ABSTRACT
Asthma is a heterogeneous disease characterized by airway inflammation resulting from complex interactions between multiple hosts as well as environmental factors. As a chronic respiratory condition, asthma exerts a significant impact on patients and the healthcare system. Per the Global Initiative for Asthma (GINA), inhaled corticosteroids (ICS) with/without long-acting beta2-agonists (LABAs) should be used as the preferred controllers for the management of asthma. Despite a range of therapeutic options, many patients with asthma remain uncontrolled, resulting in an increased risk of hospitalization and emergency room visits and a worsened quality of life. Tiotropium (Spiriva®, Boehringer Ingelheim Pharmaceuticals, Inc; 1.25 µg, two puffs, once daily), delivered via the Respimat® inhaler (Boehringer Ingelheim Pharmaceuticals, Inc.), was the first long-acting muscarinic antagonist to be approved as an add-on maintenance treatment option for patients with asthma aged ≥6 years at GINA steps 4 and 5. By binding to the muscarinic receptors M1 and M3 in the bronchial airways, tiotropium antagonizes the action of acetylcholine, leading to smooth muscle relaxation and reduced mucus secretion.
The efficacy and safety of tiotropium add-on to ICS±LABA maintenance treatment have been evaluated in randomized controlled trials (RCTs) involving patients with a range of asthma severities (mild, moderate, and severe) and across age groups (children, adolescents, and adults). Add-on tiotropium was found to be well tolerated and efficacious in all RCTs. Moreover, the findings from real-world studies complement results from RCTs, showing beneficial effects of tiotropium in reducing exacerbations, hospitalization, emergency room visits, and asthma worsening.
In this review article, we discuss the pathophysiology of asthma and the role of tiotropium in the management of asthma from the perspective of a primary care physician.
1. Introduction
Asthma is a heterogeneous condition and is usually characterized by chronic inflammation of the airways [Citation1]. The health impact of asthma in the US is substantial. In 2017, it affected >25 million Americans, including >6 million children, and caused >3,500 deaths [Citation2]. In the same year, >11 million patients with asthma suffered from an exacerbation, while in 2016, asthma accounted for >1.7 million emergency room visits [Citation2].
Asthma management involves a control-based cycle of assessing symptoms, adjusting treatment, and reviewing response [Citation1]. Building on a base of inhaled corticosteroids (ICS), either alone or in combination with long-acting beta2-agonists (LABAs) as preferred controller options, the Global Initiative for Asthma (GINA) strategy document outlines a stepwise pharmacological approach to achieve good symptom control and to minimize future risk of exacerbations [Citation1]. Besides increasing the dose of ICS+LABA, using additional controller options may be helpful for the management of asthma [Citation1]. However, these controller options (leukotriene receptor antagonists [LTRAs] and theophylline) are often limited by their reduced efficacy and risk of side effects compared with ICS [Citation3,Citation4], the need for phenotype assessment (biologics) [Citation1], and systemic side effects (oral corticosteroids [OCS]) [Citation1]. Long-acting muscarinic antagonists (LAMAs) have been successfully used in the management of chronic obstructive pulmonary disease (COPD) for many years. Treatment with LAMA alone or as part of dual or triple therapy with LABA or LABA+ICS, respectively, is recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [Citation5]. The need for new treatment options for managing patients with asthma led to the use of long-acting muscarinic LAMAs [Citation1,Citation6], which have traditionally been used in the management of COPD [Citation5].
Tiotropium (Spiriva®, Boehringer Ingelheim Pharmaceuticals, Inc.), delivered in a soft-moving mist via the Respimat® inhaler (Boehringer Ingelheim Pharmaceuticals, Inc.), was the only LAMA to be approved in 2015 by the US Food and Drug Administration (FDA) for long-term (1.25 µg, two puffs, once daily) maintenance treatment of asthma in patients aged ≥12 years and added to GINA steps 4 and 5 [Citation1,Citation7]. Subsequently, in 2017, the FDA extended the approval for tiotropium to include patients with asthma aged ≥6 years [Citation8,Citation9]. The approved dose for tiotropium Respimat® in the European Union (EU) is 2.5 µg, two puffs, once daily, for patients with asthma aged ≥6 years [Citation10].
