ABSTRACT
Objectives: To evaluate the duration of efficacy, safety, and tolerability of SHP465 mixed amphetamine salts (MAS) extended-release versus placebo and immediate-release MAS (MAS IR) in adolescents with attention-deficit/hyperactivity disorder (ADHD).
Methods: This phase 2, randomized, 3-period, 3-treatment crossover study compared SHP465 MAS (25/50 mg) with placebo and MAS IR (12.5 mg) in 13–17-year-old adolescents with ADHD having ADHD Rating Scale, Version IV (ADHD-RS-IV) total scores ≥24. A laboratory classroom served as a controlled environment during 16-hour observations, with efficacy assessed on the last day of each 7-day treatment period. The primary efficacy analysis compared SHP465 MAS with placebo on Permanent Product Measure of Performance (PERMP) total score averaged over the 16-hour postdose period using a mixed linear model. Comparisons were also conducted between MAS IR and placebo (for assay sensitivity) and between SHP465 MAS and MAS IR. PERMP problems attempted and answered correctly and ADHD symptoms based on ADHD-RS-IV; participant self-report; Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale; and Revised Conner’s Parent Rating Scale scores were also evaluated. Safety and tolerability assessments included treatment-emergent adverse events and vital signs.
Results: The intent-to-treat population included 84 participants. Least squares mean (95% CI) PERMP total score treatment differences significantly favored SHP465 MAS (combined 25/50 mg) over placebo for the average of all postdose assessment time points (41.26 [32.24, 50.29]; P < 0.0001) and each postdose assessment time point (all P < 0.0001). Similar results were observed for MAS IR versus placebo (all postdose assessment time points averaged: nominal P < 0.0001; each postdose assessment time point: all nominal P < 0.004). The safety and tolerability of SHP465 MAS were consistent with previous reports.
Conclusions: SHP465 MAS significantly improved PERMP total scores versus placebo from 2 to 16 hours postdose in adolescents with ADHD. The safety and tolerability profile of SHP465 MAS was consistent with previous reports of SHP465 MAS in individuals with ADHD.
1. Introduction
Multiple extended-release psychostimulant formulations are approved for use in adolescents with attention-deficit/hyperactivity disorder (ADHD), including osmotic controlled-release oral delivery system methylphenidate, extended-release mixed amphetamine salts (MAS XR), and lisdexamfetamine dimesylate [Citation1–Citation3]. Studies that have examined the time course of treatment effects of various extended-release formulations with laboratory classroom models have reported efficacy versus placebo ranging from 12 to 13 hours postdose based on Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Scale and Permanent Product Measure of Performance (PERMP) scores [Citation4–Citation7].
SHP465 mixed amphetamine salts (SHP465 MAS) extended-release is a once-daily, single-entity MAS product for oral administration approved in the United States for the treatment of ADHD in patients 13 years and older [Citation8]. It contains equal amounts (by weight) of four salts: dextroamphetamine sulfate, amphetamine sulfate, dextroamphetamine saccharate, and amphetamine aspartate monohydrate. This results in a 3:1 mixture of dextro- to levoamphetamine base equivalent. Each capsule contains three types of drug-releasing beads, an immediate-release bead and two different types of delayed-release beads. The efficacy and tolerability of SHP465 MAS in individuals with ADHD has been demonstrated in four phase three efficacy studies: three conducted in adults [Citation9–Citation11] and one in children and adolescents [Citation12]. Across all short-term efficacy studies, dose-optimized and fixed-dose SHP465 MAS met the primary efficacy endpoint of significant reductions in ADHD Rating Scale (ADHD-RS) total score from baseline versus placebo [Citation9–Citation12]. SHP465 MAS has also been shown to have a safety and tolerability profile consistent with other long-acting stimulants [Citation13–Citation15].
Two different SHP465 MAS studies [Citation16,Citation17], which used crossover designs similar to this study, were conducted in adults with ADHD [Citation16,Citation17]. In both studies, which utilized the Adult Workplace Environment (AWE) model [Citation18], SHP465 MAS produced significantly greater improvement than placebo in PERMP total score averaged across all postdose time assessments from 2 to 16 hours. Significantly greater PERMP total scores versus placebo were observed for 25 mg SHP465 MAS from 4 to 16 hours postdose [Citation16] and for 50/75 mg SHP465 MAS from 2 to 16 hours postdose [Citation16,Citation17]. The most frequently reported treatment-emergent adverse events (TEAEs) with SHP465 MAS in these studies were insomnia, anorexia, decreased appetite, dry mouth, and headache [Citation16,Citation17].
Because SHP465 MAS is approved for use in adolescents, it is important to understand the effects of SHP465 MAS in this population. The primary objective of this phase 2, placebo-controlled, 3-way crossover study was to examine the onset and duration of efficacy of SHP465 MAS (25 and 50 mg) versus placebo and immediate-release MAS (MAS IR; 12.5 mg) in adolescents with ADHD using the PERMP in a laboratory classroom setting. This setting provides a structured, controlled environment that monitors and quantifies stimulant effects on the performance of school-aged children and adolescents, while using the PERMP as a surrogate measure of efficacy [Citation18,Citation19]. The PERMP is a time-sensitive, skill-adjusted, 10-minute math test that requires participants to be alert and selectively attentive and to sustain their attention to complete multiple tests that are scheduled throughout the day. Because the PERMP is skill adjusted, it is not a test of math ability or learning; higher PERMP scores indicate better performance [Citation18]. The PERMP has been used in the laboratory classroom setting to evaluate the duration of efficacy of multiple psychostimulants in children and adolescents diagnosed with ADHD [Citation4–Citation6,Citation20–Citation22].
2. Materials and methods
2.1. Study design
This phase 2, randomized, multicenter, double-blind, 3-period, 3-way crossover study was conducted at five sites in the United States between 27 July 2004, and 12 February 2005. The protocol was approved by a central institutional review board (IRB; three sites) or by local IRBs (two sites). A clinical trial registry number for this study is not available because it enrolled participants before July 2005. At that time, listing this study in a registry was not required.
