ABSTRACT
Background
Thyroid fine needle aspiration (FNA) is the mainstay for diagnosis of malignancy, and is an integral part of current thyroid nodule assessment. The present study analyzes the diagnostic accuracy of palpation-directed versus ultrasound guided fine-needle aspiration in patients who underwent surgery for thyroid nodules.
Methods
A retrospective chart review of all consecutive patients who had FNA biopsy (palpation or ultrasound guided) of thyroid nodules and underwent thyroid gland surgery between 1998 and 2014 was conducted. The FNA findings of the palpation-guided and ultrasound-guided groups were compared for baseline characteristics. Moreover, the diagnostic accuracy of FNA findings and surgical histopathology results were analyzed.
Results
A total of 1174 patients were included in the study with a mean age of 46.3 ± 11.7 years and the majority were females (75.5%). Among the study population, 392 (33.4%) patients underwent US-guided FNA; 570 (48.6%) had palpation-guided FNA in clinic and no FNA was done in 212 (18%) cases. Patients underwent US-guided FNA were more likely to have suspicion of malignancy (p = 0.001), and had indeterminate findings (p = 0.001). On the other hand, palpation-guided FNA group had significantly higher frequency of benign cytology (p = 0.001). With respect to the suspicion for malignancy as well as malignancy, the US-guided group had a similar diagnostic accuracy in comparison to the palpation group. The proportion of malignancy finding on US-guided FNA (8.9%) was higher than the palpation-guided FNA (6.4%) that had been confirmed on postoperative histopathological examination (p = 0.95).
Conclusion
The present study demonstrates higher sensitivity of US-guided thyroid FNA biopsies over palpation-guided FNA for the suspicion of malignancy; however, the accuracy is comparable. Moreover, both groups showed more postoperative malignancy in the benign and unsatisfactory categories than predicted in the Bethesda system. Further prospective studies are needed to underpin a realistic correlation between FNA and final histopathology reports.
Introduction
Thyroid nodules are a commonly an encountered clinical condition. Its progressively increasing incidence is often attributed to the frequent use of neck ultrasound, which resulted in detection of more incidental and otherwise nonpalpable thyroid nodules [Citation1]. Therefore, the estimated prevalence of thyroid nodules relies primarily on the method of detection which could be palpation of the nodule on general physical examination or by radiographic examination [Citation2]. Notably, the prevalence of palpable nodules on physical examination alone in adult population is as low as 5–7% [Citation3]. On the other hand, thyroid nodules are frequently detected upon neck ultrasonography which ranges from 19% to 67%; of which only 4–7% are also palpable on physical examination [Citation4]. The reported prevalence of thyroid nodules in some studies is as high as 68% using high-resolution ultrasound in randomly chosen subjects [Citation5]. However, the clinical importance lies in identifying thyroid cancer, which is the most common endocrine malignancy [Citation6]. Earlier reports showed that around 7–15% of thyroid nodules were diagnosed to have malignancy.
Most thyroid cancers are well differentiated with good prognosis. The reported mortality is as low as 6% for papillary thyroid cancer and 10% for follicular cancer over 30-year period [Citation7]. This highlighted the importance of its early detection and diagnoses to achieve better outcome in terms of long-term survival. For accurate diagnosis, preoperative assessment and tissue sampling is of paramount importance to minimize the false negative findings and thus minimizing unnecessary surgical interventions. Fine needle aspiration (FNA) cytology of thyroid nodules is a safe nonsurgical approach with diagnostic sensitivity ranges from 65% to 85% and specificity of 72–100% [Citation8]. However, inadequate sampling or inappropriate preparation technique leads to nondiagnostic findings, multiple clinic visits for resampling and delay in definitive treatment in about 20% [Citation2]. There are different opinions about the suitability of palpation- versus ultrasound-guided FNA method for the initial diagnostic evaluation of thyroid nodule. Therefore, the present study analyses the diagnostic accuracy of palpation-directed versus ultrasound guided fine-needle aspiration in patients who underwent surgery for thyroid nodules in a tertiary care hospital.