This article reviews the role of tiotropium in managing patients with asthma by providing an overview of data from clinical and real-world studies and discusses its application to routine clinical practice. A brief discussion on the pathophysiology of asthma to explain the rationale for using LAMAs is presented along with the mechanism of action of tiotropium.
2. Pathophysiology of asthma
Asthma is described by a history of respiratory symptoms, including wheezing, shortness of breath, chest tightness, and cough, along with variable expiratory airflow limitation [Citation1]. The heterogeneous nature of asthma implies that it involves a range of phenotypes related to the age of onset, triggers, and type of inflammation (eosinophilic, neutrophilic, and pauci-granulocytic phenotypes) [Citation11]. Both host factors and environmental factors contribute to the development of asthma and involve complex, interactive mechanisms [Citation12]. The critical host factors include genetic predisposition, obesity, sex, and preterm birth, or small size for gestational age [Citation13]. The major environmental factors include indoor and outdoor allergens, occupational sensitizers, viral infections, microbiome within the host and in the surrounding environment of the host, tobacco smoke, air pollution, diet, and stress [Citation2].
Airway hyperresponsiveness is a result of various underlying factors that include thickening of the airway walls, inflammation of airways leading to excessive contraction of airway smooth muscles, and overstimulation of sensory nerves [Citation2]. Obstruction of the airways results from contraction of the airway smooth muscle, mucosal inflammation, edema, and characteristic structural changes termed as ‘airway remodeling’ [Citation14]. These structural changes include subepithelial fibrosis, increase in number and size of airway smooth muscles, increased blood vessels, mucus hypersecretion, and goblet cell hyperplasia () [Citation14]. The smooth muscle contraction is largely reversible, whereas airway thickening is not fully reversible [Citation2].
Figure 1. Pathophysiological changes in asthmatic airways (A) with underlying mechanism of action of acetylcholine (B) and tiotropium (C)
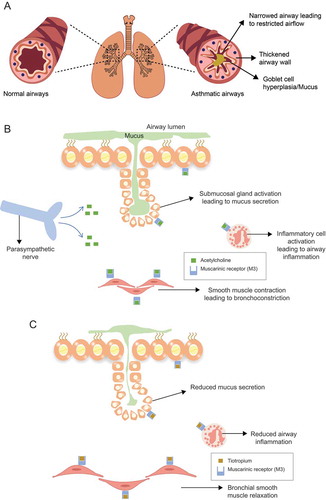
Airway hyperresponsiveness is often induced by allergens or irritants such as cigarette smoke [Citation1]. While the mechanisms leading to airway hyperresponsiveness are poorly understood, parasympathetic cholinergic pathways are thought to be involved [Citation16]. Acetylcholine, a predominant parasympathetic neurotransmitter in the airways, is also an autocrine or paracrine hormone secreted from airway epithelial cells and acts on muscarinic receptors on the airway smooth muscles [Citation16].
3. Mechanism of action of LAMAs
As parasympathetic activity is increased in asthma, anticholinergic therapies that block muscarinic receptors can be highly effective [Citation17]. Acetylcholine drives bronchial smooth muscle contraction and regulates airway tone, mucus secretion, and vasodilation by interacting with the G protein–coupled muscarinic receptors on the airway smooth muscles, glands, and pulmonary vasculature of the lungs [Citation17]. Of the three types of muscarinic receptors (M1, M2, and M3) in the airways, M3 receptors mediate the effects of acetylcholine on airway smooth muscle tone and mucus secretion from mucosal glands () [Citation17].
3.1 Bronchodilatory effects of tiotropium
LAMAs are a class of bronchodilators that have been shown to cause relaxation of the smooth muscles and reduce inflammation and asthma-related airway remodeling in preclinical asthma models [Citation18,Citation19,Citation20,Citation21]. As a LAMA, tiotropium exerts its bronchodilatory effect by blocking endogenous acetylcholine receptors in the airways (). Tiotropium binds to the muscarinic receptors (M1, M2, and M3) with equal affinity but mainly acts on the M3 anticholinergic receptors located in the airways [Citation22]. Tiotropium dissociates slowly from the M1 and M3 anticholinergic receptors, resulting in the long duration of the bronchodilator effect [Citation22]. In addition, tiotropium shows comparatively low variation of bronchodilation between the peak level and the level before the next dose administration, resulting in a stable 24-h effectiveness profile [Citation22]. LABAs achieve bronchodilation by employing a mechanism different to that of LAMAs, involving direct stimulation of smooth muscle β2-adrenoceptors [Citation23]. However, long-term use of LABAs is associated with loss of the bronchoprotective effect (or functional desensitization) [Citation23,Citation24]. By binding to the M1 and M3 receptors, tiotropium prevents the direct binding of bronchoconstrictors, such as methacholine, to muscarinic receptors and offers both bronchodilation and bronchoprotection [Citation25].
