ABSTRACT
Irritable bowel syndrome (IBS), which is characterized by recurrent abdominal pain and disordered bowel habits, is one of the most common functional bowel disorders. IBS is a substantial burden on both patient health-related quality of life and healthcare costs. Several pathophysiologic mechanisms have been postulated for the occurrence of IBS, including altered gastrointestinal motility, visceral hypersensitivity, changes in gut permeability, immune activation, gut-brain dysregulation, central nervous system dysfunction, and changes in the gut microbiota. Of note, both qualitative and quantitative differences have been observed in the gut microbiota of a population with IBS versus a healthy population. Because of the substantial interest in the gut microbiota and its role as a therapeutic target in IBS, this article provides an overview of specific interventions with the potential to modulate the gut microbiota in IBS, including elimination diets, prebiotics, probiotics, synbiotics, and nonsystemic antibiotics. Although probiotics and synbiotics are generally well tolerated, differences in the composition and concentration of different bacterial species and inclusion or exclusion of prebiotic components varies widely across studies and has prevented strong recommendations on their use in IBS. For nonsystemic antibiotics, rifaximin is indicated in the United States for the treatment of IBS with diarrhea in adults and has been shown to be efficacious and well tolerated in well-designed clinical trials. Overall, more consistent evidence is needed regarding the efficacy and safety of elimination diets, prebiotics, probiotics, and synbiotics for the treatment of patients with IBS. Furthermore, additional well-designed studies are needed that examine alterations in the gut microbiota that occur with these interventions and their potential associations with clinical symptoms of IBS.
1. Introduction
Irritable bowel syndrome (IBS) is a common functional bowel disorder characterized by recurrent abdominal pain, bloating, and changes in stool form or frequency without the presence of structural, biochemical, or major inflammatory changes [Citation1]. Overall, the reported global pooled prevalence of IBS is 11.2% [Citation2]; however, regional and cultural variations exist [Citation2,Citation3]. A 2017 meta-analysis performed by the Rome Foundation confirms significant heterogeneity and methodological variance discouraging the use of pooled data when describing prevalence rates [Citation3]. Updated Rome diagnostic criteria in 2016 () emphasized recurrent abdominal pain for the definition of IBS and set new symptom frequency criteria: on average, at least 1 day per week of abdominal pain during the last 3 months, either related to defecation or associated with a change in stool form or frequency [Citation4]. Furthermore, these 3-month criteria for IBS should be fulfilled with symptoms starting at least 6 months before the diagnosis [Citation4]. In addition, IBS is further characterized by predominant bowel type: diarrhea (IBS-D), constipation (IBS-C), or mixed (IBS-M); these different IBS types have reported rates of 26, 28, and 44%, respectively, in the United States [Citation4,Citation5]. Other symptoms such as bloating and abdominal distention may occur, and the predominant bowel symptom subtype may change (e.g. IBS-D to IBS-M) over time [Citation6].
Table 1. Rome IV criteria for irritable bowel syndrome.
IBS negatively impacts health-related quality of life (QOL) in affected individuals and is associated with decreased work productivity and increased healthcare utilization and healthcare costs [Citation7,Citation8]. For patients with IBS-C, total direct annual costs were estimated at more than $11,000 (2010 US dollars), with 53.7% of costs resulting from outpatient care (e.g. office visits, diagnostic tests, outpatient procedures); hospitalizations, medications, and emergency department visits accounted for 21.8, 19.1, and 5.4%, respectively, of total costs [Citation9]. Although primarily managed in an outpatient setting, IBS accounted for approximately 12,000 US hospitalizations annually (1997–2010), with a mean duration of 3.7 days [Citation10].
IBS can be classified as mild, moderate, or severe depending on various measures that include symptoms (e.g. abdominal pain), the presence of comorbid health conditions (e.g. fibromyalgia, anxiety/depression), health-related QOL, and healthcare utilization [Citation11–Citation13]. Although an older study estimated that 5% of patients had severe IBS [Citation14], findings from subsequent studies have suggested that severe disease may affect 15–50.4% of patients with IBS [Citation15,Citation16].
The patient population seen at various healthcare settings tends to differ by IBS severity, with patients in primary care typically presenting with milder disease [Citation17]. To help healthcare providers determine IBS severity, the Rome Foundation has developed a useful tool called the multidimensional clinical profile (MDCP). This tool can be used to assist healthcare providers in assessing IBS severity and can also be helpful for establishing a treatment approach. The MDCP defines a number of disease features, including determining whether the patient meets Rome criteria for a diagnosis of IBS, IBS subtype, psychosocial factors, effects of IBS on daily activities, and the presence of biomarkers or known physiologic modifiers associated with IBS (e.g. positive breath test results).
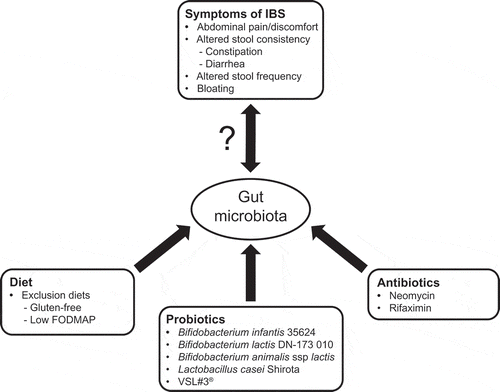
IBS is a diverse disorder, and many factors have been implicated in its pathogenesis () [Citation18]. Not all factors may be present in one individual, but genetic, central (e.g. gut-brain dysregulation), hormonal, psychological, diet, and gastrointestinal (GI; e.g. altered motility) factors have all been proposed to be potential candidates [Citation18–Citation21]. Further, it is likely that IBS is a result of various interactions between genetic and environmental factors [Citation22]. Besides the impact of diet, research has noted the role of immune-mediated factors, especially the effect of an infection on the gut microbiota (e.g. post-infectious IBS) as well as intestinal dysbiosis (e.g. small intestinal bacterial overgrowth [SIBO]) [Citation21,Citation23]. The gut microbiota appears to play an important role in IBS (), as quantitative and qualitative differences have been observed in patients with IBS compared with healthy individuals [Citation24–Citation29]. For instance, a greater magnitude of gut microbiota instability has been associated with greater intensity of symptoms in some patients with IBS [Citation24]. Also, some clinical characteristics of IBS (e.g. bloating, satiety, altered intestinal transit time) have been associated with specific gut microbiota profiles in patients with IBS [Citation30,Citation31] and increases in some proinflammatory cytokines. Because of the substantial interest in the gut microbiota and its role in disease, the purpose of this review is to provide an overview of specific interventions with the potential to modulate the gut microbiota in IBS, including elimination diets, prebiotics, probiotics, synbiotics, and antibiotics.
