ABSTRACT
Patients with type 2 diabetes (T2D) often have comorbidities, such as cardiovascular disease or chronic kidney disease, and a large and growing proportion of the T2D patient population is over 65 years. There are many therapies for the treatment of T2D but not all are suitable for patients with comorbidities. Oral semaglutide is a tablet formulation of a glucagon-like peptide-1 receptor agonist (GLP-1RA) and was recently approved for the treatment of T2D, representing an oral alternative to injectable GLP-1RAs. This article reviews data from: PIONEER 6, a phase 3a cardiovascular outcomes trial in patients at high cardiovascular risk; PIONEER 5, a phase 3a trial in patients with moderate renal impairment; a post-hoc analysis of PIONEER data by age; and pharmacokinetic trials investigating the effects of renal impairment, gastrointestinal disease, and hepatic impairment on the exposure of oral semaglutide. PIONEER 6 demonstrated the cardiovascular safety of oral semaglutide compared with placebo (hazard ratio: 0.79; 95% confidence interval [CI]: 0.57, 1.11; p < 0.001 for noninferiority), ruling out excess cardiovascular risk. In PIONEER 5, oral semaglutide was superior to placebo in decreasing glycated hemoglobin over 26 weeks (estimated treatment difference [ETD]: –0.8%; 95% CI: –1.0, –0.6; p < 0.0001) and body weight (ETD: –2.5 kg; 95% CI: –3.2, –1.8; p < 0.0001), and renal function was unchanged in both treatment groups. There was no effect of age on glycemic efficacy of oral semaglutide and the presence of upper gastrointestinal disease or hepatic impairment did not affect the pharmacokinetics of semaglutide. Across the trials, the safety profile of oral semaglutide was as expected for a GLP-1RA, with gastrointestinal adverse events most commonly reported. As such, oral semaglutide provides an effective oral GLP-1RA treatment option in older patients and/or those with comorbidities, with no requirements for dose adjustment.
Article overview and relevance to your clinical practice
Cardiovascular disease (CVD) and chronic kidney disease (CKD) are common in patients with type 2 diabetes (T2D) and often need to be considered, along with age and other common comorbidities, when selecting treatment options in the primary care setting.
This article summarizes the burden of CVD and CKD, and highlights the importance of these and other comorbidities that might influence treatment choices in T2D.
Oral semaglutide has been recently approved and offers the option of a glucagon-like peptide-1 receptor agonist treatment in a tablet.
This article reviews the efficacy and safety data from clinical studies of oral semaglutide in patients with important comorbidities such as CVD and CKD, providing a summary of key guidance for clinical practice.
In addition, we discuss patient selection and monitoring, guidance on dose initiation and escalation, and the most common side effects and how these can be managed in practice.
We also highlight the important information that patients need to understand regarding food, beverages, or other medications when taking oral semaglutide.
1. Comorbidities in patients with type 2 diabetes
The prevalence of type 2 diabetes (T2D) has been increasing in recent decades and this has led to a growth in the medical and economic burden associated with both macrovascular complications such as cardiovascular disease (CVD), and microvascular complications such as chronic kidney disease (CKD) [Citation1,Citation2]. In addition, T2D is found to affect ~20% of people aged ≥65 years, with forecasts predicting a further rise in prevalence over time [Citation3,Citation4]. Older people with T2D have higher rates of CVD when compared with those without T2D [Citation5]. CVD is a leading cause of death and disability in people with T2D, and the rate of death from CVD for those with T2D is significantly higher than for those without T2D [Citation2,Citation6,Citation7].
CKD is prevalent in people with T2D and is associated with high morbidity and mortality as well as a high economic burden [Citation4,Citation8]. Furthermore, the occurrence of CKD in people with T2D has increased over previous years [Citation2]. Data from the National Health and Nutrition Examination Survey reported a prevalence of CKD during 1999–2012 of 43.5% in patients with T2D [Citation9]. In this study, 12.9% of patients had estimated glomerular filtration rate (eGFR) 45–59 mL/min/1.73 m2 (mild-to-moderate impairment) and 9.0% had eGFR <45 mL/min/1.73 m2 (moderate-to-severe impairment). These proportions were even higher in patients aged ≥65 years (24.5% and 18.6%, respectively). In patients with T2D and renal impairment, hypertension, coronary heart disease, myocardial infarction, and congestive heart failure were the most common cardiovascular (CV) comorbidities [Citation9]. Studies have shown that lower eGFR [Citation10] and higher albuminuria in patients with T2D are associated with increased rates of death and adverse kidney and CV outcomes, including end-stage renal disease [Citation11–13].
