ABSTRACT
Objectives
Fixed-dose combination (FDC) therapy can improve outcomes in type 2 diabetes (T2D). We evaluated the bioequivalence of 2 doses of an FDC of extended-release metformin (metformin XR), empagliflozin, a sodium-glucose co-transporter 2 inhibitor, and linagliptin, a dipeptidyl peptidase-4 inhibitor, versus corresponding free tablet combinations.
Methods
Two randomized, open-label, two-way crossover studies in healthy adults compared: 2 FDC tablets of empagliflozin 5 mg/linagliptin 2.5 mg/metformin XR 1000 mg (Study 1; N = 30), 1 FDC tablet of empagliflozin 25 mg/linagliptin 5 mg/metformin XR 1000 mg (Study 2; N = 30) versus corresponding dose of free combinations. Subjects received study medication under fed conditions; washout was ≥35 days between treatments. Primary endpoints: area under the plasma concentration–time curve (AUC) from time 0 to last quantifiable data point for empagliflozin and metformin; AUC from time 0 to 72 hours for linagliptin, and peak plasma concentration (Cmax) for empagliflozin, linagliptin, and metformin. Bioequivalence was defined as adjusted geometric mean ratios (FDC: free combination) and two-sided 90% confidence intervals (CIs) of AUC and Cmax for each component within 80.00–125.00%.
Results
Study 1: 27/29 and 28/30 treated participants were included in the pharmacokinetic analysis for the FDC and free combination periods, respectively. Study 2: 29/29 treated participants were included in the pharmacokinetic analysis for both periods. The adjusted geometric mean ratios of FDCs to their respective free tablet combinations and two-sided 90% CIs were all within the predefined range. The shapes of the mean plasma concentration–time profile of empagliflozin, linagliptin, and metformin XR were similar for subjects in the FDC and free combination groups in both studies. No serious adverse events were reported.
Conclusion
The evaluated doses of empagliflozin/linagliptin/metformin XR FDC tablets were bioequivalent to the corresponding free combinations. Based on these two bioequivalence studies and existing phase 3 data, the FDA has recently approved this triple FDC to improve glycemic control in adults with T2D.
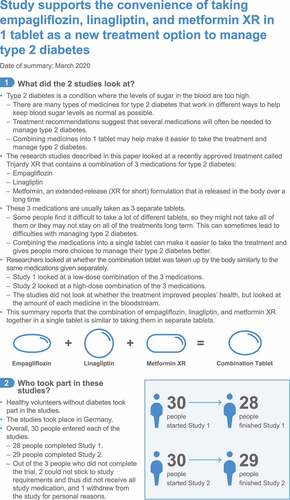
PLAIN LANGUAGE SUMMARY:
Study supports the convenience of empagliflozin, linagliptin, and metformin XR in 1 tablet as new treatment for type 2 diabetes
What is the purpose of this summary?
-
This summary is to help you understand findings from recent research.
-
This summary is about 2 studies that are part of a larger group of studies with these medications. Researchers look at many studies to understand whether a medication works, how it works, and whether it is safe to prescribe to patients.
What is known?
-
People with type 2 diabetes often need several medicines to control their blood sugar.
-
Some people find it difficult to take a lot of different tablets, and some people may not want to. This can lead to difficulties with managing type 2 diabetes.
-
Combining medicines into 1 tablet may help make it easier to take the treatment and to manage type 2 diabetes, which gives people more choices.
What is new?
-
The combination of empagliflozin, linagliptin, and metformin extended-release (metformin XR for short) in 1 tablet is approved for type 2 diabetes.
-
This combination could help people take all the treatments and better manage their type 2 diabetes.
