ABSTRACT
As the cornerstone of type 2 diabetes (T2D) management within the community, primary care providers are now faced with the challenge of not only managing diabetes itself, but also preventing hypoglycemia and weight gain associated with intensive disease management, and reducing cardiovascular risk.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are well established as efficacious treatments for T2D, and the safety/tolerability profile of this drug class is well defined. However, despite their beneficial effects, GLP-1RAs are under-utilized, highlighting the need for novel approaches to increase their use in primary care. Oral semaglutide is the first oral GLP-1RA approved for the treatment of T2D, offering glucose lowering and body weight loss, a low risk of hypoglycemia, and no increase in cardiovascular risk. Oral semaglutide represents an additional treatment option for patients not achieving their glycemic goal despite treatment with metformin, either alone or with other hypoglycemic agents. Oral semaglutide has the potential to increase usage of GLP-1RAs in the primary care setting by addressing clinician and patient concerns about injections, and may facilitate earlier initiation of GLP-1RA therapy in T2D.
Due to the formulation of oral semaglutide, clinicians need to be aware of specific considerations in order to ensure optimal use. Such considerations include dosing conditions and use of concomitant medications. This article provides practical guidance on the use of oral semaglutide in the primary care setting, based on evidence from clinical studies, including the phase 3a PIONEER program, and the authors’ clinical experience.
1. Introduction
1.1. Burden of T2D in the USA and the essential role of primary care
In the USA, there is a substantial unmet need to improve the management of type 2 diabetes (T2D) due to the high prevalence of the condition [Citation1] and poor levels of glycemic control [Citation2], both of which contribute to significant diabetes-related health-care costs (USD327 billion in 2017) [Citation3]. Primary care providers (PCPs) treat around 90% of individuals with diabetes in the USA [Citation4], and in partnership with their patients, determine the choice of medication used. PCPs can play an important role in treatment safety, such as avoidance of hypoglycemia and medication adherence, as well as in factors beyond glycemic control, including weight loss and cardiovascular disease (CVD) risk reduction [Citation4,Citation5].
1.2. Where do GLP-1RAs sit in the treatment paradigm?
Current guidelines from the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend lifestyle changes and metformin as first-line therapy for T2D [Citation6,Citation7]. Second- and further-line therapy options include oral medications (e.g. sodium-glucose co-transporter-2 inhibitors [SGLT2is], dipeptidyl peptidase-4 inhibitors [DPP-4is], sulfonylureas, or thiazolidinediones), and injectables (glucagon-like peptide-1 receptor agonists [GLP-1RAs] and insulins), with many options for combining these therapies to step‑up treatment as needed to improve glycemic control [Citation6,Citation7].
GLP-1RAs are well established as highly efficacious treatments for T2D, and the safety/tolerability profile for the class is well defined [Citation7,Citation8]. The most commonly observed adverse events (AEs) are gastrointestinal, such as vomiting and diarrhea, as well as complaints of nausea, which are usually transient and present shortly after treatment initiation or dose escalation [Citation8–16].
GLP-1RAs have an action similar to natural incretin hormones, increasing glucose-dependent insulin secretion and suppressing glucagon release, while also increasing satiety [Citation17,Citation18]. In addition to reducing glycated hemoglobin (HbA1c), GLP-1RAs reduce body weight and have a low risk of hypoglycemia (note that hypoglycemia can still occur when used in combination with insulin or an insulin secretagogue) [Citation6,Citation7]. Treatments that reduce body weight are important, since more than 80% of patients with T2D are either overweight or living with obesity [Citation1], and weight loss in T2D is associated with improvements in quality of life and glycemic control, decreased cardiovascular risk, and significant reductions in health costs [Citation19,Citation20].
GLP-1RAs directly and indirectly target more of the key physiological pathways known to contribute to the pathophysiology of T2D than other currently available treatment options, including metformin, SGLT2is, and DPP-4is [Citation21,Citation22]. Although both GLP-1RAs and DPP-4is act on the incretin system, DPP-4is do not infer all of the benefits associated with GLP-1RAs, in that they only have intermediate glucose-lowering efficacy, do not reduce body weight, and have no proven cardiovascular benefit [Citation7,Citation17]. This may be due to differences in how each class of drug acts upon the incretin system [Citation23]. DPP-4is raise plasma levels of GLP-1 by preventing the degradation of the endogenous GLP-1 peptide by DPP-4 [Citation24]. In contrast, GLP-1RAs are modified forms of native GLP-1 with enhanced resistance to DPP-4 degradation (some GLP-1RAs also include modifications that slow absorption and/or delay elimination) [Citation24]. Differences in clinical benefits may relate to greater activation of GLP-1 receptors in response to pharmacological levels of exogenously administered GLP-1RAs, compared with the more modest activation arising from stabilization of endogenous GLP-1 levels with DPP-4is [Citation17,Citation25–27].