Table 2. Proposed pathophysiologic factors in irritable bowel syndrome.
2. Methods
A PubMed search of English-language articles available through 24 August 2017 was conducted using the following key words to identify studies performed in adult humans: ‘irritable bowel syndrome AND diet,’ ‘irritable bowel syndrome AND FODMAP,’ ‘irritable bowel syndrome AND prebiotic,’ ‘irritable bowel syndrome AND probiotic,’ ‘irritable bowel syndrome AND Bifidobacterium,’ ‘irritable bowel syndrome AND Lactobacillus,’ ‘irritable bowel syndrome AND VSL 3,’ ‘irritable bowel syndrome AND antibiotic,’ and ‘irritable bowel syndrome AND rifaximin.’ Reference lists from review articles were used to identify additional studies for inclusion.
3. Role of diet in IBS
Exacerbation of symptoms among patients with IBS has been described to occur commonly in relation to food ingestion [Citation32–Citation34]. While true food allergies are considered uncommon in IBS [Citation1], food intolerances or sensitivities are frequently reported [Citation35]. In a general population study, only 19.4% (95% confidence interval [CI], 11.4–27.4%) of patients who believed they had a food intolerance had a positive reaction during a blinded food challenge [Citation36]. Over the last few years, food-related symptoms have received increased attention, and emerging evidence supports the evaluation of diet modification for patients with IBS [Citation37]. In fact, approximately 90% of patients with IBS restrict their diet to prevent or improve GI symptoms [Citation35].
One potential mechanism for the important role diet may play in IBS involves endocrine cells localized to the GI lumen. GI endocrine cells respond to stimuli from both dietary components (e.g. carbohydrates, proteins) and bacterial products by releasing hormones and influencing cell density [Citation38]. Diet has also been shown to modulate the composition and function of the gut microbiota, which, in turn, may be associated with symptoms of IBS (e.g. bloating, altered intestinal transit time) [Citation39].
3.1. Elimination diets
3.1.1. Gluten-free diets
Nonceliac wheat sensitivity is characterized by symptoms similar to those of IBS, including abdominal pain, diarrhea, constipation, nausea, vomiting, headache, and depression, with symptoms typically improving when wheat-containing products are removed from the diet [Citation40,Citation41]. However, before entertaining a diagnosis of nonceliac wheat sensitivity, patients should be properly evaluated with celiac serological screening and an upper endoscopy with biopsies to rule out celiac sprue. Because studies have shown that a gluten-free diet can improve IBS symptoms, at least in some patients, nonceliac wheat sensitivity may comprise a subset of patients with IBS [Citation40,Citation42]. A gluten-free diet was also shown to improve IBS-related QOL from baseline in some patients with IBS [Citation43]. This may partly be related to GI mucosal changes observed in patients with IBS with a suspected food intolerance; these changes include epithelial barrier disruptions, fluid secretion into the GI lumen, and an influx of intraepithelial lymphocytes [Citation44].
The component(s) of wheat responsible for this effect need to be elucidated, although proteins (e.g. gluten) and carbohydrates (e.g. fermentable oligo-, di-, and monosaccharides and polyols [FODMAPs]) are thought to play a role [Citation41]. Results of controlled studies in patients with IBS receiving gluten-free diets have been mixed [Citation42,Citation45], with some suggesting that gluten contributes little to IBS symptomatology and that fructans contained in wheat are actually the culprits [Citation45]. This was concluded in a double-blind, crossover trial of 37 patients with IBS with self-reported nonceliac wheat sensitivity who received a reduced FODMAP diet during a 2-week run-in phase before randomization to a diet high in gluten (16 g/d), low in gluten (2 g/d plus 14 g whey protein/d), or placebo for 7 days [Citation45]. Global and specific symptoms (i.e. abdominal pain, bloating, satisfaction with stool consistency, flatulence, and fatigue) improved significantly from baseline to the second week of the run-in phase (P < 0.0001, for all comparisons). However, global symptoms and abdominal pain worsened from the end of the run-in phase to day 7 of treatment [Citation45]. Conversely, a single-center, controlled study of 45 patients with IBS-D showed that patients randomly assigned to a gluten-free diet had a significant decrease in daily stool frequency from baseline compared with a diet containing gluten after 4 weeks (P = 0.04) [Citation42]. These results raise the possibility that the positive effect of a gluten-free diet in patients with IBS may be a nonspecific consequence of reducing the intake of FODMAPs, given that wheat is one of the possible sources of FODMAPs. Another possibility is the potential of the gluten-free diet to alter the composition of the gut microbiota, which has been demonstrated in healthy individuals [Citation46]. However, data are currently lacking regarding the effects of a gluten-free diet on gut microbiota of patients with IBS.
3.1.2. Low FODMAP diet
FODMAPs contain carbohydrates that are poorly absorbed in the small intestine and can pass unabsorbed into the colon where they increase luminal water levels through osmotic activity; further, these unabsorbed carbohydrates may undergo fermentation by colonic bacteria, which induces gas production [Citation47,Citation48]. Gas production, in turn, can lead to luminal distension, which induces GI symptoms in susceptible individuals [Citation47,Citation49]. Fecal samples obtained from patients with IBS who consumed a 3-week low FODMAP diet had a significantly lower absolute abundance of bacteria compared with those who consumed a typical diet (P < 0.001), including the butyrate-producing bacteria Clostridium coccoides (P < 0.001) and mucus-degrading bacteria Akkermansia muciniphila (P < 0.001); the abundance of mucus-degrading bacteria Ruminococcus torques was significantly greater with a low FODMAP diet versus a typical diet (P = 0.001) [Citation50]. Patients with IBS following a low FODMAP diet had significant decreases from baseline in the quantity of fecal bacteria (i.e. Mycoplasma hominis, Bifidobacterium, Actinobacteria) after 4 weeks [Citation51]. However, the implications of a long-term low FODMAP diet on the gut microbiota in IBS are currently unknown [Citation50].
