day 6 – Study BUP1011.
Table 2. Time-matched, placebo-adjusted QTcI change from baseline (msec) for BTDS 40 and moxifloxacin on Day 13 – Study BUP1011.
Table 3. Average QTcI interval change from baseline – Study BUP1011.
Figure 2. Mean QTcI change from baseline versus time by treatment on Day 6 (FAP) -Study BUP1011.
Time points for ECGs were 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 7, 10, 13, 18, and 23.5 hours.
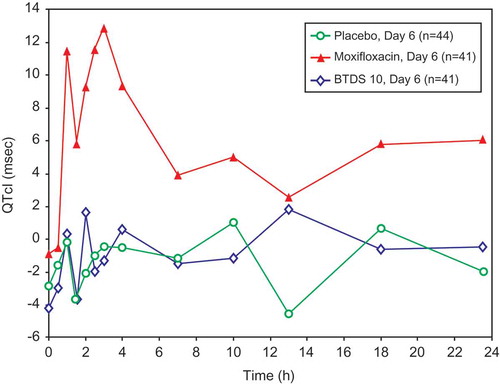
Figure 3. Mean QTcI change from baseline versus time by treatment on Day 13: FAP – Study BUP1011.
Time points for ECGs were 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 7, 10, 13, 18, and 23.5 hours.
No subject had maximum QT/QTc intervals ≥480 msec, and no subject had increases in QTcI ≥ 60 msec. Among subjects receiving moxifloxacin, changes from baseline in QTcI of 30–60 msec occurred in six (14%) and five (12.2%) on Days 6 and 13, respectively. Among subjects receiving placebo, changes in QTcI of this magnitude were observed in five (11.4%) and one (2.3%) subjects on Days 6 and 13, respectively. Among subjects receiving BTDS, two (4.5%) had such changes after BTDS 10 and three (6.8%) after BTDS 40.
3.2 Study BUP1025
Sixty-six subjects were randomized to BTDS alone, 66 to BTDS/naltrexone, 66 to naltrexone alone, 65 to placebo, and 65 to moxifloxacin. Twenty-seven subjects discontinued: eight receiving BTDS (all due to AEs), seven receiving BTDS/naltrexone (all due to AEs), five receiving naltrexone (two due to AEs), four receiving placebo (one due to AEs), and three receiving moxifloxacin (two due to AEs). One subject receiving BTDS (highest BTDS dose was 20 mcg/h) withdrew due to ventricular extra systoles. No other subjects withdrew because of ECG or other cardiovascular events. The majority of subjects in the BTDS, BTDS/naltrexone, naltrexone, placebo, and moxifloxacin groups were male (range, 55–67%) and white (range, 59–67%), with a mean age range of 32–35 years and mean BMI of 25–26 kg/m2.
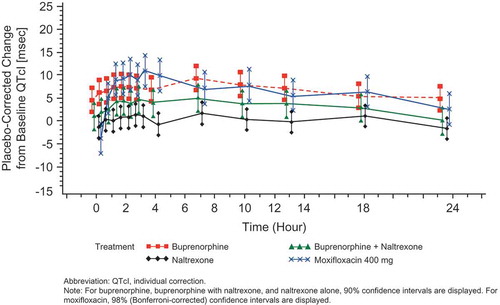
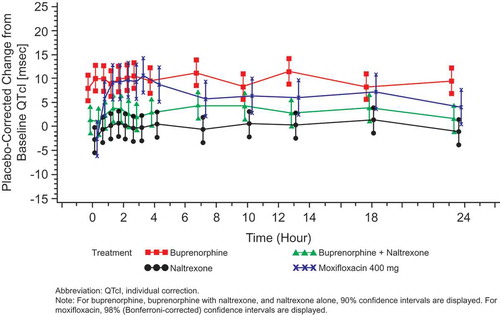
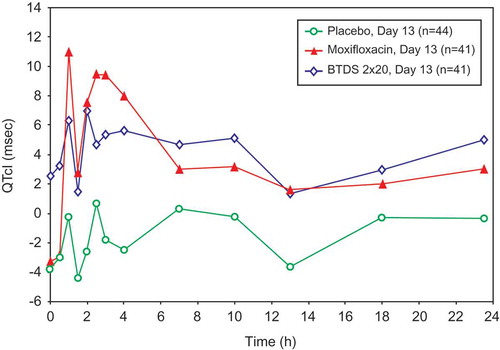
QTcI intervals seen in the BTDS-only group after treatment showed no effect on cardiac repolarization at the 10 mcg/h dose. BTDS 10 treatment resulted in a maximum two-sided 90% CI upper bound for placebo-corrected mean change from baseline for QTcI that was <10 msec at all time points. The maximum 2-sided 90% CI upper bound for placebo-corrected mean change from baseline for QTcI was >10 msec at 5 of the 13 time points for the BTDS 40 dose and at all 13 time points for BTDS 80 dose. For BTDS 40, the maximum 90% CI upper bound was 11.81 msec and ranged from 10.09 to 10.44 for the other 4 time points that exceeded 10 msec ().