Methods
It is a retrospective chart review of all consecutive patients who underwent thyroid gland surgery and had FNA biopsy (palpation or ultrasound guided) of thyroid nodules at Hamad general hospital the only tertiary hospital in the state of Qatar, between January 1998 and July 2014. Data included patients demographics (age, gender), body mass index, presenting symptoms (firm neck mass, dyspnea, pain, dysphagia, hoarseness of voice), comorbidities (hyperthyroidism, hypertension, diabetes mellitus, dyslipidemia), personal and family history of cancer, findings of ultrasonography, FNA (palpation and US-guided) and histopathology, type of excision (total thyroidectomy, hemi-thyroidectomy, subtotal thyroidectomy), and type of cancer (papillary carcinoma, medullary carcinoma, follicular carcinoma, hurthle cell carcinoma, anaplastic carcinoma, poorly differentiated carcinoma). FNA cytology findings were categorized into suspicion of malignancy, benign, indeterminate and inadequate/unsatisfactory for evaluation as per the recommended guidelines as mentioned by Can et al [Citation9]. As per the guidelines used for the present study the indeterminate fine needle aspiration cytology refers to ‘suspicious,’ ‘follicular lesion,’ or ‘follicular neoplasm,’ or cytologic features suggestive of ‘atypia’ [Citation10]. Notably, in some patients more than one FNA was done for multiple lesions which are reflected in the FNA cytology findings. Particularly, for the ultrasound-guided FNA group, the median FNA done was 1 (1–4) with a median of 2 (range 1–4) passes performed using a 23-gauge needle. On the other hand, in the palpation-guided FNA group, the median FNA done was 1 (range 1–3) with median number of needle passes to be 2 (range 1–6) with a 21-gauge needle were performed, after which the specimens were evaluated by cytotechnologist to assess for adequacy of the tissue specimen. The ultrasound device which was used for US-guided FNA in our study was by GE Healthcare (GE Medical System Ultrasound and Primary Care Diagnostics, Wauwatosa, WI, USA).
Ethical approval was granted from the medical research canter. The Institutional Review Board (IRB# 14197/14) of the Hamad Medical Corporation has been approved and granted exempt status for this retrospective study.
Statistical analysis
Data were reported as proportion, mean (± standard deviation), median, and range, when applicable. The FNA findings of the palpation-guided and ultrasound-guided groups were compared for baseline characteristics. The two groups were compared using χ2 test for categorical variables and Student’s t test was used for comparison of continuous variables. A significant difference was considered when the two-tailed p value was less than 0.05. Moreover, the diagnostic accuracy of FNA findings and surgical histopathology results were analyzed using sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio (+LR), negative likelihood ratio (-LR). +LR is the probability of a person who has the disease testing positive divided by the probability of a person who does not have the disease testing positive. -LR is the probability of a person who has the disease testing negative divided by the probability of a person who does not have the disease testing negative. Data analysis was carried out using the SPSS version 18 (SPSS Inc., Chicago, Illinois).
Results
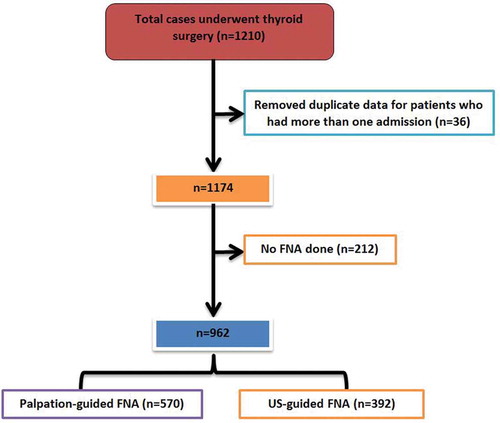
During the study period, a total of 1210 cases were registered to undergo surgery for thyroid nodules; of which 36 cases were excluded due to more than one admission (). Therefore, a total of 1174 patients were included in the final analysis. Among the study population, 392 (33.4%) patients underwent US-guided FNA; 570 (48.6%) had palpation-guided FNA in clinic and no FNA was done in 212 (18%) cases.