3.2 Anti-inflammatory activity of tiotropium in preclinical studies
Apart from the known bronchodilatory effect discussed above, anti-inflammatory properties have also been associated with tiotropium. In vitro studies and experiments in animal models have shown that tiotropium is able to control proinflammatory activity and reduce resistive breathing–induced inflammation [Citation26,Citation27,Citation28]. Moreover, experiments in mouse models of asthma have shown that tiotropium reduces airway inflammation and remodeling in the airways and may potentially prevent pathological changes in asthma by regulating apoptosis [Citation29]. This has led to the postulation of an alternative mechanism of action of tiotropium [Citation30] that may likely be the reason for tiotropium showing beneficial effects on exacerbations of COPD compared with ICS+LABA [Citation31]. However, human clinical studies in the asthmatic population are required for confirmation.
4. Place of tiotropium in the asthma treatment paradigm
The long-term goals of asthma management are to achieve good control of symptoms, minimize the future risk of exacerbations, and reduce persistent airflow limitation and medication side effects [Citation1]. As per the GINA strategy document, ICS are the preferred controllers for the management of asthma in adolescents and adults, with the use of ICS+LABA as a recommended step-up to achieve disease control () [Citation34]. Despite numerous treatment options, a high proportion of patients with asthma remain poorly controlled [Citation1,Citation32,Citation33]. According to the findings of Real-world Evaluation of Asthma Control and Treatment (REACT), a web-based survey administered to patients in the US with diagnoses of asthma for ≥1 year who were using multiple controller medications, 55% of the surveyed patients had uncontrolled asthma [Citation32]. Similar findings were also observed in the European National Health and Wellness Survey [Citation33].
Figure 2. GINA 2020 guidelines for personalized management of asthma. Figure 2 outlines the personalized asthma management strategy recommended by the Global Initiative for Asthma (GINA) to control symptoms and minimize future risk for adults and adolescents (aged 12+ years) (2A) and children aged 6 to 11 years (2B). BDP, beclomethasone dipropionate; FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; HDM, house dust mite; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL4R, interleukin 4 receptor; IL5, interleukin 5; IL5R, interleukin 5 receptor; LABA, long-acting beta2-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroids; SABA, short-acting beta2-agonist; SLIT, sublingual immunotherapy. This figure is reused with permission from What’s new in GINA 2020, 2020. [cited 10 April 2020]. Available from:https://ginasthma.org/gina-reports/ [Citation34]
![Figure 2. GINA 2020 guidelines for personalized management of asthma. Figure 2 outlines the personalized asthma management strategy recommended by the Global Initiative for Asthma (GINA) to control symptoms and minimize future risk for adults and adolescents (aged 12+ years) (2A) and children aged 6 to 11 years (2B). BDP, beclomethasone dipropionate; FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; HDM, house dust mite; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL4R, interleukin 4 receptor; IL5, interleukin 5; IL5R, interleukin 5 receptor; LABA, long-acting beta2-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroids; SABA, short-acting beta2-agonist; SLIT, sublingual immunotherapy. This figure is reused with permission from What’s new in GINA 2020, 2020. [cited 10 April 2020]. Available from:https://ginasthma.org/gina-reports/ [Citation34]](/cms/asset/d5007079-4687-4d4e-99fb-e2495a59c16b/ipgm_a_1816329_f0002_c.jpg)
The current GINA guidelines recommend several other controller options for patients with uncontrolled asthma despite use of medium- to high-dose ICS±LABA, which include high-dose ICS plus LTRA/theophylline, tiotropium, and biologics [Citation1]. Tiotropium (1.25 µg, two puffs, once daily [Citation8]) is currently recommended as an additional controller option for patients uncontrolled on medium-dose ICS+LABA (GINA step 4), or as a preferred controller option for patients uncontrolled on high-dose ICS+LABA (GINA step 5) [Citation1]. The National Asthma Education and Prevention Program (NAEPP) guidelines were last updated in 2007, and the expert panel is in the process of revising the draft guidelines, which include updates to selected topics [Citation35,Citation36]. In the current draft of the guidelines, treatment with a LAMA as an add-on is recommended as part of the alternative treatment options at steps 3 and 4 and as part of the preferred treatment at step 5 for patients with asthma ≥12 years of age [Citation9].