A randomized, single-blinded, controlled study (n = 75) published in 2015 compared a low FODMAP diet with a traditional IBS controlled diet (e.g. ingestion of three meals and three snacks daily; decreased consumption of fatty or spicy foods, coffee, alcohol, cabbage, onions, and beans; avoidance of carbonated beverages) in patients with IBS. The study reported that the intensity of IBS symptoms was significantly decreased from baseline with both interventions after 29 days (P < 0.001) [Citation52]. A meta-analysis of 10 prospective clinical studies of the low FODMAP diet in patients with IBS showed that a standard IBS diet (high FODMAP) and low FODMAP diet were both efficacious for improving IBS symptoms [Citation53]. Differences between these two groups did not achieve statistical significance, possibly because of study heterogeneity. However, a crossover clinical study of 30 patients with IBS noted that a low FODMAP diet significantly decreased overall IBS-related symptoms in as early as 7 days, with effects maintained for the duration of the intervention (up to 3 weeks), compared with a typical Australian diet (P < 0.001) [Citation54]. The low FODMAP diet also significantly improved bloating and abdominal pain and decreased dissatisfaction with stool consistency in patients with IBS compared with the typical diet [Citation54]. Furthermore, a systematic review of dietary fiber supplementation (3 studies) or a low FODMAP diet (5 studies) found these dietary interventions to be effective management options for patients with IBS-C, although the authors concluded that not all patients would benefit from these interventions because of the wide variation in both the pathophysiology of the condition and the symptoms experienced by patients [Citation55].
In general, exclusion diets (e.g. gluten-free, low FODMAP) appear to be efficacious because of improvements in global and individual IBS symptoms in patients, but more evidence is needed before dietary restriction can be strongly recommended for these patients [Citation56]. Although clinical trial data support that the reduction of FODMAPs is beneficial for patients with IBS [Citation52,Citation54,Citation57], long-term adherence to a strict low FODMAP diet is not recommended because of nutritional concerns and potential effects on the gut microbiota [Citation58]; rather, excluded components of the diet should be slowly reintroduced until satisfactory symptom control is maintained by the individual patient [Citation59]. Interestingly, a study conducted in England reported that primary care physicians (PCPs) generally recommend dietary modification first to patients with IBS [Citation60], but based on the very low quality of evidence, the American College of Gastroenterology (ACG) makes only a weak recommendation for specialized diets in the management of IBS [Citation1].
4. Prebiotics, probiotics, and synbiotics in the treatment of IBS
4.1. Prebiotics
Prebiotics are dietary products not digested in the human GI tract, but selectively fermented by specific genera of resident gut microbiota [Citation61]. Prebiotics promote the growth of host bacteria when ingested, potentially affecting the composition and function of the gut microbiota [Citation62,Citation63]. Bifidobacteria concentrations have been found to be lower in patients with IBS compared with healthy controls [Citation64,Citation65]; therefore, as a therapeutic target, specific prebiotic-stimulated growth of bifidobacteria is a potential treatment option. The two most investigated carbohydrates that fulfill the criteria for prebiotics are inulin-type fructans and the galacto-oligosaccharides (GOS) [Citation61]. Inulin-type fructans are fructose polymers linked by β bonds with a terminal α-linked glucose [Citation61,Citation66]. Longer chains are inulin, and shorter chains are oligofructose/fructo-oligosaccharides (FOS) [Citation61]. Use of a prebiotic mixture comprised of trans-GOS, obtained through enzymatic galactosylation of lactose (Clasado, Milton Keynes, UK), showed beneficial effects in a randomized, single-blinded, placebo-controlled, crossover study of patients with IBS (n = 44 completed study) [Citation67]. Patients with IBS who received the prebiotic mixture 3.5 g/d experienced significant improvements in stool consistency, flatulence, bloating, composite symptom score, and subjective global assessment (SGA) compared with baseline after 4 weeks of treatment (P < 0.05, for all vs. baseline) [Citation67]. The prebiotic mixture 7 g/d significantly improved SGA and anxiety levels from baseline in patients with IBS after 4 weeks of treatment (P < 0.05, for both vs. baseline) [Citation67]. Mild nausea was reported by one patient who received the prebiotic mixture 3.5 g/d [Citation67]. Compared to baseline levels, both doses of the prebiotic mixture significantly increased fecal levels for the beneficial bacteria Bifidobacterium (P < 0.05 for both) [Citation67]. Similarly, fecal Bifidobacterium levels significantly increased with the prebiotic mixture 3.5 g/d and 7 g/d compared with placebo after 4 weeks of treatment (P < 0.005 for both) [Citation67]. FOS obtained by enzymatic synthesis from sucrose are naturally found in onions, bananas, and artichokes [Citation61,Citation68]. A randomized, double-blind study of healthy individuals with mild functional bowel symptoms demonstrated that regular consumption of short-chain FOS 5 g/day reduced the frequency and intensity of digestive symptoms and improved intestinal discomfort and QOL compared with placebo after 6 weeks [Citation68]. Others have proposed that use of prebiotics may lead to short-term, potentially deleterious alterations in the microbiota, as was suggested by an increased number and volume of anal gas evacuations 2 days after initiation of supplementation with GOS by healthy individuals; however, after 3 weeks, the number and volume of evacuations decreased to pretreatment levels, suggesting that gut microbiota adapted to the prebiotics [Citation69]. In this study, bifidobacteria abundance increased significantly from baseline to days 3 and 21 (P = 0.04 and P = 0.03, respectively, vs. baseline) for healthy individuals with bifidobacteria abundance <0.5% of total bacteria at baseline [Citation69]. Using prebiotics to enhance the growth of bifidobacteria seems logical to reduce symptoms of IBS; however, studies are limited by the type and dose of prebiotic used. The ACG has determined that there was insufficient evidence to recommend prebiotic use in patients with IBS [Citation1].
4.2. Probiotics
Probiotics are composed of live or attenuated microorganisms (i.e. bacteria, yeast) that may be beneficial to human health when ingested by, for example, boosting host immunity and inhibiting bacterial growth and viral adhesion [Citation63,Citation70,Citation71]. Probiotics differ from each other in a number of ways, including bacterial composition, number of viable organisms, and activity [Citation70]. Bifidobacterium and Lactobacillus strains are commonly used in probiotic formulations [Citation72], with some commercially available.
A survey-based study of community and academic gastroenterologists and PCPs reported that all survey respondents considered probiotics to be safe; 98% of those surveyed believed that probiotics had a role in treating GI disorders, although some respondents also cited a lack of evidence to support the use of probiotics in patients with GI disorders [Citation72]. Overall, 98% of physicians surveyed indicated using probiotics for patients with IBS, with 91% of survey participants recommending Bifidobacterium infantis 35624 (Align®, Proctor and Gamble, Cincinnati, OH) for patients with IBS [Citation72]. Bifidobacteria have been shown to be inversely correlated with abdominal pain in healthy individuals [Citation73], and patients with IBS have lower concentrations of bifidobacteria compared with healthy individuals [Citation64,Citation65], thus supplementation with bifidobacteria may alleviate symptoms in patients with IBS.