The study was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki [Citation23], including amendments of the 29th, 35th, 41st, and 48th World Medical Assemblies. After receiving a complete study description, participants and their legally authorized representatives were required, respectively, to provide written informed assent and consent. Informed consent documents were developed in accordance with Good Clinical Practice and guidelines of the Health Insurance Portability and Accountability Act of 1996.
2.2. Participants
Eligible males and nonpregnant, nonlactating females (13–17 years old at consent) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for a primary ADHD diagnosis, established by a psychiatric interview and the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version [Citation24], and had baseline ADHD-RS, Version IV (ADHD-RS-IV) total scores ≥24 (after a washout period for those using stimulant therapy at screening to treat ADHD symptoms). Enrolled participants could be treatment naive, on prior treatment, or currently on treatment that was deemed dissatisfying to the participant for reasons including insufficient duration, but who were not considered treatment resistant. Participants were also required to have a Kaufman Brief Intelligence Test [Citation25] score ≥80 and no clinically significant abnormalities based on medical history or baseline examinations.
Key exclusion criteria included being underweight or overweight and having a comorbid severe Axis I or II psychiatric disorder controlled with prohibited medications or uncontrolled and associated with significant symptoms. Prohibited medications included anticonvulsants, antipsychotics, antihypertensives (not including diuretics), anxiolytics, benzodiazepines or benzodiazepine derivatives, clonidine and guanfacine, herbal preparations, investigational medications, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, tricyclic antidepressants, stimulants and sympathomimetics (or any stimulant-containing medication), sedatives, sedative-hypnotics, and sedating antihistamines. Bronchodilator use was allowed if needed. Additional exclusion criteria included having a condition that could confound safety assessments or lead to increased risk; having blood pressure measurements outside the 90th percentile; having a clinically significant electrocardiogram (ECG) or laboratory abnormality; having DSM-IV-TR–defined drug dependence or substance abuse within 12 months, including nicotine dependence; having a positive urine drug test at screening (excluding current stimulant medication); having used a psychoactive medication within 30 days of screening (methylphenidate or amphetamine use at screening required a 7-day washout period; atomoxetine use within 30 days of screening was exclusionary) or an over-the-counter psychoactive medication requiring a >7-day washout; having participated in a clinical trial within 30 days of screening; and having a documented allergy to, intolerance of, or history of nonresponsivity to methylphenidate, amphetamine, or MAS.
2.3. Treatment and assessment schedule
The study consisted of screening (visit 1), baseline (visit 2), and practice (visit 3) visits; a double-blind, 3-way crossover phase (visits 4–6; each separated by 7 days); and a follow-up period (). provides a listing of study assessments by visit. Screening took place up to 6 weeks before the practice visit to facilitate recruitment. Eligible participants using psychostimulant medications at screening underwent a 7-day washout before the baseline visit. The baseline visit was completed up to 5 days before the practice visit and 7 to 12 days before the first double-blind treatment visit.
Table 1. Study schedule.
Figure 1. Study design (a) and participant disposition (b).
IR = immediate-release; ITT = intent-to-treat; MAS = mixed amphetamine salts; PBO = placebo; V = visit. *Enrolled participants were those for whom all screening assessments were completed and reviewed.
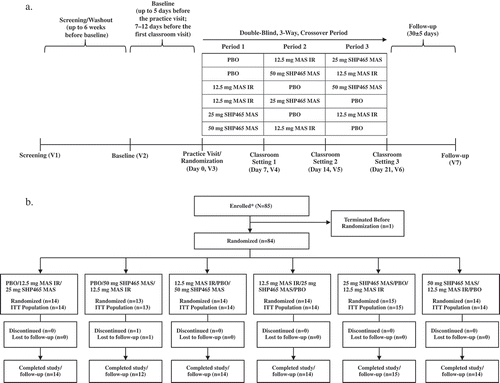
During the practice visit, participants reported to the laboratory school site as a group and were oriented to the classroom procedures. During the visit, a math pretest was completed to determine the PERMP level of difficulty to assign to each participant. As outlined in , assessments during the practice visit included 2 to 4 PERMP tests at the assigned level of difficulty, a self-report version of the ADHD-RS-IV (ADHD-SRS), and the Revised Conner’s Parent Rating Scale (CPRS-R) [Citation26]. Participants were then randomized to 1 of 6 treatment sequences, provided with study drug, and given instructions for the first treatment week (days 1–6). Treatment regimens were determined by a block randomization schedule that used two 3 × 3 Latin squares; the randomization schedule was generated by the sponsor using SAS, version 8.2 (SAS Institute; Cary, NC) with a block size of six. Computer-generated two-part labels were used to blind the investigational product.
The double-blind treatment phase consisted of three 1-week intervals (). During the first treatment week, participants received placebo (two capsules daily), 12.5 mg MAS IR (1 × 12.5 mg MAS IR capsule + 1 placebo capsule daily), or SHP465 MAS (25 mg [1 × 25 mg SHP465 MAS capsule + 1 placebo capsule daily] OR 50 mg [1 × 25 mg SHP465 MAS capsule + 1 placebo capsule for days 1–3 followed by 2 × 25 mg SHP465 MAS capsules for days 4–7]). During subsequent weeks, alternate treatments were administered. Placebo capsules were identical in appearance to SHP465 MAS capsules. The SHP465 MAS doses selected for evaluation (25 and 50 mg) were comparable to the total daily MAS dose used in adolescents with ADHD in clinical practice. The MAS IR dose used represents one-fourth to one-half of the SHP465 MAS doses under investigation. As the MAS IR treatment arm was included for assay sensitivity, the dose used in this study was not intended to be equivalent to the SHP465 MAS doses used. Rather, the MAS IR dose chosen was one that would be used clinically [Citation27] and would be consistent with product labeling. Based on the published literature, the duration of efficacy of MAS IR was expected to be approximately 5 to 10.5 hours postdose [Citation5,Citation21].
Each double-blind phase visit (visits 4, 5, and 6) was separated by 7 days and took place on treatment day 7. Visits consisted of a 17.5-hour schedule of planned activities (Supplemental Table 1). On each treatment day, participants returned to the laboratory classroom and were administered their treatment at approximately 7:00 am. Assessments at these visits () were identical and included the PERMP and SKAMP (both assessed at seven time points), the ADHD-SRS (assessed at three time points), and vital signs (assessed at four time points). At visits 4 and 5, study drug and instructions for days 1 to 6 of the following treatment week were provided.