Other LAMAs, such as aclidinium, glycopyrronium, and umeclidinium, are currently being investigated for the treatment of asthma [Citation37]. However, clinical studies for some of these agents are currently limited to adult patients and have not yet been conducted in adolescents or children [Citation37].
4.1 Clinical evidence for tiotropium in patients with asthma
Uncontrolled asthma is regarded an important risk factor for exacerbations [Citation1], may lead to hospitalization, and is associated with increased healthcare costs [Citation32]. The efficacy and safety of tiotropium as an add-on treatment to either ICS±LABA or at least ICS maintenance treatment has been demonstrated in >6,000 patients enrolled in 18 randomized controlled trials (RCTs) [Citation38–54] ().
Table 1. Key randomized clinical studies of tiotropium [Citation40–56]
Of these, 11 studies included adult patients with asthma [Citation38–46], three studies were conducted in adolescent patients with asthma [Citation48,Citation50,Citation51], while four studies were conducted in children aged ≤11 years [Citation47,Citation49,Citation52,Citation53]. These studies involved patients across all asthma severities (mild, moderate, and severe) and ranged from 8 to 52 weeks in duration (). The efficacy endpoints investigated were peak forced expiratory volume in 1 second (FEV1), trough FEV1, peak expiratory flow (PEF), symptom reduction, asthma worsening, risk of exacerbation, and time to next exacerbation.
The Phase 2 RCTs were performed using different doses (1.25 μg, 2.5 μg, 5 μg, and 10 μg) [Citation39,Citation41–44,Citation48,Citation49], while the Phase 3 studies included the 2.5 μg and 5 μg doses [Citation38,Citation40,Citation45–47,Citation50–52]; all doses were delivered via the Respimat® inhaler [Citation38,Citation40,Citation45–47,Citation50–53]. Both the 2.5 μg and 5 μg doses offered greater improvements versus placebo for most of the clinical endpoints related to lung function, symptom reduction, and exacerbations [Citation38,Citation40,Citation43,Citation45,Citation47,Citation49,Citation50,Citation53]. Across the Phase 2 and Phase 3 studies, all tiotropium doses were well tolerated, and safety results were comparable to those of placebo.
The efficacy of tiotropium was investigated in an independent study involving 210 adults with mild-to-moderate asthma receiving ICS. The efficacy was assessed by measuring the morning PEF. Use of tiotropium was found to be superior to doubling the dose of ICS and noninferior to addition of salmeterol (LABA) to ICS [Citation54].
4.2 Pediatric clinical studies of tiotropium
Current GINA guidelines recommend add-on tiotropium as an ‘other controller option’ at step 4 and ‘preferred add-on treatment’ at step 5 for patients with asthma aged ≥6 years [Citation1] (). Both the US FDA and the European Medicines Agency (EMA) have expanded the indication for tiotropium as add-on maintenance treatment for patients aged ≥6 years (approved dose: US, 2.5 μg; EU, 5 μg) based on the available clinical trial data [Citation7,Citation55]. Of the seven pediatric clinical trials, three trials each were conducted in adolescents (12–17 years old) and children (6–11 years old), while one trial with safety as the primary endpoint was conducted in children 1 to 5 years old ().
