ABSTRACT
Objectives: Evaluate the efficacy, duration of effect, and safety of 25 mg SHP465 mixed amphetamine salts (MAS) extended-release versus placebo in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods: Adults (18–55 years) with ADHD and with ADHD Rating Scale-IV (ADHD-RS-IV) scores ≥24 were randomized to treatment in a double-blind, 2-period, 2-treatment crossover study utilizing the Adult Workplace Environment (AWE), as described by Wigal and Wigal (J Atten Disord 2006;10:92–111). On day 7 of each 7-day treatment period, efficacy was assessed during a 16.5-hour postdose period. The primary endpoint, Permanent Product Measure of Performance (PERMP) total score, was analyzed in the intent-to-treat population using a mixed linear model of analysis of variance. Secondary endpoints, for which the study was not powered, included PERMP problems attempted and answered correctly, ADHD clinician ratings based on counselor observations and inputs during the Time Segment Rating System (Co-ADHD-RS TSRS), and the ADHD self-rating scale (ADHD-SRS). Safety and tolerability assessments included treatment-emergent adverse events (TEAEs) and vital signs.
Results: The least squares mean (95% CI) treatment difference (SHP465 MAS–placebo) for PERMP total score significantly favored SHP465 MAS over placebo when averaged across all postdose assessments (19.29 [10.95, 27.63]; P < 0.0001), with significant treatment differences favoring SHP465 MAS over placebo observed at 4–16 hours postdose (all P < 0.01). TEAEs observed with SHP465 MAS (≥5% of participants) included insomnia, decreased appetite, dry mouth, headache, and anorexia. Mean pulse and blood pressure increases with SHP465 MAS exceeded those of placebo.
Conclusions: SHP465 MAS (25 mg) was superior to placebo on PERMP total score, with treatment differences observed from 4 to 16 hours postdose; nominal treatment differences on the ADHD-SRS, but not the Co-ADHD-RS TSRS, were also observed. The safety and tolerability profile of SHP465 MAS was similar to previous reports for SHP465 MAS and other long-acting stimulants.
Clinical trials registry: clinicaltrials.gov (NCT00202605; https://clinicaltrials.gov/ct2/show/NCT00202605)
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) can persist from childhood into adulthood, with a reported 40–66% of individuals diagnosed with ADHD as a child continuing to show symptoms of ADHD as an adult [Citation1–Citation3]. The presence of ADHD in adults is associated with difficulties in daily functioning, including impaired educational, occupational, and social function [Citation4–Citation7], and decreased work performance [Citation8].
Psychostimulants are the most commonly prescribed pharmacotherapy in adults with ADHD [Citation9], with multiple extended-release (ER) psychostimulant formulations approved for use in adults by the US Food and Drug Administration [Citation10]. However, medication choice varies across patients and is often highly individualized. Medication attributes reported to contribute to the attractiveness of specific pharmacotherapies in adults with ADHD include the ability of the medication to improve function during the work day and into the late afternoon and evening, and a reduced number of doses that need to be taken during the day [Citation11]. In addition, as is the case with other chronic disorders [Citation12], medication adherence among adults with ADHD is negatively influenced by multiple factors that include forgetting to take medication (in part due to the executive function deficits that may be associated with ADHD [Citation13]), the complexity of the treatment regimen (e.g. the need to take multiple medications or doses throughout the day), and the stigma associated with taking medication [Citation14]. Taken together, these findings suggest some adults with ADHD may benefit from a once-daily medication with a duration of effect that allows them to more easily meet their specific, individualized environmental demands (i.e. extended work days and the need to manage familial affairs in the evening).
Based on published reviews, it is estimated that currently available long-acting psychostimulants, such as osmotic controlled-release oral delivery system (OROS) methylphenidate, mixed amphetamine salts ER (MAS XR), and lisdexamfetamine dimesylate (LDX), have durations of effect ranging from 8 to 14 h [Citation10,Citation15–Citation17]. However, the reported supplementation of long-acting ADHD medications with an additional immediate-release ADHD medication in some individuals with ADHD [Citation18,Citation19] suggests a significant proportion of adults with ADHD may desire symptom control beyond 14 h postdose.
SHP465 mixed amphetamine salts (SHP465 MAS) extended-release is a once-daily, single-entity MAS product for oral administration approved in the USA for the treatment of ADHD in patients 13 years and older. It contains equal amounts (by weight) of four salts: dextroamphetamine sulfate, amphetamine sulfate, dextroamphetamine saccharate and amphetamine aspartate monohydrate. This results in a 3:1 mixture of dextro- to levoamphetamine base equivalent. Each capsule contains 3 types of drug-releasing beads, an immediate-release bead and two different types of delayed-release beads.
In a phase I study, the area under the plasma drug concentration-time curve for amphetamine following 37.5 mg SHP465 MAS was similar to that observed after a morning dose of 25 mg MAS XR supplemented 8 h later by 12.5 mg immediate-release MAS (MAS IR) [Citation20]. In three phase III studies (1 dose-optimization study using 12.5–75 mg SHP465 MAS [Citation21] and 2 forced-dose studies using 25, 50, or 75 mg SHP465 MAS [Citation22] or 12.5 or 37.5 mg SHP465 [Citation23]), SHP465 MAS produced significantly greater reductions in ADHD Rating Scale IV (ADHD-RS-IV) or ADHD-RS with adult prompts total score than placebo. In these phase III studies [Citation21–Citation23], SHP465 MAS had a safety and tolerability profile generally consistent with other long-acting stimulants [Citation24–Citation26]. These findings suggest SHP465 MAS is a viable once-daily treatment option for adults with ADHD.