According to the ADA guidelines, the choice of second-line therapy largely depends on the presence of comorbidities including atherosclerotic CVD (ASCVD), heart failure (HF), or chronic kidney disease (CKD) [Citation7]. In patients at high risk for or with established ASCVD, HF, or CKD, the choice of second-line therapy should be considered independently of baseline HbA1c or individualized HbA1c targets [Citation7]. In large-scale cardiovascular outcomes trials (CVOTs), specific GLP-1RAs, including liraglutide [Citation28], subcutaneous semaglutide [Citation29], and dulaglutide [Citation30], have shown favorable effects on cardiovascular events in patients with T2D at high cardiovascular risk [Citation31]. For patients in whom ASCVD predominates, the preferred choice for a second-line agent is a GLP-1RA or an SGLT2i with demonstrated evidence of cardiovascular risk reduction () [Citation7]. For those patients in whom HF or CKD predominates, an SGLT2i that has been shown to reduce the progression of the relevant disease is the preferred treatment option [Citation7]. A GLP-1RA with proven cardiovascular benefit is also recommended in this setting if an SGLT2i is not appropriate for a particular patient [Citation7]. For patients without established ASCVD or CKD, the choice of a second agent should be based on several factors, including the avoidance of side effects, particularly hypoglycemia and weight gain, cost, and patient preference [Citation7].
Figure 1. The ADA and AACE/ACE 2020 recommendations for GLP-1RA therapy in the treatment of T2D
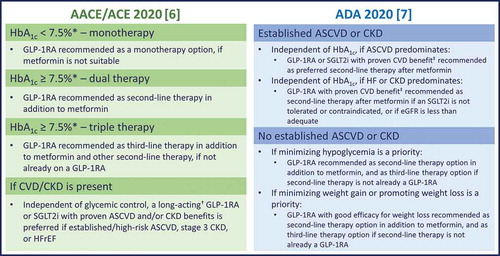
In the AACE/ACE glycemic control algorithm, GLP-1RAs are recommended as the preferred second-line therapy, closely followed by an SGLT2i, and then a DPP-4i [Citation6]. Independent of glycemic control, GLP-1RAs or SGLT2is with proven ASCVD and/or CKD benefits are recommended in patients with high risk for/established ASCVD, stage 3 CKD, or HF with reduced ejection fraction () [Citation6].
Combination therapy with a GLP-1RA and a DPP-4i is not typically recommended due to lack of clinical benefit [Citation32]. In contrast, based on synergistic effects on glycemic control and weight reduction, combination therapy with SGLT2is and GLP-1RAs is an ADA-recommended option for patients requiring additional HbA1c-lowering after treatment with one of these agents [Citation7], and has been reviewed in detail previously [Citation33].
Several injectable GLP-1RAs are currently available for the treatment of T2D in the USA, with some being available in different formulations or in combination with insulin [Citation9–11,Citation13–16,Citation34]. Although sharing the same underlying mechanism of action, they differ in terms of frequency of administration (either once or twice daily, or once weekly) and formulation.
Despite their beneficial effects, utilization rates of GLP-1RAs are low [Citation35,Citation36]. In a study conducted in the USA in 2015, less than 10% of adult patients with T2D were receiving a GLP-1RA [Citation36]. This is likely to be at least partially attributable to the fact that, until recently, all GLP-1RAs were administered by subcutaneous injection, a route that may be less preferable to some patients compared with oral administration [Citation37,Citation38]. Patients may initially be hesitant to initiate injectable glucose-lowering therapies due to injection-related concerns, such as fear of injection pain [Citation39,Citation40]. In a GLP-1RA discontinuation study conducted in 2014 when only once- or twice-daily GLP-1RAs were available, 40% of patients cited the reason for discontinuation as ‘prefer oral medication over injections’ [Citation41]. In addition, in the USA, a preference for oral medication over injections was considered the most common problem with GLP-1RA treatment by 61% of patients and 40% of physicians [Citation41]. Although patients express a preference for less frequent injections, clinicians may overestimate the degree to which fear of injection concerns patients [Citation42–44]. It is important for PCPs to discuss administration route and frequency with their patients to better understand the preferences of each individual patient and guide appropriate therapy choices.
