ABSTRACT
Context: Chronic pain is highly prevalent in most of the industrialized nations around the world. Despite the documented adverse effects, opioids are widely used for pain management. Cannabinoids, and specifically Cannabidiol, is proposed as an opioid alternative, having comparable efficacy with better safety profile.
Objectives: We aim to investigate the impact of full hemp extract cannabidiol (CBD) on opioid use and quality of life indicators among chronic pain patients.
Methods: An initial sample of 131 patients was recruited from a private pain management center’s investigative population. Ninety-seven patients completed the 8-week study. The primary inclusion criteria included patients between 30 and 65 years old with chronic pain who have been on opioids for at least 1 year. Data were collected at three different time points: baseline, 4, and 8 weeks. Opioid and other medication use were evaluated via the medication and psychiatric treatment receipt. Improvement was evaluated using four indices: Pain Disability Index (PDI-4); Pittsburgh Sleep Quality Index (PSQI), Pain Intensity and Interference (PEG); and Patient Health Questionnaire (PHQ-4).
Results: Over half of chronic pain patients (53%) reduced or eliminated their opioids within 8 weeks after adding CBD-rich hemp extract to their regimens. Almost all CBD users (94%) reported quality of life improvements. The results indicated a significant relationship between CBD and PSQI (p = 0.003), and PEG (p = 0.006). There was a trend toward improvement but no significant relationship between CBD use and PHQ and PDI.
Conclusion: CBD could significantly reduce opioid use and improve chronic pain and sleep quality among patients who are currently using opioids for pain management.
Key Message: This is a prospective, single-arm cohort study for the potential role of cannabinoids as an alternative for opioids. The results indicate that using the CBD-rich extract enabled our patients to reduce or eliminate opioids with significant improvement in their quality of life indices.
Introduction
Chronic pain is a major cause of disability worldwide with a prevalence rate of 15% to 30% in the general adult population, and more than 500 billion dollars annual costs in the United States. Unfortunately, multiple reports are showing a lack of satisfactory results with available pharmacotherapy with less than 70% of patients having pain relief [Citation1]. Currently, opioids are the mainstay for pain control for most cases including, neuropathic and cancer pain. Nonetheless, the inherent adverse events of opioids are representing major concerns [Citation2]. All too often, these patients become dependent on opioid medications, which carry a risk of tolerance and subsequent physical dependence and multiple adverse effects such as somnolence and constipation, and the potential of death from an overdose [Citation3,Citation4]. Therefore, enormous efforts are exerted to find additional approaches and to provide alternative options with a better safety profile and comparable efficacy.
Cannabis, the plant source of cannabinoids (CB), have been used for millennia for different purposes such as pain control and stress relief. Recently, the delineation of the endocannabinoid system and CB receptors in humans has paved the road for broader applications [Citation5]. Their natural source and the widespread use besides its lower risk of addiction or dependency and relative safety have flagged them for in-depth investigation for potential therapeutic roles. Two molecules have been of high-interest: cannabidiol (CBD) and tetrahydrocannabidiol (THC) [Citation6].
Recent evidence highlights cannabinoids’ efficacy and safety for pain control. Whiting et al. analyzed 28 clinical trials evaluating cannabinoids in pain control, concluding that there is a moderate-quality evidence that cannabinoids may result in marked pain reduction [Citation7]. This was consistent with its effect on neuropathic pain where cannabinoids were effective in pain relief [Citation8,Citation9]. National Academies of Science, Engineering, and Medicine conducted an extensive systematic review to evaluate cannabinoids, stating that there is ‘extensive evidence’ of cannabinoids’ efficacy in pain relief with good tolerability [Citation10].
Besides its potential direct effects on pain, cannabinoids are suggested to have a role in reducing opioid intake [Citation11,Citation12]. A recent report has highlighted the lower mortality from opioids’ overdose in states with medical cannabis legalization. Similarly, Medicare prescriptions’ reports revealed reduced opioids’ use and their consequent adverse events in the United States (U.S.) with legalized cannabis access [Citation13]. In addition, cannabinoids use may mitigate the escalation of opioid doses in patients with chronic pain with a substantial reduction in opioid intake [Citation14].