1.1. Treatments for patients with type 2 diabetes and comorbidities
Guidelines for the care of people with T2D advocate an individualized approach that includes screening for complications and comorbidities [Citation5,Citation14]. Consequently, assessment of CVD and CKD, along with other common comorbidities, is central to the appropriate management of patients with T2D and the selection of appropriate treatment options [Citation15–17]. The American Diabetes Association (ADA) also recommends that management approaches and targets in older patients with T2D should be reviewed regularly [Citation5], so it is important to understand the efficacy and tolerability profile of treatments with advancing age. A brief summary of the factors to consider when prescribing glucose-lowering medications to these patient groups is shown in .
Figure 1. A summary of factors to consider for use of glucose-lowering medications in patients with cardiovascular disease, renal impairment, and in older patients. Adapted from [Citation5,Citation15,Citation16]
![Figure 1. A summary of factors to consider for use of glucose-lowering medications in patients with cardiovascular disease, renal impairment, and in older patients. Adapted from [Citation5,Citation15,Citation16]](/cms/asset/06ffd33a-9f18-4a1b-b262-88f0119f06ea/ipgm_a_1800286_f0001_c.jpg)
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) as a class provide effective glucose and body weight reductions, with a low risk of hypoglycemia [Citation15]. Additionally, some GLP-1RA therapies confer potential reductions in CV risk [Citation18–20]. GLP-1RAs are peptide-based therapies that are subject to degradation by gastric enzymes in the stomach and have limited permeability across the gastric mucosa if administered orally [Citation21]. Until recently, the available GLP-1RAs have thus been administered by injection, which may be less desirable for some patients compared with oral administration [Citation22]. Furthermore, ensuring appropriate use of injectable therapy may be an important consideration for patients with impaired visual or motor skills that could pose a challenge with using the device, for example, if they have existing or develop medical conditions that affect their ability to follow their regimen safely [Citation5].
Oral semaglutide is the first oral GLP-1RA to be developed and has recently been approved in the US for the improvement of glycemic control in adults with T2D [Citation23]. An extensive, global, phase 3a clinical trial program (PIONEER) evaluated oral semaglutide in patients from across the spectrum of T2D (for an overview of the PIONEER program, see Brunton & Wysham [Citation24]). Oral semaglutide offers the option of a GLP-1RA in tablet form for patients with uncontrolled T2D, thereby addressing the potential challenges relating to the injection route of administration historically required for GLP-1RAs.
For successful treatment in the primary care setting, it is important for prescribers and those involved in all aspects of patient care to understand the use of oral semaglutide in patients with comorbidities, and to be able to advise on practical issues such as dosing instructions and the management of adverse events (AEs). The aim of this review is to present the clinical evidence relevant to the use of oral semaglutide in specific patient populations, including those at high risk of CV events (as studied in the PIONEER 6 CV outcomes trial [CVOT]) [Citation25], those with renal impairment (including data from PIONEER 5) [Citation26], and in older patients [Citation27]. We also review the data in patients with hepatic impairment [Citation28] and patients with upper gastrointestinal disease [Citation29] to assess the potential influence of these comorbidities on the pharmacokinetics of oral semaglutide. Finally, the implications of these data are summarized in the context of the management of patients with T2D and comorbidities in a primary care setting.
2. Cardiovascular safety of oral semaglutide: data from PIONEER 6
2.1. Trial design and patients randomized
The PIONEER 6 CVOT was an event-driven randomized, double-blind, placebo-controlled pre-approval trial conducted to determine if oral semaglutide can be used without increasing the risk of CV events in patients at high CV risk [Citation25,Citation30]. It was designed and powered in line with regulatory requirements [Citation31,Citation32] to confirm that treatment with oral semaglutide does not result in an unacceptable 80% excess in CV risk compared with placebo (both added to standard of care). Key information about the trial, its design and endpoints are shown in .
Table 1. Overview of PIONEER 5 and PIONEER 6 trial designs [Citation25,Citation26]
As with all studies in the phase 3a PIONEER clinical program, a gradual dose-escalation regimen of oral semaglutide was implemented to reduce the risk of gastrointestinal AEs until the target dose of 14 mg was achieved [Citation25]: patients were treated with 3 mg oral semaglutide for 4 weeks, then 7 mg for 4 weeks before commencing treatment with the 14 mg dose. Patients were also instructed to take oral semaglutide in the morning in a fasted state with up to 4 fl oz (120 mL) of water, at least 30 minutes before eating, drinking anything else, or taking any other oral medication.
PIONEER 6 enrolled 3183 patients with T2D with established CVD or CKD, or who were at high risk of CV events [Citation25]. Approximately 85% of patients were aged ≥50 years and had established CVD or CKD, while the remaining patients were aged ≥60 years and had CV risk factors only. Overall, mean baseline glycated hemoglobin (HbA1c) was 8.2%, mean age was 66 years, and mean duration of diabetes was approximately 15 years. Mean body weight was 90.9 kg, mean body mass index was 32.3 kg/m2, and mean eGFR at baseline was 74 mL/min/1.73 m2.