1.3. How does oral semaglutide differ from other GLP-1RAs?
Oral semaglutide is the first oral GLP-1RA approved (US Food and Drug Administration [FDA] in September 2019 and the European Union in April 2020) for the improvement of glycemic control in adults with T2D [Citation12,Citation45,Citation46]. The availability of oral semaglutide provides a once-daily oral GLP-1RA option [Citation12] that enables individualization of treatment for patients who may benefit from a GLP-1RA therapy, but who prefer oral medication. Orally delivered peptides typically have low bioavailability and oral semaglutide has therefore been co-formulated with an absorption enhancer, sodium N‑(8‑[2‑hydroxybenzoyl] amino) caprylate (SNAC), to help protect against proteolytic degradation and aid absorption across the gastric mucosa [Citation47]. Although SNAC enhances the absorption of oral semaglutide, absolute bioavailability remains low [Citation12]. As such, there are some specific recommendations regarding dosing conditions and use of concomitant medications that need to be followed to avoid decreasing absorption and reducing the effectiveness of treatment. This article will review key data from the development of oral semaglutide and lessons from the extensive phase 3a Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) global clinical trial program, and provide practical guidance on its use.
2. Clinical efficacy and safety of oral semaglutide in T2D
A summary of the key efficacy and safety findings from the global PIONEER clinical trials is provided in . The PIONEER program also included a pre-approval CVOT for oral semaglutide (PIONEER 6 [Citation48]), a trial evaluating the efficacy and safety of a flexible-dose adjustment of oral semaglutide versus sitagliptin (PIONEER 7 [Citation49]), as well as two additional trials that investigated the efficacy and safety of oral semaglutide in Japanese populations (PIONEER 9 and 10 [Citation50,Citation51]).
Table 1. Key efficacy endpoints for PIONEER trials 1–5, 7, and 8
The efficacy and safety of oral semaglutide were established versus placebo in patients with T2D insufficiently controlled with diet and exercise in PIONEER 1. At 26 weeks, once-daily oral semaglutide monotherapy demonstrated superior and clinically relevant improvements in HbA1c (at 3, 7, and 14 mg) and body weight loss (14 mg) versus placebo, and significantly more patients achieved target HbA1c () [Citation52].
International head-to-head studies compared the efficacy of oral semaglutide with commonly used agents in patients uncontrolled on metformin with or without a second agent [Citation53–55]. These studies indicate significantly greater HbA1c reductions and greater likelihood of achieving target HbA1c after 26 weeks’ treatment with oral semaglutide 14 mg compared with the SGLT2i empagliflozin 25 mg (PIONEER 2) [Citation53] and with oral semaglutide 7 and 14 mg compared with the DPP-4i sitagliptin 100 mg (PIONEER 3) () [Citation54]. In a trial evaluating the efficacy of oral semaglutide 14 mg versus the injectable GLP-1RA liraglutide 1.8 mg and placebo (PIONEER 4), reductions in HbA1c were not significantly different for oral semaglutide versus liraglutide at week 26 (–1.2% versus –1.1%); however, by the end of the 52-week study, reductions were significantly greater with oral semaglutide than liraglutide (–1.2% versus –0.9%; estimated treatment difference: –0.3% [95% confidence interval (CI): –0.5, –0.1]; p = 0.0002) [Citation55] (). Body weight was significantly reduced after 26 weeks with oral semaglutide 14 mg compared with liraglutide 1.8 mg in PIONEER 4 [Citation55], and with oral semaglutide 7 and 14 mg compared with sitagliptin 100 mg in PIONEER 3 [Citation54]. Weight reductions were similar to oral semaglutide 14 mg and empagliflozin 25 mg in PIONEER 2 [Citation53].
When studied in Japanese patients with T2D, 26 weeks’ oral semaglutide monotherapy (3, 7, and 14 mg) significantly reduced HbA1c compared with placebo, and the 14 mg dose provided greater reductions in body weight versus placebo (PIONEER 9) [Citation51]. In addition, oral semaglutide 14 mg significantly reduced HbA1c and body weight compared with both open-label liraglutide 0.9 mg when used as monotherapy (PIONEER 9) and open-label dulaglutide 0.75 mg when added to an oral background therapy (PIONEER 10) [Citation50,Citation51]. These studies used the maximum approved doses of liraglutide and dulaglutide in Japan; higher doses are approved in the US [Citation14,Citation15,Citation50,Citation51]. These results suggest that the glucose- and body weight-lowering effects of oral semaglutide extend to east Asian populations.