See for a full infographic version of this summary
1. Introduction
Type 2 diabetes mellitus (T2D) is an important cause of morbidity and mortality, with a growing prevalence worldwide [Citation1]. A consensus statement from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) advises that the goals of treatment for patients with T2D are to prevent or delay the development of complications while maintaining quality of life. This involves the control of glycemia and cardiovascular (CV) risk factor management, and a patient-centered approach to care [Citation2]. While treatment of T2D typically begins with metformin monotherapy, unless it is contraindicated or not tolerated, the ADA recommends initial combination therapy be considered for newly diagnosed patients with HbA1c ≥ 1.5% above the glycemic target [Citation3]. The choice of pharmacologic agents should take account of comorbidities, in particular, the presence of atherosclerotic CV disease (ASCVD), heart failure (HF), and chronic kidney disease (CKD), in addition to the risk of hypoglycemia, impact on weight, cost, potential for side effects, and patient preferences [Citation3]. Since T2D is a major risk factor for CV disease (CVD), which is the most common cause of death in patients with T2D [Citation4], the impact of treatment on CV risk is an important consideration. Recent years have seen the publication of several clinical trials that have demonstrated the CV benefits of treatment with sodium-glucose cotransporter 2 (SGLT2) inhibitors [Citation5–Citation8] and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) [Citation9–Citation11] that have led to changes in clinical practice. Updated guidelines from the ADA [Citation12] and a consensus report from the ADA and EASD [Citation2] recommend the addition of one of these agents with proven CV benefit irrespective of glycemic goals. For patients who have ASCVD or indicators of high-risk, established kidney disease or heart failure, an SGLT2 inhibitor or GLP-1 RA with demonstrated CV benefit is recommended, independent of baseline or HbA1c target [Citation2,Citation12]. For patients requiring additional treatment for control of glycemia, similar consideration should guide the addition of a third agent, which may include a dipeptidyl peptidase-4 (DPP-4) inhibitor (if not on a GLP-1 RA or excluding saxagliptin in the setting of HF) [Citation2,Citation3]. The use of an SGLT2 inhibitor, GLP-1 RA, or DPP-4 inhibitor may also be added alone or in combination in patients without ASCVD, HF, or CKD if there is a need to minimize hypoglycemia [Citation3].
Adherence to the prescribed regimen is an important predictor of clinical outcomes [Citation13], and patient adherence is known to decline as the number of medications increases [Citation14]. One study found that patients with T2D were 4 times less likely to adhere to their medications with each unit increase in the number of prescribed medications, and 9 times less likely to adhere to treatment if they received more than once-daily dosing of glucose-lowering medication [Citation15]. In a systematic review of data from 7 studies that compared fixed-dose combination (FDC) therapy versus the corresponding agents in free combination for the treatment of T2D, the use of FDC was associated with a 13% increase in the rate of compliance [Citation16]. Since the management of T2D frequently requires combination therapy, the use of FDC therapy can reduce the pill burden leading to a simplified treatment regimen, reduce medication costs to patients, and improve adherence to therapy [Citation14,Citation17,Citation18]. This can translate into clinical benefits resulting from improved patient satisfaction and compliance with FDC therapy, leading to improved glycemic control and an increased likelihood of achieving glycemic targets [Citation19–Citation21]. FDCs are increasingly used in clinical practice for a range of chronic disorders that require the use of multiple treatments, such as chronic obstructive pulmonary disease [Citation22], hypertension [Citation23,Citation24], hepatitis C [Citation25,Citation26], and HIV infection [Citation27,Citation28], with the aim of simplifying treatment regimens and improving clinical outcomes. Similarly, in the field of T2D management, the use of FDCs of effective and well-tolerated therapies can offer a useful treatment option to help optimize therapy for individual patients, and several FDC therapies for T2D are available for clinical use [Citation29].