At baseline, 93.9% of patients were taking antihypertensive medication, 85.2% lipid-lowering medication, and 79.4% antiplatelet or antithrombotic medication [Citation25]. Most patients were taking metformin (77.4%) or insulin (60.6%), with 32.3% taking sulfonylureas and 9.6% sodium-glucose co-transporter-2 inhibitors (SGLT2is). More patients in the placebo group started or intensified glucose-lowering therapy during the trial than in the oral semaglutide group, including greater use of SGLT2is (7.0% versus 3.1%).
Baseline characteristics and the profile of CV risk factors including blood pressure, low-density lipoprotein cholesterol, smoking, and renal function were similar between treatment groups [Citation25]. The median time in the trial was 15.9 months (range 0.4–20.0 months).
2.2. Cardiovascular safety
PIONEER 6 confirmed that CV events did not occur more frequently in patients treated with oral semaglutide when compared with placebo [Citation25]. The trial achieved its primary endpoint by demonstrating noninferiority (p < 0.001) for time to first major adverse CV event (MACE; a composite of CV death, nonfatal myocardial infarction, and nonfatal stroke) for oral semaglutide compared with placebo (hazard ratio [HR]: 0.79; 95% confidence interval [CI]: 0.57, 1.11) (). The trial was not designed to demonstrate superiority, and superiority of oral semaglutide versus placebo could not be confirmed (p = 0.17 for superiority).
Figure 2. Composite primary outcome in PIONEER 6 [Citation25]
![Figure 2. Composite primary outcome in PIONEER 6 [Citation25]](/cms/asset/b9d95ee2-d826-4e3a-9208-762a17aaa8af/ipgm_a_1800286_f0002_b.gif)
PIONEER 6 also investigated the incidence of individual components of the primary outcome, including death from CV causes, as well as for all-cause mortality, although it was not powered to show a difference between treatments. It was reported that death from CV causes occurred in 0.9% (15/1591) of patients in the oral semaglutide group and 1.9% (30/1592) in the placebo group; death from any cause occurred in 1.4% (23/1591) of patients in the oral semaglutide group and 2.8% (45/1592) in the placebo group; HRs were 0.49 (95% CI: 0.27, 0.92) and 0.51 (95% CI: 0.31, 0.84), respectively [Citation25]. The HR for the expanded MACE outcome (consisting of the primary outcome plus unstable angina resulting in hospitalization or heart failure resulting in hospitalization) was similar to that of the primary outcome (HR: 0.82 [95% CI: 0.61, 1.10]) [Citation25].
PIONEER 6 demonstrated that oral semaglutide did not result in an increase in CV risk compared with placebo [Citation25], a finding that is supported by a post-hoc analysis of CV events across the PIONEER glycemic efficacy trials [Citation39]. The trial was an event-driven trial designed and powered to assess noninferiority of oral semaglutide to placebo, and continued until at least 122 MACE occurred, with no predefined minimum duration. This design differs to that of other GLP-1RA CVOTs [Citation30], such as SUSTAIN-6 with subcutaneous semaglutide, LEADER with liraglutide, and REWIND with dulaglutide, which reported significant reductions in risk of MACE with these agents versus placebo [Citation18–20] (for an in-depth overview of the designs and outcomes of PIONEER 6 and SUSTAIN-6, see Husain et al., 2020 [Citation39]). Like PIONEER 6, SUSTAIN-6 and LEADER were conducted primarily to establish noninferiority; however, these trials were both time- and event-driven and conducted for longer than PIONEER 6, accruing more MACE than originally planned (for example, MACE occurred in 254 patients in SUSTAIN-6, versus 137 in PIONEER 6), and therefore had greater power to evaluate the potential superiority of the study drug to placebo [Citation18,Citation19]. In contrast, the REWIND CVOT for dulaglutide was primarily conducted and powered to establish superiority, and as such had a longer duration than PIONEER 6, was conducted in a larger population, and accrued substantial more MACEs [Citation20]. Following the results of REWIND, LEADER and SUSTAIN-6, dulaglutide, liraglutide and subcutaneous semaglutide are now approved for reducing the risk of CV events in patients with established CVD (dulaglutide is also indicated for reducing this risk in patients with T2D and multiple CV risk factors) [Citation33–38] and guidelines recommend that patients with established CVD should be treated with agents with proven CV benefit [Citation15–17]. With proven CV safety, oral semaglutide can be used in patients without established CVD, but it is not currently indicated for the reduction in CV risk in patients with established CVD. A larger, long-term CVOT (the SOUL trial) is ongoing to determine whether oral semaglutide provides CV benefit in patients with T2D and CVD or CKD [Citation40].