Establishing the cardiovascular safety of new glucose-lowering agents is an FDA requirement for all new drug approvals. For once-weekly subcutaneous semaglutide, cardiovascular safety was demonstrated in the SUSTAIN-6 trial, in which the risk of a first major adverse cardiovascular event (MACE) (cardiovascular death, nonfatal myocardial infarction, or non‑fatal stroke) over the 2-year study period was significantly lower among patients on subcutaneous semaglutide than those receiving placebo (hazard ratio [HR]: 0.74 [95% CI: 0.58, 0.95]; p < 0.001 for noninferiority) [Citation29]. The oral semaglutide CVOT (PIONEER 6) met its primary endpoint and demonstrated a lack of excess cardiovascular risk with oral semaglutide compared with placebo, both added to standard of care, over a median of 15.9 months in patients at high cardiovascular risk (aged ≥ 50 years with established CVD or CKD, or aged ≥ 60 years with cardiovascular risk factors only) [Citation48]. In this study, MACEs were reported in 3.8% of patients with oral semaglutide versus 4.8% with placebo (HR: 0.79 [95% CI: 0.57, 1.11]; p < 0.001 for noninferiority) [Citation48]. Neither SUSTAIN-6 nor PIONEER 6 were designed to show a cardiovascular benefit (i.e. superiority versus placebo). Nevertheless, in a post hoc analysis of SUSTAIN-6, subcutaneous semaglutide demonstrated cardiovascular benefit (p = 0.02 for superiority versus placebo), and is now FDA-approved for reducing the risk of MACE in adults with T2D and established CVD [Citation11]. Although PIONEER 6 did not show superiority for the three-point MACE composite endpoint (p = 0.17 for superiority), reductions in cardiovascular death (HR: 0.49 [95% CI: 0.27, 0.92]) and all-cause mortality (HR: 0.51 [95% CI: 0.31, 0.84]) were observed with oral semaglutide versus placebo [Citation48]. These findings should be interpreted with caution because the trial was not powered to evaluate these individual outcomes and there were relatively small numbers of patients evaluated for each endpoint.
In all trials within the PIONEER program, the safety profile of oral semaglutide was consistent with that expected of a GLP-1RA, including in the studies conducted in Japanese patients [Citation48–57]. Nausea, diarrhea, and vomiting were the most commonly reported side effects (), with these events generally being mild-to-moderate in severity, transient in nature, and mostly occurring during dose initiation and escalation [Citation12,Citation48–57]. In a pooled analysis of two placebo-controlled PIONEER trials, gastrointestinal events occurred in 32% and 41% of patients receiving oral semaglutide 7 and 14 mg, respectively, versus 21% with placebo, with the majority being reported during dose-escalation periods [Citation12]. More patients receiving oral semaglutide 7 mg (4%) and oral semaglutide 14 mg (8%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (1%) [Citation12].
The tolerability of oral semaglutide is similar to that seen with injectable GLP-1RAs [Citation9–12,Citation14,Citation15,Citation34,Citation55]. The proportions of patients experiencing AE-related premature discontinuations with oral semaglutide 14 mg [Citation12,Citation50–57] were generally similar to those previously observed with subcutaneous semaglutide 1 mg once weekly and other GLP-1RAs [Citation25–27,Citation50,Citation51,Citation55,Citation58]. In PIONEER 4, AE-related premature discontinuations occurred in 11% of patients in both the oral semaglutide 14 mg groups, 8% in the liraglutide 1.8 mg group and 4% in the placebo group [Citation55].
A flexible dosing regimen, allowing physicians to switch patients between the 3, 7, and 14 mg doses of oral semaglutide, according to efficacy and tolerability criteria, was investigated in the PIONEER 7 trial () [Citation49]. The primary endpoint, HbA1c < 7% at week 52, was achieved by 58% of patients randomized to oral semaglutide versus 25% randomized to sitagliptin 100 mg. Among the patients receiving oral semaglutide who were on treatment at week 52, 30% were receiving the 7 mg dose and 59% were receiving 14 mg [Citation49]. These findings suggest that, in clinical practice, dosage adjustments could be individualized based on tolerability and efficacy.
