ABSTRACT
Objective: Patients with chronic kidney disease (CKD) have increased cardiovascular disease (CVD) risk, likely driven by atherogenic and inflammatory markers beyond low-density lipoprotein cholesterol (LDL-C). The objective of this hypothesis-generating post hoc subgroup analysis was to explore the effects of icosapent ethyl at 2 or 4 g/day (prescription pure ethyl ester of the omega-3 fatty acid eicosapentaenoic acid [EPA]) on atherogenic lipid, apolipoprotein, inflammatory parameters (high-sensitivity C-reactive protein [hsCRP], lipoprotein-associated phospholipase A2 [Lp-PLA2]), and oxidative parameters (oxidized-LDL [ox-LDL]) in statin-treated patients from ANCHOR with stage 3 CKD.
Methods: The 12-week ANCHOR study evaluated icosapent ethyl in 702 statin-treated patients at increased CVD risk with triglycerides (TG) 200–499 mg/dL despite controlled LDL-C (40–99 mg/dL). This post-hoc analysis included patients from ANCHOR with stage 3 CKD (estimated glomerular filtration rate [eGFR] ≤60 mL/min/1.73 m2 for ≥3 months) randomized to icosapent ethyl 4 g/day (n = 19), 2 g/day (n = 30), or placebo (n = 36).
Results: At the prescription dose of 4 g/day, icosapent ethyl significantly reduced TG (−16.9%; P = 0.0074) and other potentially atherogenic lipids/lipoproteins, ox-LDL, hsCRP, and Lp-PLA2, and increased plasma and red blood cell EPA levels (+879% and +579%, respectively; both P < 0.0001) versus placebo. Icosapent ethyl did not significantly alter eGFR or serum creatinine. Safety and tolerability were similar to placebo.
Conclusions: In patients with stage 3 CKD at high CVD risk with persistent high TG despite statins, icosapent ethyl 4 g/day reduced potentially atherogenic and other cardiovascular risk factors without raising LDL-C, with safety similar to placebo. These findings suggest prospective investigation may be warranted.
1. Introduction
Chronic kidney disease (CKD) is independently associated with increased risk of cardiovascular (CV) morbidity and mortality [Citation1–Citation3]. Patients with CKD are much more likely to die from CV disease (CVD) than from progressive kidney disease [Citation4] and CKD is identified as a coronary heart disease–risk equivalent. Statins are generally recommended as first-line therapy for the management of dyslipidemia to reduce CVD risk [Citation5,Citation6] and can reduce risk of major CV events and mortality by approximately 20% in patients with CKD [Citation7,Citation8]. However, statins (with or without ezetimibe) have not been shown to significantly reduce CV risk in patients on hemodialysis [Citation7,Citation9–Citation11]. The residual CVD risk remaining after statin therapy is likely driven, in part, by lipid-related factors beyond low-density lipoprotein cholesterol (LDL-C) [Citation11], and CKD is marked by impairments in lipid and lipoprotein metabolism that result in atherogenic changes such as dyslipidemia and increased levels of oxidized LDL-C and apolipoprotein B–containing particles [Citation12,Citation13]. Dyslipidemia in CKD is characterized by increased levels of triglycerides (TG) and TG-rich lipoproteins, with only minor changes in LDL-C [Citation13,Citation14]. TG-rich lipoproteins may predict CVD events in patients with CKD and diabetes [Citation15]. Elevated TG are associated with new-onset kidney disease in non-hypertensive patients with diabetes [Citation16,Citation17]. Modifying lipid risk factors beyond LDL-C may provide a viable approach for reducing residual CVD risk in patients with CKD.
Eicosapentaenoic acid (EPA) is an omega-3 fatty acid that reduces TG and TG-rich lipoproteins [Citation18]. EPA is incorporated into cell membrane components, such as phospholipids, and atherosclerotic plaque, and produces beneficial effects on a wide variety of inflammatory and oxidative mechanisms involved in atherosclerosis [Citation19,Citation20]. EPA and the ratio of EPA to the principal omega-6 fatty acid, arachidonic acid (AA), are reduced in patients on hemodialysis [Citation21]. Serum EPA levels are inversely correlated with proinflammatory cytokine levels, and the EPA/AA ratio seems to be inversely associated with proteinuria in patients with CKD and with adverse CV events in hemodialysis cohorts [Citation21,Citation22].