Results of a systematic review and meta-analysis of three clinical studies showed that tiotropium as add-on compared with placebo significantly improved peak and trough FEV1 (P < 0.001), increased Asthma Control Questionnaire 7 (ACQ7) score (P = 0.04), and decreased the number of exacerbations (P = 0.002) in approximately 1,000 adolescents (mean age, 14 years) with moderate-to-severe asthma. Additionally, treatment with tiotropium showed no significant difference in adverse events compared with placebo; therefore, indicating that tiotropium as an add-on to ICS±controller medications is efficacious and well tolerated [Citation56]. Compared with placebo, tiotropium was associated with a significant reduction in the percentage of patients who experienced an asthma worsening episode (measured by the ACQ 7) and the number of patients with at least one exacerbation [Citation56]. Based on another systematic review of three RCTs involving approximately 900 school-age children with asthma, treatment with tiotropium resulted in a significant improvement in lung function compared with placebo [Citation57]. Moreover, tiotropium significantly reduced the number of patients with ≥1 exacerbation compared with placebo [Citation57]. According to the results of a pooled analysis of safety data from clinical trials in adolescents and children with symptomatic moderate asthma, the most commonly reported adverse events with tiotropium were asthma worsening, exacerbations, decreased PEF, nasopharyngitis, viral respiratory tract infection, and respiratory tract infection [Citation58,Citation59].
4.3 Real-world data for tiotropium
Findings from RCTs involving a highly selective population and complementary real-world data were similar with add-on tiotropium demonstrating beneficial effects such as reducing exacerbations, hospitalization, and emergency room visits, in addition to decreasing the ICS dose and antibiotic prescriptions for respiratory infections in patients with asthma [Citation38,Citation52,Citation60,Citation61] (). In a retrospective analysis of 64 patients with severe asthma receiving high-dose ICS+LABA, tiotropium add-on treatment significantly decreased the number of emergency room visits and the number of hospitalizations (P < 0.05) [Citation60]. In another retrospective analysis of 2,042 adult patients with asthma in the UK, add-on tiotropium resulted in a significant decrease in the incidence of exacerbations and antibiotic prescriptions for lower respiratory tract infections in the following year [Citation61].
Table 2. Key real-world studies with tiotropium [Citation63,Citation64,Citation71]
This concordance of results across different types of studies provides reassurance that treatment with add-on tiotropium is not only effective in a select group of patients from RCTs but also representative of patients routinely encountered in real-world clinical practice.
4.4 Safety profile of tiotropium
Tiotropium is well tolerated and may be an alternate treatment option for patients with uncontrolled asthma on ICS± add-on therapy or for those patients where other controller treatments may not be appropriate [Citation62,Citation63]. However, the trials excluded patients with comorbidities such as heart conditions and moderate-to-severe renal impairment [Citation40,Citation47–49,Citation52,Citation53].
Overdose of tiotropium may be associated with a temporary increase in heart rate [Citation64]; however, cardiac safety of the drug has been demonstrated in patients with COPD [Citation65,Citation66] and has also been assessed during the clinical studies in patients with asthma [Citation38,Citation40,Citation46].
The most common adverse reactions observed in patients receiving tiotropium include pharyngitis, headache, bronchitis, dry mouth, and sinusitis [Citation8]. Tiotropium at a dose of 5 µg led to palpitations, constipation; gastroesophageal reflux disease; oropharyngeal candidiasis, dizziness, dysphonia, pruritus, rash, and urinary tract infections. The incidence of dry mouth was lower in patients with asthma than in patients with COPD receiving tiotropium (0.83% versus 2.9%) [Citation7]. For patients with asthma, dry mouth was not considered serious and did not lead to discontinuation in any patient [Citation67]. The adverse reactions were usually mild to moderate and the overall safety profile was comparable to that of the placebo [Citation67].
The major contraindication for tiotropium is hypersensitivity. Tiotropium has been used concomitantly with short-acting and long-acting sympathomimetic (beta2-agonists) bronchodilators, methylxanthines, oral and inhaled steroids, antihistamines, mucolytics, leukotriene modifiers, cromones, and anti-immunoglobulin E (anti-IgE) treatment without any additional adverse events [Citation8]. Owing to a potential additive interaction, coadministration of tiotropium with other anticholinergic drugs is not recommended [Citation8]. In addition, tiotropium could cause urinary retention and worsening of narrow-angle glaucoma; therefore, close monitoring is recommended for patients with moderate-to-severe renal impairment and narrow-angle glaucoma receiving tiotropium. Additionally, tiotropium should be cautiously prescribed in patients suffering from prostatic hyperplasia or bladder-neck obstruction [Citation8]. Therefore, elderly patients with asthma should be prescribed tiotropium with caution.