The above studies reference the effects of both THC and CBD, the two most abundant and frequently used cannabinoids. THC is a psychogenic molecule responsible for eliciting a ‘high’ sought out in recreational marijuana use. CBD is not intoxicating and therefore possesses an arguably better safety profile than THC [Citation15–Citation17]. Because of these differences in intoxication and abuse potential, the THC and CBD experience varying regulatory paths. Despite increasing state-level legalization, cannabis plants with higher levels (>0.3%) of THC are considered ‘marijuana’ and are federally illegal in the U.S [Citation18]. Low THC (<0.3%) cannabis plants, known as hemp, and its extracts have been recently deemed federally legal in the U.S. via pilot programs in Section 7606 of the 2014 Farm Bill, subsequently made permanent via the 2018 Farm Bill [Citation19–Citation21]. Therefore, hemp-derived cannabis extracts, low in THC and high in CBD, have become increasingly available as over-the-counter products and subject to widespread consumer use across diverse populations [Citation22].
Aiming to control the opioid epidemic, CBD has been investigated for its potential to reduce the addiction risk and physiological dependence features of opioid use while subsequently managing pain [Citation23]. Preclinical models demonstrate CBD's ability to decrease relapse risk by reducing opioid seeking behavior [Citation24]. Early human trials confirm CBD's potential in reducing opioid withdrawal symptoms [Citation25]. A recent survey study concluded that 44% of hemp CBD users reported it helped reduce the use of their opioid pain medication [Citation25]. CBD was found to reduce the craving, anxiety and psychological manifestations significantly in drug-abstinent individuals with previous opioid dependency [Citation26,Citation27]. Emerging literature supports evidence for CBD in pain relief and opioid reduction, but no studies to date have evaluated the effects of readily available hemp CBD in chronic pain and opioid use in a single cohort.
In the present study, we aim at investigating the impact of hemp CBD use on opioid use in chronic pain, disability, physical and psychosocial symptoms, sleep, and motivation to taper opioids. We believe that such a study will fill the existing gap and highlight potentially applicable roles for CBD therapeutic indications.
Methods
Study design and ethical considerations
Our study is a prospective, single-arm cohort study that was carried out at Murphy Pain Center, U.S. The study was granted an Institutional Review Board approval from Advarra. All study procedures were conducted in accordance with the declaration of Helsinki. In addition, all enrolled participants had to sign an IRB approved informed consent form at a standard of care visit. For confidentiality, all participants’ data have been coded and stored separately from participants’ identifiers and contact information. The crosswalk between the study ID and participant identifiers has been stored separately from participant data in a password-protected file within a password-protected folder on a secure server system.
Participants’ recruitment
Between September and December 2018, patients were informed about the study aims and procedures at their standard care visits at Murphy Pain Center. Patients were enrolled if they met the following eligibility criteria: (1) age between 30 and 65 years old, (2) has moderate to severe chronic pain for at least 3 years, and (3) has been stable on opioids for at least 1 year (defined as less than 10% change in its severity). The Morphine Equivalent Daily Dose (MEDD) of the administered opioid had to be at least 50 to be enrolled in the study.
Participants were excluded if they had (1) any history of substance use disorder, (2) any psychotic disorder, (3) abnormal drug screen over the last 12 months, (4) history of non-fatal overdose, (5) any epileptic activity in the last 12 months, (6) incapacitating systemic disorder (cardiac, renal or hepatic), or (7) any known allergy to cannabis-based products.