Icosapent ethyl, a pure ethyl ester formulation of EPA (Vascepa®; Amarin Pharma Inc., Bedminster, NJ), is a prescription omega-3 fatty acid indicated as an adjunct to diet for reducing TG in adults with severe hypertriglyceridemia (TG ≥500 mg/dL) [Citation23]. The ANCHOR study in adults with residually high TG (200–499 mg/dL) and high CV risk despite stable statin therapy, demonstrated that, in addition to significantly reducing TG without raising LDL-C compared with placebo, icosapent ethyl treatment elicited beneficial changes in non-high-density lipoprotein cholesterol (non-HDL-C), total cholesterol (TC), very-low-density lipoprotein cholesterol (VLDL-C), very-low-density lipoprotein TG (VLDL-TG), remnant lipoprotein cholesterol (RLP-C), apolipoprotein B (Apo B), apolipoprotein C-III (Apo C-III), oxidized LDL (ox-LDL), lipoprotein-associated phospholipase A2 (Lp-PLA2), and high-sensitivity C-reactive protein (hsCRP) [Citation24–Citation28]. Data on the effects of icosapent ethyl treatment on such parameters in the CKD population are limited. This hypothesis-generating post hoc analysis explored the effects of icosapent ethyl on various atherogenic lipids, apolipoproteins, ox-LDL, hsCRP, and Lp-PLA2 in a subgroup of patients from ANCHOR who had stage 3 CKD.
2. Methods
2.1. Trial design and participants
Methodologic details of the ANCHOR study (NCT01047501) have been reported [Citation24]. Briefly, in this phase 3, multicenter, placebo-controlled, randomized, double-blind, 12-week study, adults who had high CVD risk and residually high TG (200–499 mg/dL) despite controlled LDL-C (40–99 mg/dL) on stable statin therapy (with or without ezetimibe) received icosapent ethyl 4 g/day (the approved prescription dose), 2 g/day, or placebo. Exclusion criteria related to renal function included known nephrotic range (>3 g/day) proteinuria, history or evidence of major and clinically significant renal disease that would interfere with the conduct of the study or interpretation of the data, and requirement for peritoneal dialysis or hemodialysis for renal insufficiency. The present post hoc subgroup analysis includes the subset of patients from ANCHOR who had available blood samples and CKD defined by an estimated glomerular filtration rate (eGFR) ≤60 mL/min/1.73 m2 at baseline and 12 weeks (≥3 months); no lower limit for eGFR was specified. ANCHOR was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The appropriate institutional review boards approved the protocol, and all patients provided written informed consent.
2.2. Efficacy assessments
The primary efficacy variable was the median difference in percent change in fasting plasma TG from baseline to week 12 between icosapent ethyl and placebo. Additional efficacy assessments included the median difference in percent change from baseline to week 12 between icosapent ethyl and placebo in plasma levels of LDL-C, non-HDL-C, TC, HDL-C, VLDL-C, VLDL-TG, RLP-C, Apo B, Apo C-III, ox-LDL, Lp-PLA2, and hsCRP, and also plasma and red blood cell (RBC) concentrations of EPA. These parameters were measured as previously described [Citation25,Citation28–Citation32]. The protocol prespecified that EPA levels were to be measured with liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology in the first approximately 216 patients in ANCHOR with complete sample datasets. To convert from mg/dL to the International System unit of mmol/L, multiply cholesterol by 0.0259 and multiply TG by 0.0113. To convert ApoB and ApoC-III levels to the SI unit of g/L, multiply by 0.01. To convert hsCRP to SI units of nmol/L, multiply by 9.524.