The primary objective of this study was to evaluate the efficacy of SHP465 MAS versus placebo and to characterize the postdose duration of effect of SHP465 MAS versus placebo in adults with ADHD in the Adult Workplace Environment (AWE) using the Permanent Product Measure of Performance (PERMP). The AWE is a structured, controlled environment, designed to monitor and quantify treatment effects on the performance of adults during activities simulating those that occur during a typical work day [Citation27,Citation28]; it is based on the childhood laboratory school protocol model [Citation28]. The AWE is a useful model for assessing ADHD symptoms because the structured tasks that are performed during the study day focus on deficits in attention in adults with ADHD. The PERMP is a time-sensitive, skill-adjusted, 10-min math test that is administered following an 8-min level-finding test for proper placement [Citation28]; it measures aspects of sustained attention, including the ability to initiate and perform effortful work, and stay on task, but not math ability [Citation28]. The PERMP is considered a valid tool to investigate drug response and has been used in other studies evaluating efficacy in stimulants [Citation27,Citation29–Citation31].
2. Materials and methods
2.1. Study design
This phase II, randomized, multicenter, double-blind, two-period, two-treatment crossover study (ClinicalTrials.gov registry number NCT00202605) was conducted at 4 US sites between 29 September 2005, and 6 January 2006. Before study initiation, the protocol was approved by a central institutional review board (IRB) for 3 sites and a local IRB for 1 site. The study was managed by Quintiles, Inc. (San Diego, CA). Quintiles was responsible for conducting the study and submitting the protocol and amendments, informed consent documents, relevant supporting information, and all participant recruitment information to the central IRB for review for all study sites except the 1 site that used the local IRB.
The study was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki [Citation32], including amendments of the 29th, 35th, 41st, and 48th World Medical Assemblies. All participants received a complete study description and provided written informed consent before participating. Informed consent documents were written in accordance with applicable regulations, good clinical practice, and guidelines established by the Health Insurance Portability and Accountability Act of 1996.
2.2. Participants
Eligible participants were men or nonpregnant/nonlactating women (18–55 years [inclusive]) who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for a primary ADHD diagnosis (with ≥6 of 9 subtype criteria met) as established by a psychiatric interview (the Structured Clinical Interview for the DSM-IV-TR [SCID][Citation33]), and who had a baseline ADHD-RS-IV total score ≥24. All eligible participants also had an intelligence quotient ≥80 based on the Kaufman Brief Intelligence Test [Citation34] and a satisfactory medical assessment, with no clinically significant or relevant abnormalities. All participants understood and were able and willing to comply with the study procedures and restrictions.
Key exclusion criteria included body mass index <18.5 or >34 kg/m2; the presence of comorbid psychiatric disorders (defined by the SCID) controlled with prohibited medications or uncontrolled and associated with significant symptoms; an illness or an unstable medical condition that could confound safety assessments, lead to increased risk, or make it difficult to comply with the study protocol; a history of intellectual disability or severe learning disability; a history of seizures (excluding infantile febrile seizures), any tic disorder or a current diagnosis and/or family history of Tourette disorder; known cardiac abnormalities or any condition affecting cardiac performance or a history of hypertension (systolic blood pressure [SBP] ≥140 mmHg and/or diastolic blood pressure [DBP] ≥90 mmHg) at screening; a clinically significant electrocardiogram (ECG) or laboratory abnormality at baseline or screening; drug dependence or substance abuse disorder defined by the SCID (excluding nicotine dependence) within 12 months before screening; a positive urine drug test at screening or baseline (excluding current stimulant medication); and a documented allergy, intolerance, or history of nonresponsivity to methylphenidate, amphetamine, or MAS. The use of psychoactive prescription medications (≤30 days of screening), over-the-counter medications requiring more than a 7-day washout (participants using a methylphenidate or amphetamine at screening underwent a 7-day washout period), antihypertensive agents (excluding diuretics), or investigational products (≤30 days before screening) was also prohibited. Participation in a clinical trial (including a SHP465 MAS trial) ≤30 days before screening was also exclusionary.
2.3. Treatment and assessment schedule
The study consisted of a screening visit, a baseline visit, an orientation visit, a double-blind crossover phase consisting of 2 visits (visits 4 and 5) separated by 7 days, and a follow-up period ().
Figure 1. Schematic diagram of (a) the study design and (b) participant disposition.
AWE = adult workplace environment; ITT = intent to treat; MAS = mixed amphetamine salts; PERMP = Permanent Product Measure of Performance; PP = per protocol; V = visit.*Enrolled participants were those for whom all screening assessments were completed and reviewed.
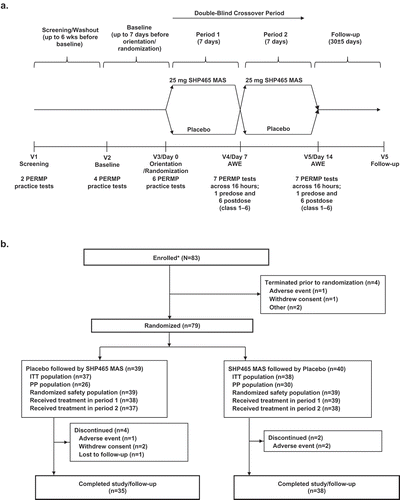
During the screening visit (visit 1), participants completed 2 practice PERMP tests. Screening could take place up to 6 weeks before the orientation visit to facilitate participant recruitment. Participants taking sedating antihistamines or using a psychostimulant for ADHD underwent a 1-week washout period before the baseline visit. Other screening assessments included a serum pregnancy test, physical examination, biochemistry, hematology and thyroid-stimulating hormone tests, urinalysis and urine drug screening, vital signs, height and weight measurement, and a single ECG recording.