Table 4. Placebo-corrected change from baseline – estimates from the mixed-effect model for QTcI (msec) for BTDS only (FAP for ECG) – Study BUP1025.
The dose-related increase in time-matched QTcI was noted between BTDS 10 and BTDS 80. The largest time-matched placebo-corrected mean QTcI values were 3.36, 9.16, and 11.46 msec for the BTDS 10, BTDS 40, and BTDS 80 doses, respectively. The 30-mcg/h escalation in BTDS dose from BTDS 10 to BTDS 40 was associated with an increase in the largest time-matched mean QTcI of 5.80 msec, while the larger 40-mcg/h escalation from BTDS 40 to BTDS 80 was associated with a smaller increase in QTcI of 2.30 msec.
No subjects in the placebo, moxifloxacin, or BTDS treatment groups had a >60-msec change from baseline in QTcI, new >500 msec absolute QTc duration, or new abnormal U wave with the exception of one subject who had an abnormal U wave while receiving BTDS 40. For subjects randomized to BTDS/naltrexone, the largest time-matched mean QTcI values were 5.37, 5.12, and 4.47 msec for BTDS 10, BTDS 40, and BTDS 80, respectively. Under naltrexone blockade (50 mg q12 h), the 30-mcg/h escalation in dose from BTDS 10 to BTDS 40 was associated with a decrease in largest time-matched mean QTcI of 0.25 msec, while the 40-mcg/h escalation from BTDS 40 to BTDS 80 was associated with a decrease in QTcI of 0.65 msec. Compared to results with BTDS alone, the largest time-matched mean QTcI values for BTDS/naltrexone were 2.01 msec higher, 4.04 msec lower, and 6.99 msec lower with BTDS 10, BTDS 40, and BTDS 80 doses, respectively. Coadministration of naltrexone with buprenorphine suppressed the QTc prolongation produced by BTDS 40 and BTDS 80 when given alone ().
Table 5. Placebo-corrected change from baseline – estimates from the mixed-effect model for QTcI (msec) for BTDS with naltrexone (FAP for ECG) – Study BUP1025.
For subjects randomized to naltrexone alone, the largest time-matched mean QTcI values were 4.34, 1.81, and 1.50 msec on Days 6, 13, and 17, respectively (), values that are 1.03, 3.31, and 2.97 msec lower than the corresponding values seen with BTDS/naltrexone.
Table 6. Placebo-corrected change from baseline – estimates from the mixed-effect model for QTcI (msec) for naltrexone alone (FAP for ECG) – Study BUP1025.