CBD-rich hemp-extract use
Participants were offered a free sixty-count bottle of hemp-derived (15 mg), CBD-rich soft gels at baseline, and weeks 4, and 8, which were provided for free by Ananda Professional. Each soft gel contained 15.7 mg CBD, 0.5 mg THC, 0.3 mg cannabidivarin (CBDV), 0.9 mg cannabidiolic acid (CBDA), 0.8 mg cannabichrome (CBC), and >1% botanical terpene blend. Participants were educated on safe CBD use, and ultimately elected whether or not to use CBD and self-titrated their dose of CBD. Of the 97 participants who completed the study, 94 chose to use the CBD soft gels. Almost all participants (91) used two soft gels (~30 mg) daily. One participant supplemented his free bottle of CBD soft gels and consumed four soft gels (~60 mg) daily. Two participants reported using only one soft gel (~15 mg) daily.
Three participants chose not to use CBD. Two initiated the CBD but reported the adverse effect of drowsiness and stopped using the soft gels. One participant declined CBD and expressed his concern in that he could not afford to pay out of pocket for the product after the end of the study if it was successful. One participant reported that CBD ‘made her heart race’ and combined twice-daily dosing into one dose to manage the side effect. One participant reported nausea from CBD but continued using the product. One participant reported ‘heart burn and dry mouth’ after initiating CBD. One participant reported CBD increased her nighttime anxiety and disturbed sleep. No significant adverse events were reported.
Outcomes’ assessment and follow-up points
The primary outcome of the study was the effectiveness of the CBD-rich extract to reduce the dependence on opioids for pain control measured via the opioids’ dose. Secondary outcomes included the pain-related quality of life (QoL) changes which were assessed by Pain Disability Index (PDI), 4-item Patient Health Questionnaire (PHQ-4), Pittsburgh Sleep Quality Index (PSQI), 3-item scale assessing Pain Intensity and Interference (PEG). The willingness of the patients to taper their opioid medications by administering the readiness to Taper Visual Analog scale was also included. Three data collection points were assigned for each participant: baseline, week 4, and week 8 with corresponding levels of CBD-rich extract use at each interval. The outcomes’ assessment at each of the three timepoints was conducted via phone or in-person interviews on the web or paper-based questionnaires and/or scales according to the patient’s preference. In addition, open-ended comments were allowed and documented for qualitative analysis.
Patient-reported side effects were collected at each clinic visit and at the time of follow-up questionnaire completion. Side effects were considered serious if they were life-threatening, resulting in hospitalization or emergency department visits, or required medical intervention for resolution. No serious side effects were reported.
Data analysis
Data analysis was conducted using SPSS v.24 software (SPSS Inc., Chicago, IL). Continuous variables are presented as mean ± SD. Cronbach’s alpha was calculated for each of the included scales to assess the reliability of indices. Normal distribution of the data was checked by the Kolmogorov–Smirnov test. If normally distributed, ANOVA test was used to compare the means of the outcomes between the three levels of CBD-rich extract use. For categorical variables, chi-Square test was used. Otherwise, the corresponding non-parametric tests were used. P value <0.05 was considered statistically significant in all these tests.
Results
The study recruited 131 participants, 97 of whom completed the 8-week follow-up period. All of the 97 participants, 31 males & 66 females, had a documented diagnosis of chronic pain and were on a stable on opioid dose for at least 2 years. The mean age of the study population was 56.1 years (range from 39 to 70 years). Ninety-four (96.9%) out of the 97 participants who completed the 8-week follow-up period used CBD hemp extract.
Primary outcomes
Fifty of the 94 (53.2%) participants using the CBD hemp extract were able to reduce opioid medications at week 8. Additional reductions in polypharmacy on the medication receipt were noted; six participants reported reducing or eliminating their anxiety medications, and four participants reported reducing or eliminating their sleep medication. None of the three participants who declined to use CBD hemp extract reduced their opioid medication at any interval.
Secondary outcomes
Eighty-nine (94%) of the hemp CBD users reported improved quality of life outcomes on subjective, open-ended questions. Quality of life was further evaluated by the four indices and/or questionnaires PDI, PHQ-4, PSQI, and PEG. For each of these indices, the reliability was measured through the Cronbach’s alpha which was relatively high for all of them, indicating good reliability. Cronbach’s alpha was 0.88, 0.77, 0.63, and 0.89 for the PDI, PHQ-4, PSQI, and PEG, respectively.