A new FDC of extended-release metformin (metformin XR) plus an SGLT2 inhibitor, empagliflozin, and a DPP-4 inhibitor, linagliptin, has recently been evaluated and approved by the FDA for the treatment of T2D. These agents are attractive choices for patients needing glucose lowering with multiple agents, in line with recommended treatment options for treatment intensification for T2D [Citation2,Citation3]. Both linagliptin and empagliflozin are effective and well-tolerated treatments, with a low risk of hypoglycemia (as monotherapy or in combination with metformin) and are not associated with weight gain [Citation2]. Furthermore, empagliflozin is indicated to reduce the risk of CV death in patients with T2D and CVD based on the results of the EMPA-REG OUTCOME trial (EMPAgliflozin Removal of Excess Glucose: Cardiovascular OUTCOME Event Trial in Type 2 Diabetes Mellitus Patients) [Citation5]. This trial also demonstrated a reduction in the risk for the prespecified secondary outcomes of hospitalization for heart failure (hHF), and the progression of kidney disease (incident or worsening nephropathy) versus placebo, when added to standard care. The DPP-4 inhibitors have a neutral effect on ASCVD when added to standard care for patients with T2D and elevated CV risk or established CVD. Recent findings from the CARMELINA trial (Cardiovascular and Renal Microvascular Outcome Study with Linagliptin) confirmed the safety of linagliptin when used in a high CV and renal risk population with T2D and showed no increase in risk of CV events or hHF [Citation30]. CARMELINA also demonstrated the long-term renal safety of linagliptin, with no increase in risk of the key secondary renal composite outcome of death due to kidney disease, progression to end-stage kidney disease, or sustained ≥40% decrease in estimated glomerular filtration rate from baseline for patients who received linagliptin versus placebo [Citation31]. Specifically, linagliptin may also be a suitable choice to improve glycemic control for patients with declining renal function; linagliptin does not require dose adjustment in patients with renal impairment since it has a primarily non-renal route of excretion [Citation2,Citation3]. Furthermore, linagliptin has an excellent safety profile, a low risk of hypoglycemia, and is very well tolerated even among vulnerable patient populations, including those who are elderly [Citation32].
Four different tablet strengths of the FDC tablet have recently been approved by the FDA (January 2020) ().
Table 1. Approved doses of FDCs of empagliflozin/linagliptin/metformin XR.
We report data from 2 recently completed studies that evaluated the bioequivalence of the highest and lowest doses of the FDC tablets in relation to the corresponding free combinations of tablets. Studies have previously been published to demonstrate the safety and efficacy of the combination of empagliflozin, linagliptin, and metformin in patients with T2D [Citation33–Citation37]. The findings of the bioequivalence studies will be discussed within the context of the previously published clinical data and their relevance to the application of guideline-directed management of patients with T2D. A plain language summary of this article can be found in .
2. Methods
In accordance with regulatory guidance, two open-label, randomized, single-dose, two-period, two-sequence crossover studies were conducted in healthy volunteers to evaluate the bioequivalence of an FDC tablet compared with that of a free combination of separate tablets. The design of both studies was a cross-over design allowing for intraindividual comparison of treatments. Study 1 (https://clinicaltrials.gov/show/NCT02821910) compared 2 FDC tablets, each containing empagliflozin 5 mg/linagliptin 2.5 mg/metformin XR 1000 mg versus the corresponding free combination of tablets, which included one empagliflozin 10 mg tablet, one linagliptin 5 mg tablet, and 4 metformin XR 500 mg tablets. Study 2 (https://clinicaltrials.gov/show/NCT03259490) compared a single FDC tablet that contained empagliflozin 25 mg/linagliptin 5 mg/metformin XR 1000 mg versus a corresponding free combination of tablets that included one empagliflozin 25 mg tablet, one linagliptin 5 mg tablet, and two metformin XR 500 mg tablets. For metformin XR, the free formulation used in both studies as the reference product was metformin XR 500 mg. This is the same reference product as used for the dual extended-release FDC clinical trial programs (involving linaglpitin + metformin XR or empaglflozin + metformin XR). The appropriate number of tablets were given to achieve a dose that matched the total dose of metformin XR in the FDC. In both studies, participants were randomly assigned to receive a single dose of either the FDC tablet or the free combination of tablets followed by a washout period of ≥35 days before crossing over to a single dose of the opposite treatment. Participants received a standard high-fat, high-calorie breakfast prior to the administration of study medication. (Further details of Methods are provided in the Supplementary Materials).
Participants were eligible for inclusion if they were aged 18 to 55 years, with a body mass index of 18.5 to 29.9 kg/m2. Volunteers were excluded if they had repeated measures of systolic blood pressure outside the range of 90 to 140 mmHg, diastolic blood pressure outside the range of 50 to 90 mm Hg, or a pulse rate outside the range of 45 to 90 beats per minute. Also excluded were those with concomitant disease considered clinically relevant by the investigator, or significant gastrointestinal, hepatic, renal, or respiratory disorders, or a history of relevant chronic or acute infections.