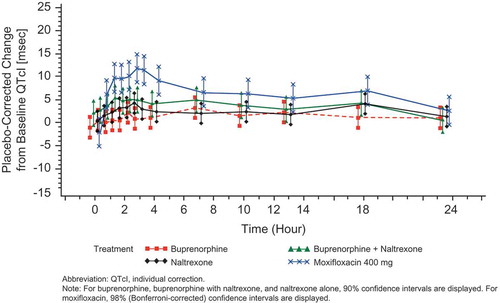
For subjects randomized to moxifloxacin, the time-matched analysis for the QTcI end point revealed that the moxifloxacin group on all 3 days met the assay sensitivity criteria and had the typical profile, as shown in –. In addition, the criteria that the lower Bonferroni-corrected CI of at least one of the preselected time points (at hours 1, 2, 2.5, 3, or 4) for moxifloxacin must be >5 msec was met for each day of moxifloxacin administration (). For moxifloxacin, the largest time-matched mean QTcI values on Days 13 and 17 were 10.68 and 10.78 msec; corresponding values for BTDS 40 and BTDS 80 (9.16 and 11.46 msec) are consistent with these. These results for the positive control treatment indicate that QTc prolongation is at the threshold of regulatory concern of approximately 5 msec and could be reliably detected, thereby confirming the sensitivity of the assay used for this study.
Table 7. Placebo-corrected change from baseline – estimates from the mixed-effect model for QTcI (msec) for moxifloxacin (FAP for ECG) – Study BUP1025.
Figure 4. Placebo-corrected change from baseline QTcI (msec) versus time on Day 6 – buprenorphine 10 mcg/h (FAP for ECG) – Study BUP1025.
Time points for ECGs were 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 7, 10, 13, 18, and 23.5 hours.
3.3 HR, PR, and QRS results
3.3.1 Study BUP1011
BTDS 10 and BTDS 40 were not associated with changes in HR with placebo-corrected mean change from baseline values of −1 and +1 bpm, respectively. The moxifloxacin administrations produced placebo-corrected mean change from baseline values of +1 and +3 bpm mean change, reflecting the variability between two different determinations for this agent, and were clinically irrelevant.
Neither BTDS nor moxifloxacin was associated with significant effects on PR or QRS durations with placebo-corrected mean change from baseline duration changes of <6 msec for PR and <1 msec for QRS.
3.3.2 Study BUP1025
For BUP1025, the mean placebo-corrected changes from baseline for HR for the BTDS 10, BTDS 40, and BTDS 80 groups were all within 0–1 bpm. For the BTDS/naltrexone 10-, 40-, and 80-mcg/h dose groups, the mean placebo-corrected change from baseline for HR was a reduction of 3–4 bpm. The mean placebo-corrected change from baseline for HR was a reduction of approximately 2 bpm for naltrexone alone for Days 6, 13, and 17. None of these changes was clinically meaningful.
The mean placebo-corrected change from baseline for PR duration for the 10-, 40-, and 80-mcg/h BTDS dose groups ranged from 2 to 4 msec. For the 10-, 40-, and 80-mcg/h BTDS/naltrexone dose groups, the mean placebo-corrected change from baseline for PR ranged from 2 to 3 msec; for the naltrexone alone dose group, changes ranged from 1 to 2 msec on Days 6, 13, and 17. These changes were considered clinically unimportant.
The mean placebo-corrected change from baseline for QRS duration for BTDS 10, BTDS 40, and BTDS 80 was <1 msec. For the 10-, 40-, and 80-mcg/h BTDS/naltrexone dose groups, the mean placebo-corrected change from baseline for QRS was also <1 msec; for naltrexone alone, it was approximately 1 msec on Days 6, 13, and 17. These changes were considered clinically unimportant.
The only new morphological findings that were observed were in one or two subjects in each treatment group (except placebo) who developed nonspecific ST depression and T-wave inversion and were not clinically relevant.
3.4 Concentration response relationship (PK/PD)
3.4.1 Study BUP1011
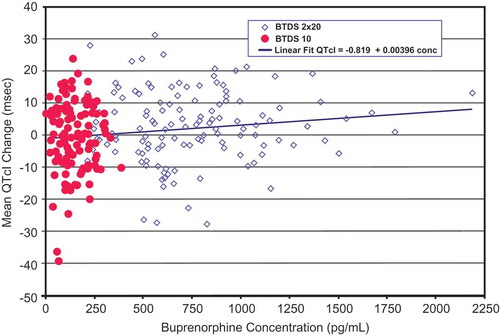
For subjects receiving BTDS, concentrations of buprenorphine increased predictably with increased dosage; however, no clinically relevant effects on vital signs or ECGs were seen except QT intervals. The increased plasma concentrations of buprenorphine following dosing with BTDS 40 were associated with slight increases in QTcI, as expected (). The relationship between buprenorphine concentration and QTcI was adequately described by a simple linear regression model.