During the baseline visit (visit 2), participants completed a math pretest and 4 practice PERMP tests. Baseline assessments were completed up to 7 days before the orientation visit. Other baseline assessments included an ECG, vital signs and body weight measurement, an ADHD-RS-IV assessment, a recording of adverse events (AEs) and concomitant medication use, a urine pregnancy test for females of childbearing potential, biochemistry and hematology testing (if >30 days elapsed since screening), and an abbreviated physical examination (if >14 days elapsed since screening).
During the orientation visit (visit 3), participants were familiarized with the AWE and study procedures in a group setting and randomized to a treatment sequence (25 mg SHP465 MAS followed by placebo or placebo followed by 25 mg SHP465 MAS). Participants also completed 6 additional PERMP practice tests (a total of 12 PERMP practice tests were completed before the double-blind crossover phase). Treatment regimens were determined by a block randomization schedule using a 1-balanced 2 × 2 Latin Square with 2 treatment sequences. Computer-generated 2-part labels were used for blinding the treatment. Other orientation visit assessments included ADHD clinician ratings based on counselor observations and inputs during Time Segment Rating System (Co-ADHD-RS TSRS), the ADHD self-rating scale (ADHD-SRS), the Pittsburgh Sleep Quality Index (PSQI), a urine pregnancy test, vital signs and weight measurement, and a recording of AEs and concomitant medications. During this visit, the first 6 days of medication was provided along with administration instructions.
Visits during the double-blind crossover phase (visits 4 and 5) were separated by 7 days to allow treatment stabilization. On day 7 of each 1-week treatment period, participants returned to the AWE and took their medication simultaneously as a group at the AWE site at approximately 7:00 am. Assessments during visits 4 and 5 included the PERMP (assessed at 7 time points [classes 0–6]: –0.5 h predose and 2, 4, 8, 12, 14, and 16 h postdose), the Co-ADHD-RS TSRS (assessed during 3 sequential 5.5-h cycles: cycle A, up to 5.5 h postdose; cycle B, 5.5−11 h postdose; cycle C, 11−16.5 h postdose), the ADHD-SRS (completed toward the end of each Co-ADHD-RS TSRS cycle), and the PSQI (before dosing). In addition, vital sign (predose and approximately 4, 8, and 16 h [±45 min] postdose), ECG (6 ± 1 h postdose), body weight, biochemistry and hematology, drug accountability and adherence, and AEs and concomitant medication use data were collected. Participants reported to the AWE as a group, but participant-specific schedules guided timing of Co-ADHD-RS TSRS activities and safety assessments to ensure that all participants could take part in group activities. At visit 4, the last 6 days of medication were provided along with administration instructions.
During the follow-up period, telephone contact was initiated (30 ± 5 days following the last study drug dose) to collect information on any ongoing, new, or serious AEs. All AEs were followed until an outcome was reached or the event was otherwise explained, resolved, or stabilized.
2.4. Study endpoints
2.4.1. Efficacy
The PERMP is a 10-min skill-adjusted math test consisting of 5 pages with 80 math problems per page [Citation28]. The difficulty level of the PERMP was adjusted based on the math pretest performance; in addition, different versions were available so participants did not take the same test more than once during the study. PERMP total score (primary efficacy endpoint) was defined as the sum of the number of problems attempted and the number of problems answered correctly across the postdose period; the number of problems attempted and the number of problems answered correctly across the postdose period and at each time point were secondary efficacy endpoints.
Other secondary efficacy endpoints included the ADHD-SRS and Co-ADHD-RS TSRS. The Co-ADHD-RS TSRS is an 18-item clinician-rated scale based on DSM-IV-TR ADHD criteria and derived from the ADHD-RS-IV. For the purpose of this study, this scale was modified such that counselors (nonclinical staff) were trained to collect systematic observations during the TSRS tasks. This information was provided to clinicians who would utilize this input and the objective results of the TSRS tasks to enter Co-ADHD-RS TSRS scores. Symptom frequency and severity on the Co-ADHD-RS TSRS are measured on 4-point scales (range, 0–3). The ADHD-SRS is an 18-item self-report scale based on DSM-IV-TR ADHD diagnostic criteria; symptom severity is measured on a 4-point scale (range, 0–3). For both the Co-ADHD-RS TSRS and ADHD-SRS, total score and subscale scores for hyperactivity/impulsivity and inattentiveness were collected.
2.4.2. Safety and tolerability
All AEs were recorded from the time of informed consent until treatment ended; AEs were categorized according to their relatedness to treatment, severity, and seriousness. Vital signs (SBP, DBP, and pulse) were recorded after the participant had been seated for 5 min. Sleep quality was assessed using a modified PSQI. Individual PSQI items can be used to generate a global score (range, 0–21) and 7 component scores [Citation35,Citation36], with higher scores reflecting worse sleep quality. Other safety and tolerability assessments included 12-lead ECGs and clinical laboratory evaluations.
2.5. Data presentation and statistical analysis
Sample size was estimated using an effect size of 0.357 for PERMP total score. It was estimated that approximately 64 participants needed to complete the double-blind crossover phase to obtain 80% power at a two-sided α level of 0.05 using a paired t test. Assuming 10% dropout and a balanced 2 × 2 Latin Square design with 2 treatment sequences, the study planned to randomize approximately 72 participants.