3. Practical guidance for initiation and maintenance of treatment with oral semaglutide
3.1. Dosing/administration of oral semaglutide
Patients should be instructed to swallow the oral semaglutide tablet whole, upon waking, in a fasting state, with no more than 4 fluid ounces (120 mL) of plain water (approximately half a glass), and to wait ≥ 30 minutes before taking their first food, drink, or other oral medications that day [Citation12,Citation48,Citation49,Citation52–57]. As the presence of food, fluid or other oral medications in the stomach may impair absorption of oral semaglutide [Citation12,Citation47,Citation59,Citation60] it is important that patients closely follow these dosing instructions in order to optimize the therapeutic effects of oral semaglutide.
Many patients with T2D take multiple medications. In pharmacokinetic studies, absorption of oral semaglutide was found to be reduced by co-administration with multiple (five) placebo tablets [Citation60], which is addressed by the dosing guidance recommendation that other oral medication should be dosed at least 30 minutes after oral semaglutide administration [Citation12]. In other pharmacokinetic studies with oral semaglutide, no clinically relevant interactions were observed with omeprazole on the exposure of oral semaglutide [Citation61]. Oral semaglutide did not have clinically relevant effects on the exposure of lisinopril, warfarin, metformin, digoxin [Citation62], furosemide, or rosuvastatin [Citation63], or the combined oral contraceptive, ethinylestradiol/levonorgestrel [Citation64].
Levothyroxine is a commonly used oral medication with similarities in dosing conditions to oral semaglutide [Citation65]. In a drug–interaction study, levothyroxine (600 µg) exposure was increased by 33% when co-administered with oral semaglutide 14 mg at steady state [Citation12,Citation60]. Monitoring of thyroid parameters should, therefore, be considered when treating patients with oral semaglutide in combination with levothyroxine [Citation65]. Prescribing information states that levothyroxine should be administered in the morning on an empty stomach [Citation65] with recommendations suggesting that it can be taken at bedtime, at least 3 hours after the evening meal as an alternative [Citation66]. None of the studies investigating bedtime dosing of levothyroxine included dosing of oral semaglutide; however, morning dosing of oral semaglutide and evening dosing of levothyroxine could be considered, and is aligned with current evidence [Citation12,Citation67,Citation68].
When co-administering other oral medications, it is important to adhere to the administration instructions for oral semaglutide, and consider increased monitoring for medications that have a narrow therapeutic index or that require clinical monitoring [Citation12].
Unlike some other GLP-1RAs (i.e. lixisenatide [Citation13,Citation34] and exenatide [Citation9,Citation10]), where caution or monitoring of renal function is required when escalating doses in patients with renal impairment, no dose adjustment appears necessary when oral semaglutide is used in patients with renal impairment [Citation12,Citation69]. Indeed, renal function was unchanged during treatment with oral semaglutide in patients with moderate renal impairment (estimated glomerular filtration rate 30–59 mL/min/1.73 m2) included in PIONEER 5 [Citation56]. There were no apparent pharmacokinetic differences in subjects with different degrees of hepatic impairment [Citation12,Citation70] or patients with upper gastrointestinal disease (i.e. chronic gastritis and/or gastro-esophageal reflux disease) [Citation71] when oral semaglutide was administered, suggesting that there is no need for dose adjustment in patients with these comorbidities.
3.2. Managing expectations of patients initiating treatment with oral semaglutide
The choice of second-line treatment will depend on multiple factors, including the extent of hyperglycemia, vulnerability to hypoglycemia, and comorbidities. In all cases, however, treatment decisions must take a patient-centered approach that acknowledges multimorbidity, benefit and risk of treatments, and patient preference [Citation7].
Based on the results of the PIONEER program, patients can be reassured that there is good evidence for the efficacy and safety of oral semaglutide across a broad range of background medications, baseline HbA1c levels, and diabetes disease durations [Citation48,Citation49,Citation52–57]. Patients should, however, be made aware that the 3 mg dose of oral semaglutide is intended for treatment initiation and is not the target dose [Citation12].
Although oral semaglutide has been shown to lead to weight loss in the PIONEER trials, patients should be made aware that it is not approved or marketed as a weight loss drug [Citation12,Citation23]. Patients should expect to lose weight within the first 6 months of treatment with oral semaglutide, which may continue over a more sustained time course, albeit to a lesser extent [Citation49,Citation52–57].