Results of a meta-analysis of 11 randomized, controlled clinical studies reported that short-term (i.e. 10–28 days) probiotic therapy could reduce intestinal transit time, although the magnitude of treatment effect was dependent on the bacterial strain of the probiotic, the presence of constipation, and older age of the patient [Citation74]. Overall, the ACG concluded that probiotics improve symptoms of IBS; however, given the lack of consistent data among individual species, strains, and preparations, the ACG weakly recommended the use of probiotics in patients with IBS [Citation1].
4.2.1. Bifidobacterium infantis 35624
Results from three randomized, controlled studies of B infantis 35624 indicated that some baseline symptoms of IBS improved after a 4- or 8-week course of daily B infantis 35624 therapy [Citation75–Citation77]. In a double-blind, placebo-controlled study, patients with IBS who were randomly assigned to receive B infantis 35624 capsules daily did not experience significant improvements in individual symptoms of IBS (i.e. abdominal pain, bloating, urgency, incomplete evacuation, straining, gas) compared with placebo after 8 weeks of treatment; furthermore, only fecal levels of the C coccoides-Eubacterium rectale group of bacteria, but not other bacteria evaluated, were significantly higher compared with placebo after 8 weeks of treatment (P = 0.02) [Citation75]. In this study, the overall intensity of symptoms improved from baseline in both groups, but differences between groups were not significant [Citation75]. B infantis 35624 was generally well tolerated, with the greatest frequency of adverse events (AEs) reported by patients with IBS receiving placebo (38%) compared with 33 and 32% of patients with IBS or healthy individuals receiving B infantis 35624, respectively [Citation75].
In a randomized, double-blind, placebo-controlled, dose-ranging study of B infantis 35624 capsule administered once daily (range, 1 × 106 to 1 × 1010 cells/dose) for 4 weeks, females with IBS (n = 362) who received B infantis 1 × 108 cells/dose (n = 90) experienced significant improvement from baseline in abdominal pain/discomfort after 4 weeks of treatment (P = 0.02) [Citation76]. Further, patients who received B infantis 1 × 108 cells/dose experienced significant improvement from baseline in bloating/distension, sense of incomplete evacuation, flatulence, straining, and satisfaction with bowel habit compared with placebo [Citation76]. In a separate study, patients with IBS randomly assigned to receive a once-daily malted milk drink containing B infantis 35624 (1 × 1010 live cells/dose) experienced lower composite symptom scores for pain/discomfort, bloating/distension, and bowel movement difficulty for most weeks during the 8-week treatment course compared with patients assigned to receive a drink containing either Lactobacillus salivarius UCC4331 or placebo (i.e. malted milk drink alone) [Citation77].
4.2.2. Bifidobacterium lactis DN-173 010
Bifidobacterium lactis DN-173 010 (Activia®, Dannon, White Plains, NY) was shown to be efficacious in a female IBS-C population [Citation78] and a healthy population with digestive symptoms [Citation79,Citation80]. A randomized, double-blind, controlled study examined the efficacy of fermented milk-containing B lactis DN-173 010 administered twice daily for 4 weeks in females with IBS-C (n = 34) who experienced bloating at least twice weekly [Citation78]. Compared with patients who received a control solution, patients who received B lactis had an improvement from baseline in median percentage change of maximal abdominal distension (P = 0.02), decreases in orocecal (P = 0.049) and colonic (P = 0.03) transit times, and reduced intensity of IBS-related abdominal pain/discomfort (P = 0.04) and global IBS symptoms (P = 0.03) after 4 weeks of treatment.
In a randomized, double-blind, single-center, controlled study in generally healthy females with ≥1 minor digestive symptom (abdominal pain/discomfort, bloating, flatulence, rumbling stomach) in the previous month, fermented milk containing B lactis DN-173 010 (n = 100) or a nonfermented dairy product (n = 97) was administered twice daily for 4 weeks [Citation79]. B lactis significantly improved overall GI well-being from baseline in a greater percentage of individuals compared with control during ≥2 of 4 weeks of treatment (52.0 vs. 36.1%, respectively; P = 0.02). Further, a randomized, controlled, open-label study of healthy adults with abdominal discomfort and stool frequency of 3–21 weekly stools who received either one (n = 144) or two (n = 147) 125-g servings of fermented milk containing B lactis DN-173 010 had significant improvement in digestive comfort compared with the control group (n = 69) after 2 weeks of treatment (82.5 and 84.3% vs. 2.9%, respectively) [Citation80]. None of these three studies of B lactis DN-173 010 examined alterations in the gut microbiota.
4.2.3. Bifidobacterium animalis ssp lactis
Published data regarding the efficacy of B animalis ssp lactis in patients with IBS are lacking. In a study of healthy volunteers, individuals with a low frequency of weekly bowel movements (i.e. 2–4 d/wk) and abdominal discomfort were randomly assigned to receive 4 weeks of treatment with a probiotic containing B animalis ssp lactis BB-12 (1 billion colony-forming units [CFUs; n = 343] and 10 billion CFUs [n = 452]) or placebo (n = 453) [Citation81]. Probiotic administration increased the likelihood of improving the frequency of weekly bowel movements during ≥2 of 4 weeks of treatment (odds ratio [OR], 1.3; 95% CI, 1.0–1.8). In addition, when the two probiotic formulations were analyzed together, probiotic administration significantly improved the mean number of bowel movements per week from baseline during the 4-week treatment period (P = 0.007); in addition, significant improvement versus placebo occurred during each of the 4 weeks, but varied by probiotic dose (1 billion CFUs, weeks 2–4 [P < 0.01]; 10 billion CFUs, weeks 1–2 [P < 0.05]). Authors noted no apparent differences in the frequency of AEs between groups. This study also did not evaluate potential alterations in the gut microbiota.
4.2.4. Lactobacillus casei Shirota
Two studies that examined the efficacy of Lactobacillus casei Shirota (Yakult®, Yakult USA Inc., Fountain Valley, CA) in patients with IBS reported mixed results, with patients achieving improvement in some, but not all, GI-related symptoms; these studies did not assess alterations in the gut microbiota [Citation82,Citation83]. A randomized, double-blind, placebo-controlled study of adults with IBS (n = 80) who received a solution containing L casei Shirota administered twice daily for 8 weeks did not achieve a significant improvement from baseline of ≥30% in mean symptom score nor did they achieve significant improvements in individual symptom scores for discomfort, pain, bloating, and flatulence, after 8 weeks of treatment [Citation82]. However, patients who received the probiotic had an improvement from baseline of ≥30% in mean symptom scores for discomfort and flatulence (P ≤ 0.05) after 8 weeks posttreatment (i.e. 16 weeks) [Citation82]. In a separate study, most (64%) patients with IBS with SIBO (n = 18) who received a solution containing L casei Shirota once daily no longer met the criteria for a SIBO diagnosis after 6 weeks. However, although flatulence improved significantly from baseline at 6 weeks of treatment (P = 0.04), no significant change from baseline was reported in overall abdominal symptoms (P = 0.3) [Citation83].