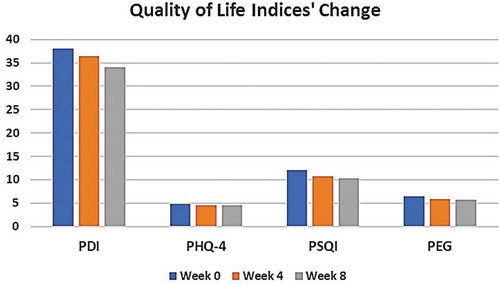
The first index, PDI, was assessed with its seven components and showed no significant changes over the study duration, starting from 38.02 (95% CI 35.38–40.66) at baseline, declining to 36.4 (95% CI 34.15–38.73) and 34.1 (95% CI 31.61–36.58) at weeks 4 and 8, respectively (p = 0.09). In addition, the change in the PHQ-4 for the CBD-rich extract showed no statistically significant difference over the follow-up period (4.8 [95% CI 4.18–5.41] at baseline and 4.5 at week 4 [95% CI 3.95–5.12] and week 8 [95% CI 3.79–5.14], p = 0.7).
Sleep quality was assessed via the PSQI. The mean score value significantly changed from 12.09 (95% CI 11.37–12.80) at baseline to 10.7 (95% CI 9.99–11.44) and 10.3 (95% CI 9.48–11.20) at week 4 and week 8, respectively (p = 0.03). Similarly, the PEG scale showed significant difference among the follow-up points (6.5 [95% CI 6.16–6.81], 5.9 [95% CI 5.55–6.25]and 5.7 [95% CI 5.31–6.12] at baseline, week 4 and week 8, respectively, p = 0.006). and show the change in the quality of life indices.
Table 1. Quality of life indices’ change over the study duration.
The willingness to reduce the opioid dose was evaluated through the readiness to taper the visual analog scale. The average score was 4.6 (95% CI 4.1–5.3) at baseline, and 4.4 at weeks 4 (95% CI 3.7–5.1) and 8 (95% CI 3.6–5.2) (p = 0.8) with no significant change.
To assess the impact of gender, all parameters were compared between males and females in our study population. No statistically significant differences between both genders were detected for all time points, except for PHQ-4 score which showed a significant decline in males compared with females at week 8 (indicating better effectiveness in males). However, this significant difference was not present at baseline or week 4.
Discussion
The results of this study suggest that using CBD-rich hemp extract oil may help reduce opioid use and improve quality of life, specifically in regards to pain and sleep, among chronic pain patients. This is consistent with emerging literature on the topic, which has concluded that CBD is an effective analgesic, and one that helps reduce barriers to opioid reduction, such as physiological withdrawal symptoms [Citation22–Citation31]. Recently, Wiese et al. summarized the evidence for different used of the cannabinoids with opioids [Citation13]. Haroutounian et al. evaluated the pain in 308 patients using S-TOPS pain score. The score was significantly improved from 83.8 to 75, with 65.9% of patients reported pain improvement. In addition, sleep quality showed significant improvement [Citation28]. Additional studies showed significant improvement in pain symptoms using CBD as augmentation for opioids [Citation9,Citation25–Citation31].
Limitations
At the beginning of the study, the investigator hypothesized that participants may report reduced opioid use in order to appear agreeable to the interviewers, but this concern changed with experience. In reality, many participants disclosed that they were hesitant to report any reduction in opioid use due to the potential consequences of changes in prescriptions. If CBD-rich hemp extract helped their pain and resulted in decreased opioid use, this would result in limited prescription opioids. They often questioned what would happen at the conclusion of the study if the patient could not access affordable CBD-rich hemp extract and their pain returned to baseline. Opioids are likely covered by insurance, but CBD-rich hemp extract is not.