4.5 Cost-effectiveness of tiotropium
Several cost-effectiveness studies conducted in the context of both the UK and US healthcare systems have shown that tiotropium also provides economic value in asthma management [Citation68–71], potentially leading to favorable healthcare access. The cost-effectiveness of tiotropium and omalizumab as add-on therapies to ICS+LABA for patients with uncontrolled allergic asthma in the US was compared using a probabilistic Markov model. Total costs (in 2013 US $) and health outcomes of three interventions, including standard therapy (ICS+LABA), add-on therapy with tiotropium, and add-on therapy with omalizumab, were calculated over a 10-year time horizon. The 10-year discounted costs and quality-adjusted life years (QALYs) for add-on therapy with tiotropium and with omalizumab were 41,535 USD and 6.88 and 217,847 USD and 7.17, respectively [Citation68]. Compared with tiotropium, greater improvements in QALYs and reduction in exacerbations associated with omalizumab were achieved at substantially higher costs [Citation68].
Treatment with biologics is associated with a significant economic burden [Citation69]; tiotropium thus provides a prudent step-up treatment option before switching to biologics [Citation68,Citation70,Citation71]. Other real-world studies conducted in a number of other European countries, such as Spain, Portugal, Poland, and the UK, have also shown that tiotropium offers a cost-effective treatment option added to standard of care [Citation71–74]. Hence, the use of tiotropium before the use of more expensive individualized treatment options such as biologic add-on therapies may also be considered for patients with uncontrolled asthma.
5. Using tiotropium in routine clinical practice
Medications for managing asthma can be classified as either rescue (or reliever) or controller (maintenance) medications [Citation1]. Short-acting beta2-agonists (SABAs) and short-acting muscarinic antagonists (SAMAs) have been used as rescue medications to provide quick relief during asthma attacks [Citation1,Citation37]. However, due to concerns associated with the use of SABAs in asthma, such as asthma exacerbations, decline in lung function, and an increase in airway hyperresponsiveness [Citation75], SABA-only treatment is not recommended in the current GINA guidelines [Citation1].
Controller medications provide long-term symptom control and include ICS±LABA and add-on treatments such as LTRAs, biologics, and tiotropium [Citation1]. However, use of controller options for managing patients with uncontrolled asthma is often limited by a number of factors, a few of which are described below. High-dose ICS is significantly associated with adrenal suppression in children and therefore, not a viable option [Citation76]. Although ICS offers an effective means of reducing airway inflammation [Citation77], it is also associated with osteoporosis, pneumonia risk, suppression of the hypothalamic-pituitary-adrenal axis, and growth suppression [Citation77,Citation78]. Moreover, a meta-analysis of 12 clinical studies suggests that adding LABA to a moderate dose of ICS provides greater clinical benefit in symptomatic adults than increasing the ICS dose [Citation79]. Notably, tiotropium is noninferior to LABA with regard to lung function, exacerbation frequency, and asthma control. Additionally, the safety profile is comparable for both the treatment options. Therefore, tiotropium may be a suitable alternative add-on in patients where LABAs are not well tolerated or are ineffective [Citation80].
Use of OCS such as prednisone is often associated with corticosteroid-related adverse events [Citation81]. Moreover, OCS use is associated with an increase in healthcare resource use [Citation82]. Furthermore, LTRA as a single-agent asthma treatment was found to be less effective than ICS and was associated with an increased risk of withdrawal due to poor asthma control [Citation83]. Findings from a systematic review of literature showed that tiotropium provided greater improvements in lung function than LTRA as an add-on to ICS in adults as well as in children and adolescents with asthma [Citation84,Citation85]. Use of biologics as a controller treatment option for managing asthma is limited by the need for phenotype assessment [Citation1] with a focus on identifying ways to predict response to biologics [Citation86].
Given the concerns associated with the use of controller treatment options, there has been an interest in exploring the use of LAMAs, such as tiotropium, in asthma. Tiotropium is currently the only LAMA licensed for use in children, adolescents, and adults with asthma (≥6 years of age) [Citation7,Citation55], and clinical trials have shown its efficacy and safety across all asthma severities [Citation38,Citation40,Citation43,Citation45–54]. It is an effective add-on treatment option for patients across all age groups, including children [Citation47,Citation49,Citation52,Citation53], in whom caution should be exercised before increasing the ICS dose [Citation78]. In patients with uncontrolled asthma, addition of tiotropium resulted in greater improvements in lung function, better asthma control, and reduction in the number of exacerbations compared with increasing the dose of ICS [Citation54]. An exploratory subgroup analysis of four RCTs suggested that tiotropium added on top of ICS±LABA in patients with symptomatic asthma leads to improvements in lung function, reduction in exacerbation risk, and improvements in symptom control independent of the patients’ T2 phenotype [Citation87]. In addition, tiotropium is as effective as LAMA in patients with asthma with the B16-Arg/Arg genotype [Citation41].