During the follow-up period, a telephone call was conducted 30 ± 5 days following the last study drug dose to collect information on adverse events.
2.4. Study endpoints
2.4.1. Efficacy
The prespecified primary efficacy endpoint was PERMP total score (the sum of the number of problems attempted and the number of problems answered correctly) averaged across all postdose assessment time points. The PERMP consisted of five pages with 80 math problems per page [Citation18]. Different versions of the PERMP were generated with random listings of nonrepeating problems so participants did not take the same test more than once; there was little chance of a participant seeing the same problem sequence multiple times. For each participant, PERMP difficulty was adjusted based on the practice day math pretest performance. Study participants were tested on the PERMP 23 to 25 times during the study (2–4 times during the practice visit; 7 times each during the double-blind treatment visits).
Prespecified secondary efficacy endpoints included the number of problems attempted and the number of problems answered correctly on the PERMP and score changes on the ADHD-RS-IV, ADHD-SRS, SKAMP, and CPRS-R. The ADHD-RS-IV is an 18-item clinician-rated scale based on DSM-IV-TR criteria for ADHD [Citation28]; items are rated on a 4-point scale (0–3), with higher scores indicating increased severity (total score range: 0–54). The ADHD-SRS is a patient-reported version of the ADHD-RS-IV that allowed for the collection of individual participant observations in three 5.5-hour segments. The 13-item SKAMP has been validated in school-aged children in a laboratory school setting; it is used to identify and measure classroom manifestations of ADHD through a total score and four subscales (attention, deportment, quality of work, and compliance) [Citation29]. The SKAMP is completed by trained, independent behavioral observers (see Wigal and Wigal [Citation18] for a review of the laboratory study protocol); total score ranges from 0 to 78, with higher scores indicating worse impairment. The 27-item short version of the CPRS-R also assesses behavior using a total score and four subscales (oppositionality, cognitive problems, hyperactivity, and ADHD index) [Citation26,Citation30]. Although the CPRS-R typically assesses behavior during the previous month, the present study asked about behavior during the previous week.
2.4.2. Safety and tolerability
Adverse events were recorded from the time of informed consent. Vital signs (systolic blood pressure [SBP], diastolic blood pressure [DBP], and pulse) were assessed after the participant was seated for 5 minutes. Sleep quality was assessed using an 18-item self-report version of the Pittsburgh Sleep Quality Index (PSQI) that was modified for weekly assessment. Individual PSQI items can be used to generate a global score and seven component scores [Citation31,Citation32]. The global scores reported in this study range from 0 to 21, with higher scores reflecting worse sleep quality. Other safety and tolerability assessments were based on the 12-lead ECG, including changes in Fridericia-corrected QT (QTcF) interval [Citation33].
2.5. Data presentation and statistical analysis
Limited data regarding effect size for duration of efficacy were available in adolescents at the time this study was conducted. As a result, sample size for this study was estimated based on a conservative effect size of 0.35 for the treatment difference between SHP465 MAS (25 and 50 mg combined: 25/50 mg) and placebo on PERMP total score averaged across all postdose assessment time points. It was estimated that approximately 70 participants needed to complete the double-blind phase to obtain >83% power at the level of 0.05 (two-sided) using a paired t test. Assuming a 15% dropout rate and two 3 × 3 Latin square designs with six treatment sequences, it was determined that approximately 84 participants needed to be randomized.
Statistical analyses of the primary and secondary efficacy endpoints were conducted using the intent-to-treat population (randomized participants taking ≥1 study drug dose and having ≥1 postbaseline primary efficacy assessment), with significance set at a two-sided P < 0.05. Assessment of PERMP total score (primary efficacy endpoint) was conducted using a mixed linear model for the average of assessment time points 1 to 6 (postdose hours 2–16) and at each postdose assessment time point (2, 4, 8, 12, 14, and 16 hours postdose). The model included sequence, period, and treatment as fixed effects and participant within sequence as a random effect. Treatment was considered to separate from placebo if the average of all postdose assessment time points (2–16 hours postdose) for PERMP total score was statistically greater for SHP465 MAS than placebo. The primary hypotheses of onset of efficacy and duration of efficacy of SHP465 MAS versus placebo were defined as follows: onset of efficacy was considered the assessment time point when the difference between the two treatments first became statistically significant; duration of efficacy was considered the last assessment time point beyond which a statistically significant difference was no longer evident. In this study, the first assessment was at 2 hours postdose, and the last assessment was at 16 hours postdose. Therefore, duration of efficacy could be demonstrated up to 16 hours.
Additional secondary analyses included comparisons of 12.5 mg MAS IR and placebo (to evaluate assay sensitivity), comparisons between treatments (12.5 mg MAS IR and 25/50 mg SHP465 MAS), and comparisons between individual SHP465 MAS doses (25 and 50 mg) and placebo. These analyses were conducted using the same mixed linear model used for the primary efficacy analysis. The study was not powered for assessment of secondary analyses. Therefore, all reported P values for secondary analyses are nominal (unadjusted) and provided for descriptive purposes only.
Safety and tolerability endpoints for the randomized population (all enrolled participants who were randomized to treatment) are reported using descriptive statistics.
3. Results
3.1. Participant disposition and demographics
Participant disposition is summarized in ; demographic and clinical characteristics are summarized in . Most participants were white (71.4% [60/84]) and were male (75% [63/84]); mean ± SD age was 14.5 ± 1.3 years. Most participants were diagnosed as having the combined ADHD subtype (66.7% [56/84]); no participant was diagnosed with the hyperactive/impulsive ADHD subtype. The mean ± SD duration of ADHD diagnosis and age of ADHD onset, respectively, were 5.9 ± 3.4 and 5.2 ± 1.0 years.
Table 2. Baseline demographic and clinical characteristics (randomized population).