The primary endpoints in both studies were the area under the plasma concentration‒time curve from time 0 to the last quantifiable data point (AUC0-tz) for empagliflozin and metformin; the area under the plasma concentration‒time curve from time 0 to 72 hours (AUC0-72) for linagliptin, and the peak plasma concentration (Cmax) for empagliflozin, linagliptin, and metformin. The statistical model used for bioequivalence analysis was a linear mixed model with fixed effects for treatment, treatment sequence and treatment period, and random effect for participants within sequences. Each endpoint was log-transformed prior to fitting the model. Log treatment differences estimated from that model were afterward back-transformed to original scale to obtain the geometric mean ratio of FDC to free combination.
Bioequivalence would be met if the adjusted geometric mean ratios (FDC to free combination) and two-sided 90% confidence intervals (CIs) of AUC and Cmax for each component were within 80.00‒125.00%.
For determination of sample size, data from previous trials were evaluated to estimate the expected treatment differences (ratio of geometric means) and the variations (gCV) of the primary endpoints. The overall power to reject the null hypothesis of bioinequivalence for all analytes in favor of equivalence at the 5% level of significance is given by the product of the individual power values for each of the analytes, assuming that all three analytes are independent of each other. For Study 1, the maximal observed gCV for either Cmax or AUC from a former trial was 13% for linagliptin, 14% for empagliflozin and 11% for metformin. Assuming a treatment difference of 6% and using a sample size of 26 subjects, the overall power would be 98.8%. If the treatment difference was 10%, the power would still be 83.5%. Assuming that up to 4 subjects would have non-evaluable data for the pharmacokinetic analysis, 30 subjects were planned to be included in Study 1. For Study 2, the 80% upper CI limit of the pooled gCV from up to 9 former trials was estimated to be 13.5% for empagliflozin, 13.9% for linagliptin and 10.9% for metformin for Cmax. For AUC, the gCVs were generally lower. Using these estimates for gCV and treatment differences of 5% for linagliptin and empagliflozin, and 8% for metformin and a sample size of 24 subjects, provides an overall power of 98.6%. Using the maximal observed gCVs (14% for empagliflozin and metformin, and 21% for linagliptin), the overall power would still be 83%. Assuming that up to 6 subjects would have non-evaluable data for the pharmacokinetic analysis, 30 subjects were planned to be included in Study 2.
The studies were conducted in compliance with the clinical trial protocol, in accordance with the Declaration of Helsinki, and in accordance with the International Council for Harmonization Guideline for Good Clinical Practice. All participants provided written informed consent prior to participating in the studies. The trial was conducted at the Human Pharmacology Center (HPC) of Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany.
3. Results
Both studies enrolled 30 participants. During Study 1, 27 of 29 treated participants were included in the pharmacokinetic (PK) analysis for the FDC period and 28 of 30 treated participants were included in the free combination period. In Study 2, a total of 29 of 29 participants (different study population from Study 1) were treated and included in the PK analysis for both treatment periods. shows that the adjusted geometric mean ratios of FDCs to their respective free combinations of single tablets and two-sided 90% CIs for AUC and Cmax were all within the predefined acceptance range of 80.00‒125.00%. The shapes of the mean plasma concentration‒time profile of empagliflozin, linagliptin, and metformin XR were similar for subjects who received FDC and those who received single tablets in the two studies ().
Figure 2. Study 1: Mean plasma concentration‒time profiles of empagliflozin (a), linagliptin (b), and metformin (c) after single oral administration of low-dose FDC (2x [empagliflozin 5 mg/linagliptin 2.5 mg/metformin 1000 mg XR]) or corresponding free tablets. Study 2: Mean plasma concentration‒time profiles of empagliflozin (d), linagliptin (e), and metformin (f) after single oral administration of high-dose FDC (empagliflozin 25 mg/linagliptin 5 mg/metformin 1000 mg XR) or corresponding free tablets.
![Figure 2. Study 1: Mean plasma concentration‒time profiles of empagliflozin (a), linagliptin (b), and metformin (c) after single oral administration of low-dose FDC (2x [empagliflozin 5 mg/linagliptin 2.5 mg/metformin 1000 mg XR]) or corresponding free tablets. Study 2: Mean plasma concentration‒time profiles of empagliflozin (d), linagliptin (e), and metformin (f) after single oral administration of high-dose FDC (empagliflozin 25 mg/linagliptin 5 mg/metformin 1000 mg XR) or corresponding free tablets.](/cms/asset/78508c7b-efd6-418a-a3ab-972224fdb150/ipgm_a_1750228_f0002_b.gif)
Table 2. Pharmacokinetic profiles of FDC vs free combination of empagliflozin/linagliptin/metformin XR in healthy subjects under fed conditions.