4.2.5. Saccharomyces
Saccharomyces boulardii, used in food processing and for the treatment of IBS, was shown to significantly improve IBS symptom scores from baseline in patients with IBS-D (n = 12) after 30 days of treatment (P = 0.003) [Citation84]. A meta-analysis of individual data from 579 patients with IBS included in two randomized, double-blind, placebo-controlled studies of Saccharomyces cerevisiae CNCM I-3856 showed that abdominal pain/discomfort and bloating were significantly improved with probiotic therapy versus placebo during month 2 of treatment (P = 0.01, for both comparisons) [Citation85]. However, data are currently lacking to demonstrate a direct effect of yeast on the gut microbiota of patients with IBS.
4.2.6. Multispecies probiotics: VSL#3®
The medical food probiotic mixture VSL#3 (4 species of Lactobacillus, 3 species of Bifidobacterium, and Streptococcus thermophilus; VSL Pharmaceuticals, Inc., Gaithersburg, MD) has been shown to alter the bacterial profile of the rectal mucosa (i.e. 15 cm from the anal verge) of patients with IBS, with twice daily treatment for 4 weeks, leading to an increase from baseline in average abundance of Bifidobacterium, Lactobacillus, and Streptococcus and a decrease from baseline in Bacteroides average abundance to levels comparable with that of the mucosa of healthy individuals [Citation86]. However, Michail et al. [Citation87] showed no differences from baseline in the number of bacteria or the diversity of bacterial species after 4- or 8-week treatment with VSL #3. The efficacy of the probiotic VSL #3 has been examined in four clinical studies of patients with IBS [Citation87–Citation90]; overall, the efficacy of VSL#3 was similar to that of placebo, although flatulence improved significantly with VSL#3 versus placebo after 4 and 8 weeks in one study () [Citation87–Citation90].
Table 3. Summary of clinical studies of VSL#3 in patients with IBS.
4.2.7. Other multispecies probiotics
Clinical studies of probiotics containing a mixture of bacterial species in patients with IBS have reported mixed results () [Citation91–Citation100]. Overall, multispecies probiotics appear to have a favorable safety profile. Levels of B lactis, Lactobacillus rhamnosus, and Streptococcus thermophilus increased significantly from baseline after 4-week treatment with a multispecies probiotic mixture (P < 0.01, P < 0.01, and P = 0.04, respectively); authors postulated that the multispecies probiotics were associated with a decrease in abdominal pain and bloating. However, further analyses are needed to definitively demonstrate this potential association between alterations in the gut microbiota and improvement in specific IBS symptoms. Kajander et al. [Citation100] reported that treatment with a multispecies probiotic stabilized the gut microbiota compared with placebo; during the second half of the 5-month treatment period, the stability of the gut microbiota increased with probiotic supplementation and decreased with placebo (P = 0.002). However, a limitation of this study was the small number of patients with samples who could be included in the analysis of gut microbiota (n = 20) [Citation100].
Table 4. Summary of clinical studies of multispecies probiotics in IBS.
4.3. Synbiotics
Synbiotics contain a mixture of prebiotics and probiotics [Citation63]. While synbiotics are safe for consumption, a number of randomized, controlled, and open-label clinical studies of patients with IBS who received synbiotics have shown mixed results regarding efficacy () [Citation63,Citation101–Citation103]. Findings appear to depend on the microorganisms examined, the duration of treatment, and the design of the study. Of note, the clinical studies of synbiotics presented in this review did not evaluate the gut microbiota. The ACG concluded that there was insufficient evidence to recommend the use of synbiotics in IBS [Citation1].
Table 5. Summary of clinical studies of synbiotics in IBS.
5. Antibiotics
Off-label administration of some antibiotics (e.g. neomycin) has been shown to improve symptoms of IBS; however, adverse effects and the development of antibiotic resistance or Clostridium difficile infection have been associated with systemically absorbed agents [Citation1,Citation6,Citation104–Citation107]. The nonsystemic antibiotic rifaximin (Xifaxan®, Salix Pharmaceuticals, Bridgewater, NJ) was approved in 2015 by the US Food and Drug Administration for the treatment of IBS-D in adults [Citation108]. Rifaximin 550 mg three times daily for 2 weeks has been evaluated in two phase III, identically designed, randomized, placebo-controlled IBS-D trials [Citation109]. A significantly greater percentage of patients who received rifaximin either achieved adequate relief of global IBS symptoms for ≥2 of the first 4 weeks posttreatment or achieved adequate relief of bloating for ≥2 of the first 4 weeks posttreatment versus placebo (; combined population) [Citation109]. Rifaximin and placebo had comparable safety profiles through 10 weeks posttreatment, with serious AEs reported in 1.6% of patients in the rifaximin group and 2.4% of patients in the placebo group; no patients developed C. difficile infection [Citation109]. A pooled analysis of safety data from these two phase III studies and a phase IIb study reaffirmed the favorable safety profile of rifaximin compared with placebo; no patients in these 3 studies developed C. difficile infection while receiving treatment [Citation110]. In the pooled analysis, the most common AEs with rifaximin 550 mg (n = 1008) vs. placebo (n = 829) were headache (5.5 vs. 6.2%), upper respiratory tract infection (4.5 vs. 5.7%), nausea (4.1 vs. 3.7%), and abdominal pain (4.0 vs. 4.7%) [Citation110]. A meta-analysis determined that one individual with IBS-D would have an AE with rifaximin for every 846 individuals who would benefit (number needed to harm = 8,971; number needed to treat = 10.6) [Citation111].
Figure 2. Patients achieving adequate relief of global irritable bowel syndrome (IBS) symptomsa and adequate relief of bloatingb during ≥2 of the first 4 weeks posttreatment (combined population from two phase 3 trials).
aYes response to the following question, which was asked weekly: ‘In regard to all your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?’ bYes response to the following question, which was asked weekly: ‘In regard to your symptoms of bloating, as compared with the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptom of bloating?’ Data from Pimentel M et al. N Engl J Med 2011;364:22–32 [Citation109].