3.2. Prior/concomitant medication
In the randomized population, 52.4% of the participants (44/84) reported prior use of an ADHD medication. ADHD medications used by participants before entering the study were branded MAS XR (28.6% [24/84]), methylphenidate hydrochloride (17.9% [15/84]), branded MAS IR (3.6% [3/84]), dexmethylphenidate hydrochloride (3.6% [3/84]), and dexamfetamine sulfate 1.2% [1/84]. In the randomized population, 17.9% of the participants (15/84) reported concomitant medication use (used by ≥2% of the participants: ibuprofen, 9.5% [8/84]; paracetamol/acetaminophen, 4.8% [4/84]; tetracycline, 2.4% [2/84]).
3.3. Extent of exposure
The mean ± SD number of exposure days was 7.0 ± 0 for placebo and 12.5 mg MAS IR, and 7.0 ± 0.1 for 25/50 mg SHP465 MAS (25 mg SHP465 MAS, 7.0 ± 0; 50 mg SHP465 MAS, 7.0 ± 0.2). Mean ± SD adherence (number of capsules taken divided by the number of capsules that should have been taken) over the entire study was 98.8% ± 3.97% for placebo, 99.2% ± 3.16% for 12.5 mg MAS IR, and 98.6% ± 4.77% for 25/50 mg SHP465 MAS (25 mg SHP465 MAS, 97.7% ± 6.18%; 50 mg SHP465 MAS, 99.7% ± 2.23%).
3.4. PERMP total score (primary efficacy endpoint)
Mean ± SD PERMP total scores at each assessment time point are reported in Supplemental Table 2. The least squares (LS) mean (95% CI) treatment difference versus placebo (SHP465 MAS – placebo) for PERMP total score significantly favored 25/50 mg SHP465 MAS for the average of all postdose assessment time points (P < 0.0001), with significant differences observed at each assessment time point from 2 to 16 hours postdose (all P < 0.0001; ). Secondary analyses revealed that LS mean (95% CI) treatment differences versus placebo also favored 12.5 mg MAS IR for the average of all postdose assessment time points (nominal P < 0.0001) and each assessment time point from 2 to 16 hours postdose (nominal P < 0.01; ).
Table 3. Least squares mean (95% CI) treatment differences for primary and secondary efficacy endpoints (ITT population).
Secondary analyses of individual SHP465 MAS doses indicated that LS mean (95% CI) treatment differences versus placebo for PERMP total score favored 25 mg and 50 mg SHP465 MAS for the average of all postdose assessment time points (both nominal P < 0.0001; Supplemental Table 3) and each postdose assessment time point (all nominal P < 0.01). Furthermore, the LS mean (95% CI) treatment difference (SHP465 MAS – MAS IR) favored 25/50 mg SHP465 MAS over 12.5 mg MAS IR for the average of all postdose assessment time points (nominal P = 0.0403), with treatment differences observed at 12 and 16 hours postdose (both nominal P < 0.05). When examined at each postdose assessment time point, LS mean (95% CI) treatment differences did not favor 25 mg SHP465 MAS over 12.5 mg MAS IR at any assessment time point; the treatment difference at 16 hours postdose favored 50 mg SHP465 MAS over 12.5 mg MAS IR (nominal P = 0.0008; Supplemental Table 3).
3.5. PERMP problems attempted and answered correctly
depicts the time course of PERMP problems attempted and answered correctly. The mean ± SD numbers of problems attempted and answered correctly on the PERMP at each assessment time point are reported in Supplemental Table 2. LS mean (95% CI) treatment differences versus placebo for the average of all postdose assessment time points for problems attempted and answered correctly favored both 25/50 mg SHP465 MAS and 12.5 mg MAS IR, with nominally significant treatment differences observed at each postdose assessment time point (all nominal P < 0.01; ). Analysis of the individual SHP465 MAS doses indicated that LS mean (95% CI) treatment differences versus placebo favored 25 and 50 mg SHP465 MAS for problems attempted and problems answered correctly for the average of all postdose assessment time points and each individual postdose assessment time point (all nominal P < 0.01; Supplemental Table 3).
Figure 2. Mean ± SD PERMP number of problems attempted (a) and number of problems answered correctly (b) in the ITT population. IR = immediate-release; ITT = intent-to-treat; MAS = mixed amphetamine salts; PBO = placebo; PERMP = Permanent Product Measure of Performance.
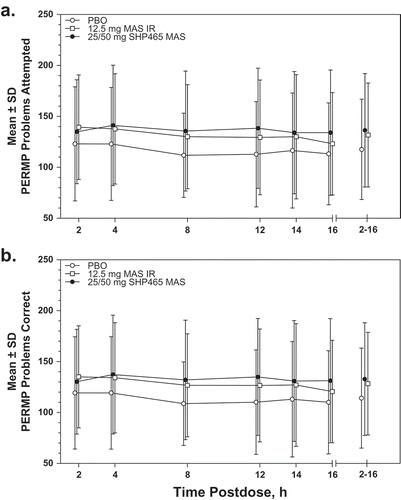
The LS mean (95% CI) treatment differences for problems attempted and problems answered correctly nominally favored 25/50 mg SHP465 MAS over 12.5 mg MAS IR for the average of all postdose assessment time points and at 12 and 16 hours postdose (all nominal P < 0.05; ). Treatment differences between 50 mg SHP465 MAS and 12.5 mg MAS IR for problems attempted and answered correctly were observed at 16 hours postdose (both nominal P ≤ 0.001), with treatment effects favoring SHP465 MAS over MAS IR. No statistically significant treatment differences were observed between 25 mg SHP465 MAS and 12.5 mg MAS IR at any postdose assessment time point (Supplemental Table 3).