2.3. Safety assessments
The evaluation of safety included monitoring of treatment-emergent adverse events (TEAEs), defined as any adverse events (AEs) that began after receiving the first dose of study medication or that worsened in severity during the double-blind treatment period. TEAEs in this analysis included total TEAEs and those occurring in >3% of patients in any treatment group of the overall ANCHOR population (ie, nausea, diarrhea, nasopharyngitis, and arthralgia) [Citation24].
2.4. Statistical analyses
This subgroup analysis of patients with CKD was not prespecified per the ANCHOR protocol. Like the analyses in the full study population, these post hoc efficacy analyses were primarily done in the intent-to-treat (ITT) population (randomized patients who received ≥1 dose of study medication and had efficacy measurements at baseline and post-randomization). Median difference in percent change from baseline between icosapent ethyl and placebo for the primary efficacy variable and additional assessments were estimated with the Hodges-Lehmann method (P values from the Wilcoxon rank-sum test for treatment comparisons) when departures from normal distribution were observed; for normally distributed parameters, an analysis of covariance model was used with least squares (LS) means and standard errors (SE). Missing values were imputed using the last-observation-carried-forward method. For all post hoc analyses, the alpha for statistical significance was 0.05. Safety analyses were based on all randomized patients who received at least one dose of study drug. These analyses were conducted with SAS/STAT 9.22 software.
3. Results
3.1. Patients
ANCHOR randomized 702 statin-treated patients to icosapent ethyl 4 g/day, icosapent ethyl 2 g/day, or placebo [Citation24]. The present post hoc subgroup analysis included 85 patients with CKD (ie, eGFR ≤60 mL/min/1.73 m2 for ≥3 months) randomized to receive icosapent ethyl 4 g/day (n = 19), 2 g/day (n = 30), or placebo (n = 36). One patient each in each of the groups discontinued early from the study and they were excluded from efficacy analyses (but not safety analyses). EPA levels in plasma and RBC were measured in a subset of CKD patients in the icosapent ethyl 4 g/day (n = 7), 2 g/day (n = 12), and placebo groups (n = 16). Baseline characteristics of the CKD subgroup are shown in .
Table 1. Baseline Characteristics (CKD Subset of ANCHOR ITT Population).
3.2. Effects on atherogenic parameters
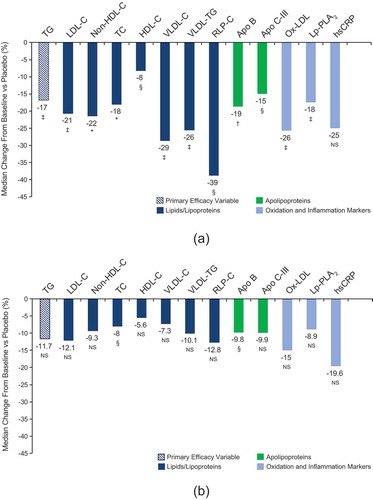
Icosapent ethyl 4 g/day significantly reduced fasting TG (primary endpoint) by 16.9% (P = 0.007) compared with placebo in patients with CKD (, )). Icosapent ethyl 4 g/day also significantly reduced LDL-C, non-HDL-C, TC, HDL-C, VLDL-C, VLDL-TG, and RLP-C compared with placebo (P values ranging from <0.05 to <0.0001), as well as both Apo B and Apo C-III (P = 0.0002 and P = 0.0168, respectively) (, )). Icosapent ethyl 4 g/day significantly reduced ox-LDL and Lp-PLA2 compared with placebo (both P = 0.0068). The reduction in hsCRP with icosapent ethyl 4 g/day versus placebo did not reach statistical significance (P = 0.1209). Reductions of a lesser magnitude were also observed across the parameters in the 2 g/day group versus placebo but only reached statistical significance for total cholesterol and Apo B (, )).
Table 2. Effects of Icosapent Ethyl on Lipids/Lipoproteins, Apolipoproteins, ox-LDL, hsCRP, and Lp-PLA2 (CKD Subset of ANCHOR ITT Population).