Gastrointestinal side effects can be a limiting factor with the use of GLP-1RAs; however, there are ways to minimize these. Gastrointestinal effects appear to be dose-related, and there is evidence that initiating oral semaglutide at a low dosage and escalating it slowly can improve tolerability [Citation72]. Patients should start treatment with oral semaglutide at the 3 mg dose for 30 days, then increase to 7 mg once daily. After 30 days on the 7 mg dose, depending on response and glycemic target, the dose may be increased to 14 mg once daily [Citation12].
As has been suggested for other GLP-1RAs [Citation23], patients receiving oral semaglutide should be educated about the possibility of experiencing gastrointestinal side effects. However, they should also be reassured that these do not affect the majority of patients, are likely to be only mild-to-moderate in severity, and are typically transient in nature [Citation12,Citation49,Citation52–57]. For this reason, patients experiencing these side effects should be encouraged to continue taking oral semaglutide as recommended, and to discuss with a health-care professional any side effects that are particularly bothersome, severe or that continue for a prolonged period. According to the prescribing information, patients should be carefully monitored for signs and symptoms of pancreatitis (e.g. severe abdominal pain, with or without vomiting) after initiating treatment with oral semaglutide, and if pancreatitis is suspected, oral semaglutide should be discontinued and appropriate management initiated [Citation12].
To help minimize nausea, patients could be advised to eat smaller meals and stop when they feel full, and to avoid meals with a high-fat content [Citation23,Citation73,Citation74]. If gastrointestinal side effects are observed, dose adjustments could help improve tolerability (based on clinical experience with other GLP-1RAs) and therefore could also be considered at the discretion of the treating physician.
Hypoglycemia may be a cause for concern for many patients with T2D. However, the risk of severe (requiring assistance of another person [Citation75]) or blood glucose-confirmed (blood glucose concentration < 56 mg/dL) hypoglycemia is low with oral semaglutide [Citation52–56]. The risk of hypoglycemia may be increased when oral semaglutide is used in combination with sulfonylureas or insulin [Citation12,Citation53,Citation57], and therefore a lower dose of the secretagogue or insulin may be needed to reduce the risk in this setting [Citation12]. If oral semaglutide is being prescribed as an add-on to basal insulin therapy, the authors recommend that prescribers consider reducing the insulin dose by 10–20% depending upon the HbA1c level, and increase as needed after steady-state with oral semaglutide has been achieved based on monitoring of blood glucose.
It is recommended that patients switching from subcutaneous semaglutide once weekly at a dose of 0.5 mg can be transitioned onto oral semaglutide at a dose of 7 or 14 mg once daily, up to 7 days after their last injection of subcutaneous semaglutide; but there is no equivalent oral dose for those switching from subcutaneous semaglutide 1 mg [Citation12]. No data or official guidance are currently available regarding the dose of oral semaglutide if it is being prescribed for a patient switching from an injectable GLP-1RA other than subcutaneous semaglutide. The authors therefore recommend that patients should start at either the 3 or 7 mg dose of oral semaglutide (depending on their previous treatment), as gastrointestinal tolerability should already have been established with the previous treatment, but noting that a temporary worsening of glycemic control may result with the 3 mg dose.
A summary of the practical considerations when initiating a patient on oral semaglutide is included in .
Figure 2. Summary of practical guidance for initiating patients on oral semaglutide [Citation12]
![Figure 2. Summary of practical guidance for initiating patients on oral semaglutide [Citation12]](/cms/asset/6adb75b6-73b3-44a9-9403-f63e0b579444/ipgm_a_1788340_f0002_c.jpg)
4. Summary
The number of patients with T2D and the burden of disease is increasing in the USA. To achieve effective glycemic control, treatment guidelines advocate GLP-1RAs among various options in patients with T2D who are uncontrolled on metformin. Oral semaglutide represents an important therapeutic advance by making a GLP-1RA available in oral form, with effective glucose lowering and body weight loss, a low risk of hypoglycemia, and no significant increase in cardiovascular risk.
For patients and physicians who may be reluctant to initiate or intensify therapy by injection, an oral GLP-1RA allows earlier initiation of GLP-1RA therapy in the T2D treatment continuum of care. Practical issues including dosing instructions and gastrointestinal AEs are important to discuss with patients, and education is needed in order to ensure optimal efficacy and to minimize adverse effects.