3.6. ADHD-SRS total score
Mean ± SD ADHD-SRS total scores at the practice visit (visit 3) are summarized in , and mean ± SD ADHD-SRS total scores at each postdose assessment time point are reported in Supplemental Table 2. LS mean (95% CI) treatment differences versus placebo for ADHD-SRS total score nominally favored SHP465 MAS for the average of all postdose assessment time points and at 5.5 and 16.5 hours postdose (all nominal P < 0.05; ). Analysis of the individual SHP465 MAS doses revealed that 50 mg SHP465 MAS was superior to placebo for the average of all postdose assessment time points and for each postdose assessment time point (all nominal P < 0.05); 25 mg SHP465 MAS did not differ from placebo at any assessment time point (Supplemental Table 3). LS mean (95% CI) treatment differences for ADHD-SRS total score nominally favored MAS IR over placebo for the average of all postdose assessment time points and at 5.5 hours postdose (both P < 0.05; ). There were no treatment differences between 25/50 mg SHP465 MAS and 12.5 mg MAS IR or between individual SHP465 MAS doses and 12.5 mg MAS IR.
3.7. SKAMP total score
Mean ± SD SKAMP total scores at each assessment time point are reported in Supplemental Table 2. LS mean (95% CI) treatment differences versus placebo for SKAMP total score nominally favored 25/50 mg SHP465 MAS and 12.5 mg MAS IR for the average of all postdose assessment time points (both nominal P < 0.0001), with treatment differences observed at each postdose assessment time point for 25/50 mg SHP465 MAS and at each assessment time point except 16 hours postdose for 12.5 mg MAS IR (all nominal P < 0.05; ). LS mean (95% CI) treatment differences nominally favored 25/50 mg SHP465 MAS over 12.5 mg MAS IR for the average of all postdose assessment time points (nominal P = 0.0025) and each assessment time point from 8 to 16 hours postdose (all nominal P < 0.05; ).
3.8. CPRS-R total score
Mean ± SD CPRS-R total scores at the practice visit are summarized in . Mean ± SD CPRS-R total scores for each treatment are reported in Supplemental Table 2. The LS mean (95% CI) treatment difference versus placebo for CPRS-R total score nominally favored 25/50 mg SHP465 MAS (nominal P < 0.0001; ), with similar findings observed for 25 and 50 mg SHP465 MAS (both nominal P < 0.0001; Supplemental Table 3) and 12.5 mg MAS IR (nominal P = 0.0069; ). The LS mean (95% CI) treatment difference for CPRS-R total score also nominally favored 25/50 mg SHP465 MAS over 12.5 mg MAS IR (nominal P < 0.0001; ), with a similar finding observed for 50 mg SHP465 MAS (nominal P < 0.0001) but not 25 mg SHP465 MAS (Supplemental Table 3).
3.9. ADHD-RS-IV total score
Mean ± SD ADHD-RS-IV total scores at baseline are summarized in . Mean ± SD ADHD-RS-IV total scores during each treatment are reported in Supplemental Table 2. The LS mean (95% CI) treatment difference versus placebo for ADHD-RS-IV total score nominally favored 25/50 mg SHP465 MAS (nominal P < 0.0001; ), with similar findings observed for 25 and 50 mg SHP465 MAS (both nominal P < 0.0001; Supplemental Table 3) and 12.5 mg MAS IR (nominal P < 0.0001; ). The LS mean (95% CI) treatment difference for ADHD-RS-IV total score also nominally favored 25/50 mg SHP465 MAS over 12.5 mg MAS IR (nominal P < 0.0001; ), with similar findings observed for 25 and 50 mg SHP465 MAS (both nominal P < 0.05; Supplemental Table 3).
3.10. Safety and tolerability
Higher percentages of participants reported TEAEs with SHP465 MAS than with 12.5 mg MAS IR or placebo (), with a numerically higher frequency of TEAEs observed with 50 mg SHP465 MAS than 25 mg SHP465 MAS. Across all treatment arms, most TEAEs were of mild or moderate severity. Among TEAEs reported by ≥5% of the participants with any dose of SHP465 MAS (), insomnia was reported most frequently. Most individual TEAEs were reported more frequently with 50 mg SHP465 MAS than 25 mg SHP465 MAS (). Middle insomnia was reported as a TEAE in two participants treated with MAS IR; no participant reported initial insomnia, terminal insomnia, or early morning awakening as TEAEs. No serious TEAEs, TEAEs leading to discontinuation, or deaths occurred during the study.
Table 4. Summary of TEAEs (randomized population).
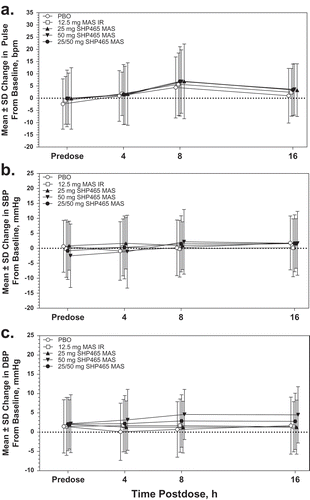
Mean ± SD baseline values for pulse, SBP, and DBP in all randomized participants (n = 84) were 77.4 ± 10.8 bpm, 112.2 ± 9.2 mmHg, and 65.6 ± 6.6 mmHg, respectively. Mean increases in pulse, SBP, and DBP were observed across treatment arms (). Pulse, SBP, and DBP increases with 25/50 mg SHP465 MAS and 12.5 mg MAS IR were numerically larger than with placebo, and increases with 25/50 mg SHP465 MAS were numerically larger than with 12.5 mg MAS IR. There were no notable differences in pulse between 25 mg and 50 mg SHP465 MAS, but SBP and DBP increases with 50 mg SHP465 MAS were numerically greater than with 25 mg SHP465 MAS.
Figure 3. Mean ± SD pulse (a), systolic blood pressure (b), and diastolic blood pressure (c) over time in the randomized population. bpm = beats per minute; DBP = diastolic blood pressure; IR = immediate-release; MAS = mixed amphetamine salts; PBO = placebo; SBP = systolic blood pressure.
The mean ± SD baseline QTcF interval in all randomized participants (n = 84) was 386.0 ± 16.3 msec. Mean ± SD changes in QTcF (msec) from baseline at end of treatment were −0.3 ± 13.6 for placebo, −1.5 ± 15.0 for 12.5 mg MAS IR, and 0.2 ± 15.4 for 25/50 mg SHP465 MAS (25 mg SHP465 MAS, −1.8 ± 16.9; 50 mg SHP465 MAS, 2.4 ± 13.3). During the study, no participant had a change from baseline QTcF ≥60 msec or a QTcF interval ≥500 msec.