Statistical assessments of the primary and secondary efficacy endpoints were conducted in the intent-to-treat (ITT) population (randomized participants taking ≥1 study drug dose and having ≥1 postbaseline primary efficacy assessment). Sensitivity analyses were conducted in the per-protocol (PP) population (ITT population participants who completed the study and were deemed protocol compliant). Statistical significance for all analyses was set at P < 0.05. The study was not powered for assessment of secondary endpoints, so all reported P values are nominal (unadjusted and reported for descriptive purposes only).
Statistical assessment of PERMP total score (the primary endpoint) was conducted using a mixed linear model analysis of variance (ANOVA) for the average of classes 1 to 6 (postdose 2–16 h) and at each postdose class (2, 4, 8, 12, 14, and 16 h postdose). The model included sequence (placebo followed by 25 mg SHP465 MAS versus 25 mg SHP465 MAS followed by placebo), period (visit 4 versus visit 5), and treatment (placebo versus 25 mg SHP465 MAS) as fixed effects and participant within sequence as a random effect. SHP465 MAS was considered superior to placebo on PERMP total score if the average of 2–16 h postdose (classes 1–6) was statistically different between 25 mg SHP465 MAS and placebo. Duration of effect was then identified as the last time point beyond which a statistically significant difference was no longer evident. If the last time point was statistically significant, duration of effect was demonstrated at 16 h. Statistical assessment of the secondary efficacy endpoints used the same mixed linear ANOVA model used for the primary endpoint.
Safety and tolerability endpoints are described in the randomized safety population (randomized participants who took ≥1 study drug dose or who were randomized and dispensed study medication but immediately lost to follow-up) using descriptive statistics.
3. Results
3.1. Participant disposition and demographics
Participant disposition is summarized in ; demographic and clinical characteristics are summarized in . In general, most participants were white and the majority was men; 48.7% (38/78) of participants were 18–25 years old. Most study participants were diagnosed as having the combined ADHD subtype (1 participant was diagnosed with the hyperactivity/impulsivity subtype); the mean ± standard deviation (SD) duration of ADHD diagnosis and age at ADHD onset, respectively, was 5.6 ± 8.4 and 5.4 ± 0.6 years.
Table 1. Demographics and baseline clinical characteristics, randomized safety population.
3.2. Prior/concomitant medication
In the randomized safety population, 17.9% (14/78) of participants reported use of ADHD medications within 30 days before screening (MAS IR [2.6% (2/78)], MAS XR [7.7% (6/78)], dexamphetamine [2.6% (2/78)], and methylphenidate [7.7% (6/78)]). The use of concomitant medications was reported by 29.9% (23/77) of participants. Concomitant medications used by ≥2% of participants were acetaminophen (11.7% [9/77]), ibuprofen (9.1% [7/77]), loratadine (2.6% [2/77]), and naproxen (2.6 [2/77]).
3.3. Extent of exposure
Mean ± SD exposure days were 7.0 ± 0.3 for placebo and 6.9 ± 0.3 for 25 mg SHP465 MAS in the randomized safety population. Mean ± SD compliance (calculated as the number of capsules taken divided by the number of capsules that should have been taken over the entire treatment period) was 98.9 ± 11.9% for placebo and 101.9 ± 8.73% for 25 mg SHP465 MAS.
3.4. Efficacy
3.4.1. PERMP total score (primary efficacy endpoint)
Predose mean ± SD PERMP total scores were 226.9 ± 61.7 for placebo and 217.5 ± 59.6 for 25 mg SHP465 MAS. PERMP total scores for the average of classes 1 to 6 (postdose 2–16 h) were 248.8 ± 62.2 for placebo and 267.9 ± 65.5 for 25 mg SHP465 MAS. The least squares (LS) mean (95% confidence interval [CI]) treatment difference (SHP465 MAS–placebo) for PERMP total score significantly favored 25 mg SHP465 MAS over placebo for the average of all postdose time points (P < 0.0001) in the ITT population (), with LS mean (95% CI) treatment differences favoring 25 mg SHP465 MAS over placebo at 4 h postdose through 16 h postdose (). Similar findings were observed for PERMP total score in the PP population for the average of all postdose time points and for each postdose time point ().
Table 2. LS mean (95% CI) and P values for treatment differences in PERMPa, Co-ADHD-RS TSRSb, and ADHD-SRSb.
3.4.2. Secondary efficacy endpoints
3.4.2.1. PERMP problems attempted and PERMP problems answered correctly
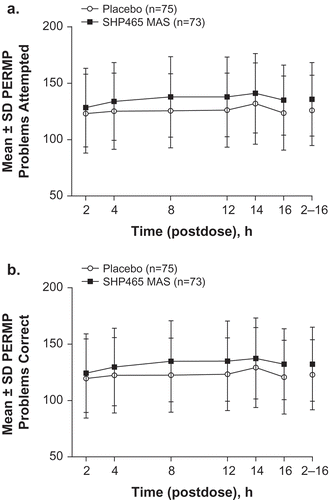
Mean ± SD predose values for PERMP problems attempted and problems answered correctly were 115.1 ± 31.0 and 111.8 ± 30.8 with placebo and 111.0 ± 29.5 and 106.4 ± 31.2 with 25 mg SHP465 MAS in the ITT population, respectively. depict the time course of mean ± SD PERMP problems attempted and answered correctly. The LS mean (95% CI) treatment difference for the average of all postdose time points for PERMP problems attempted (P < 0.0001) and problems answered correctly (P < 0.0001) nominally favored 25 mg SHP465 MAS over placebo (). The LS mean (95% CI) treatment differences nominally favored 25 mg SHP465 MAS over placebo for problems attempted and problems answered correctly at 4 h postdose through 16 h postdose (). Comparable findings were observed in the PP population for the average of all postdose time points and for each individual postdose time point (), with the exception of the number of problems answered correctly not being nominally superior for 25 mg SHP465 MAS until 8 h postdose.