Declaration of interest
Dr Morales is on the speakers bureau, advisory boards, and is a consultant for Abbott Laboratories, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Mylan, and Novo Nordisk; and serves on advisory boards for Bayer and Intarcia.
Dr Shubrook serves on an advisory board for Bayer, Eli Lilly, Intarcia, Novo Nordisk, and Sanofi.
Dr Skolnik reports receiving non-financial support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Sanofi; and receiving personal fees and serving on advisory boards of AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Intarcia, Janssen Pharmaceuticals, Merck, Mylan, Sanofi, and Teva Pharmaceutical.
A reviewer on this manuscript has disclosed that they are on the speakers bureau for Novo Nordisk. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Social media
LinkedIn: Jay Shubrook and Javier Morales [email protected].
Take-home points
Around 90% of the care given to patients with type 2 diabetes (T2D) is provided in the primary care setting in the United States.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are under-utilized, despite well-established efficacy and safety profiles.
Oral semaglutide is the first oral GLP-1RA approved for the treatment of T2D, and demonstrates glucose lowering, body weight loss, a low risk of hypoglycemia, and no significant increase in cardiovascular events.
Oral semaglutide offers access to the benefits of treatment with a GLP-1RA, in terms of glycemic control, weight loss, and low risk of hypoglycemia.
To ensure optimal efficacy and to minimize adverse effects with oral semaglutide, it is important for primary care providers to discuss practical issues with patients, such as dosing instructions and gastrointestinal adverse events.
Acknowledgments
This article was supported by Novo Nordisk Inc., who performed a medical accuracy review. Medical writing and editorial support were provided by Laura Ward and Paul Barlass of Axis, a division of Spirit Medical Communications Group Limited (funded by Novo Nordisk Inc.) under the direction of J. Morales, J.H. Shubrook, and N. Skolnik.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC). National diabetes statistics report. 2017; [cited 2020 Apr 29]. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Carls G, Huynh J, Tuttle E, et al. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–873.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928.
- Davidson JA. The increasing role of primary care physicians in caring for patients with type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 Suppl):S3–4.
- Giugliano D, Maiorino MI, Bellastella G, et al. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J Endocrinol Invest. 2019;42(5):495–503.
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the american association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26(1):107–139.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–110.
- Nauck MA, Meier JJ. Management of endocrine disease: are all GLP-1 agonist equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181(6):R211–34.
- Bydureon® prescribing information [updated Feb 2020; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022200s030lbl.pdf
- Byetta® prescribing information [updated Feb 2020; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021773s043lbl.pdf
- Ozempic® prescribing information [updated 2020 Jan; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf
- Rybelsus® prescribing information [updated 2020 Jan; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213182s000,213051s001lbl.pdf
- Soliqua® 100/33 prescribing information [updated Nov 2019; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208673s008s009lbl.pdf
- Trulicity® prescribing information [updated Feb 2020; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s033lbl.pdf
- Victoza® prescribing information [updated 2019 Jun; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022341s031lbl.pdf
- Xultophy® 100/3.6 prescribing information [updated 2019 Nov; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208583s014s015lbl.pdf
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705.
- Morales J, Assumpcao-Morales M. The use of SGLT2 inhibitors and GLP-1 receptor agonists, a worthwhile physiologic combination in managing type 2 diabetes while reducing cardiovascular risk. J Cardiol Curr Res. 2019;12(5):104–110.
- Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–2556.
- Karkare S, Fridman M, Dang-Tan T, et al. Effect of weight change on economic outcomes among persons with type 2 diabetes mellitus in the United States: beyond glycemic control. J Manag Care Spec Pharm. 2019;25(6):658–668.
- Abdul-Ghani M, DeFronzo RA. Is it time to change the type 2 diabetes treatment paradigm? Yes! GLP-1 RAs should replace metformin in the type 2 diabetes algorithm. Diabetes Care. 2017;40(8):112–117.
- DeFronzo RA, Triplitt CL, Abdul-Ghani M, et al. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100–112.
- Reid TS. Practical use of glucagon-like peptide-1 receptor agonist therapy in primary care. Clin Diabetes. 2013;31(4):148–157.
- Meier J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742.
- Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354.
- Nauck M, Weinstock RS, Umpierrez GE, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–2158.
- Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130.
- American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–34.
- Lajthia E, Bucheit JD, Nadpara PA, et al. Combination therapy with once-weekly glucagon like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a case series. Pharm Pract (Granada). 2019;17(4):1588.