Additionally, there is a small amount (<0.3%) of delta-9-THC, the intoxicating compound abundant in marijuana plants, in CBD-rich hemp extract. This poses a risk that participants could fail a drug test at work or in other pain management settings, compromising their employment, livelihood, and medical care. They could not risk this consequence. It is important to consider that while the study population did not include participants with dual diagnoses or a history of substance use disorders, but nevertheless several participants described themselves as ‘addicted to’ their opioid pain medications.
According to the World Health Organization, the public health risk of CBD is considered limited, but cannabis use is not absent of abuse risk or addiction potential [Citation32,Citation33]. Cannabis derived from hemp, including the product used in this study, is high in CBD and low in THC and is considered less harmful than the alternative [Citation34]. Still, cannabis use disorder is real and some studies show cannabis may perpetuate the cycle of addiction or lead to other substance abuse [Citation35,Citation36]. Additionally, even with cannabis products with low THC concentration, the aroma of cannabis itself could present the risk of cue-induced drug-seeking behavior in those with previous substance use disorder.
Our study limitations include a lack of a randomized, placebo-controlled design. The short length of the study, lack of control group, and relatively small sample size limit conclusions. The attrition rate was moderate and was influenced by external factors such as changes in or loss of patient insurance plans and alteration in insurance policies at the primary clinic over the course of the study. Several participants’ phone service was canceled over the course of the study which contributed to the attrition rate. As with all voluntary participation studies, the potential differences in those who agreed to participate versus those who declined to participate may influence the study conclusion.
Pain diagnoses, comorbidities, type of opioid, opioid dose and CBD dose also varied among the participants. Several patients used fentanyl patches and therefore could not self-titrate to reduce opioid intake, as was possible with short-acting oral medications, such as oxycodone/acetaminophen and oxycodone controlled release. Variables such as changes in weather may contribute to pain, as the study began in warm months and concluded in colder months, a factor many participants reported as influencing their pain. Several participants noted significant life events that likely influenced pain and medication use, such as a car accident or surgery, during the study period. A final average change in MEQ over time would have been a valuable data point, but it was not available as many prescriptions did not officially change due to short study duration. Two participants reported completely eliminating opioid use over the 8-week period, while others reported deliberately skipping or forgetting doses of opioid medication. Some reported skipping or missing doses every day, whereas others did so irregularly. Still, more detailed data on average MEQ change over time would have improved clarity of study outcomes.
Finally, due to their subjective natures, the variables of pain, sleep, and mood are difficult variables to assess, even with validated instruments. The risk of confirmation and response biases during interviews cannot be ignored.
Future research should expand on these findings and include larger, randomized, placebo-controlled trials. These results also signal a need for improved clinical education on the topic, particularly in the pain management specialty, and potential adjustments to drug-test policies within clinics and across employers.
Conclusion
This study concludes that using CBD for chronic pain in patients using opioids has a significant effect on reducing opioid intake, reducing pain and improving QoL. Over half of the participants who added CBD hemp extract reduced or eliminated opioids over the course of 8 weeks, and almost all CBD users reported improvements in QoL.
Data sharing
The data that support the findings of this study are available from the corresponding author, AC, upon reasonable request.
Reviewer disclosure
One of the reviewers is a Consultant/Speaker and Researcher for US World Meds, BDSI, Salix, Enalare, Scilex, and Neumentum. They have no relationship with this specific research.
Declaration of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download MS Word (19.7 KB)Acknowledgments
James Patrick Murphy, MD
James Murphy granted access to his patient population for this study and allowed enrollment and data collection in his clinic, Murphy Pain Center. He has agreed to be acknowledged in this study.
Data analysis and editorial assistance were provided by Omar Aboshady a medical writer and freelancer at Upwork.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Gatchel RJ, McGeary DD, McGeary CA, et al. Interdisciplinary chronic pain management: past, present, and future. Am Psychologist. 2014;69:119.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8:287–313.