3.4.2 Study BUP1025
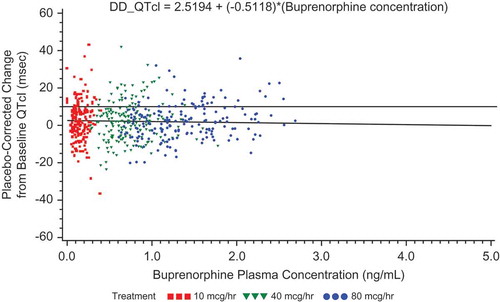
The PK/PD model showed that the slope for QTcI for the BTDS-only treatment was positive in contrast to the slightly negative slope for the BTDS/naltrexone treatment. The upper 90% confidence bounds for QTcI at steady-state concentrations of Cmax for the BTDS-only and BTDS/naltrexone treatment groups were 12.1 msec and 3.7 msec, respectively ( and ).
Figure 8. Placebo-corrected change from baseline QTcI versus mean buprenorphine plasma concentration – estimates from the mixed-effects model regression – BTDS only (PK/PD analyses) – Study BUP1025.
The colors are only to highlight the distributions of the doses; the PK/PD model does not include dose.
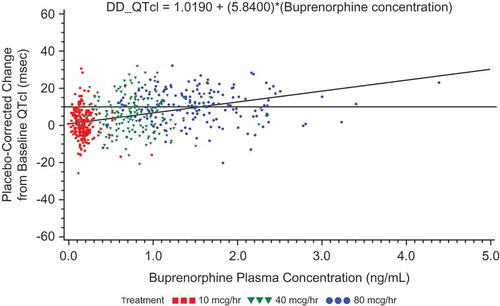
Figure 9. Placebo-corrected change from baseline QTcI versus mean buprenorphine plasma concentration – estimates from the mixed-effects model regression – BTDS with naltrexone (PK/PD analyses) – Study BUP1025.
The colors are only to highlight the distributions of the doses; the PK/PD model does not include dose.
Mean Cmax of buprenorphine increased steadily from Day 6 to Day 17 for both the BTDS-only (mean Cmax: 0.201, 0.880, and 1.704 ng/mL) and BTDS/naltrexone (0.170, 0.813, and 1.516 ng/mL) treatment groups, respectively. Mean Cmax of buprenorphine was 15.4%, 7.6%, and 11.0% lower for the BTDS/naltrexone treatment compared with the BTDS-only treatment on Days 6, 13, and 17, respectively. Mean Cmin also increased steadily from Day 6 to Day 17 for both the BTDS-only (mean Cmin: 0.149, 0.707, and 1.385 ng/mL) and BTDS/naltrexone (0.129, 0.667, and 1.267 ng/mL) treatment groups. Mean Cmin of buprenorphine was 13.4%, 5.7%, and 8.5% lower for the BTDS/naltrexone treatment group compared with the BTDS-only treatment group on Days 6, 13, and 17, respectively (). As BTDS-only and BTDS/naltrexone are separate treatment groups, it is unclear whether or not naltrexone coadministration contributed to these small differences in Cmax and Cmin.
Table 8. Summary of mean plasma pharmacokinetic metrics of buprenorphine (FAP) – Study BUP1025.
3.5 Safety
No unexpected adverse effects occurred in these two studies in these healthy subjects. There were no treatment-emergent serious adverse events; however, one subject randomized to BTDS only in study BUP1025 experienced a serious adverse event of infrequent bowel movements beginning prior to study drug administration which resulted in discontinuation of BTDS treatment and hospitalization. Other AEs experienced by this subject resolved within 48 h with no treatment.