2.3. Efficacy outcomes
The efficacy of oral semaglutide compared with placebo was demonstrated in PIONEER 6 (these outcomes were not statistically analyzed) [Citation25]. For example, from baseline to the end of the trial, oral semaglutide was associated with greater reductions in HbA1c versus placebo (–1.0% versus –0.3%, respectively), despite more patients initiating or intensifying glucose-lowering therapy in the placebo group than in the oral semaglutide group during the trial. Oral semaglutide also resulted in greater body weight reductions versus placebo (–4.2 kg versus –0.8 kg, respectively). These reductions in HbA1c and body weight were comparable with results reported in other studies within the PIONEER program [Citation26,Citation41–46].
2.4. Adverse events and safety
AEs occurring during the trial are shown in . More patients permanently discontinued oral semaglutide (11.6%) than placebo (6.5%) due to AEs, with differences mainly due to gastrointestinal AEs, primarily nausea (2.9% versus 0.5%), vomiting (1.5% versus 0.3%), and diarrhea (1.4% versus 0.4%), which were mostly nonserious [Citation25]. Premature treatment discontinuation rates due to AEs were 11.5–14.5% with subcutaneous semaglutide and 5.7–7.6% with placebo over 2.1 years in SUSTAIN-6 [Citation18] and 9.5% with subcutaneous liraglutide and 7.3% with placebo over 3.8 years in LEADER [Citation19].
Figure 3. Incidence of adverse events during PIONEER 5 and 6 (on-treatment data) [Citation25,Citation26]
![Figure 3. Incidence of adverse events during PIONEER 5 and 6 (on-treatment data) [Citation25,Citation26]](/cms/asset/a552b190-50a1-4a21-a275-fa3e25c8bdc5/ipgm_a_1800286_f0003_c.jpg)
In PIONEER 6, the percentage of patients with severe hypoglycemia (defined in accordance with ADA criteria as a hypoglycemic event requiring assistance of another person [Citation47]) was 1.4% for oral semaglutide and 0.8% for placebo, all of which occurred in those receiving concomitant insulin or sulfonylureas. AEs related to diabetic retinopathy occurred in 7.1% of patients treated with oral semaglutide and 6.3% with placebo, most of which were nonproliferative and were identified during scheduled eye examinations; 75.7% resulted in no new treatment [Citation25]. Due to a higher risk of diabetic retinopathy complications with subcutaneous semaglutide in SUSTAIN-6 [Citation18], patients with proliferative retinopathy or maculopathy resulting in active treatment were excluded from PIONEER 6 [Citation25].
Meta-analyses, as well as information collected from CVOTs involving long-term follow-up of thousands of patients, have consistently found insufficient evidence to support an increased risk of acute pancreatitis or cancer (including thyroid cancers) associated with GLP-1RAs as a class [Citation48–50]. This was also suggested by data from PIONEER 6, in which acute pancreatitis was confirmed in one patient treated with oral semaglutide, and three patients receiving placebo [Citation25].
Malignant neoplasms occurred in 2.6% of patients receiving oral semaglutide and 3.0% receiving placebo, with no evidence of clustering in any organ system. Medullary thyroid cancer was reported in one patient in the oral semaglutide group who had an elevated baseline calcitonin level and pre-existing thyroid nodules; another patient had a recurrence of a previous thyroid cancer [Citation25].
Overall, the safety results from PIONEER 6 were as expected for the GLP-1RA class, with no unexpected AEs identified. These results support the use of oral semaglutide for the treatment of T2D in patients either with CVD or with risk factors for CVD [Citation25].
Key clinical take-home points: CV safety of oral semaglutide
Patients with T2D at risk of, or with, CVD can receive treatment with oral semaglutide; the CV safety of oral semaglutide has been confirmed in a clinical trial.
Oral semaglutide reduces HbA1c and body weight in patients with T2D and CVD, with a safety profile as expected for a GLP-1RA.
Three GLP-1RAs (dulaglutide, liraglutide, and subcutaneous semaglutide) are currently indicated for reducing the risk of CV events in patients with T2D and established CVD; dulaglutide is also indicated for reducing this risk in patients with T2D and multiple CV risk factors.
The ongoing SOUL trial will determine whether oral semaglutide provides CV benefit in patients with T2D and CVD or CKD.