3.4.2.2. Co-ADHD-RS TSRS
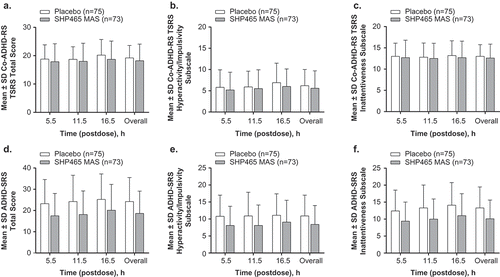
Mean ± SD Co-ADHD-RS TSRS total scores and subscale scores averaged across cycles A through C (i.e. at 5.5, 11, and 16.5 h) and for each individual cycle in the ITT population are summarized in . LS mean (95% CI) treatment differences for the average of all postdose cycles for the counselor-administered Co-ADHD-RS TSRS total score (P = 0.0783), hyperactivity/impulsivity subscale score (P = 0.0984), and inattentiveness subscale score (P = 0.2397) were not statistically different (). The LS mean (95% CI) treatment difference for Co-ADHD-RS TSRS total score at 16.5 h postdose nominally favored 25 mg SHP465 MAS (P = 0.0301); treatment differences for the hyperactivity/impulsivity or inattentive subscale scores did not differ at any individual postdose cycle (). Similar findings were generally observed in Co-ADHD-RS TSRS total and subscale scores in the PP population for the average of all cycles and for each postdose cycle (). In the PP protocol population, the LS mean (95% CI) treatment difference at 16.5 h postdose nominally favored 25 mg SHP465 MAS for the total (P = 0.0184) and hyperactivity/impulsivity subscale (P = 0.0200) scores.
Figure 3. Co-ADHD-RS TSRS (a–c) and ADHD-SRS (d–f) total and subscale scores.
ADHD = attention-deficit/hyperactivity disorder; Co-ADHD-RS TSRS = ADHD clinician ratings based on counselor observations and input during the Time Segment Rating System; ADHD-SRS = ADHD self-rating scale; MAS = mixed amphetamine salts; SD = standard deviation.
3.4.2.3. ADHD-SRS
Mean ± SD ADHD-SRS total and subscale scores averaged across cycles A through C and for each individual cycle in the ITT population are summarized in . The LS mean (95% CI) treatment differences nominally favored 25 mg SHP465 MAS over placebo for ADHD-SRS total, hyperactivity/impulsivity, and inattentiveness subscale scores when averaged across cycles (all P < 0.0001) and at each postdose cycle (all P < 0.01). Similar findings were observed for ADHD-SRS total and subscale scores in the PP population ().
3.5. Safety and tolerability
3.5.1. Adverse events
The frequency of treatment-emergent AEs (TEAEs) with 25 mg SHP465 MAS treatment was higher than with placebo. More than half of the TEAEs with 25 mg SHP465 MAS were deemed to be related to study drug as judged by the investigator; a small proportion of TEAEs were considered to be severe (). Insomnia was the only severe TEAE reported by ≥2 participants (25 mg SHP465 MAS, n = 3; placebo, n = 0). One case of severe treatment-emergent insomnia resulted in study discontinuation. The other instance of study discontinuation was due to treatment-emergent, moderate nasopharyngitis (n = 1) in a participant receiving 25 mg SHP465 MAS. Both severe TEAEs resolved following discontinuation. There were no serious TEAEs or deaths. The most frequently reported TEAEs reported by ≥5% of participants during 25 mg SHP465 MAS treatment were insomnia, decreased appetite, dry mouth, headache, and anorexia.
Table 3. Summary of TEAEs, randomized safety population.
3.5.2. Vital signs
Mean SBP, DBP, and pulse increased with 25 mg SHP465 MAS (). Most increases from predose to postdose were small; however, outlier analyses indicated that SBP increases from baseline of ≥15 mmHg were reported by 15% (12/78) and 24% (19/78) of participants with placebo and 25 mg SHP465 MAS, respectively; SBP values ≥140 mmHg were reported in 12% (9/78) and 10% (8/78) of participants with placebo and 25 mg SHP465 MAS, respectively. Outlier analyses indicated that DBP increases ≥10 mmHg from baseline were reported in 24% (19/78) and 28% (22/78) of participants with placebo and 25 mg SHP465 MAS, respectively; DBP values ≥90 mmHg were reported in 1% (1/78) and 3% (2/78) of participants with placebo and 25 mg SHP465 MAS, respectively. Outlier analyses indicated that pulse values ≥110 bpm were reported in 3% (2/78) and 0% of participants with placebo and 25 mg SHP465 MAS, respectively.
Table 4. Summary of vital signs at baseline and during each treatment arm, randomized safety population.
3.5.3. Other safety and tolerability endpoints
Mean ± SD baseline Fridericia-corrected QT interval (QTcF) and ECG-determined heart rate were 389.9 ± 18.3 msec and 65.2 ± 10.0 bpm, respectively, in the overall randomized safety population. Mean ± SD changes from baseline for QTcF and heart rate, respectively, at the end of the treatment were 4.7 ± 13.0 msec and 5.4 ± 10.3 bpm with placebo and 4.6 ± 11.3 msec and 6.6 ± 9.9 bpm with 25 mg SHP465 MAS. During the study, no participant had a change from baseline QTcF ≥60 msec.