Subjects in the BTDS-only treatment groups experienced the expected adverse effects typically associated with opioid administration, the most frequent of which were nausea, headache, constipation, vomiting, and dizziness. All AEs in each study were mild or moderate in intensity. As anticipated, in study BUP1025, these AEs were reduced in frequency in the BTDS/naltrexone treatment group as compared to the BTDS alone treatment group, typically by 33–50%. Within the BTDS-only treatment group in study BUP1025, AEs on the three 24-h ECG recording periods (Day 6 for BTDS 10, Day 13 for BTDS 40, Day 17 for BTDS 80) showed an increase in frequency following the dose increase from BTDS 10 to BTDS 40; however, the dose increase from BTDS 40 to BTDS 80 showed no increase in AE frequency.
4. Discussion
Results of both studies indicate that BTDS at 10 mcg/h does not prolong QTc. At supratherapeutic doses of 40 and 80 mcg/h, BTDS produces modest QTc prolongations comparable in magnitude to those seen with a single 400-mg dose of moxifloxacin. In study BUP1025, coadministration of buprenorphine with naltrexone reduced the prolongation seen with buprenorphine alone. Therefore, evaluation of effects of buprenorphine on prolongation in presence of naltrexone may not be appropriate or valid.
The ICH E14 Guideline has established specific recommendations for conducting and assessing clinical studies to evaluate the potential of a drug to delay cardiac repolarization. Based on these guidelines, a positive QTc prolongation result is one in which the upper bound of the largest time-matched, placebo-corrected change from baseline QTc CI is ≥10 msec [Citation17]. Additionally, the use of concurrent positive and placebo controls is important to establish assay sensitivity and a high degree of confidence in the ability of the study to detect differences of clinical significance. The guidelines recommend that a valid positive control should have an effect on the mean QT/QTc interval of about 5 msec. Moxifloxacin is commonly used as the positive control arm in a TQT study as it reliably causes QTc prolongation with a magnitude that is consistent with the threshold of regulatory concern.
Adequate evaluation of QTc effects typically involves study of a range of doses including both therapeutic and supratherapeutic exposures. Multiple factors affect the selection doses in studies of QTc effects, including safety and tolerability of contemplated doses as well as consideration of factors such as drug–drug or drug–food interactions that could lead to significant increases in drug concentrations. In the case of transdermal delivery of buprenorphine from BTDS, food effects are not a consideration. Further, systemic buprenorphine exposure following BTDS dosing has been shown to be unchanged by CYP3A4 inhibition [Citation18]. Therefore, the primary determinants of the maximum doses for QTc studies of BTDS were the safety and tolerability of the doses and the practicality of prolonged treatment periods, given the need for gradual dose escalation.
Furthermore, the guidelines recommend a parallel group design, rather than a crossover design, when multiple doses and treatment groups are to be compared. There is a marked spontaneous variability in QTc durations both within and between subjects over time, and the effect of this intrinsic variability in QTc can be reduced through the collection of multiple ECGs at baseline and during the studies. The design and analysis of both clinical studies presented followed these three guideline recommendations.
On Days 6 and 13, the lower bound of the moxifloxacin 90% two-sided CI exceeded 5.0 msec at multiple time points establishing assay sensitivity of the positive control. The placebo group’s change from baseline for QTcI was –1 to –2 msec, showing excellent control of background QTc variability. Results of the PK/PD evaluation for buprenorphine were consistent with these QTcI changes. In BUP1011, there was no QTcI prolongation with BTDS 10. The QTcI prolongation with BTDS 40 was similar to that with moxifloxacin. Thus, BUP1011 demonstrated that administration of the available therapeutic dose of BTDS 10 approved in the United States results in no signal of any ECG effect in QTcI duration.