![Figure 2. Patients achieving adequate relief of global irritable bowel syndrome (IBS) symptomsa and adequate relief of bloatingb during ≥2 of the first 4 weeks posttreatment (combined population from two phase 3 trials).aYes response to the following question, which was asked weekly: ‘In regard to all your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?’ bYes response to the following question, which was asked weekly: ‘In regard to your symptoms of bloating, as compared with the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptom of bloating?’ Data from Pimentel M et al. N Engl J Med 2011;364:22–32 [Citation109].](/cms/asset/bec04850-c078-4865-aeb7-9eeddbb020f4/ipgm_a_1383819_f0002_b.gif)
Because rifaximin is administered as short-course therapy (i.e. 2 weeks) for IBS-D, a phase III retreatment study was conducted to evaluate if patients with IBS-D who initially responded to rifaximin and then experienced symptom recurrence would respond to 2-week repeat treatment [Citation112]. Patients with IBS-D who responded to an open-label, 2-week course of rifaximin and then relapsed during follow up (up to 18 weeks) were randomly assigned to double-blind treatment with rifaximin (n = 328) or placebo (n = 308). A significantly greater percentage of patients in the rifaximin group (38.1%) responded (≥30% decrease from baseline in mean weekly pain score for abdominal pain and ≥50% decrease from baseline in the number of days with mushy/watery stools [Bristol Stool Scale type 6 or 7]) for ≥2 of 4 weeks posttreatment compared with placebo (31.5%; P = 0.03) [Citation112]. Repeat treatment with rifaximin was generally well tolerated. The most commonly reported AEs during a 22-week period (which included two 2-week treatment courses) occurring more often with rifaximin versus placebo were nausea (3.7 vs. 2.3%), upper respiratory tract infection (3.7 vs. 2.6%), and nasopharyngitis (3.0 vs. 2.9%). C. difficile infection occurred in one patient receiving rifaximin repeat treatment after 37 days; however, this patient had previous infection with C. difficile and had received 10-day treatment with cefdinir for a urinary tract infection immediately before developing this C. difficile infection. The ACG stated that rifaximin is effective at reducing total IBS symptoms and bloating in IBS-D (weak recommendation based on moderate quality of evidence) [Citation1].
In a case report, a patient with IBS-C with constipation and excess methane production was treated with rifaximin and had improved stool frequency and form [Citation113]. Further, a retrospective study of patients with IBS with a positive breath test for methane showed that the combination of rifaximin and neomycin resulted in greater clinical response (85%) compared with neomycin alone (63%; P = 0.2) or rifaximin alone (56%; P = 0.01) [Citation114]. It is possible that antibiotic therapy reduces the effects of methanogenic bacteria, given that patients with IBS, particularly IBS-C, were shown to have increased fecal concentrations of the methanogen Methanobrevibacter smithii compared with healthy individuals; further, methane producers by breath testing had greater concentrations of M. smithii [Citation115]. However, a retrospective study of patients with IBS receiving antibiotic treatment reported that the best therapeutic response occurred in patients who had no increase in measured hydrogen and methane gases in a lactulose breath test [Citation116]. Thus, while some evidence suggests that methanogenic bacteria may play a role in response to antibiotics, further study is needed.
The effect of a nonsystemic antibiotic on the gut microbiota is viewed as a safety concern of rifaximin. The mechanism of action of rifaximin in IBS is unclear, but studies in patients with non–IBS-C receiving a 2-week course of treatment with rifaximin experienced only modest, although detectable, changes in fecal microbial profiles [Citation117,Citation118]. Additional preclinical [Citation119] studies and human IBS fecal sample analysis [Citation118,Citation120,Citation121] studies of rifaximin have supported that any changes in the fecal microbial profile are modest and not sustained. Overall, data supported short courses of therapy (2 weeks per course) with rifaximin in the treatment of patients with IBS-D.
Although these studies currently raise no red flags, future research should continue to monitor bacterial antibiotic resistance and the incidence of C. difficile infection, especially in patients who require repeat courses of rifaximin and potentially other antibiotics. Advances in molecular techniques, such as RNA sequencing, may offer further information regarding the safety of antibiotic therapy in the treatment of IBS.
6. Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) provides the most powerful means of modifying the microbiota from a presumed healthy donor. FMT received FDA approval for the treatment of recurrent C. difficile infection, with a meta-analysis of 611 patients showing pooled efficacy (i.e. cure) to be 91.2% [Citation122]. There is a growing interest in using FMT for a number of GI diseases that are linked to a disturbed gut microbiota composition, including IBS. Reestablishing a healthy intestinal microbiota could be a promising novel treatment option for IBS; indeed, a small, open-label study of FMT in patients with IBS (n = 10) demonstrated significant improvement from baseline in the reestablishment of fecal microbiota diversity 4 weeks after treatment (P = 0.03) and efficacy of FMT was dependent on increased concentrations of bifidobacteria in the donor stool [Citation123]. A small number of clinical trials are currently registered under ClinicalTrials.gov, and to date, there are no published randomized clinical trials investigating the impact of FMT on IBS. A 2017 review compiled the limited number of studies, the majority of which were conference abstracts that included a small number of patients (range, n = 9 to n = 13) [Citation124]. Because of differences in methods of administration, inclusion criteria, assessment of symptoms, and control groups, conclusions must be made cautiously. The authors concluded that there is an ‘at least temporary improvement’ in a large percentage (58%) of FMT-treated patients with IBS without any significant complications. However, there is concern that more than desired microbes are being transmitted during FMT and that donors need to be carefully screened [Citation125,Citation126]. In one study, germ-free mice receiving fecal microbiota samples from patients with IBS-D exhibited increased GI transit, activation of innate immunity, and altered intestinal barrier function after 3 weeks compared with mice receiving fecal microbiota from healthy individuals [Citation127]. Anxiety-like behavior was evident in mice receiving FMT from patients with IBS-D with anxiety, but not in mice receiving FMT from patients with IBS-D without anxiety or from healthy individuals. FMT appears promising; however, further research is needed before FMT can be recommended as a therapeutic modality for patients with IBS.
7. Conclusions
IBS is a common chronic condition characterized by alterations in the gut microbiota. Treatment of patients with IBS may include a number of agents with the potential to modulate the gut microbiota (i.e. dietary modifications, prebiotics, probiotics, synbiotics, and nonsystemic antibiotics). However, the number and diversity of different bacterial species for probiotics or synbiotics, used either alone or in combination, are inconsistent among studies, as is the duration of treatment. Thus, it is not entirely surprising that efficacy findings across studies vary widely. Further research should place an emphasis on identifying species- and strain-specific effects, rather than drawing generalized conclusions from studies with limited comparability.