A mean ± SD weight increase was observed at the end of the placebo treatment periods (1.2 ± 2.9 lb) and a decrease was observed at the end of 25 mg SHP465 MAS treatment periods (–0.8 ± 2.9 lb). No participants exhibited weight loss ≥7% from baseline during either treatment.
Mean ± SD PSQI total score at the orientation visit in the overall randomized safety population was 6.0 ± 3.07 (n = 78), which was above the proposed cutoff of 5 for poor sleep quality [Citation35]. Mean ± SD changes were –0.5 ± 2.41 at the end of placebo treatment (n = 76; 2 missing values) and 0.6 ± 3.74 at the end of 25 mg SHP465 MAS treatment (n = 74; 4 missing values).
4. Discussion
The primary finding of this study is that 25 mg SHP465 MAS produced significantly greater PERMP total score improvement than placebo in adults with ADHD in the AWE. As measured in this study, 25 mg SHP465 MAS produced significantly greater improvements in PERMP total score than placebo from 4 h postdose through 16 h postdose, which was the last time point measured. Although the magnitude of the overall treatment effect in the study was small, possibly due to a practice effect in both treatment groups that led to a small increase in PERMP scores across the day independent of treatment status, the mean PERMP total score changes from predose values averaged across all postdose time points in this study (approximate increases of 50 points with 25 mg SHP465 MAS and 22 points with placebo) were roughly comparable to the changes observed with LDX [Citation27] and multilayer-release methylphenidate hydrochloride (MLR-MPH) [Citation31] in the AWE model. The effects of 25 mg SHP465 MAS on secondary efficacy endpoints were generally consistent with the findings for PERMP total score, with the exception of the counselor-administered rating scale (Co-ADHD-RS TSRS). The alignment of these results with those of other ADHD medications assessed in the AWE model further support the use of this model and use of the PERMP to measure drug effects. However, methodological differences (including differences in baseline ADHD-RS-IV total scores and the use of open-label dose-optimization phases) among studies limit the ability to make direct comparisons between LDX, MLR-MPH, and SHP465 MAS, so any comparisons should be interpreted with caution.
The perceived improvement on the ADHD-SRS is important in light of previous studies that have reported that individuals with ADHD inconsistently report their symptoms and tend to overestimate their competence [Citation37–Citation39]. This finding extends the results of the study from improvement on an objectively determined measure (PERMP total score) to improvement on a subjective self-report. In contrast to the findings of the objective PERMP and subjective ADHD-SRS, 25 mg SHP465 MAS did not significantly improve scores on the piloted subjective, clinician-rated Co-ADHD-RS TSRS. The Co-ADHD-RS TSRS scale has not previously been used within the AWE model. Although the lack of an observed treatment effect is disappointing, it is not surprising. With this scale, ratings were compiled by nonclinical staff who would not be expected to discern the occurrence of ADHD symptoms as well as experts, despite being trained to use the scale before the study. This finding underscores the need to use experienced and expert clinicians to rate ADHD symptoms outside the classroom and within the AWE model. Overall, these findings point to the importance of utilizing objectively rated measures and self-reports in conjunction with clinician-rated tools when assessing the efficacy of an ADHD medication to ensure that dose optimization based on physician assessment can maximize the benefit of treatment. However, all data from the secondary ADHD-SRS and Co-ADHD-RS-TSRS endpoints should be interpreted cautiously because the study was only powered to assess treatment differences in PERMP total score.
The short-term safety and tolerability profile of 25 mg SHP465 MAS was generally consistent with previously published findings for SHP465 MAS in adults with ADHD [Citation21–Citation23] and for other long-acting stimulants assessed in adults within the AWE model [Citation27,Citation31]. The most frequently reported TEAEs (reported by ≥5% of participants) in this study included insomnia, decreased appetite, dry mouth, headache, and anorexia. Mean changes from baseline across the 16-h postdose assessment period (SHP465 MAS versus placebo) were greater with 25 mg SHP465 MAS than with placebo for SBP (2.0 to 4.3 mmHg versus –0.5 to 2.6 mmHg), DBP (1.1 to 3.1 mmHg versus –1.1 to 1.1 mmHg), and pulse (3.9 to 8.0 bpm versus 3.4 to 5.5 bpm).
Insomnia was the most frequently reported TEAE and the most frequently reported severe TEAE; insomnia also accounted for 1 of the 2 TEAEs leading to study discontinuation. The frequency of insomnia-related TEAEs (initial insomnia, 1.3%; insomnia, 25.0%; middle insomnia, 1.3%; early morning awakenings, 1.3%) with 25 mg SHP465 MAS in the current study was similar to the frequency of insomnia-related TEAEs (combined preferred terms of insomnia, initial insomnia, middle insomnia, early morning awakenings, and terminal insomnia) reported in a phase III, dose-optimization study of SHP465 MAS (29.2% across all SHP465 MAS doses) [Citation21] but was lower than the frequency reported in a phase III, forced-dose study of SHP465 MAS (40.4% with 25 mg SHP465 MAS) [Citation22]. When comparing insomnia-related TEAEs occurring with SHP465 MAS in the current study with other long-acting psychostimulants in adults within the context of the AWE model [Citation27,Citation31], it is important to consider the use of a dose-optimization phase in the study designs. In the current study, which did not include a dose-optimization phase, the frequency of insomnia-related TEAEs with SHP465 MAS was higher than the frequencies reported for LDX (2.6%) and MLR-MPH (insomnia [6%] and initial insomnia [4%]) during the double-blind treatment phases that followed initial open-label dose-optimization phases of those studies [Citation27,Citation31]. The frequencies of insomnia-related TEAEs for LDX (insomnia: 18.3%) and MLR-MPH (insomnia [30.5%], initial insomnia [28.8%], middle insomnia [16.9%]) were considerably higher during the initial open-label dose-optimization phases of those studies [Citation27,Citation31]. This suggests the lack of a titration phase in this study may have contributed to the higher frequency of insomnia-related TEAEs in the current study. However, a detailed analysis of sleep architecture is required to better understand the effects of SHP465 MAS on insomnia. Overall, these findings support the importance of dose titration and dose optimization for providing the best balance between efficacy and tolerability for each individual patient.