Figure 1. Percent change in lipids/lipoproteins, apolipoproteins, ox-LDL, hsCRP, and Lp-PLA2 in patients with CKD (CKD subset of ANCHOR ITT population). Values represent median difference in percent change from baseline to week 12 for (a) icosapent ethyl 4 g/day vs placebo and (b) icosapent ethyl 2 g/day vs placebo. IQRs are available for each parameter in . *P < 0.0001; †P < 0.001; ‡P < 0.01; §P < 0.05; NS, not significant versus placebo. Apo C-III, apolipoprotein C-III; Apo B, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; ox-LDL, oxidized low-density lipoprotein; RLP-C, remnant lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; VLDL-C, very-low-density lipoprotein cholesterol; VLDL-TG, very-low-density lipoprotein triglycerides.
3.3. Plasma and RBC levels of EPA
Icosapent ethyl 4 g/day significantly increased mean (standard deviation [SD]) plasma EPA levels from 22.8 (7.0) µg/mL at baseline to 233.4 (82.1) µg/mL at week 12; the LS mean (SE) increase was 879.2% (75.8%) with icosapent ethyl 4 g/day compared with placebo (P < 0.0001). Mean (SD) plasma EPA levels increased from 28.2 (11.0) µg/mL at baseline to 131.4 (40.3) µg/mL at week 12 with icosapent ethyl 2 g/day; compared with placebo, the LS mean (SE) increase was 441.7% (65.7%) with icosapent ethyl 2 g/day (P < 0.0001). In RBC, icosapent ethyl 4 g/day significantly increased mean (SD) EPA levels from 10.6 (2.9) µg/mL at baseline to 72.3 (34.0) µg/mL at week 12; the LS mean (SE) increase was 579.4% (73.0%) with icosapent ethyl 4 g/day compared with placebo (P < 0.0001). Mean (SD) RBC EPA levels increased from 11.5 (6.1) µg/mL at baseline to 52.9 (15.8) µg/mL at week 12 with icosapent ethyl 2 g/day; compared with placebo, the LS mean (SE) increase was 487.5% (61.0%) with icosapent ethyl 2 g/day (P < 0.0001).
3.4. Safety
TEAEs were reported in 11 of 19 (57.9%) patients with CKD in the icosapent ethyl 4 g/day group, 12 of 30 (40.0%) patients in the icosapent ethyl 2 g/day group, and 20 of 36 (55.6%) patients in the placebo group. Among TEAEs reported most frequently in the overall ANCHOR population (n = 702), diarrhea was reported by 2 patients with CKD (10.5%) in the icosapent ethyl 4 g/day group, 1 patient with CKD (3.3%) in the icosapent ethyl 2 g/day group, and 2 patients with CKD (5.6%) in the placebo group. Nausea, nasopharyngitis, and arthralgia were each reported by 1 patient with CKD (3.3%) in the icosapent ethyl 2 g/day group, and were not reported by any patients with CKD in the icosapent ethyl 4 g/day or placebo groups.