Although the pharmacogenetic factors influencing response to tiotropium are not known, evidence suggests that the single nucleotide polymorphism (SNP) of Arg16Gly in the ADRB2 gene (coding the beta2 adrenoreceptor) is significantly associated with response to tiotropium [Citation88]. Further studies are required to identify SNPs that can predict response to tiotropium treatment.
Most patients with asthma are managed in the primary care setting, with some patients requiring referral to a specialist due to persistent uncontrolled asthma and exacerbations or need for phenotyping for prescribing biologics. Such referrals can help in increasing the diagnostic certainty, leading to effective asthma management and thus impacting disease prognosis and the patient’s health status [Citation89].
6. Overview of the Respimat® (soft-mist inhaler)
Tiotropium inhalation spray is delivered in a soft-moving mist via the Respimat® inhaler [Citation8], which uses mechanical energy of a coiled spring to generate a slow-moving aerosol () [Citation90]. The inhaler was designed to overcome some of the limitations of pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs) by generating a long-lasting, soft-moving soft mist. This feature reduces the need for coordinating inhalation with inhaler actuation and decreases the dependence on patients’ inspiratory efforts [Citation91]. Generally, particles <5 µm in size are considered easily respirable and are referred to as the fine particle fraction (FPF) [Citation92]. More than 60% of the drug released from the Respimat® inhaler lies within the easily respirable FPF of ≤5 µm, leading to high drug deposition in the lungs [Citation91].
Figure 3. Respimat® inhaler (https://player.vimeo.com/video/169737753?autoplay=1) Figure 3 shows the major components of the Respimat® inhaler which is the soft mist inhaler used to deliver tiotropium. A link (https://player.vimeo.com/video/169737753?autoplay=1) to a video explaining the correct technique to use the Respimat® inhaler has also been provided
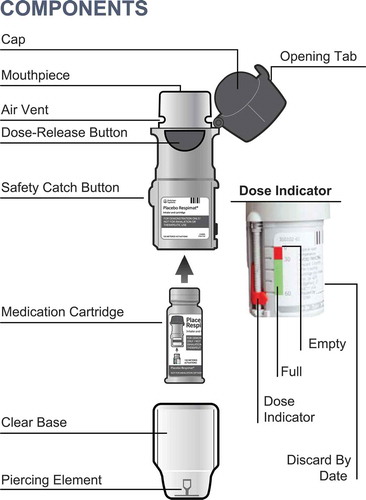
Tiotropium delivered via the HandiHaler® (a DPI) is approved for long-term maintenance treatment of COPD but is not approved for the management of asthma [Citation55]. All RCTs evaluating the efficacy and safety of tiotropium in asthma have been conducted using the Respimat® inhaler. To understand the steps for using the Respimat® inhaler, please review the Spiriva® Respimat® prescribing information [Citation86] or see this video (https://player.vimeo.com/video/169737753?autoplay=1).
7. Conclusions
Use of add-on treatments is recommended for the management of asthma that is uncontrolled with ICS therapy. Currently, tiotropium (1.25 µg, two puffs, once daily), delivered via the Respimat® inhaler, is the only LAMA approved for treatment of patients with uncontrolled asthma (GINA steps 4 and 5) in the US. Tiotropium improves lung function and asthma control and reduces exacerbation risk across various age groups, independent of patient characteristics.
Substantial evidence from RCTs and real-world studies demonstrates that tiotropium is a cost-effective treatment option that improves lung function parameters, reduces risk of and time to asthma exacerbation and asthma worsening, and has a safety profile comparable to that of placebo in patients with symptomatic asthma despite ICS±LABA therapy. Moreover, preclinical evidence suggests that tiotropium may prevent pathological changes in asthma by mechanisms beyond bronchodilation, involving anti-inflammatory activity.
Awareness among primary care physicians as well as patients regarding the available add-on treatment options for asthma uncontrolled on ICS is crucial given the significant burden associated with the disease.