- Smith HS, Kirsh KL, Passik SD. Chronic opioid therapy issues associated with opioid abuse potential. J Opioid Manag. 2009;5(5):287–300.
- Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004 Dec 1;112(3):372–380.
- Whittle BA, Guy GW, Robson P. Prospects for new cannabis-based prescription medicines. J Cannabis Ther. 2001;1:183–205.
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180.
- Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–1385.
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. Jama. 2015;313:2456–2473.
- Serpell M, Ratcliffe S, Hovorka J, et al. A double‐blind, randomized, placebo‐controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999–1012.
- Meng H, Johnston B, Englesakis M, et al. Selective cannabinoids for chronic neuropathic pain: a systematic review and meta-analysis. Anesthesia Analg. 2017;125:1638–1652.
- Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the national academies of sciences, engineering and medicine report. Eur J Intern Med. 2018;49:7–11.
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179.
- Wiese B, Wilson-Poe AR. Emerging evidence for cannabis’ role in opioid use disorder. Cannabis Cannabinoid Res. 2018;3:179–189.
- Bradford AC, Bradford WD, Abraham A, et al. Association between US state medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Intern Med. 2018;178:667–672.
- Birnbaum A. How high can patients get on CBD? Epilepsy Curr. 2019 Sep 16:1535759719874408.
- Dill JL, Kurkowski A. CBD: considerations for use within the health system. Hosp Pharm. 2019. doi:10.1177/0018578719873870.
- Crippa JA, Guimarães FS, Campos AC, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. doi:10.3389/fimmu.2018.02009.
- Dragone D, Prarolo G, Vanin P, et al. Crime and the legalization of recreational marijuana. J Econ Behav Organiz. 2019 Mar;1(159):488–501.
- Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017 May;1(70):288–291.
- Malone T, Gomez K. Hemp in the United States: a case study of regulatory path dependence. Appl Econ Perspect Policy. 2019 Mar 8;41(2):199–214.
- Mead A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front Plant Sci. 2019;10. doi:10.3389/fpls.2019.00697.
- Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018 Jul 1;3(1):152–161.
- Khan SP, Pickens TA, Berlau DJ. Perspectives on cannabis as a substitute for opioid analgesics. Pain Manag. 2019 Jan 25;9(2):191–203.
- Ren Y, Whittard J, Higuera-Matas A, et al. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009 Nov 25;29(47):14764–14769.
- Moeller-Bertram T, Schilling J, Hughes C, et al. (360) can CBD reduce the use of pain medication? Lessons from a survey in a pain clinic environment. J Pain. 2019 Apr 1;20(4):S64.
- Hurd YL, Yoon M, Manini AF, et al. Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics. 2015 Oct 1;12(4):807–815.
- Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019 May 21. doi:10.1176/appi.ajp.2019.18101191
- Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain. Clin J Pain. 2016 Dec 1;32(12):1036–1043.
- Knopf A. CBD may help prevent relapse in abstinent heroin addicts. Alcohol Drug Abuse Weekly. 2019 Jun 3;31(22):3–4.
- Johnson JR, Lossignol D, Burnell-Nugent M, et al. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218.
- Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–154.
- World Health Organization. Cannabidiol (CBD) pre-review report agenda item 5.2. Expert Committee on Drug Dependence Thirty-ninth Meeting; Geneva; 2017.
- Lau N, Sales P, Averill S, et al. A safer alternative: cannabis substitution as harm reduction. Drug Alcohol Rev. 2015;34(6):654–659.
- Curran HV, Freeman TP, Mokrysz C, et al. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci. 2016;17(5):293.
- Zehra A, Burns J, Liu CK, et al. Cannabis addiction and the brain: a review. FOCUS J Am Psychiatr Assoc. 2019;17(2):169–182.
- Simpson AK, Magid V. Cannabis use disorder in adolescence. Child Adolesc Psychiatr Clin N Am. 2016;25(3):431–443.