- Goncalves E, Bell DSH. Combination treatment of SGLT2 inhibitors and GLP-1 receptor agonists: symbiotic effects on metabolism and cardiorenal risk. Diabetes Ther. 2018;9(3):919–926.
- Adlyxin® prescribing information [updated 2016 Jul; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471Orig1s000lbl.pdf
- Montvida O, Shaw J, Atherton JJ, et al. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69–78.
- Weng W, Tian Y, Kong SX, et al. The prevalence of cardiovascular disease and antidiabetes treatment characteristics among a large type 2 diabetes population in the United States. Endocrinol Diabetes Metab. 2019;2(3):e00076.
- DiBonaventura MD, Wagner JS, Girman CJ, et al. Multinational internet-based survey of patient preference for newer oral or injectable Type 2 diabetes medication. Patient Prefer Adherence. 2010;4:397–406.
- Marchesini G, Pasqualetti P, Anichini R, et al. Patient preferences for treatment in type 2 diabetes: the Italian discrete-choice experiment analysis. Acta Diabetol. 2019;56(3):289–299.
- Kruger DF, LaRue S, Estepa P. Recognition of and steps to mitigate anxiety and fear of pain in injectable diabetes treatment. Diabetes Metab Syndr Obes. 2015;8:49–56.
- Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–2545.
- Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–412.
- Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12.
- Nakar S, Yitzhaki G, Rosenberg R, et al. Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220–226.
- Santos Cavaiola T, Kiriakov Y, Reid T. Primary care management of patients with type 2 diabetes: overcoming inertia and advancing therapy with the use of injectables. Clin Ther. 2019;41(2):352–367.
- US Food and Drug Administration. FDA approves first oral GLP-1 treatment for type 2 diabetes. 2019. [cited 2020 Mar 9]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-glp-1-treatment-type-2-diabetes
- Novo Nordisk. Rybelsus® (oral semaglutide) approved for the treatment of adults with type 2 diabetes in the EU, 2020. [cited 2020 Apr 9]. Available from: https://www.novonordisk.com/media/news-details.2277630.html
- Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10:467.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851.
- Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539.
- Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8:392–406.
- Yamada Y, Katagiri H, Hamamoto Y, et al. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8:377–391.
- Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732.
- Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281.
- Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–1480.
- Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
- Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–527.
- Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–2271.
- Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266.
- Bækdal TA, Borregaard J, Donsmark M, et al. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide (abstract). Diabetes. 2017;66(Suppl 1):1179–P.
- Hauge C, Breitschaft A, Hartoft-Nielsen ML, et al. A drug-drug interaction trial of oral semaglutide with levothyroxine and multiple coadministered tablets (abstract). J Endocr Soc. 2019;3(Suppl1):SAT–140.
- Bækdal TA, Breitschaft A, Navarria A, et al. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869–877.
- Bækdal TA, Borregaard J, Hansen CW, et al. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58(9):1193–1203.
- Bækdal TA, Albayaty M, Maniigandan E, et al. A trial to investigate the effect of oral semaglutide on the pharmacokinetics of furosemide and rosuvastatin in healthy subjects (abstract). Diabetologia. 2018;61(Suppl 1):714.
- Jordy A, Breitschaft A, Christiansen E, et al. Oral semaglutide does not affect the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel (abstract). Diabetologia. 2018;61(Suppl 1):713.
- Levoxyl® prescribing information [updated Dec 2018; cited 2020 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021301s038lbl.pdf
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751.
- Bolk N, Visser TJ, Nijman J, et al. Effects of evening vs morning levothyroxine intake: a randomized double-blind crossover trial. Arch Intern Med. 2010;170(22):1996–2003.
- Geer M, Potter DM, Ulrich H. Alternative schedules of levothyroxine administration. Am J Health Syst Pharm. 2015;72(5):373–377.
- Granhall C, Søndergaard FL, Thomsen M, et al. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571–1580.
- Bækdal TA, Thomsen M, Kupčová V, et al. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–1323.
- Meier J, Granhall C, Hoevelman U, et al. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes (abstract). Diabetes. 2019;68(Suppl 1):1013–P.
- Davies M, Pieber TR, Hartoft-Nielsen ML, et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460–1470.
- Gomez-Peralta F, Abreu C. Profile of semaglutide in the management of type 2 diabetes: design, development, and place in therapy. Drug Des Devel Ther. 2019;13:731–738.
- Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202–210.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American diabetes association and the endocrine society. Diabetes Care. 2013;36(5):1384–1395.