In our cohort the majority were females (75.5%). The mean age at presentation was 46.3 ± 11.7 years (). Presence of a firm neck mass (35.1%), dysphagia (19.5%) and dyspnea (17.5%) were the most common presenting symptoms. Only 6.0% patients presented with hoarseness, and few had palpable lymph nodes (2.9%). The ultrasonographic characteristics of thyroid nodules showed ill-defined margin in 26 (2.4%), hypoechogenicity in 341 (31.1%), hyperechogenicity in 134 (12.3%), the presence of microcalcification in 89 (8.1%), and intranodular vascularity in 297 (27.1%) cases. Hyperthyroidism (15.1%), hypertension (14.2%), and diabetes mellitus (12.2%) were the most prevalent comorbidities followed by dyslipidemia (6.9%) and hypothyroidism (4.6%). Thyroid disease as part of multiple endocrine neoplasia syndrome was found in only 6 (0.5%) cases and history of exposure to neck radiation was present in only one patient. Family history of thyroid cancer was identified in 19 (1.6%) cases. Most of the nodules were mixed solid/cystic or solid hypoechoic on ultrasound investigation.
Table 1. Demographics, presentation, predisposing factors and procedures performed in thyroid gland surgery (n = 1174).
Thyroid cancer on postoperative histopathology was identified in 264 (22.8%) patients (). Papillary carcinoma (89.4%) was the most frequent cancer type followed by medullary (4.2%) and follicular (3.0%) carcinomas. The majority of malignancy were limited to one lobe (70.7%), 23.6% of cancers were involving both thyroid lobes and 5.7% were located in the isthmus. Adenomas were identified in 134 (11.4%) patients.
Table 2. FNA and histopathological characterization (n = 1174).
shows the comparison of US-guided FNA versus palpation-guided FNA cases. The two groups were comparable for age, gender and BMI. Patients underwent US-guided FNA were more likely to have suspicion of malignancy (25.9% vs. 16.3%, p = 0.001), had indeterminate findings (14.8% vs. 9.2%; p = 0.007) and had frequently diagnosed with adenomas (16.3% vs. 11.6%; p = 0.03) postoperatively as compared to those who underwent palpation-guided FNA. On the other hand, palpation-guided FNA group had significantly higher frequency of benign cytology (70.1% vs. 55.4%; p = 0.001). However, the rate of unsatisfactory cytology (p = 0.07), malignancy on cytology (p = 0.09), and postoperative histopathology finding of cancer (p = 0.07) did not differ significantly between the two groups.
Table 3. Comparison of US-guided FNA vs. palpation-guided FNA cases.
demonstrates the diagnostic accuracy of FNA cytology (US-guided vs. Palpation-guided) for suspicion of malignancy for confirmed diagnosis of cancer. With respect to the suspicion of malignancy, the US-guided group had better sensitivity (69.7% vs. 52.3%) and positive predictive value (75.3% vs. 73.1%) in comparison to the palpation group. However, the specificity (94.3% vs. 91.1%) and positive likelihood ratio (9.21 vs. 7.8) was slightly higher in the palpation group. Moreover, the negative predictive values and accuracy were comparable among the two groups. The diagnostic accuracy of malignancy based on cytology was also assessed for the palpation-guided and US-guided FNA groups. Similarly, the US-guided FNA have slightly higher sensitivity as compared to palpation-guided FNA (36.7% vs. 33.1%). However, the positive likelihood ratio and negative likelihood ratio was greater for palpation-guided FNA group in comparison to US-guided FNA group.