No significant changes in serum creatinine, blood urea nitrogen (BUN), or eGFR from baseline to week 12 were detected among patients with CKD in the icosapent ethyl group compared with placebo at either the 4 g/day or 2 g/day dose. The median (interquartile range [IQR]) serum creatinine concentration in the icosapent ethyl 4 g/day group was 1.2 (0.3) mg/dL at baseline and 1.2 (0.4) mg/dL at week 12, 1.2 (0.4) mg/dL at baseline and 1.1 (0.3) mg/dL at week 12 in the icosapent ethyl 2 g/day group, and 1.3 (0.3) mg/dL at baseline and 1.1 (0.3) mg/dL at week 12 in the placebo group. In the icosapent ethyl 4 g/day and 2 g/day groups, respectively, the median difference in serum creatinine change from baseline compared with placebo was 6.5% (P = 0.1983) and 3.3% (P = 0.2662). Median (IQR) BUN was 24.5 (12.0) mg/dL at baseline and 22.5 (11.0) mg/dL at week 12 in the icosapent ethyl 4 g/day group, 26.5 (11.0) mg/dL at baseline and 24.0 (5.0) mg/dL at week 12 in the icosapent ethyl 2 g/day group, and 23.0 (7.0) mg/dL at baseline and 23.0 (11.0) mg/dL at week 12 in the placebo group. The median difference in BUN change from baseline compared with placebo was 1.0 mg/dL (P = 0.5471) or 5.5% (P = 0.5291) in the icosapent ethyl 4 g/day group and −1.0 (P = 0.4365) or −3.7% (P = 0.4938) in the icosapent ethyl 2 g/day group. Median (IQR) eGFR was 55.0 (13.8) mL/min/1.73 m2 at baseline and 56.0 (12.9) mL/min/1.73 m2 at week 12 in the icosapent ethyl 4 g/day group, 50.5 (12.4) mL/min/1.73 m2 at baseline and 56.1 (13.8) mL/min/1.73 m2 at week 12 in the icosapent ethyl 2 g/day group, and 52.4 (11.2) mL/min/1.73 m2 at baseline and 58.8 (14.5) mL/min/1.73 m2 at week 12 in the placebo group. The median difference in eGFR change from baseline compared with placebo was −7.8% (P = 0.1983) for the icosapent ethyl 4 g/day group and −4.7% (P = 0.2662) for the icosapent ethyl 2 g/day group. Baseline and end-of-treatment levels of serum albumin were similar in all 3 groups, and no significant median changes from baseline in serum albumin occurred in any group over the 12-week study.
4. Discussion
The present hypothesis-generating post hoc analysis in statin-treated patients with CKD and residually elevated TG showed that icosapent ethyl, at the prescription dose of 4 g/day, significantly reduced TG (−16.9%) and other potentially atherogenic parameters, with lesser reductions observed at the 2 g/day dose. The results in this CKD subgroup of ANCHOR were generally similar to those in the entire ANCHOR population [Citation24,Citation25,Citation27,Citation28]. Although icosapent ethyl 4 g/day significantly reduced a battery of potentially atherogenic parameters, CV outcomes studies are needed to ascertain whether these changes will translate into lower CVD morbidity and mortality. The increasing prevalence of diabetic kidney disease and the shared atherogenic lipid profile between kidney disease and diabetes in this comorbid state underscores the importance of recognizing dyslipidemia in such patient subgroups. Statins are recommended as first-line treatment for dyslipidemia in CKD patients as in patients with diabetes, and significantly reduce CVD events [Citation7,Citation8,Citation33–Citation35]. However, residual CVD risk may still remain despite statin therapy, and likely reflects the contributions of other atherogenic parameters.
The findings of this analysis of high-purity prescription EPA are generally consistent with those of other reports of the effects of omega-3 fatty acids on lipids and atherogenic parameters. In a systematic review and meta-analysis of 1461 patients with end-stage renal disease from 20 randomized controlled trials (RCTs), omega-3 fatty acid supplementation (0.9–13.5 g/day) was found to significantly reduce TG, LDL-C, and hsCRP, with no significant effects on TC or HDL-C [Citation36]. In another meta-analysis of 444 patients with CKD from 9 RCTs, omega-3 supplementation (1.8–10 g/day) had little or no effect on eGFR or serum creatinine clearance; notably, high-dose supplementation was associated with reduced risk of end-stage renal disease [Citation37]. It may be worth noting that the actual dose of omega-3 fatty acids may have been lower than the reported doses in some studies because some omega-3 supplements may contain other ingredients.
The overall safety and tolerability profile of icosapent ethyl was similar to placebo in the CKD subgroup over the 12-week course of the ANCHOR study, consistent with the safety profile in the entire ANCHOR cohort and other clinical studies [Citation24,Citation29].