Some aspects of this study may limit the ability to generalize these findings. First, only a single dose was evaluated in this study so it is not known how the observed effects would translate across doses. Second, the length of treatment period was only 1 week so it is not possible to interpret the observed effects in terms of long-term effectiveness or in terms of the long-term safety and tolerability profile of 25 mg SHP465 MAS. It should also be noted that unlike in the AWE studies of LDX and MLR-MPH [Citation27,Citation31], this study did not include a dose-optimization phase and it is not known how the incorporation of dose optimization into the current study design would have influenced the efficacy and safety profile of SHP465 MAS in this model. The lack of a dose-optimization phase may increase treatment response variability between participants. Because an optimal dose was not determined, some participants on a given dose may have been under medicated, which resulted in decreased efficacy, whereas others may have been over medicated, which resulted in poor tolerability. This poor tolerability could account for the overall frequency of insomnia-related TEAEs in this study. Additionally, the inclusion of a dose-optimization phase would further increase the generalizability of these findings because a dose-optimization treatment strategy more closely mimics clinical practice conditions. Furthermore, despite the finding of a statistically greater improvement in PERMP total score with 25 mg SHP465 MAS than with placebo, it should be noted that the presence of a practice effect during the early portions of the AWE may have reduced the overall magnitude of the treatment effect. Last, the study was not powered for assessment of secondary endpoints so interpretation of these findings is limited by the fact that all reported P values for secondary endpoints are nominal (unadjusted and descriptive). Based on experience with this model since the time when this study was conducted, future studies in the AWE model should include longer dose-optimization periods, incorporate additional exposure to the PERMP before the initiation of treatment to minimize practice effects, involve only expert raters who have extensive experience in ADHD, include more self-rating scales, and assess treatment effects at shorter time intervals to mitigate some of these limitations.
5. Conclusions
SHP465 MAS (25 mg) produced statistically significantly greater increases in PERMP total score in a simulated AWE compared with placebo, with efficacy observed 4 h postdose through the last postdose assessment time of 16 h. In addition, treatment differences nominally favored 25 mg SHP465 MAS over placebo on the self-reported ADHD-SRS but not on the counselor-administered and clinician-rated Co-ADHD-RS TSRS, potentially due to a lack of discriminating output on the Co-ADHD-RS TSRS. Overall, these findings point to the importance of utilizing both objectively rated measures, such as the PERMP, and subjective rating measures (e.g. self-reported scales and clinician-rated scales) when assessing the efficacy of an ADHD medication. These findings also point to the importance of appropriate titration and dose optimization for achieving clinically relevant effects of medication on ADHD symptomatology. While the frequencies of some adverse events were higher than those noted in the dose-optimized AWE studies [Citation27,Citation31], the short-term safety and tolerability profile of 25 mg SHP465 MAS was generally similar to previous reports for SHP465 MAS and other long-acting stimulants, with 25 mg SHP465 MAS being associated with higher rates of insomnia-related TEAEs in this study than other long-acting stimulants [Citation24–Citation26,Citation40]. Overall, the choice and dosage strength of stimulants in adults with ADHD should be determined by physicians based on the risk benefit for each individual patient.
Declaration of interest
T Wigal is a consultant to, member of scientific advisory boards, or received speaker fees and/or has received research support from Johnson & Johnson, Eli Lilly, Ironshore, Neurovance, NLS, Noven, McNeil, Purdue, Rhodes, Shire, and Sunovion. A Childress has served on advisory boards for Arbor, Ironshore, Neos, Neurovance, Noven, Pfizer, Rhodes, and Tris; has been a consultant for Arbor, Ironshore, Neos, Neurovance, Rhodes, Shire, and Sunovion; has been a speaker at Arbor, Pfizer, and Shire; has received research support from Alcobra, Arbor, Eli Lilly, Forest, Ironshore, Lundbeck, Medgenics, Neos, Neurovance, Noven, Otsuka, Pearson, Pfizer, Purdue, Rhodes, Shire, Sunovion, Tris, and Akili; and has received writing support from Arbor, Ironshore, Neos, Rhodes, Shire, and Pfizer. S Wigal is a consultant to, member of scientific advisory boards, or received speaker fees and/or has received research support from Akili, Arbor, Attentiv, Eli Lilly, Ironshore, Neos, Neurovance, NLS, Noven, Pfizer, Purdue, Rho, Rhodes, Shire, Sunovion, and Tris. G Frick is a former employee of Shire and holds stock and/or stock options in Shire. M Madhoo and B Yan are employees of Shire and hold stock and/or stock options in Shire. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
Under the direction of the authors, writing assistance was provided by Madhura Mehta, PhD, and Craig Slawecki, PhD (employees of Complete Healthcare Communications, LLC [CHC]; West Chester, PA, an ICON plc Company). Editorial assistance in the form of proofreading, copyediting, and fact checking was also provided by CHC. Shailesh Desai, PhD, from Shire, reviewed and edited the manuscript for scientific accuracy. The authors would like to acknowledge Colleen Anderson, MEd, for her contributions to the clinical development program for SHP465 MAS and for her insightful comments on this manuscript.