Table 4. Diagnostic accuracy of FNA cytology (US-guided vs. palpation-guided) for suspicion of malignancy with confirmed diagnosis of cancer by histopathology.
shows the overview of the FNA findings in both groups (US-guided vs. palpation-guided FNA) with its final surgical pathology findings. compares the findings of US-guided FNA and palpation-guided FNA with post-operative histopathology findings. In the US-guided FNA group, the findings of benign cytology, suspicion of malignancy and malignancy were confirmed by postoperative histopathology in 75.3%, 75.2%, and 88.9% cases, respectively. Similarly, in the palpation-guided FNA group, the findings of benign cytology, suspicion of malignancy and malignancy were confirmed by post-operative histopathology in 76.4%, 73.1%, and 91.5% cases, respectively which was comparable among the two groups. However, the proportion of indeterminate finding on palpation-guided FNA (32.2%) was higher than the US-guided FNA (19.0%) to have confirmed diagnosis of malignancy post-operatively.
Figure 2. Shows the overview of FNA findings in both groups with its final surgical pathology findings (P-FNA: palpation-guided FNA; US-FNA: ultrasound-guided FNA).
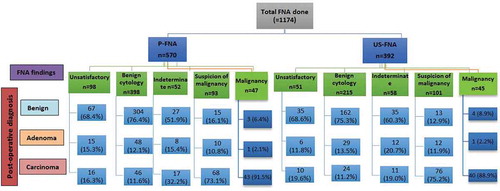
Table 5. Comparison of US-guided FNA findings with postoperative diagnosis.
Discussion
The present study reports the diagnostic accuracy of palpation- versus US-guided thyroid fine-needle aspiration cytology in a tertiary care hospital. Up to our knowledge, the sensitivity analysis and accuracy of FNA cytology have not been well documented in the Arabian Gulf region. There are several key findings in the present study. Patients underwent US-guided FNA were more likely to have suspicion of malignancy and indeterminate findings whereas; palpation-guided FNA group had significantly higher association with benign cytology. Particularly, the US-guided FNA could be useful to predict the suspicion of malignancy with higher sensitivity and positive predictive value as compared to the palpation-guided FNA but both the two groups have similar accuracy. Notably, the proportion of indeterminate finding on palpation-guided FNA was two-fold higher than the US-guided FNA to have confirmed diagnosis of malignancy post-operatively.
The increased usage and experience with ultrasound of the neck resulted in detection of more incidental and non-palpable thyroid nodules [Citation11]. The prevalence of thyroid nodules ranges between 19% and 68% in patients who underwent random ultrasonography of the neck [Citation4]. This high prevalence of thyroid nodules requires an evidence-based approach for appropriate diagnosis and management which may lead to reduction of the unnecessary surgical intervention and its associated complications [Citation1].
For thyroid diseases, FNA cytology is a useful diagnostic modality for clinical decision making and distinction from benign and malignant nodules in adult population [Citation12]. FNA cytology is considered to be an accurate (sensitivity: 76–98% and specificity: 71–100%) and cost-effective method for evaluating thyroid nodules [Citation13]. This is consistent with our finding for suspicion of malignancy with an accuracy of 85%. It can be performed with the help of ultrasonography or by palpation in the clinic. For thyroid nodules which are difficult to palpate due to posterior location or smaller size, US-guided FNA is usually preferred [Citation14]. In the present study, 33.4% patients underwent US-guided FNA and 48.6% had palpation-guided FNA in the outpatient clinic. However, FNA findings of the nodule could be challenging especially with cytology revealed to have suspicious, inadequate, or indeterminate findings [Citation15].