The present data analysis has several limitations. This post hoc analysis was hypothesis-generating as the data are derived from a CKD subgroup with a small sample size, with twice as many patients in the placebo group as in the icosapent ethyl 4 g/day group. By design, all of the patients met the criteria for CKD, but many also had diabetes mellitus. While this is a limitation of the study, it is also reflective of the rising prevalence of diabetic kidney disease. The analysis was not designed or powered to determine potential benefits of icosapent ethyl in CKD, and ANCHOR was not designed to test the effects of icosapent ethyl on CVD outcomes. While serum albumin levels suggested that nutritional status was not compromised, data were not available for urine albumin or urine albumin-to-creatinine ratio to assess proteinuria, as these parameters were not collected in ANCHOR. As ANCHOR was a 12-week study, information regarding the long-term safety and efficacy of icosapent ethyl in CKD patients is still needed given that this population is being treated for a chronic condition. While this analysis did not examine DHA levels, no significant changes in DHA are expected in the population of patients with CKD. In the full ANCHOR population, there was no significant change in DHA levels vs placebo (−1.1%; P = 0.7820) and EPA increases in the full ANCHOR population were similar to the EPA increases observed in this subset of patients with CKD [Citation38].
Outcomes data with EPA are not available in CKD cohorts, although related data come from several Japanese studies conducted with low EPA doses in hemodialysis patients [Citation21]. For example, EPA (1.8 g/day) significantly reduced CV death (hazard ratio [HR]: 0.20; P = 0.04), CV events including acute myocardial infarction, stroke, and aortic disease–related events (HR: 0.50; P = 0.04), and the combined outcome (HR: 0.49; P = 0.02) over 2 years compared with placebo in a randomized study of 179 chronic hemodialysis patients [Citation39]. In 2 other studies of maintenance hemodialysis patients, EPA 1.8 g/day significantly reduced all-cause mortality (n = 176; HR: 0.42; P = 0.03 [Citation40] and n = 459; HR: 0.53; P = 0.02 [Citation41]). One of these studies also conducted an analysis of CVD mortality and also found EPA to have benefit (HR: 0.41; P = 0.03 [Citation41]). Further studies are needed to corroborate these results.
The data presented herein raise the potential hypothesis that icosapent ethyl 4 g/day may be a treatment option for statin-treated patients with CKD and residually high TG. However, larger trials specifically designed to address this hypothesis are needed.
The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT; NCT01492361) evaluated the potential CV benefit of icosapent ethyl 4 g/day in 8179 patients who had high CVD risk, elevated TG, and controlled LDL-C on stable statin therapy, similar to the ANCHOR study population [Citation42]. A statistically significant relative risk reduction of 25% (HR, 0.75; 95% confidence interval [CI], 0.68–0.83; P < 0.001) in the first occurrence of a major adverse CV event (primary composite endpoint) was demonstrated for icosapent ethyl 4 g/day versus placebo over a median follow-up time of 4.9 years [Citation42]. Although REDUCE-IT was not specifically powered or designed to evaluate the safety and efficacy of icosapent ethyl in patients with CKD, the trial included over 1800 patients with CKD (eGFR <60 mL/min/1.73 m2 in 905 and 911 patients in the icosapent ethyl 4 g/day and placebo groups, respectively). Subgroup analysis of occurrence of the REDUCE-IT primary endpoint based on baseline eGFR revealed a non-significant interaction P value of 0.41, suggesting similar benefits in patients with baseline eGFR <60, ≥60 to <90, and ≥90 mL/min/1.73 m2. Recently, based on the findings of REDUCE-IT, the American Diabetes Association recommended that icosapent ethyl be considered for reducing CV risk in statin-treated patients with controlled LDL-C, elevated TG (135–499), diabetes, and atherosclerotic CVD or other cardiac risk factors [Citation34].
In summary, the present post hoc hypothesis-generating analysis of statin-treated patients from ANCHOR with stage 3 CKD, high CVD risk, and persistent high TG demonstrates that icosapent ethyl 4 g/day significantly reduces TG and other potentially atherogenic parameters, including markers of inflammation. Icosapent ethyl was well tolerated in this population, with a safety profile similar to that of the full ANCHOR population. On the basis of these hypothesis-generating data, prospective studies with icosapent ethyl in the CKD population may be warranted.