Overall, in studies that evaluated safety, dietary interventions, prebiotics, probiotics, and synbiotics were associated with a favorable safety profile. For nonsystemic antibiotics, rifaximin has been shown to be efficacious and well tolerated in well-designed clinical trials. However, it is unclear how often a 2-week course of rifaximin repeat treatment might be needed to manage symptoms of IBS, and thus repeat treatment courses should be individualized based on patient symptoms. It also bears mentioning that, for other nonsystemic antibiotics, there are an inadequate number of IBS-D studies in the literature. Furthermore, more consistent evidence is needed regarding the efficacy and safety of elimination diets, prebiotics, probiotics, and synbiotics for the treatment of IBS. Additional well-designed studies that examine alterations in the gut microbiota and potential associations with the clinical symptoms of IBS are also warranted.
Declaration of Interests
LA Harris reports serving as a consultant for Salix Pharmaceuticals, Ironwood Pharmaceuticals, Inc., Allergan plc, Synergy Pharmaceuticals Inc., Synthetic Biologics, Inc., IM HealthScience, QOL Pharmaceuticals, and Napo Pharmaceuticals; as well as performing research with Alvine Pharmaceuticals and Rhythm Pharmaceuticals, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
Technical editorial assistance was provided, under the direction of the authors, by Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, Pennsylvania, USA. Funding for this support was provided by Salix Pharmaceuticals, Bridgewater, NJ, USA.
Additional information
Funding
References
- Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–S26.
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.
- Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075–1082.
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407.
- Su AM, Shih W, Presson AP, et al. Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterol Motil. 2014;26:36–45.
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. Jama. 2015;313:949–958.
- Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004;10:299–309.
- Paré P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726–1735.
- Doshi JA, Cai Q, Buono JL, et al. Economic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured population. J Manag Care Spec Pharm. 2014;20:382–390.
- Sethi S, Wadhwa V, LeClair J, et al. In-patient discharge rates for the irritable bowel syndrome - an analysis of national trends in the United States from 1997 to 2010. Aliment Pharmacol Ther. 2013;38:1338–1346.
- Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–1759.
- Drossman DA, Chang L, Schneck S, et al. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig Dis Sci. 2009;54:1532–1541.
- Drossman DA, Morris CB, Schneck S, et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43:541–550.
- Drossman DA, Thompson WG. The irritable bowel syndrome: review and a graduated multicomponent treatment approach. Ann Intern Med. 1992;116:1009–1016.
- Coffin B, Dapoigny M, Cloarec D, et al. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2004;28:11–15.
- Ricci J, Jhingran P, Harris W, et al. Impact of differences in severity of irritable bowel syndrome (IBS) on patients’ well being and resource use. Gastroenterology. 2001;120:A406.
- Bijkerk CJ, de Wit NJ, Stalman WA, et al. Irritable bowel syndrome in primary care: the patients’ and doctors’ views on symptoms, etiology and management. Can J Gastroenterol. 2003;17:363–368.
- Harris LA, Umar SB, Baffy N, et al. Irritable bowel syndrome and female patients. Gastroenterol Clin North Am. 2016;45:179–204.
- Eswaran S, Goel A, Chey WD. What role does wheat play in the symptoms of irritable bowel syndrome? Gastroenterol Hepatol (NY). 2013;9:85–91.
- Quigley EM. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis. 2007;8:2–7.
- Crowell MD, Harris L, Jones MP, et al. New insights into the pathophysiology of irritable bowel syndrome: implications for future treatments. Curr Gastroenterol Rep. 2005;7:272–279.
- Halland M, Saito YA. Irritable bowel syndrome: new and emerging treatments. Bmj. 2015;350:h1622.
- Srivastava D, Ghoshal U, Mittal RD, et al. Associations between IL-1RA polymorphisms and small intestinal bacterial overgrowth among patients with irritable bowel syndrome from India. Neurogastroenterol Motil. 2014;26:1408–1416.
- Durbán A, Abellán JJ, Jiménez-Hernández N, et al. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol Ecol. 2013;86:581–589.
- Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807.
- Durbán A, Abellán JJ, Jimenéz-Hernández N, et al. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep. 2012;4:242–247.
- Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745.
- Zhuang X, Xiong L, Li L, et al. Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38.
- Ganji L, Alebouyeh M, Shirazi MH, et al. Dysbiosis of fecal microbiota and high frequency of Citrobacter, Klebsiella spp., and Actinomycetes in patients with irritable bowel syndrome and gastroenteritis. Gastroenterol Hepatol Bed Bench. 2016;9:325–330.
- Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006.
- Tap J, Derrien M, Tornblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152:111–123, e118.
- Cuomo R, Andreozzi P, Zito FP, et al. Irritable bowel syndrome and food interaction. World J Gastroenterol. 2014;20:8837–8845.
- Posserud I, Strid H, Storsrud S, et al. Symptom pattern following a meal challenge test in patients with irritable bowel syndrome and healthy controls. United European Gastroenterol J. 2013;1:358–367.
- Shekhar C, Monaghan PJ, Morris J, et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749–757.
- Hayes P, Corish C, O’Mahony E, et al. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27:36–47.
- Young E, Stoneham MD, Petruckevitch A, et al. A population study of food intolerance. Lancet. 1994;343:1127–1130.
- Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–1214.
- El-Salhy M, Mazzawi T, Hausken T, et al. Interaction between diet and gastrointestinal endocrine cells. Biomed Rep. 2016;4:651–656.
- Rajilić-Stojanović M, Jonkers DM, Salonen A, et al. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol. 2015;110:278–287.
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr J. 2015;14:92.
- De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169–178.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911.
- Barmeyer C, Schumann M, Meyer T, et al. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int J Colorectal Dis. 2017;32:29–39.
- Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147:1012–1020.
- Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328.
- Bonder MJ, Tigchelaar EF, Cai X, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:45.
- Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–717.
- Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y). 2014;10:164–174.
- Ringel-Kulka T, Choi CH, Temas D, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110:1339–1346.
- Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100.
- Bennet SMP, Bohn L, Storsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs [published online ahead of print April 17, 2017]. Gut. 2017.
- Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407.
- Varju P, Farkas N, Hegyi P, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS One. 2017;12:e0182942.
- Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.
- Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270.
- Moayyedi P, Quigley EM, Lacy BE, et al. The effect of dietary intervention on irritable bowel syndrome: a systematic review. Clin Transl Gastroenterol. 2015;6:e107.
- Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111:1824–1832.
- Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32(Suppl 1):16–19.
- Muir JG, Gibson PR. The low FODMAP diet for treatment of irritable bowel syndrome and other gastrointestinal disorders. Gastroenterol Hepatol (N Y). 2013;9:450–452.
- Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome–an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
- Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–63.
- Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176.
- Andriulli A, Neri M, Loguercio C, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42:S218–S223.