No serious adverse events or adverse events (AEs) leading to study discontinuation were reported. During Study 1, all AEs were rated as mild or moderate in intensity. A total of 14 participants reported AEs, of which 9 participants reported events during the FDC administration period and 8 participants during the free combination period. During the treatment periods, 10 subjects (33.3%) reported AEs that were assessed by the investigator as drug-related; 7 subjects (24.1%) had drug-related AEs in the treatment period with the FDC and 6 subjects (20.0%) had drug-related AEs in the treatment period with the free combination (). Drug-related AEs included nausea (5 subjects, 16.7%), headache (5 subjects, 16.7%), decreased appetite (3 subjects,10.0%), vomiting (2 subjects, 6.7%), and diarrhea (1 subject, 3.3%). During Study 2, on-treatment AEs were reported for a total of 17 subjects (56.7%). Fourteen subjects (48.3%) reported AEs in the treatment period with the FDC tablet and 7 subjects (24.1%) in the treatment period with the free combination. Severe nasopharyngitis was reported in 1 participant treated with the FDC tablet; all other AEs were mild or moderate in intensity. Treatment-related AEs were reported in 9 subjects (FDC, n = 6; free combination, n = 4). Drug-related AEs included headache (4 subjects, 13.3%), nausea (3 subjects, 10.0%), dyspepsia, vomiting, dizziness, energy increased, fatigue, and euphoric mood (reported for 1 subject each, 3.3%) ().
Table 3. Frequency of subjects with drug-related AEs.
4. Discussion
These two studies demonstrated bioequivalence of the two evaluated strengths of empagliflozin/linagliptin/metformin XR FDC tablets and the corresponding free dose combinations in healthy volunteers when administered under fed conditions. The tolerability of the combination was also established, and no new safety concerns were raised. Based on these data, which analyzed the highest and lowest available tablet strengths of empagliflozin/linagliptin/metformin XR FDC tablets, bioequivalence may also be extrapolated across the range of commercially approved doses.
Based on the totality of data available, including the two bioequivalence studies reported here, and previously published phase 3 studies of the combination of these three medications in patients with T2D [Citation33–Citation37], the FDA concluded that bioequivalence, clinical efficacy, and safety have been demonstrated and that an additional phase 3 program is not warranted. The FDA recently approved the triple FDC of empagliflozin/linagliptin/metformin XR to improve glycemic control in adults with T2D.
The combination of empagliflozin and linagliptin as second-line therapy has been evaluated in patients with T2D inadequately controlled on metformin alone. The FDC of empagliflozin 25 mg/linagliptin 5 mg or empagliflozin 10 mg/linagliptin 5 mg was compared with monotherapy involving the individual components. At Week 24, HbA1c levels were significantly reduced in the empagliflozin/linagliptin FDC groups compared with monotherapy groups (p < 0.001) [Citation34]. Evaluation of the safety and tolerability of the FDC of empagliflozin/linagliptin reported that the frequency of AEs was similar, including the occurrence of hypoglycemia, compared with the individual components [Citation34].
In a study of 482 patients with T2D who had inadequate glycemic control on metformin plus open-label empagliflozin, 10 mg or 25 mg, for 16 weeks, the addition of linagliptin was evaluated using two different doses of dual-therapy FDCs [Citation37]. Evaluated treatments were empagliflozin 25 mg/linagliptin 5 mg or empagliflozin 10 mg/linagliptin 5 mg, versus empagliflozin 10 mg/placebo or empagliflozin 25 mg/placebo plus metformin for 24 weeks. The addition of FDC empagliflozin/linagliptin to metformin significantly reduced mean HbA1c from baseline at Week 24 (p ≤ 0.001), and significantly more patients achieved HbA1c < 7.0% at Week 24 versus patients treated with either dose of empagliflozin/placebo plus metformin (p ≤ 0.001), and the triple combination was well tolerated [Citation37].