Mean ± SD baseline weight in all randomized participants (n = 84) was 136.2 ± 35.3 lb. At end of treatment, mean ± SD increases in weight were observed with placebo (1.6 ± 11.0 lb), and decreases were observed with 25/50 mg SHP465 MAS (−2.1 ± 3.3 lb) and 12.5 mg MAS IR (−0.7 ± 2.4 lb). Weight decreases were similar with 25 mg (−2.0 ± 3.4 lb) and 50 mg (−2.2 ± 3.2 lb) SHP465 MAS.
Mean ± SD PSQI total score in all randomized participants (n = 84) was 5.2 ± 2.85 at the practice visit. At end of treatment, mean ± SD PSQI total scores were 4.0 ± 2.69 with placebo, 4.1 ± 2.87 with 12.5 mg MAS IR, and 5.5 ± 3.34 with 25/50 mg SHP465 MAS (25 mg SHP465 MAS, 5.0 ± 2.78; 50 mg SHP465 MAS, 6.2 ± 3.77).
4. Discussion
The primary finding of this study is that SHP465 MAS produced significantly greater PERMP total score improvements than placebo in adolescents with ADHD in a laboratory classroom setting when averaged over a 2- to 16-hour postdose period. The effects of SHP465 MAS (for the combined 25/50 mg dose group) were statistically significant at the first postdose assessment time point (2 hours postdose) and lasted until the last postdose assessment time point (16 hours postdose). Although the primary efficacy analysis assessed the combined effects of 25 and 50 mg SHP465 MAS, secondary analyses by dose indicated that both 25 and 50 mg SHP465 MAS were nominally superior to placebo on PERMP total score over the postdose assessment period. The observed effects of SHP465 MAS on secondary efficacy endpoints generally complemented the primary endpoint, with nominal superiority of SHP465 MAS over placebo observed for PERMP problems attempted and answered correctly and for ADHD-SRS, SKAMP, CPRS-R, and ADHD-RS-IV scores.
The findings for SHP465 MAS from this study are partially consistent with those of similarly designed studies in adults that used the AWE model [Citation16,Citation17], with 25 mg SHP465 MAS and 50/75 mg SHP465 MAS producing significantly greater PERMP total score improvements than placebo when averaged over a 16-hour postdose period. Although onset of efficacy was observed at 2 hours postdose with 50/75 mg SHP465 MAS in adults with ADHD [Citation17], the onset of efficacy was observed at 4 hours postdose with 25 mg SHP465 MAS in adults with ADHD [Citation16]. The reason for the later onset of efficacy in adults treated with 25 mg SHP465 MAS is unknown at this time. The superiority of 12.5 mg MAS IR over placebo observed in this study is also consistent with previous findings from an AWE study of SHP465 MAS, in which 25 mg MAS IR was nominally superior to placebo from 2 to 16 hours postdose [Citation17]. Furthermore, in a laboratory classroom study of children diagnosed with ADHD, 10 mg MAS IR improved performance significantly more than placebo on the SKAMP (1.5–7.5 hours postdose for attention; 1.5–10.5 hours postdose for deportment) and on the PERMP (1.5–9.0 hours postdose for number attempted and number correct) [Citation5]. From these data, it is apparent that both formulation and dose contribute to the overall duration of efficacy for MAS. For 25/50 mg SHP465 MAS, the data from the current study indicate reliable and consistent efficacy up to 16 hours postdose.
The effects of the different SHP465 MAS doses were not statistically assessed in this study. However, the pattern of findings suggests that 25 mg and 50 mg SHP465 MAS had roughly comparable effects, which is consistent with findings from a phase 3 clinical study of SHP465 MAS in adults [Citation10] that did not demonstrate a significant dose response on ADHD-RS-IV total score reductions across 25, 50, and 75 mg doses of SHP465 MAS. However, they do differ slightly from the findings from an AWE study of SHP465 MAS [Citation17] where the magnitude of the treatment effects of 75 mg SHP465 MAS tended to be numerically lower than those of 50 mg SHP465 MAS.
To assess SHP465 MAS treatment effect magnitude, mean PERMP total score changes from predose baseline (averaged across all postdose time points) were examined. Mean PERMP total score change with 25/50 mg SHP465 MAS in this study was approximately 55 points (vs 3 points with placebo). The magnitude of the PERMP total score treatment response in this study cannot be compared directly with other published reports in children and adolescents because previously published studies did not report PERMP total score data in the same way as this study. Furthermore, comparisons across studies would be complicated by the different age ranges employed across studies, especially those that included individuals younger than 13 years old, an age group which has empirically been shown to not exhibit a practice effect [Citation18]. However, these findings are generally consistent with published laboratory classroom studies of long-acting stimulants in pediatric populations aged 6 to 14 years that have reported efficacy versus placebo on the PERMP for up to 12 hours postdose in children and adolescents with ADHD [Citation4–Citation6,Citation20,Citation22]. The onset of effect at 2 hours postdose for SHP465 is also consistent with a previous lisdexamfetamine study [Citation4]. However, other published studies that have utilized earlier assessment times (e.g. 1 hour postdose [Citation22] and 1.5 hours postdose [Citation5,Citation6]) have demonstrated an onset of effect before 2 hours postdose.
Improvements in PERMP total score with 25/50 mg SHP465 MAS in the current study were nominally superior to those of 12.5 mg MAS IR for the average of all postbaseline assessment time points, with similar findings observed for secondary efficacy endpoints, with the exception of ADHD-SRS total score. These nominal differences were seen at later assessment time points (12 and 16 hours postdose for PERMP total score, number attempted, and number correct; 8 through 16 hours postdose for SKAMP total score). Because this study was not powered to compare 25/50 mg SHP465 MAS and 12.5 mg MAS IR – the MAS IR treatment arm was included only to evaluate assay sensitivity – conclusions regarding potential differences in duration of efficacy between these treatment arms should be made with caution. However, as previously noted, these data indicate that both formulation and dose contribute to the overall duration of efficacy of MAS.