Results of BUP1025 were consistent with those of BUP1011 at corresponding doses and provide confirmation of the cardiac safety of recommended doses of buprenorphine. In BUP1025, QTcI intervals after BTDS showed no effect on cardiac repolarization at the 10-mcg/h dose; the maximum two-sided 90% CI upper bound for placebo-corrected mean change from baseline for QTcI was <10 msec at all time points. On Days 6, 13, and 17, the lower bound of the moxifloxacin 90% two-sided CI exceeded 5.0 msec at multiple time points establishing assay sensitivity of the positive control. The increase in time-matched QTcI between 10 and 80 mcg/h was linear but not proportional. In BUP1025, there was no QTcI prolongation with BTDS 10. The QTcI prolongation with BTDS 40 and BTDS 80 was similar to that with moxifloxacin. This second study reiterated that administration of the available therapeutic dose of BTDS 10 approved in the United States results in no effect on repolarization.
The time-matched values of QTcI with 400-mg doses of moxifloxacin met the prespecified conditions to establish assay sensitivity. Thus, this well-established positive control provided a high degree of confidence in the ability of the studies to detect clinically relevant changes in QTc. Placebo-corrected values were used throughout to determine the best estimate of QT intervals with the active treatments. The changes in QTcI duration at the BTDS 40 (9.16 for Day 13) and BTDS 80 (11.46 for Day 17) dose were similar to those with moxifloxacin (10.68 for Day 13 and 10.78 for Day 17) and demonstrated a linear increase from BTDS 10.
The opioid receptor antagonist, naltrexone, has been approved by the FDA for the treatment of opioid dependence. Naltrexone administered alone at q12 h from Day 4 to Day 17 had no effect on cardiac repolarization, but when coadministered with BTDS, it reduced the QTcI prolongation seen with the supratherapeutic doses of BTDS.
The PK/PD model revealed that the slopes were positive for QTcI changes as concentrations of BTDS increased for BTDS only, but flat to slightly negative for BTDS/naltrexone.
No effect on HR, AV conduction, or cardiac depolarization as measured by the PR and QRS interval durations was observed. There were no new clinically relevant morphological changes demonstrating a signal of concern.
The QT prolongations seen with supratherapeutic doses of BTDS in the two studies reported here are of similar magnitude to that seen with moxifloxacin.
The results of BUP1025 show that naltrexone coadministered with buprenorphine blocks the dose-dependent increase in QTcI that is seen with buprenorphine alone. This result for the BTDS/naltrexone treatment is consistent with a previously reported study by Darpo et al. [Citation19], in which buprenorphine administered by a buccal soluble film was coadministered with naltrexone also showed no meaningful increase in QTc at buprenorphine concentrations higher than those associated with BTDS 40 or BTDS 80. In that study [Citation19], the authors speculate that pronounced opioid-induced adverse effects such as nausea, vomiting, and sedation may result in QTc interval prolongation. They argued that a study of the effects of buprenorphine on QTc is best conducted in healthy subjects under naltrexone coverage to allow for sufficiently high doses without confounding indirect marked adverse effects. This hypothesis is not consistent with the results of BUP1025. In particular, the dose increase from BTDS 40 to BTDS 80 in the BTDS-only treatment group was associated with an increase in QTcI interval; however, this increase in dose was not accompanied by an increase in the frequency or intensity of common opioid-induced adverse effects. Therefore, there is no clear evidence that the QTc prolongation observed in BUP1025 was due primarily to confounding opioid-induced adverse effects.
A signal of disproportionate reporting of cardiovascular adverse events was not identified for transdermal buprenorphine formulations approved in the United States at strengths up to 20 mcg/h and outside the United States at strengths up to 70 mcg/h using the FDA Adverse Event Reporting System through March 2013.
5. Conclusions
At the therapeutic dose of 10 mcg/h, BTDS has no meaningful effect on QTcI interval duration. At supratherapeutic doses of 40 and 80 mcg/h, BTDS treatment produces prolongation of QTcI similar to that associated with 400 mg doses of moxifloxacin. The QTc prolongation noted with BTDS at doses of 40 and 80 mcg/h is not large enough to be considered likely to be associated with proarrhythmic effects. At present, there is no clear evidence that buprenorphine’s effect on QTc intervals is associated with proarrhythmic effects. Further study is needed to characterize the effects of buprenorphine on QTc at concentrations above those associated with BTDS 80. Concomitant administration of naltrexone and BTDS reduces the QTc prolongation seen with BTDS alone by an unknown mechanism, and BTDS used concomitantly with naltrexone can therefore confound the QT prolongation effects.