The Bethesda system for reporting FNA cytology classified results into six categories such as unsatisfactory, benign, atypia of undetermined significance, follicular neoplasm, suspicious for malignancy and malignant [Citation16]. In case of unsatisfactory cytology, the FNA needs to be repeated [Citation14] which ultimately results in increased cost (clinic revisits) and inconvenience to the patient from delayed management. Moreover, attaining multiple inadequate samples could eventually direct the surgeon toward performing thyroid lobectomy which might turn out to be negative for malignancy or may require another visit to the operating theater for completion thyroidectomy should lobectomy prove to be malignant.
In order to decrease the rate of non-diagnostic FNA, several authors advised the routine use of US-guided FNA for sampling [Citation17–Citation20]. Danese et al. [Citation18] retrospectively studied 9683 patients with thyroid nodules and reported that the rate of inadequate cytology was much lower with US-guided FNA (3.5%) in comparison to palpation-guided FNA (8.7%). Our findings are consistent with the earlier reports which showed inadequate FNA smears in 9–47% of palpation-guided and 4–21% of ultrasound-guided cases [Citation9]. Our study demonstrated slightly lower rate of unsatisfactory sampling by US-guided FNA (13%) than palpation guided-FNA (17.3%). A previous study also demonstrated that US-guided FNA results in lesser unsatisfactory findings as compared to palpation-guided FNA [Citation9]. In contrast, Choong et al. demonstrated similar rate of indeterminate and false negative FNA findings for US-guided as well as palpation-guided FNA [Citation21]. Notably, an earlier study suggested better sensitivity and accuracy of US-guided FNA as compared to palpation-guided FNA for smaller thyroid nodules (<2 cm in diameter) [Citation22,Citation23]. Our study also demonstrated higher sensitivity of US-guided FNA for the diagnosis of malignancy but the overall accuracy was comparable among the two groups. These findings are supported by an earlier systematic review and meta-analysis which suggested a higher diagnostic accuracy of US-guided FNA and lower rate of inadequate samples in comparison to palpation-guided FNA [Citation24].
A recent study suggested the superiority of US-guided core needle biopsy over US-guided FNA with lesser false negativity and better sensitivity for large thyroid nodules (≥2.0 cm) to diagnosis thyroid malignancy [Citation25]. An earlier study assessed the diagnostic accuracy of US-guided FNA based on size and US findings [Citation26]. The authors suggested that patients with smaller nodule size (≤5 mm) are more likely to have false-positive findings; while the chances of false-negative FNA findings is more with larger thyroid nodules (>20 mm) and remarkable US features. The ideal maximum rate of unsatisfactory cytology was suggested by some authors not to exceed 10% [Citation27]. In order to minimize this rate even further, some authors advocated the performance of on-site cytological evaluation [Citation14]. Other options to include cell block preparation which includes centrifugation of the FNA sample to decrease cell dispersion of the smears. When performed, rate of unsatisfactory cytology was reported to be as low as 4.3% [Citation27].
In our study, the 23-gauge needle was frequently used in ultrasound-guided group, whereas, FNA was performed by 21-gauge needle in the palpation-guided group. Consistent with our findings, it has been shown that fine (22- to 27-gauge) needles are most commonly used for FNA biopsy; particularly aspiration with a 27-gauge needle yield adequate tissue specimen for appropriate cytopathologic examination [Citation28].
In the present study, 70% of palpation-guided and 55% of ultrasound-guided FNA smears had benign cytology. This is in agreement with other reports showed benign cytology (around 70%) to be the most frequent findings of the FNA biopsies [Citation1]. Furthermore, the reported risk of malignancy among cases with benign cytology on FNA was low (0–3%) [Citation1]. Therefore, patients with benign nodules on US-guided FNA are mostly treated nonoperatively; without further immediate diagnostic work-up [Citation14]. With respect to benign cytology results, our study showed that the rate of malignancy was comparable among the US-guided and palpation-guided groups. This shows that there is a risk of false negative results associated with cytology reporting which needs to be carefully considered for long-term management and follow-up [Citation29].