3. Oral semaglutide in patients with renal impairment
3.1. Pharmacokinetics and safety
It has been established that dose adjustment is not necessary when oral semaglutide is used in patients with different levels of renal impairment [Citation23,Citation51]. In a study of people with either normal renal function (n = 24), mild renal impairment (eGFR 60–89 mL/min/1.73 m2; n = 12), moderate renal impairment (eGFR 30–59 mL/min/1.73 m2; n = 12), severe renal impairment (eGFR 15–29 mL/min/1.73 m2; n = 12), or end-stage renal disease requiring hemodialysis (n = 11), the pharmacokinetics of oral semaglutide (5 mg for 5 days followed by 10 mg for 5 days) were unaffected [Citation51]. Oral semaglutide was also well tolerated and the severity of renal impairment did not seem to have an impact on the number of AEs reported.
3.2. Clinical outcomes
PIONEER 5 evaluated the efficacy and safety of oral semaglutide 14 mg once daily versus placebo in patients with T2D and moderate renal impairment (eGFR 30–59 mL/min/1.73 m2). Key information about the trial, its design and endpoints are shown in [Citation26]. In PIONEER 5, dose escalation of oral semaglutide was used to reduce the risk of gastrointestinal AEs. Notably, this was the same dosing strategy used in non-CKD patients in the other PIONEER trials [Citation25,Citation41–44,Citation46]. Of the 324 patients randomized (mean age 70 years; mean duration of diabetes 14 years), 60% had stage 3a (eGFR 45–59 mL/min/1.73 m2) CKD and the remainder had stage 3b (eGFR 30–44 mL/min/1.73 m2) CKD. At baseline, 75% of patients were taking metformin, 40% sulfonylureas, and 35% basal insulin.
Efficacy data in PIONEER 5 were primarily analyzed regardless of study drug discontinuation or use of rescue medication. After 26 weeks of treatment, oral semaglutide was superior to placebo in reducing HbA1c (estimated treatment difference [ETD]: –0.8%; 95% CI: –1.0, –0.6; p < 0.0001) and body weight (ETD: –2.5 kg; 95% CI: –3.2, –1.8; p < 0.0001) [Citation26].
AEs (on assigned treatment) were reported by 74% of patients with oral semaglutide and 65% with placebo, and the proportions of patients reporting serious AEs were similar for both groups (10% for oral semaglutide and 11% with placebo in the on-treatment period; ). The rate of AEs in PIONEER 5 was similar to that seen in other PIONEER trials that involved add-on to 1–2 oral therapies or to insulin-based treatment [Citation42–46], despite the patient population having renal impairment. The most common AE with oral semaglutide was nausea (reported in 19% of patients with oral semaglutide and in 7% with placebo), which was usually transient and mild or moderate in severity [Citation26].
The proportion of trial product discontinuations due to AEs was 15% with oral semaglutide 14 mg and 5% with placebo, mainly due to gastrointestinal events. Nausea appeared to be more common in patients with better renal function (i.e. stage 3a versus stage 3b CKD), but the clinical significance of this could not be established due to low patient numbers [Citation26]. For patients reporting severe adverse gastrointestinal reactions, it is advised to monitor renal function when initiating or escalating doses of oral semaglutide [Citation23].
There were low rates of symptomatic hypoglycemia confirmed by blood glucose in patients randomized to oral semaglutide (), and no severe hypoglycemic events occurred. Diabetic retinopathy-related AEs were infrequent with both oral semaglutide (3%) and placebo (1%). Most were identified at routine examination and none required treatment or led to treatment discontinuation [Citation26].
Renal function was unchanged throughout the trial in both treatment groups, demonstrating that oral semaglutide treatment is not likely to worsen renal function. Minor improvements in urinary albumin to creatinine ratio were observed with oral semaglutide; however, due to the relatively small population and lack of stratification to urinary albumin to creatinine ratios at randomization, further study is needed to confirm the effect of oral semaglutide in association with albuminuria and clinical outcomes in patients with CKD.
A glycemic efficacy and safety trial has not been conducted with subcutaneous semaglutide in patients with T2D and renal impairment; however, similar results to PIONEER 5 were observed when liraglutide was compared with placebo in the LIRA-RENAL trial in patients with T2D and moderate renal impairment [Citation52].
Key clinical take-home points: renal impairment
Based on a clinical study in moderate renal impairment and pharmacokinetic data in patients with varying severity of renal impairment, no dose adjustment is recommended for oral semaglutide in patients with renal impairment.
Renal function did not change during treatment with oral semaglutide in patients with moderate renal impairment.
The most common AEs with oral semaglutide were gastrointestinal, including nausea, vomiting, and diarrhea.
Gastrointestinal AEs were often transient, mild-to-moderate in severity and were mostly associated with dose escalation.
The incidence of hypoglycemia was low in patients with moderate renal impairment on oral semaglutide, and similar to those on placebo.