The short-term safety and tolerability of SHP465 MAS in adolescents with ADHD in the current study are consistent with data from short-term phase 3 efficacy studies of SHP465 MAS in adults with ADHD [Citation9–Citation11] and children/adolescents with ADHD [Citation12], with phase 2 AWE studies of SHP465 MAS in adults with ADHD [Citation16,Citation17], and with other duration-of-efficacy studies for long-acting stimulants in pediatric populations (aged 6–12 years) with ADHD [Citation4–Citation6,Citation21,Citation22]. In this study, the most frequently reported TEAEs with SHP465 MAS (insomnia, anorexia, headache, and decreased appetite) were the same as those reported in similarly designed AWE studies of SHP465 MAS [Citation16,Citation17]. The overall frequency of insomnia as a TEAE in the present study (25 mg SHP465 MAS, 20.9%; 50 mg SHP465 MAS, 56.1%) generally fell within a range observed for other long-acting psychostimulants in pediatric populations from laboratory classroom studies that used a dose-optimization phase (lisdexamfetamine, 27.1% [Citation6]; multilayer release methylphenidate, 30.8% [Citation22]) and with another study that did not include a dose-optimization phase (MAS XR, 12.5–32% [Citation5]). It should also be noted that untreated adolescents exhibiting ADHD symptoms report higher insomnia rates than adolescents not exhibiting ADHD symptoms (33.7% vs 11.4%) [Citation34]. It is unknown whether pre-existing insomnia may have contributed to the insomnia rates observed in this study because baseline insomnia was not assessed.
This study was not powered for assessment of differences between SHP465 MAS doses. However, 50 mg SHP465 MAS was generally associated with a higher frequency of TEAEs (including insomnia) and with larger magnitude changes in pulse and blood pressure than 25 mg SHP465 MAS. This apparent dose response is consistent with SHP465 studies in adults with ADHD [Citation9,Citation10,Citation17]. However, further analyses are required to elucidate SHP465 MAS dose responses related to safety and tolerability.
These findings should be considered in light of potential limitations. First, the study was powered for assessment of the treatment differences between the combined doses of SHP465 MAS and placebo for PERMP total score. Therefore, the reported P values for secondary measures are nominal (unadjusted) and are descriptive in nature. Furthermore, MAS IR was included only for assay sensitivity and the MAS IR dose used was not chosen to be dose equivalent to the SHP465 MAS doses. As such, comparisons between the active treatment arms should be interpreted cautiously. Second, the study did not include a dose-optimization phase to titrate individuals to optimized doses of SHP465 MAS or MAS IR. This may have increased treatment response variability because participants may have been over medicated or under medicated. It is not known how the inclusion of an open-label dose-titration phase would have influenced the efficacy and safety profile of SHP465 MAS or MAS IR in this model. It should also be noted that despite the findings of statistically greater PERMP total scores with SHP465 MAS versus placebo, a practice effect during the early portions of testing may have reduced the overall treatment magnitude in this study. Thus, future studies of adolescents in the laboratory school setting should include additional practice PERMP tests beyond those included in this study before measuring treatment effects to eliminate this nuisance variable. Third, the study did not include a washout period between treatment sequences, so it is not known if crossover effects contributed to the observed results. However, the half-life of amphetamine is approximately 10 to 13 hours across MAS formulations [Citation35,Citation36]. Therefore, on the test day (which took place after 7 days of treatment), more than 12 half-lives elapsed. Furthermore, with only two exceptions (PERMP total score at 8 hours postdose [P = 0.0465] and PERMP number of problems attempted at 8 hours postdose [P = 0.0413]), there were no nominally significant effects of treatment sequence for any endpoints reported in the study. Fourth, it is important to note that the laboratory classroom model evolved from the understanding of the actions of stimulants in individuals with ADHD and the rapid onset of their effects. By design, this model is a tool to measure efficacy, onset, time response, and duration of effect [Citation18] that are difficult to obtain in naturalistic, real-world settings. As such, study outcomes are not intended to be translated to academic achievement as a specific measure of ecological validity. Lastly, participants were only treated with active drug for 3 weeks during the study. Therefore, safety and tolerability findings are indicative of short-term exposure only and cannot be extrapolated to the effects of long-term exposure.
5. Conclusions
In adolescents with ADHD, SHP465 MAS (25/50 mg) demonstrated statistically significantly greater PERMP total score improvements than placebo in a laboratory classroom setting. The onset of efficacy for SHP465 MAS was observed at the first postdose assessment time point of 2 hours, and the duration of efficacy lasted until the last assessment at 16 hours postdose. Although the primary efficacy analysis assessed the overall effects of SHP465 MAS (25 mg and 50 mg combined), secondary analyses indicated that 25 and 50 mg SHP465 MAS were nominally superior to placebo and exhibited response patterns similar to the primary analysis. The overall short-term safety and tolerability profile of SHP465 MAS was comparable with previous reports for SHP465 MAS in ADHD [Citation9–Citation12,Citation16,Citation17], with insomnia being among the most frequently reported TEAEs, and with reports for other long-acting stimulants in pediatric populations aged 6 to 12 years with ADHD assessed in the laboratory classroom setting [Citation4–Citation6,Citation21,Citation22]. This study and similar duration-of-efficacy studies in adults [Citation16,Citation17] supported the development of the phase 3 clinical program for SHP465 MAS for ADHD [Citation9–Citation12,Citation37].
Declaration of interest
S. Wigal is a consultant to, member of the scientific advisory boards of, or received speaker fees and/or research support from Akili, Arbor, Attentiv, Cingulate Therapeutics, Eli Lilly, Ironshore, Neos, Neurovance, NLS, Noven, Otsuka, Pfizer, Purdue, Rho, Rhodes, Shire, Sunovion, Supernus, TouchPoint, and Tris. F. Lopez has served as a consultant to and received speaker fees and/or research support from Eli Lilly, GSK, Ironshore, Neos, Novartis, Noven, Pfizer, Shire, Sunovion, Supernus, and Tris. G. Frick is a former employee of Shire, a member of the Takeda group of companies. B. Yan, B. Robertson, and M. Madhoo are employees of Shire, a member of the Takeda group of companies, and hold Takeda stock. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Under the direction of the authors, writing assistance was provided by Madhura Mehta, PhD, and Craig Slawecki, PhD (employees of CHC). In conjunction with the authors, editorial assistance in the form of proofreading, copyediting, and fact-checking was also provided by CHC. Shailesh Desai, PhD, from Shire, a member of the Takeda group of companies, assisted in reviewing and editing the manuscript for scientific accuracy. Peer reviewers on this manuscript have no relevant financial relationships to disclose.