Declaration of interest
S Harris, S Colucci, and S Ripa are employees of Purdue Pharma L.P. J Morganroth and M Thorn were consultants at Purdue Pharma L.P. at the time the studies were conducted. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors would like to thank Kenneth Robinson, BA, for his editorial support of this manuscript.
Additional information
Funding
References
- Kitzmiller JP, Barnett CJ, Steiner NS, et al. Buprenorphine: revisiting the efficacy of transdermal delivery system. Ther Deliv. 2015;6:419–422.
- Raffa RB, Ding Z. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonist. Acute Pain. 2007;9:145–152.
- Kögel B, Christoph T, Straßburger W, et al. Interaction of µ-opioid receptor agonists and antagonists with the analgesic efficacy of buprenorphine in mice. Eur J Pain. 2005;9:599–611.
- Steiner D, Munera C, Hale M, et al. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J Pain. 2011;12(11):1163–1173.
- Steiner DJ, Sitar S, Wen W, et al. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naïve patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manag. 2011;42(6):903–917.
- Landau CJ, Carr WD, Razzetti AJ, et al. Buprenorphine transdermal delivery system in adults with persistent noncancer-related pain syndromes who require opioid therapy: a multicenter, 5-week run-in and randomized, double-blind maintenance-of-analgesia study. Clin Ther. 2007;29(10):2179–2193.
- Munera C, Drehobl M, Sessler NE, et al. A randomized, placebo-controlled, double-blinded, parallel-group, 5-week study of buprenorphine transdermal system in adults with osteoarthritis. J Opioid Manag. 2010;6(3):193–202.
- Breivik J, Ljosaa TM, Stengaard-Pedersen K, et al. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naïve to potent opioids. Scand J Pain. 2010;1:122–141.
- Conaghan PG, O’Brien CM, Wilson M, et al. Transdermal buprenorphine plus oral paracetamol vs an oral codeine-paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthr Cartil. 2011;19:930–938.
- Gordon A, Callaghan D, Spink D, et al. Buprenorphine transdermal system in adults with chronic low back pain: a randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Ther. 2010;32(5):844–860.
- Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010 May;15(3):169–178.
- James IG, O’Brien CM, McDonald CJ. A randomized, double-blind, double-dummy comparison of the efficacy and tolerability of low-dose transdermal buprenorphine (BuTrans® seven-day patches) with buprenorphine sublingual tablets (Temgesic®) in patients with osteoarthritis pain. J Pain Symptom Manag. 2010;40(2):266–278.
- Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 mcg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Ther. 2009;31(3):503–513.
- CredibleMeds QT-prolonging drugs list; [cited 31 Nov 2016]. Available from: https://crediblemeds.org/
- Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the food and drug administration. Addiction. 2015;110:1468–1475.
- Sessler NE, Walker E, Chickballapur H, et al. Disproportionality analysis of buprenorphine transdermal system and cardiac arrhythmia using FDA and WHO postmarketing reporting system data. 2017;129(1):62–68.
- International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs: ICH E14. 12 May 2005. [cited 2016 Dec 7]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf.
- Kapil RP, Cipriano A, Michels GH, et al. Effect of ketoconazole on the pharmacokinetic profile of buprenorphine following administration of a once-weekly buprenorphine transdermal system. Clin Drug Investig. 2012;32:583–592.
- Darpo B, Zhou M, Bai SA, et al. Differentiating the effect of an opioid agonist on cardiac repolarization from µ-receptor-mediated, indirect effects on the QT interval: a randomized, 3-way crossover study in healthy subjects. Clin Ther. 2016;38(2):315–326.