4. The effect of patient age on the efficacy and safety of oral semaglutide
An exploratory analysis of PIONEER 1–5, 7, and 8 evaluated the effect of patient age on the efficacy and safety of oral semaglutide [Citation27]. Efficacy endpoints were analyzed for patients with T2D aged <45 (n = 582), ≥45–<65 (n = 3392), or ≥65 (n = 1683) years. Overall, HbA1c reductions were comparable across age groups, demonstrating the efficacy of oral semaglutide regardless of patient age (). When compared with other glucose-lowering therapies (including an SGLT2i, a dipeptidyl peptidase-4 inhibitor [DPP-4i], and an injectable GLP-1RA), reductions in HbA1c were generally greater with oral semaglutide irrespective of age (). There did not appear to be a clear relationship between age and reductions in body weight, although reductions were greater for oral semaglutide than with comparators.
Table 2. Change from baseline in HbA1c by age subgroup in select PIONEER studies comparing oral semaglutide 14 mg with placebo or an active comparator [Citation27]
The safety profile of oral semaglutide was as expected for a GLP-1RA, with similar proportions of patients with AEs across the three age groups; however, there was a tendency for higher rates of premature trial product discontinuation due to AEs with increasing age [Citation27]. For example, in the PIONEER 3 trial [Citation43], among patients receiving any dose of oral semaglutide (added to background therapy of metformin with or without a sulfonylurea), 86% of those aged ≥65 years reported AEs compared with 76% of those aged ≥45–<65 years and 80% of those aged <45 years [Citation27]. In this trial, discontinuation of treatment due to AEs occurred in 9% and 7% of patients aged ≥65 and <65 years, respectively. Increases in the proportion of patients with AEs in those aged ≥65 years versus the younger age groups were also observed for most comparator groups across the PIONEER trials. Similar to the approach in other patient populations, a slower dose escalation could be used in older patients to help mitigate any AEs that could lead to discontinuing treatment, although it should be noted that this is not specifically included in the prescribing information for this particular patient population. Treatment strategies should be individualized to patients as recommended in guidelines.
5. Oral semaglutide in patients with other comorbidities
It has been demonstrated that there are no changes in semaglutide exposure or pharmacokinetic parameters following administration of oral semaglutide in patients with hepatic impairment [Citation23,Citation28]. In an open-label, parallel-group trial, Bækdal et al. [Citation28] studied the effects of once-daily oral semaglutide (5 mg for 5 days followed by 10 mg for 5 days) in subjects with either: normal hepatic function (n = 24), or mild (n = 12), moderate (n = 12), or severe (n = 8) hepatic impairment (according to Child-Pugh criteria) [Citation28]. The incidence and severity of AEs did not appear to be influenced by hepatic function, and reported AEs were in line with those observed in the PIONEER program and for other GLP-1RAs [Citation25,Citation26,Citation41–46,Citation53]. Headache was the most frequently reported AE (14.3%), followed by dyspepsia (8.9%), vomiting (7.1%), decreased appetite (7.1%), and diarrhea (5.4%) [Citation28]. Based on these results, no dose adjustment of oral semaglutide is recommended for patients with T2D and hepatic impairment [Citation23].
Meier et al. [Citation29] studied 36 subjects aged 18–80 years with T2D and with either upper gastrointestinal disease (chronic gastritis [n = 5], gastroesophageal reflux disease [n = 8], or both [n = 23]) or without upper gastrointestinal disease (n = 19), who received oral semaglutide 3 mg once daily for 5 days followed by 7 mg for 5 days in an open-label, parallel-group trial [Citation29]. In this study, upper gastrointestinal disease had no significant effect on semaglutide exposure, and other pharmacokinetic parameters were similar in those with and without upper gastrointestinal disease. There were no serious AEs and no discontinuations due to AEs, suggesting that oral semaglutide could be tolerated in patients with upper gastrointestinal disease; however, this was a study in a small number of subjects and used a different dose-escalation regimen as well as a lower maximum dose to that used in the PIONEER trial program [Citation29]. Oral semaglutide has not been specifically studied in patients with gastroparesis, nor in those who have undergone bariatric surgery.
Key clinical take-home points: patient age and patients with other comorbidities
There is no need to make dose adjustments of oral semaglutide based on age alone.
No dose adjustment is recommended for patients with hepatic impairment.
Patients should be made aware of the occurrence of gastrointestinal AEs with GLP-1RAs.
6. Ongoing studies with oral or subcutaneous semaglutide in patients with comorbidities
Several studies were initiated in 2019 that will assess the long-term effects of oral or subcutaneous semaglutide in patients with comorbidities. The SOUL trial is a long-term, placebo-controlled CVOT investigating whether oral semaglutide (up to 14 mg daily) provides CV benefit in patients with CV comorbidities [Citation40]. This event-driven trial has an estimated recruitment of 9642 patients and is expected to last for around 5 years.