With recent practice of FNA, the percentage of thyroid nodules excised that were malignant is reported to exceed 50% in comparison to 14% before the routine use of FNA [Citation30]. The American Thyroid Association recommended FNA for all thyroid nodules >1 cm with high or intermediate suspicion of malignancy and for nodules >1.5 cm with low suspicion sonographic pattern [Citation14]. In our cohort, the cytological finding of suspicious malignancy was significantly higher in US-guided FNA than those with palpation-guided FNA. Similarly, the postoperative histopathological results also showed higher rate of malignancy in the US-FNA as compared to palpation-FNA that did not reach statistical significance. In line with our findings, earlier studies showed a significant correlation between FNA cytology findings of suspicious malignancy and the final diagnosis [Citation13,Citation31]. Therefore, US-FNA findings of suspicious malignancy could be utilized for considering the need and extent of surgical intervention [Citation32].
The current literature reported variable findings for the FNA categories as per the Bethesda system and the predicted risk of malignancy () [Citation33–Citation38]. Our study showed higher malignancy rate in the benign category for both the groups (11%) in comparison to the predicted risk of malignancy by Bethesda system (0–3%) as well as higher malignancy rate in the unsatisfactory (16.3% and 19.6%) and indeterminate (32% and 19.0%) categories against the predicted risk of 1–4% and 5–15%, respectively. Whereas, lower rate of histology confirmed malignancy was identified in the cytology based malignancy category (91.5% and 88.9%) in comparison to the predicted risk by Bethesda system (97–99%). On the other hand, Jo et al. [Citation34] reported malignancy rates which correlated well with the Bethesda risk for the benign FNA (1.1%) but higher for unsatisfactory (8.9%) FNA samples that eventually reported as malignant in postoperative histopathology. Conversely, Williams et al [Citation35] reported 16% malignancy rate in the benign FNA category and 18.2% malignancy rate in the unsatisfactory FNA category. This could be related to the fact that until recently, there were significant differences in the way FNA samples were reported which highlighted the importance of unified reporting criteria among cytopathologists and could also be attributable to the selection bias. Our findings support that classifying thyroid lesions plays an important role in stratifying patients for the malignancy risk based on initial diagnosis of FNA cytology that will help in clinical decision-making for the appropriate management. As the diagnostic work-up of indeterminate lesions of the thyroid remains difficult by cytopathology, so the introduction of novel molecular testing has promising role in better stratification and prognostication for Bethesda III and IV [Citation39]. Therefore, molecular testing should be considered as a potential alternative for follow-up in patients with indeterminate thyroid FNAs which might eventually help in minimizing unnecessary risk associated with surgery and cost of treatment [Citation40].
Table 6. Comparison of FNA findings by the Bethesda system for predicted risk of malignancy.
The present study has certain limitations attributed to the retrospective observational design and diagnostic accuracy of thyroid FNAs that necessitates careful investigations. Expertise, preferences and practical differences between surgeons might account for the variability in the approach of FNA cytology (US-guided vs. palpation-guided) and in turn findings of the diagnostic cytology and rates of malignancy. Notably, our institution did not follow the exact Bethesda System to categorize findings of the thyroid FNA which may affect generalizability. Furthermore, there might be chances of under-representation of the FNA categories as unlike Bethesda system we have only five categories and did not report ‘Suspicious’ for follicular neoplasm category in our series. Also, in the available data we lack information for patients that underwent US-guided FNA and had palpable masses. Finally, there was an inconsistency in the risk factor profile of our patients that was collected over a considerable period of time for this study which limits our understating of the clinico-pathological outcomes. The initial rate of unsatisfactory samples was high, for long time ago we were having only onsite technologist we fix and collect samples (not for sample adequacy), and if sample came inadequate we call the patient again to repeat the process. Later on the hospital quality assurance assigned an onsite cytologist to assess for sample adequacy. To reduce the false results, the number of pass can be increased to 4 and to the address the multinodularity.