Supplemental Material
Download Zip (154.5 KB)Acknowledgments
Under the direction of the authors, writing assistance was provided by Madhura Mehta, PhD, and Craig Slawecki, PhD (employees of Complete Healthcare Communications [CHC], a CHC group company; North Wales, PA). In conjunction with the authors, editorial assistance in the form of proofreading, copyediting, and fact-checking was also provided by CHC. Shailesh Desai, PhD, from Shire, a member of the Takeda group of companies, assisted in reviewing and editing the manuscript for scientific accuracy.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Concerta® (methylphenidate hydrochloride extended-release tablets). Full Prescribing Information [package insert]. Titusville (NJ): McNeil Pediatrics; 2008.
- Adderall XR® (mixed salts of a single-entity amphetamine product). Full Prescribing Information [package insert]. Lexington (MA): Shire US Inc.; 2017.
- Vyvanse® (lisdexamfetamine dimesylate). Full Prescribing Information [package insert]. Lexington (MA): Shire US Inc.; 2017.
- Biederman J, Boellner SW, Childress A, et al. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62(9):970–976.
- McCracken JT, Biederman J, Greenhill LL, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(6):673–683.
- Wigal SB, Kollins SH, Childress AC, et al. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3(1):17.
- Wigal SB, Childress AC, Belden HW, et al. NWP06, an extended-release oral suspension of methylphenidate, improved attention-deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. J Child Adolesc Psychopharmacol. 2013;23(1):3–10.
- Mydayis® extended-release capsules (mixed salts of a single-entity amphetamine product). Full Prescribing Information [package insert]. Lexington (MA): Shire US Inc.; 2017.
- Weisler RH, Greenbaum M, Arnold V, et al. Efficacy and safety of SHP465 mixed amphetamine salts in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled, forced-dose clinical study. CNS Drugs. 2017;31(8):685–697.
- Frick G, Yan B, Adler LA. Triple-bead mixed amphetamine salts (SHP465) in adults with ADHD: results of a phase 3, double-blind, randomized, forced-dose trial. J Atten Disord. 2017. Epub ahead of print. DOI:10.1177/1087054717696771
- Spencer TJ, Adler LA, Weisler RH, et al. Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry. 2008;69:1437–1448.
- Brams M, Childress AC, Greenbaum M, et al. SHP465 mixed amphetamine salts in the treatment of attention-deficit/hyperactivity disorder in children and adolescents: results of a randomized, double-blind placebo-controlled study. J Child Adolesc Psychopharmacol. 2018;28(1):19–28.
- Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373.
- Adler LA, Zimmerman B, Starr HL, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29(3):239–247.
- Weisler RH, Biederman J, Spencer TJ, et al. Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr. 2006;11(8):625–639.
- Wigal T, Childress A, Frick G, et al. Effects of SHP465 mixed amphetamine salts in adults with ADHD in a simulated adult workplace environment. Postgrad Med. 2018;130(1):111–121.
- Wigal T, Brams M, Frick G, et al. A randomized, double-blind study of SHP465 mixed amphetamine salts extended-release in adults with ADHD using a simulated adult workplace design. Postgrad Med. 2018;130(5):481–493.
- Wigal SB, Wigal TL. The laboratory school protocol: its origin, use, and new applications. J Atten Disord. 2006;10(1):92–111.
- Swanson JM, Lerner M, Wigal T, et al. The use of a laboratory school protocol to evaluate concepts about efficacy and side effects of new formulations of stimulant medications. J Atten Disord. 2002;6(Suppl. 1):S73–88.
- Dopfner M, Gerber WD, Banaschewski T, et al. Comparative efficacy of once-a-day extended-release methylphenidate, two-times-daily immediate-release methylphenidate, and placebo in a laboratory school setting. Eur Child Adolesc Psychiatry. 2004;13(Suppl. 1):I93–101.
- Swanson JM, Wigal S, Greenhill LL, et al. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37(5):519–526.
- Wigal SB, Greenhill LL, Nordbrock E, et al. A randomized placebo-controlled double-blind study evaluating the time course of response to methylphenidate hydrochloride extended-release capsules in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24(10):562–569.
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373–374.
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-age Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988.
- Naugle RI, Chelune GJ, Tucker GD. Validity of the Kaufman Brief Intelligence Test. Psychol Assess. 1993;5(2):182.
- Conners C. Conners’ Rating Scales-Revised Technical Manual. North Tonawanda (NY): Multi-Health Systems; 2000.
- AACAP Work Group on Quality Issues, Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921.
- DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: checklists, norms, and clinical interpretation. New York (NY): Guilford Press; 1998.
- Wigal SB, Gupta S, Guinta D, et al. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull. 1998;34(1):47–53.
- Conners CK, Sitarenios G, Parker JD, et al. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268.
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
- Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740.
- Tse G, Chan YW, Keung W, et al. Electrophysiological mechanisms of long and short QT syndromes. Int J Cardiol Heart Vasc. 2017;14:8–13.
- Hysing M, Lundervold AJ, Posserud MB, et al. Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: results from a large population-based study. Behav Sleep Med. 2015;14:550–564.
- Tulloch SJ, Zhang Y, McLean A, et al. SLI381(Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparison of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22(11):1405–1415.
- Ermer JC, Shojaei A, Pennick M, et al. Bioavailability of triple-bead mixed amphetamine salts compared with a dose-augmentation strategy of mixed amphetamine salts extended release plus mixed amphetamine salts immediate release. Curr Med Res Opin. 2007;23(5):1067–1075.
- Adler LA, Frick G, Yan B. A long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD. J Atten Disord. 2017. Epub ahead of print. DOI:10.1177/1087054717696770