The SELECT trial is a long-term placebo-controlled CVOT that will evaluate subcutaneous semaglutide in patients with established CVD and obesity, but without T2D [Citation54]. Since the setting is obesity rather than T2D, the maximum subcutaneous semaglutide dose being investigated in the SELECT trial is 2.4 mg once weekly rather than the maximum dose of 1.0 mg once weekly approved for T2D treatment [Citation35]. The FLOW trial is a placebo-controlled study of subcutaneous semaglutide in patients with T2D and CKD [Citation55] and FOCUS is a long-term placebo-controlled study of the effects of subcutaneous semaglutide on diabetic retinopathy complications [Citation56]. Results from these trials will provide further information on the efficacy and safety of semaglutide in patients with common comorbidities such as obesity and CKD, and important T2D complications such as diabetic retinopathy.
7. Implications for the use of oral semaglutide in the primary care setting
A summary of key implications for the use of oral semaglutide in patients with comorbidities is shown in . Oral semaglutide is an important new option to consider in the management of patients with CVD or CKD, common comorbidities in patients with T2D.
Figure 4. Summary of key clinical implications for use of oral semaglutide in patients with comorbidities. CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; GI, gastrointestinal
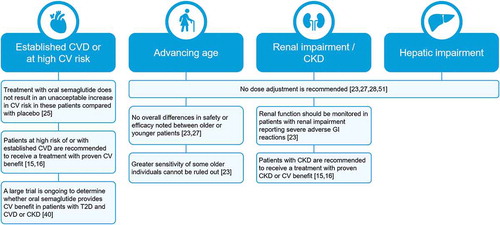
Clinicians have a number of treatment options available for the management of patients with T2D. However, there are limitations with some of these agents when taking patient comorbidities and other risk factors into account. In the management of patients with T2D and renal impairment, metformin should not be used in patients with eGFR <30 mL/min/1.73 m2 and SGLT2is have limited efficacy when eGFR is <45 mL/min/1.73 m2 [Citation16], although some SGLT2is have been shown to reduce CKD progression in CVOTs and are recommended if eGFR is adequate [Citation15]. GLP-1RAs and DPP-4is can be used in patients with CKD, although unlike oral semaglutide, some require dose adjustment. However, DPP-4is have neutral effects on body weight [Citation16] and atherosclerotic CVD risk [Citation15].
The presence of food and fluid in the stomach impairs absorption of oral semaglutide [Citation57,Citation58]. To ensure optimal efficacy, it is important to explain the reason for these dosing instructions so patients understand the need to adhere to them. Consequently, practical issues relating to treatment with oral semaglutide, including administration instructions, are important to consider. Patients should take oral semaglutide in the morning in a fasted state, with up to 4 fl oz (120 mL) of water, at least 30 minutes before eating, drinking anything else, or taking any other oral medication [Citation23].
As many patients with T2D take multiple medications, when co-administering other oral medications it is important to adhere to the administration instructions for oral semaglutide, and consider increased monitoring for medications that have a narrow therapeutic index or that require clinical monitoring [Citation23]. See Brunton et al. [Citation59] for more information and practical recommendations on a range of potential concomitant medications including omeprazole, lisinopril, warfarin, digoxin, metformin, furosemide, rosuvastatin, levothyroxine, and the combined oral contraceptive ethinylestradiol/levonorgestrel [Citation59].
To summarize, the clinical studies conducted to date have shown that oral semaglutide provides an oral GLP-1RA treatment option for T2D patients of advanced age and/or who suffer with burdensome comorbidities, such as CVD or renal impairment, with no requirements for dose adjustment. Not only does oral semaglutide provide highly effective glucose-lowering and weight-loss effects, with low risk of hypoglycemia and no increase in CV risk, this oral formulation has the added advantage of addressing concerns related to initiation of injectable therapy.
Declaration of interest
O.M. reports: advisory board for AstraZeneca, Boehringer Ingelheim, BOL Pharma, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; research grant support through Hadassah Hebrew University Hospital: AstraZeneca and Novo Nordisk; speaker’s bureau: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi.
E.M. reports: advisory board and speaker’s bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi.
M.W. reports: advisory board and speaker’s bureau for Eli Lilly, Novo Nordisk, and Sanofi; speaker’s bureau for AstraZeneca and Merck; research support from AstraZeneca, Eli Lilly, Novo Nordisk, and Sanofi.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Under the direction of the authors, medical writing and editorial support were provided by Nicola Beadle of Axis, a division of Spirit Medical Communications Group Ltd. (funded by Novo Nordisk Inc.). The authors were involved with drafting and/or critically editing all drafts during the development of the article, and all authors provided their final approval for submission.
Additional information
Funding
References
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928.