Conclusion
The present study demonstrates a relatively higher sensitivity of US-guided thyroid FNA biopsies over palpation-guided FNA for the suspicion of malignancy, however, the accuracy is comparable. Nodule with suspicious for malignancy on either palpation or US-guided FNA cytology are more likely to be diagnosed to have malignancy on postoperative histopathology. Moreover, both groups showed more postoperative malignancy in the benign and unsatisfactory categories than predicted in the Bethesda system. Further prospective studies are needed to determine whether more attention and careful selection of FNA sampling areas would yield less non diagnostic samples and realistic correlation between FNA cytology and final histopathology findings.
Authors’ contributions
IT, HA, AE, MA, MA and AT: All authors contributed to the study design, the analysis and interpretation of data, and manuscript writing. All authors have read and approved the final manuscript.
Consent for publication
This study granted ethical approval from the medical research center and institutional review board of Hamad Medical Corporation, Doha, Qatar
Declaration of interest
The contents of the paper and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication.
Ethics approval and consent to participate
This study granted ethical approval from the medical research center and institutional review board of Hamad Medical Corporation, Doha, Qatar (IRB#14197/14).
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We thank the surgery department of Hamad General Hospital in Doha, Qatar for their kind cooperation. The publication of this article was funded by the Qatar National Library.
References
- Tamhane S, Gharib H. Thyroid nodule update on diagnosis and management. Clin Diabetes Endocrinol. 2016;2:17.
- Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329–349.
- Zamora EA, Khare S, Cassaro S. Thyroid nodule. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan [Updated 2019 Dec 2]. Available from http://www.ncbi.nlm.nih.gov/books/NBK535422/
- Dauksiene D, Petkeviciene J, Klumbiene J, et al. Factors associated with the prevalence of thyroid nodules and goiter in middle-aged euthyroid subjects. Int J Endocrinol. 2017;2017. DOI:10.1155/2017/8401518.
- Prochazka A, Gulati S, Holinka S, et al. Classification of thyroid nodules in ultrasound images using direction-independent features extracted by two-threshold binary decomposition. Technol Cancer Res Treat. 2019;18:1533033819830748.
- Nguyen QT, Lee EJ, Huang MG, et al. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015;8(1):30–40.
- Huang L-Y, Lee Y-L, Chou P, et al. Thyroid fine-needle aspiration biopsy and thyroid cancer diagnosis: a nationwide population-based study. PLoS One. 2015;10(5):e0127354.
- Isaac A, Jeffery CC, Seikaly H, et al. Predictors of non-diagnostic cytology in surgeon-performed ultrasound guided fine needle aspiration of thyroid nodules. J Otolaryngol Head Neck Surg. 2014;43(1):1–5.
- Can AS, Peker K. Comparison of palpation-versus ultrasound-guided fine-needle aspiration biopsies in the evaluation of thyroid nodules. BMC Res Notes. 2008;1:12.
- Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142.
- Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43(2):229–38, vii.
- Dean DS, Gharib H. Fine-needle aspiration biopsy of the thyroid gland. In: Feingold KR, Anawalt B, Boyce A, et al. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [Updated 2015 Apr 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK285544/.
- Lee MJ, Hong SW, Chung WY, et al. Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: emphasis on correlation with sonographic findings. Yonsei Med J. 2011;52(5):838–844.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Alshaikh S, Harb Z, Aljufairi E, et al. Classification of thyroid fine-needle aspiration cytology into Bethesda categories: an institutional experience and review of the literature. Cytojournal. 2018;15:4. eCollection 2018.
- Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the national cancer institute thyroid fine-needle aspiration state of the science conference. Diagn Cytopathol. 2008;36(6):425–437.
- Cai XJ, Valiyaparambath N, Nixon P, et al. Ultrasound-guided fine needle aspiration cytology in the diagnosis and management of thyroid nodules. Cytopathology. 2006;17(5):251–256.