Declaration of interest
KV has received honoraria from Amarin Pharma Inc., Amgen, AstraZeneca, Aventyn, Baylor Research Institute, Boehringer Ingelheim, Legacy Heart, Novartis, Novo Nordisk, and ZS, and has received salary from Abrazo and the Scottsdale Cardiovascular Center.
HMS has received research grants from Akebia, Bayer, BioPorto, and La Jolla Pharmaceutical; serves on the speakers bureau for Astute Medical; has received honoraria from the Cardiorenal Society; has ownership interest in Amgen, Gilead, Merck, and Pfizer; is a consultant to and member of the advisory board of La Jolla Pharmaceutical; and has received product royalties/licensing fees from UptoDate.
CMB has received research/grant support from Abbott Diagnostics, Amarin Pharma Inc., Amgen, Esperion, Ionis, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, NIH, ADA, and AHA (all paid to institution, not individual), and is a consultant for Abbott Diagnostics, Amarin Pharma Inc., Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esperion, Ionis, Matinas Biopharma, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo.
Neither HEB nor his affiliated research center & weight management center own pharmaceutical stocks or patents. In the past 12 months, HEB’s research site has received research grants from Amarin Pharma Inc., Amgen, Alere, Allergan, AstraZeneca, Boehringer Ingelheim, Bristol Meyers Squibb, Catabasis, Dr. Reddy, Eisai, Elcelyx, Eli Lilly, Esperion, Ferrer/Chiltern, Gemphire, Gilead, GSK, Home Access, iSpecimen, Ionis, Janssen, Johnson and Johnson, Merck, Nektar, Nichi-Iko, Novartis, NovoNordisk, Omthera, Pfizer, Regeneron, Sanofi, Selecta, Takeda, and TIMI. In the past 12 months, HEB has served as a consultant/advisor for Alnylam, Akcea, Amgen, AstraZeneca, Eisai, Eli Lilly, Esperion, Ionis, Janssen, Johnson & Johnson, Kowa, Merck, Novartis, Prosciento, Regeneron, and Sanofi. In the past 12 months, HEB has served as a speaker for Amarin Pharma Inc., Amgen, Eisai, Kowa, Orexigen, Regeneron, and Sanofi.
SP, RTD Jr, RAJ, and CG are employees and stock shareholders in Amarin Corporation, the parent company of Amarin Pharma Inc.
Medical writing and editorial assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Amarin Pharma Inc., Bedminster, NJ.
PGM peer reviewers on this manuscript have no disclosures of interest to report.
Additional information
Funding
References
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305.
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352.
- Mok Y, Ballew SH, Matsushita K. Prognostic value of chronic kidney disease measures in patients with cardiac disease. Circ J. 2017;81(8):1075–1084.
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663.
- Sarnak MJ, Bloom R, Muntner P, et al. KDOQI US commentary on the 2013 KDIGO clinical practice guideline for lipid management in CKD. Am J Kidney Dis. 2015;65(3):354–366.
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American College of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease: executive summary. Endocr Pract. 2017;23(4):479–497.
- Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192.
- Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5:CD007784.
- Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248.
- Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407.
- Epstein M, Vaziri ND. Statins in the management of dyslipidemia associated with chronic kidney disease. Nat Rev Nephrol. 2012;8(4):214–223.
- Bulbul MC, Dagel T, Afsar B, et al. Disorders of lipid metabolism in chronic kidney disease. Blood Purif. 2018;46(2):144–152.
- Ferro CJ, Mark PB, Kanbay M, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. 2018;14(12):727–749.
- Choudhury D, Tuncel M, Levi M. Disorders of lipid metabolism and chronic kidney disease in the elderly. Semin Nephrol. 2009;29(6):610–620.
- Nguyen SV, Nakamura T, Uematsu M, et al. Remnant lipoproteinemia predicts cardiovascular events in patients with type 2 diabetes and chronic kidney disease. J Cardiol. 2017;69(3):529–535.