- Harding JL, Pavkov ME, Magliano DJ, et al. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16.
- National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes in America. 3rd ed. 2018 [cited 2020 May 25]. Available from: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/diabetes-in-america-3rd-edition#CandF
- International Diabetes Federation. IDF Atlas: 9th ed. 2019 [cited 2020 May 25]. Available from: http://www.diabetesatlas.org
- American Diabetes Association. 12. Older adults: Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020;43(Suppl 1):S152–S162.
- Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–2440.
- Taylor KS, Heneghan CJ, Farmer AJ, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care. 2013;36(8):2366–2371.
- Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):414–422D.
- Bailey RA, Wang Y, Zhu V, et al. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415.
- Wang Y, Katzmarzyk PT, Horswell R, et al. Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int. 2014;85(5):1192–1199.
- De Cosmo S, Lamacchia O, Pacilli A, et al. Normoalbuminuric renal impairment and all-cause mortality in type 2 diabetes mellitus. Acta Diabetol. 2014;51(4):687–689.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340.
- Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104.
- American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020;43(Suppl 1):S37–S47.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020;43(Suppl 1):S98–S110.
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107–139.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and The European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–493.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130.
- Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047.
- DiBonaventura MD, Wagner JS, Girman CJ, et al. Multinational internet-based survey of patient preference for newer oral or injectable type 2 diabetes medication. Patient Prefer Adherence. 2010;4:397–406.
- Rybelsus® (semaglutide) prescribing information [updated Jan 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213182s000,213051s001lbl.pdf
- Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad Med. 2020;132(S2):3–14.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851.
- Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–527.
- Aroda VR, Bauer R, Herts CL, et al. Efficacy and safety of oral semaglutide by baseline age in the PIONEER clinical trial program. Diabetes. 2020;69(Suppl 1):932-P.
- Bækdal TA, Thomsen M, Kupčová V, et al. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–1323.
- Meier J, Granhall C, Hoevelmann U, et al. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes. Diabetes. 2019;68(Suppl 1):1013–P.
- Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21(3):499–508.
- US Food and Drug Administration. Appendix 1. Guidance for industry: diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008 [cited 2020 May 25]. Available from: https://www.fda.gov/media/121272/download
- Bydureon® (exenatide extended-release) prescribing information [updated Feb 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022200s030lbl.pdf
- Trulicity® (dulaglutide) prescribing information [updated Feb 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s033lbl.pdf
- Victoza® (liraglutide) prescribing information [updated June 2019; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022341s031lbl.pdf
- Ozempic® (semaglutide) prescribing information [updated Jan 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf
- Invokana® (canagliflozin) prescribing information [updated Jan 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204042s036lbl.pdf
- Jardiance® (empagliflozin) prescribing information [updated Jan 2020; cited 2020 May 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204042s011lbl.pdf
- Tella SH, Rendell MS. DPP-4 inhibitors: focus on safety. Expert Opin Drug Saf. 2015;14(1):127–140.
- Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22:442–451.
- ClinicalTrials.gov. A heart disease study of semaglutide in patients with type 2 diabetes (SOUL), identifier NCT03914326 [ cited 2020 May 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03914326
- Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732.
- Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281.
- Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–1480.
- Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
- Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539.
- Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–2271.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395.
- Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113.
- Kristensen SL, Røth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785.
- Liu Y, Zhang X, Chai S, et al. Risk of malignant neoplasia with glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes: a meta-analysis. J Diabetes Res. 2019;2019:1534365.
- Granhall C, Søndergaard FL, Thomsen M, et al. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57:1571–1580.
- Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222–230.
- Lyseng-Williamson KA. Glucagon-like peptide-1 receptor analogues in type 2 diabetes: their use and differential features. Clin Drug Investig. 2019;39:805–819.
- ClinicalTrials.gov. Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT), identifier NCT03574597 [ cited 2020 May 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03574597
- ClinicalTrials.gov. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW), identifier NCT03819153. [ cited 2020 May 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03819153
- ClinicalTrials.gov. A research study to look at how semaglutide compared to placebo affects diabetic eye disease in people with type 2 diabetes (FOCUS), identifier NCT03811561 [ cited 2020 May 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03811561
- Donsmark M, Borregaard J, Breitschaft A, et al. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide. Diabetologia. 2017;60(Suppl 1):S1–S608. Abstract 792.
- Maarbjerg SJ, Borregaard J, Breitschaft A, et al. Evaluation of the effect of food on the pharmacokinetics of oral semaglutide. Diabetologia. 2017;60(Suppl 1):S1–S608. Abstract 148.
- Brunton SA, Mosenzon O, Wright EE. Integrating oral semaglutide into clinical practice in primary care: for whom, when, and how? Postgrad Med. 2020;132(S2):48–60.