- Danese D, Sciacchitano S, Farsetti A, et al. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8(1):15–21.
- Kaliszewski K, Zubkiewicz-Kucharska A, Wojtczak B, et al. Ultrasound guided fine-needle aspiration biopsy of thyroid nodules: does radiologist assistance decrease the rate of unsatisfactory biopsies? Adv Clin Exp Med. 2016;25(1):93–100.
- Izquierdo R, Arekat MR, Knudson PE, et al. Comparison of palpation-guided versus ultrasound-guided fine-needle aspiration biopsies of thyroid nodules in an outpatient endocrinology practice. Endocr Pract. 2006;12(6):609–614.
- Choong KC, Khiyami A, Tamarkin SW, et al. Fine-needle aspiration biopsy of thyroid nodules: is routine ultrasound-guidance necessary? Surgery. 2018 Oct;164(4):789–794.
- Hatada T, Okada K, Ishii H, et al. Evaluation of ultrasound-guided fine-needle aspiration biopsy for thyroid nodules. Am J Surg. 1998;175(2):133–136.
- Khalid AN, Quraishi SA, Hollenbeak CS, et al. Fine-needle aspiration biopsy versus ultrasound-guided fine-needle aspiration biopsy: cost-effectiveness as a frontline diagnostic modality for solitary thyroid nodules. Head Neck. 2008;30(8):1035–1039.
- Matz J, Abdolell M, Hayden J, et al. A systematic review and meta-analysis of palpation versus ultrasound-guided fine needle aspiration of thyroid nodules. DMJ. 2014;41(1):14.
- Lee HJ, Kim YJ, Han HY, et al. Ultrasound-guided needle biopsy of large thyroid nodules: core needle biopsy yields more reliable results than fine needle aspiration. J Clin Ultrasound. 2019 Jun;47(5):255–260.
- Dong Y, Mao M, Zhan W, et al. Ultrasound features affecting results of ultrasound-guided fine-needle aspiration of thyroid nodules. J Ultrasound Med. 2018 Jun;37(6):1367–1377.
- Cristo APD, Goldstein HF, Faccin CS, et al. Increasing diagnostic effectiveness of thyroid nodule evaluation by implementation of cell block preparation in routine US-FNA analysis. Arch Endocrinol Metab. 2016;60(4):367–373.
- Zhang L, Liu Y, Tan X, et al. Comparison of different-gauge needles for fine-needle aspiration biopsy of thyroid nodules. J Ultrasound Med. 2018 Jul;37(7):1713–1716.
- Baloch ZW, Cibas ES, Clark DP, et al. The national cancer institute thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008;5:6.
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2017;6(6):217–222.
- Kwak JY, Kim EK, Kim MJ, et al. The role of ultrasound in thyroid nodules with a cytology reading of “suspicious for papillary thyroid carcinoma”. Thyroid. 2008;18:517–522.
- Rahimi M, Farshchian N, Rezaee E, et al. To differentiate benign from malignant thyroid nodule comparison of sonography with FNAC findings. Pak J Med Sci. 2013;29(1):77–80.
- Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665.
- Jo VY, Stelow EB, Dustin SM, et al. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2010;134(3):450–456.
- Williams BA, Bullock MJ, Trites JR, et al. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42:61.
- Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315.
- Wu HH-J, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: an experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399–403.
- Tepeoğlu M, Bilezikçi B, Bayraktar SG. A histological assessment of the Bethesda system for reporting thyroid cytopathology (2010) abnormal categories: a series of 219 consecutive cases. Cytopathology. 2014;25(1):39–44.
- Rossi ED, Pantanowitz L, Faquin WC. The role of molecular testing for the indeterminate thyroid FNA. Genes (Basel). 2019 Sep 23;10(10):pii: E736.
- Vargas-Salas S, Martínez JR, Urra S, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. 2018 Mar;25(3):R163–R177.