- Russo GT, De Cosmo S, Viazzi F, et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD annals initiative. Diabetes Care. 2016;39(12):2278–2287.
- Tao-Chun L. Hypertriglyceridemia is a residual risk factor associated with new-onset DKD in type 2 diabetic patients without hypertension [abstract 536-P]. Diabetes. 2017;66(suppl 1):A140.
- Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118(1):138–145.
- Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357–366.
- Nelson JR, Wani O, May HT, et al. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul Pharmacol. 2017;91:1–9.
- Borow KM, Mason RP, Vijayaraghaven K. Eicosapentaenoic acid (EPA) as a potential therapeutic approach to reduce cardiovascular risk in patient with end-stage renal disease on hemodialysis: a review. Cardiorenal Med. 2018;8:18–30.
- Hitoshi S, Chieko N, Hiroaki I, et al. An increase in the EPA/AA ratio is associated with reduction of proteinuria in CKD patients [abstract PS3-202]. Nephrology. 2014;19(suppl 2):166.
- Vascepa [package insert]. Bedminster, NJ: Amarin Pharma Inc.; 2017.
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–992.
- Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13(1):37–46.
- Ballantyne CM, Braeckman RA, Bays HE, et al. Effects of icosapent ethyl on lipoprotein particle concentration and size in statin-treated patients with persistent high triglycerides (The ANCHOR Study). J Clin Lipidol. 2015;9(3):377–383.
- Ballantyne CM, Bays HE, Philip S, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis. 2016;253:81–87.
- Ballantyne CM, Bays HE, Braeckman RA, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on plasma apolipoprotein C-III levels in patients from the MARINE and ANCHOR studies. J Clin Lipidol. 2016;10(3):635–645.
- Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–690.
- Brinton EA, Ballantyne CM, Bays HE, et al. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12(1):100.
- Braeckman RA, Manku MS, Bays HE, et al. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostaglandins Leukot Essent Fatty Acids. 2013;89(4):195–201.
- Braeckman RA, Stirtan WG, Soni PN. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin Pharmacol Drug Dev. 2014;3(2):101–108.
- Chung CM, Lin MS, Hsu JT, et al. Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J Clin Lipidol. 2017;11(2):422–431.e2.
- American Diabetes Association® issues critical updates to the 2019 standards of medical care in diabetes Arlington, VA2019 [ cited 2019 March 27]. Available from: http://www.diabetes.org/newsroom/press-releases/2019/ada-issues-critical-updates-to-2019-standards-of-care.html.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125.
- Xu T, Sun Y, Sun W, et al. Effect of omega-3 fatty acid supplementation on serum lipids and vascular inflammation in patients with end-stage renal disease: a meta-analysis. Sci Rep. 2016;6:39346.
- Hu J, Liu Z, Zhang H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: a meta-analysis. Clinics (Sao Paulo). 2017;72(1):58–64.
- Ballantyne CM, Manku MS, Bays HE, et al. Icosapent ethyl effects on fatty acid profiles in statin-treated patients with high triglycerides: the randomized, placebo-controlled ANCHOR study. Cardiol Ther. 2019;8(1):79–90.
- Nasu M, Seino K, Tamura Y, et al. Eicosapentaenoic acid restrains the development of the cardiovascular events independent of triglyceride and C-reactive protein reduction in Japanese hemodialysis patients [abstract P1428]. Eur Heart J. 2013;34(suppl 1):271.
- Inoue T, Okano K, Tsuruta Y, et al. Eicosapentaenoic acid (EPA) decreases the all-cause mortality in hemodialysis patients. Intern Med. 2015;54(24):3133–3137.
- Umemoto N, Ishii H, Sakamoto T, et al. Administration of eicosapentaenoic acid reduces cardiovascular and all-cause mortality in chronic hemodialysis patients [abstract P3753]. Eur Heart J. 2015;36(suppl 1):662.
- Bhatt DL, Steg G, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22.