- Kerckhoffs AP, Samsom M, van der Rest ME, et al. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887–2892.
- Balsari A, Ceccarelli A, Dubini F, et al. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194.
- Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. 2017;32:64–68.
- Silk DB, Davis A, Vulevic J, et al. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–518.
- Paineau D, Payen F, Panserieu S, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr. 2008;99:311–318.
- Mego M, Manichanh C, Accarino A, et al. Metabolic adaptation of colonic microbiota to galactooligosaccharides: a proof-of-concept-study. Aliment Pharmacol Ther. 2017;45:670–680.
- Whorwell PJ. Do probiotics improve symptoms in patients with irritable bowel syndrome? Therap Adv Gastroenterol. 2009;2:37–44.
- Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics – Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767–778.
- Williams MD, Ha CY, Ciorba MA. Probiotics as therapy in gastroenterology: a study of physician opinions and recommendations. J Clin Gastroenterol. 2010;44:631–636.
- Jalanka-Tuovinen J, Salonen A, Nikkila J, et al. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011;6:e23035.
- Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:4718–4725.
- Charbonneau D, Gibb RD, Quigley EM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4:201–211.
- Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590.
- O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551.
- Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114.
- Guyonnet D, Schlumberger A, Mhamdi L, et al. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double-blind, parallel, controlled study. Br J Nutr. 2009;102:1654–1662.
- Guyonnet D, Woodcock A, Stefani B, et al. Fermented milk containing Bifidobacterium lactis DN-173 010 improved self-reported digestive comfort amongst a general population of adults. A randomized, open-label, controlled, pilot study. J Dig Dis. 2009;10:61–70.
- Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638–1646.
- Thijssen AY, Clemens CH, Vankerckhoven V, et al. Efficacy of Lactobacillus casei Shirota for patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2016;28:8–14.
- Barrett JS, Canale KE, Gearry RB, et al. Probiotic effects on intestinal fermentation patterns in patients with irritable bowel syndrome. World J Gastroenterol. 2008;14:5020–5024.
- Bafutto M, Almeida JR, Leite NV, et al. Treatment of diarrhea-predominant irritable bowel syndrome with mesalazine and/or Saccharomyces boulardii. Arq Gastroenterol. 2013;50:304–309.
- Cayzeele-Decherf A, Pelerin F, Leuillet S, et al. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: an individual subject meta-analysis. World J Gastroenterol. 2017;23:336–344.
- Ng SC, Lam EF, Lam TT, et al. Effect of probiotic bacteria on the intestinal microbiota in irritable bowel syndrome. J Gastroenterol Hepatol. 2013;28:1624–1631.
- Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of VSL#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3:1–7.
- Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696.
- Wong RK, Yang C, Song GH, et al. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–194.
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904.
- Mezzasalma V, Manfrini E, Ferri E, et al. A randomized, double-blind, placebo-controlled trial: the efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. Biomed Res Int. 2016;2016:4740907.
- Yoon H, Park YS, Lee DH, et al. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr. 2015;57:129–134.
- Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52–59.
- Ki Cha B, Mun JS, Hwan CC, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227.
- Min YW, Park SU, Jang YS, et al. Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J Gastroenterol. 2012;18:4563–4569.
- Sondergaard B, Olsson J, Ohlson K, et al. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–672.
- Ringel-Kulka T, Palsson OS, Maier D, et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45:518–525.
- Simrén M, Öhman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227.
- Williams EA, Stimpson J, Wang D, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103.
- Kajander K, Myllyluoma E, Rajilic-Stojanovic M, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57.
- Bittner AC, Croffut RM, Stranahan MC, et al. Prescript-assist probiotic-prebiotic treatment for irritable bowel syndrome: an open-label, partially controlled, 1-year extension of a previously published controlled clinical trial. Clin Ther. 2007;29:1153–1160.
- Bittner AC, Croffut RM, Stranahan MC. Prescript-Assist™ probiotic-prebiotic treatment for irritable bowel syndrome: a methodologically oriented, 2-week, randomized, placebo-controlled, double-blind clinical study. Clin Ther. 2005;27:755–761.
- Colecchia A, Vestito A, La RA, et al. Effect of a symbiotic preparation on the clinical manifestations of irritable bowel syndrome, constipation-variant. Results of an open, uncontrolled multicenter study. Minerva Gastroenterol Dietol. 2006;52:349–358.
- Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419.
- Pimentel M, Chatterjee S, Chow EJ, et al. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51:1297–1301.
- Basseri RJ, Weitsman S, Barlow GM, et al. Antibiotics for the treatment of irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2011;7:455–493.
- Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother. 2009;63:238–242.
- Salix Pharmaceuticals. Xifaxan® (rifaximin) tablets, for oral use. Bridgewater (NJ): Salix Pharmaceuticals; 2015.
- Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32.
- Schoenfeld P, Pimentel M, Chang L, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2014;39:1161–1168.
- Shah E, Kim S, Chong K, et al. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med. 2012;125:381–393.
- Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2016;151:1113–1121.
- Ghoshal UC, Srivastava D, Verma A, et al. Slow transit constipation associated with excess methane production and its improvement following rifaximin therapy: a case report. J Neurogastroenterol Motil. 2011;17:185–188.
- Low K, Hwang L, Hua J, et al. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547–550.
- Ghoshal U, Shukla R, Srivastava D, et al. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut Liver. 2016;10:932–938.
- Kasir R, Zakko S, Zakko P, et al. Predicting a response to antibiotics in patients with the irritable bowel syndrome. Dig Dis Sci. 2016;61:846–851.
- Acosta A, Camilleri M, Shin A, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol. 2016;7:e173.
- Soldi S, Vasileiadis S, Uggeri F, et al. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol. 2015;8:309–325.
- Kim MS, Morales W, Hani AA, et al. The effect of rifaximin on gut flora and Staphylococcus resistance. Dig Dis Sci. 2013;58:1676–1682.
- Pimentel M, Fodor AA, Golden PL, et al. Characterization of stool microbiota in subjects with IBS-D receiving repeat treatments with rifaximin in the TARGET 3 study. Gastroenterology. 2015;148:S–655.
- Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, et al. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes. 2016;7:397–413.
- Li YT, Cai HF, Wang ZH, et al. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43:445–457.
- Mizuno S, Masaoka T, Naganuma M, et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion. 2017;96:29–38.
- Halkjaer SI, Boolsen AW, Gunther S, et al. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol. 2017;23:4112–4120.
- Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580.
- König J, Siebenhaar A, Hogenauer C, et al. Consensus report: faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther. 2017;45:222–239.
- De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:1–14.