A study of 333 patients with T2D evaluated empagliflozin as add-on therapy to linagliptin plus metformin over 24 weeks. Participants with inadequate glycemic control on metformin plus open-label linagliptin for 16 weeks were randomized to receive an FDC of empagliflozin 10 mg/linagliptin 5 mg, an FDC of empagliflozin 25 mg/linagliptin 5 mg, or placebo/linagliptin 5 mg, as add-on therapy to metformin [Citation36]. The addition of either dose of the FDC of empagliflozin/linagliptin to metformin was associated with significant improvements (p < 0.001) from baseline in HbA1c at Week 24 compared with those who received placebo/linagliptin plus metformin, and the treatment was well tolerated [Citation36].
Although data reported from single-dose bioequivalence studies in healthy volunteers cannot necessarily be extrapolated to clinical populations, the evaluated doses of empagliflozin/linagliptin/metformin XR were well tolerated in the bioequivalence studies reported. Furthermore, the efficacy and safety of the empagliflozin/linagliptin FDC in combination with metformin has been established in clinical trials of patients with T2D [Citation34,Citation36,Citation37]. It can be expected, therefore, that the characteristics of the 3 components can be translated to the FDC formulation.
For future clinical practice, this FDC of empagliflozin/linagliptin/metformin XR may provide an additional treatment option for guideline-directed management of T2D, including intensification of treatment. The triple FDC may be suitable across a range of patients, including those with established ASCVD or indicators of high risk, established HF or CKD due to the tolerability of the combination in addition to the potential benefits of empagliflozin on CV and renal outcomes, and no increased risk of heart failure with either agent. Since patients with T2D may be at risk of nonadherence due to the complexity of treatment with multiple medications for the condition, in addition to the need for treatments for comorbid conditions, and the requirement for self-care activities (i.e., diet, exercise, foot care, blood glucose monitoring), the use of an FDC therapy could simplify individual treatment regimens for T2D and reduce the risk of nonadherence. Thus, this approach has the potential to increase the number of patients adhering to treatment and achieving their glycemic targets [Citation19,Citation20], which could translate into a lower long-term risk of complications of T2D, including micro- and macrovascular disease.
5. Conclusion
In conclusion, our data demonstrated that the 2 evaluated doses of the empagliflozin/linagliptin/metformin XR FDC tablets were bioequivalent to the corresponding free dose combinations. The FDC was well tolerated, with safety profiles similar to the known safety profiles of the individual agents. These results add to existing phase 3 clinical data on the safety and efficacy of the combination of empagliflozin, linagliptin, and metformin XR in patients with T2D. Collectively, these data suggest that this triple FDC therapy could provide an alternative treatment option to the traditional stepwise treatment approach to T2D management. In addition, this triple-dose FDC could help improve adherence and attain glycemic goals and outcomes for patients with T2D who need multiple glucose-lowering therapies.
Declaration of interest
Ildiko Lingvay has received consulting fees from AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, Lilly, NovoNordisk, Sanofi, TARGET Pharma, and Valeritas, and funding for research from GI Dynamics, Merck, Mylan, Novartis, NovoNordisk and Pfizer.
Nadine Beetz, Regina Sennewald, Annette Schuler-Metz, Julia Bertulis, Christina Loley, Benjamin Lang, Caroline Lippert, Jisoo Lee, Linda Shapiro Manning, and Derek Terada are all employees of Boehringer Ingelheim.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data sharing
The sponsor of the two bioequivalence studies (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website (https://trials.boehringer-ingelheim.com/).
Supplemental Material
Download MS Word (24.4 KB)Acknowledgments
The authors would like to thank Jennifer Garrett of Elevate Scientific Solutions for providing writing support which was contracted and compensated by BIPI.
Supplementary materials
The supplementary data for this article can be accessed here.
Additional information
Funding
References
- WHO. Diabetes. Key Facts; 2018. [cited 2019 Oct 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‒2498.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S98‒S110.
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28‒e292.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
- Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516‒2527.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‒357.
- Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323‒334.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130.
- Verma S, Bhatt DL, Bain SC, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179‒2183.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‒1844.