Additional information
Funding
References
- Barkley RA, Fischer M, Smallish L, et al. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279–289.
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165.
- Lara C, Fayyad J, De Graaf R, et al. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65:46–54.
- Barkley RA, Cunningham CE, Gordon M, et al. ADHD symptoms vs. impairment: revisited. ADHD Rep. 2006;14:1–9.
- De Graaf R, Kessler RC, Fayyad J, et al. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: results from the WHO World Mental Health Survey Initiative. Occup Environ Med. 2008;65:835–842.
- Biederman J, Faraone SV, Spencer TJ, et al. Functional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry. 2006;67:524–540.
- Fredriksen M, Dahl AA, Martinsen EW, et al. Childhood and persistent ADHD symptoms associated with educational failure and long-term occupational disability in adult ADHD. Atten Defic Hyperact Disord. 2014;6:87–99.
- Kessler R, Adler L, Ames M, et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med. 2005;47:565–572.
- Castle L, Aubert RE, Verbrugge RR, et al. Trends in medication treatment for ADHD. J Atten Disord. 2007;10:335–342.
- McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173:960–966.
- Glenngard AH, Hjelmgren J, Thomsen PH, et al. Patient preferences and willingness-to-pay for ADHD treatment with stimulants using discrete choice experiment (DCE) in Sweden, Denmark and Norway. Nord J Psychiatry. 2013;67:351–359.
- Sabate E. Adherence to long-term therapies: policy for action. Geneva, Switzerland: World Health Organization; 2001.
- Brown TE. Executive functions and attention deficit hyperactivity disorder: implications of two conflicting views. Int J Disability, Development, Educ. 2006;53:35–46.
- McCarthy S. Pharmacological interventions for ADHD: how do adolescent and adult patient beliefs and attitudes impact treatment adherence? Patient Prefer Adherence. 2014;8:1317–1327.
- Brams M, Moon E, Pucci M, et al. Duration of effect of oral long-acting stimulant medications for ADHD throughout the day. Curr Med Res Opin. 2010;26:1809–1825.
- Thomas M, Rostain A, Prevatt F. ADHD diagnosis and treatment in college students and young adults. Adolesc Med State Art Rev. 2013;24:659–679.
- Jain R, Katic A. Current and investigational medication delivery systems for treating attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2016;18(4). DOI:10.4088/PCC.16r01979
- Hodgkins P, Sasane R, Christensen L, et al. Treatment outcomes with methylphenidate formulations among patients with ADHD: retrospective claims analysis of a managed care population. Curr Med Res Opin. 2011;27(suppl 2):53–62.
- Setyawan J, Hodgkins P, Guerin A, et al. Comparison of therapy augmentation and deviation rates from the recommended once-daily dosing regimen between LDX and commonly prescribed long-acting stimulants for the treatment of ADHD in youth and adults. J Med Econ. 2013;16:1203–1215.
- Ermer JC, Shojaei A, Pennick M, et al. Bioavailability of triple-bead mixed amphetamine salts compared with a dose-augmentation strategy of mixed amphetamine salts extended release plus mixed amphetamine salts immediate release. Curr Med Res Opin. 2007;23:1067–1075.
- Spencer TJ, Adler LA, Weisler RH, et al. Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry. 2008;69:1437–1448.
- Frick G, Yan B, Adler LA. Triple-bead mixed amphetamine salts (SHP465) in adults with attention-deficit/hyperactivity disorder: results of a phase 3, double-blind, randomized, forced-dose trial. J Atten Disord. 2017. e-publication ahead of print. DOI:10.1177/1087054717696771
- Weisler RH, Greenbaum M, Arnold V, et al. Efficacy and safety of SHP465 mixed amphetamine salts in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled, forced-dose clinical study. CNS Drugs. 2017;31(8):685–697.
- Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1364–1373.
- Adler LA, Zimmerman B, Starr HL, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29:239–247.
- Weisler RH, Biederman J, Spencer TJ, et al. Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr. 2006;11:625–639.
- Wigal T, Brams M, Gasior M, et al. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct. 2010;6:34.
- Wigal SB, Wigal TL. The laboratory school protocol: its origin, use, and new applications. J Atten Disord. 2006;10:92–111.
- McCracken JT, Biederman J, Greenhill LL, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:673–683.
- Swanson JM, Wigal S, Greenhill LL, et al. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37:519–526.
- Wigal SB, Wigal T, Childress A, et al. The time course of effect of multilayer-release methylphenidate hydrochloride capsules: a randomized, double-blind study of adults with ADHD in a simulated adult workplace environment. J Atten Disord. 2016. e-publication ahead of print. DOI:10.1177/1087054716672335.
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–374.
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. Arlington, VA: American Psychiatric Publishing; 1997.
- Naugle RI, Chelune GJ, Tucker GD. Validity of the Kaufman Brief Intelligence Test. Psychol Assessment. 1993;5:182.
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740.
- Manor I, Vurembrandt N, Rozen S, et al. Low self-awareness of ADHD in adults using a self-report screening questionnaire. Eur Psychiatry. 2012;27:314–320.
- Knouse LE, Bagwell CL, Barkley RA, et al. Accuracy of self-evaluation in adults with ADHD: evidence from a driving study. J Atten Disord. 2005;8:221–234.
- Hoza B, Gerdes AC, Hinshaw SP, et al. Self-perceptions of competence in children with ADHD and comparison children. J Consult Clin Psychol. 2004;72:382–391.
- Spencer TJ, Adler LA, McGough JJ, et al. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1380–1387.