- American Diabetes Association. 10. cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S111‒S134.
- DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794‒811.
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31:213‒224.
- Jarab AS, Almrayat R, Alqudah S, et al. Predictors of non-adherence to pharmacotherapy in patients with type 2 diabetes. Int J Clin Pharm. 2014;36:725‒733.
- Hutchins V, Zhang B, Fleurence RL, et al. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27:1157‒1168.
- Xie L, Frech-Tamas F, Marrett E, et al. A medication adherence and persistence comparison of hypertensive patients treated with single-, double- and triple-pill combination therapy. Curr Med Res Opin. 2014;30:2415‒2422.
- Lokhandwala T, Smith N, Sternhufvud C, et al. A retrospective study of persistence, adherence, and health economic outcomes of fixed-dose combination vs. loose-dose combination of oral anti-diabetes drugs. J Med Econ. 2016;19:203‒212.
- Benford M, Milligan G, Pike J, et al. Fixed-dose combination antidiabetic therapy: real-world factors associated with prescribing choices and relationship with patient satisfaction and compliance. Adv Ther. 2012;29:26‒40.
- Blonde L, Wogen J, Kreilick C, et al. Greater reductions in A1C in type 2 diabetic patients new to therapy with glyburide/metformin tablets as compared to glyburide co-administered with metformin. Diabetes Obes Metab. 2003;5:424–431.
- Bajaj HS, Ye C, Jain E, et al. Glycemic Improvement with a Fixed-dose combination of DPP-4 inhibitor + metformin in patients with Type 2 diabetes (GIFT study). Diabetes Obes Metab. 2018;20:195‒199.
- Lopez-Campos JL, Carrasco-Hernandez L, Quintana-Gallego E, et al. Triple therapy for COPD: a crude analysis from a systematic review of the evidence. Ther Adv Respir Dis. 2019;13:1753466619885522.
- Mazza A, Townsend DM, Schiavon L, et al. Long-term effect of the perindopril/indapamide/amlodipine single-pill combination on left ventricular hypertrophy in outpatient hypertensive subjects. Biomed Pharmacother. 2019;120:109539.
- Simon A, Dezsi CA. Treatment of hypertensive and hypercholesterolaemic patients with the triple fixed combination of atorvastatin, perindopril and amlodipine: the results of the CORAL study. Adv Ther. 2019;36:2010‒2020.
- Soriano V, Benitez-Gutierrez L, Arias A, et al. Evaluation of sofosbuvir, velpatasvir plus voxilaprevir as fixed-dose co-formulation for treating hepatitis C. Expert Opin Drug Metab Toxicol. 2017;13:1015–1022.
- Morisawa N, Koshima Y, Kuriyama S, et al. Effectiveness of a fixed combination formula of ombitasvir/paritaprevir/ritonavir for hepatitis C virus infection in patients on maintenance haemodialysis. Nephrology (Carlton). 2017;22:562‒565.
- Troya J, Bascunana J. Safety and tolerability: current challenges to antiretroviral therapy for the long-term management of HIV infection. AIDS Rev. 2016;18:127‒137.
- Comi L, Maggiolo F. Abacavir + dolutegravir + lamivudine for the treatment of HIV. Expert Opin Pharmacother. 2016;17:2097‒2106.
- Abdulsalim S, Peringadi Vayalil M, Miraj SS. New fixed dose chemical combinations: the way forward for better diabetes type II management? Expert Opin Pharmacother. 2016;17:2207‒2214.
- McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139:351–361.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69‒79.
- Cooper ME, Rosenstock J, Kadowaki T, et al. Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: a prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA® trial. Diabetes Obes Metab. 2020. DOI:10.1111/dom.13995.
- DeFronzo RA, Lee C, Kohler S. Safety and tolerability of combinations of empagliflozin and linagliptin in patients with type 2 diabetes: pooled data from two randomized controlled trials. Adv Ther. 2018;35:1009‒1022.
- DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384‒393.
- Jain RK. Empagliflozin/linagliptin single-pill combination therapy for patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18:545‒549.
- Softeland E, Meier JJ, Vangen B, et al. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40:201‒209.
- Tinahones FJ, Gallwitz B, Nordaby M, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab. 2017;19:266‒274.