Declaration of interest
Alan Kaplan has received personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, NovoNordisk, Merck Frosst, Novartis, Pfizer, Teva, Trudel, and Sanofi. He also receives non-financial support from Boehringer Ingelheim and Trudel. Ku-Lang Chang reports no conflicts of interest.
Supplemental Material
Download MS Word (63.3 KB)Supplemental Material
Download MP3 Audio (13 MB)Acknowledgments
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Jidnyasa Mulekar, PhD, and Praveen Kaul, PhD, of Cactus Life Sciences (part of Cactus Communications), which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Notes on contributors
Alan Kaplan
Alan Kaplan: Dr. Alan Kaplan is a board-certified Family Physician working in York Region, Ontario, Canada and the Chairperson of the Family Physician Airways Group of Canada (www.fpagc.com), the Past-Chairperson of the Respiratory Section of the College of Family Physicians of Canada, and Senate member of the International Primary Care Respiratory Group. He co-chaired the Community Standards of COPD program for Health Quality Ontario. He is the medical director of the Pulmonary Rehabilitation program for the local health integration network.
Ku-Lang Chang: Dr. Chang is board certified in Family Medicine and a Fellow of the American Academy of Family Physicians. Dr. Chang is involved in asthma and COPD research. She is a Medical Review Officer and Medical Director for UF Health Gainesville Occupational Health.
References
- Global Initiative for Asthma [Internet]. Global strategy for asthma management and prevention. 2020. [ cited 2020 Apr 10]. Available from: https://ginasthma.org/gina-reports/
- Centers for Disease Control and Prevention [Internet]. Most recent national asthma data. [ cited 2019 Nov 20]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
- Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
- Dahl R, Larsen BB, Venge P. Effect of long-term treatment with inhaled budesonide or theophylline on lung function, airway reactivity and asthma symptoms. Respir Med. 2002;96(6):432–438.
- Global Initiative for Chronic Obstructive Lung Disease [Internet]. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease. 2020. [ cited 2020 Feb 18]. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
- Cazzola M, Ora J, Rogliani P, et al. Role of muscarinic antagonists in asthma therapy. Expert Rev Respir Med. 2017;11(3):239–253.
- FDA [Internet]. Spiriva® Respimat® new drug application approval by FDA. [ cited 2019 Nov 20]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/207070Orig1s000ltr.pdf.
- Boehringer Ingelheim Pharmaceuticals Inc. [Internet]. SPIRIVA® RESPIMAT® [Package Insert]. [ cited 2019 Sep 10]. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf.
- Boehringer Ingelheim Pharmaceuticals Inc. [Internet]. FDA expands approval of SPIRIVA® RESPIMAT® (tiotropium bromide) inhalation spray for maintenance treatment of asthma in children. [ cited 2019 Nov 20]. Available from: https://www.boehringer-ingelheim.us/press-release/fda-expands-approval-spiriva-respimat-tiotropium-bromide-inhalation-spray.
- Datapharm [Internet]. Spiriva® Respimat® summary of product characteristics. [ cited 2019 Dec 31]. Available from: https://www.medicines.org.uk/emc/product/407/smpc.
- Cottini M, Asero R. Asthma phenotypes today. Eur Ann Allergy Clin Immunol. 2013;45(1):17–24.
- Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999;104(6):1139–1146.
- Global Initiative for Asthma [Internet]. Global strategy for asthma management and prevention, 2020 Appendix. [ cited 2020 Apr 10]. Available from: https://ginasthma.org/reports/.
- Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007;56(4):341–348.
- Gosens R, Gross N. The mode of action of anticholinergics in asthma. Eur Respir J. 2018;52(4):1701247.
- Proskocil BJ, Sekhon HS, Jia Y, et al. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology. 2004;145(5):2498–2506.
- Novelli F, Malagrinò L, Dente FL, et al. Efficacy of anticholinergic drugs in asthma. Expert Rev Respir Med. 2012;6(3):309–319.
- Ohta S, Oda N, Yokoe T, et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin Exp Allergy. 2010;40(8):1266–1275.
- Buels KS, Fryer AD. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol. 2012;208:317–341.
- Bos IS, Gosens R, Zuidhof AB, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30(4):653–661.
- Kistemaker LE, Hiemstra PS, Bos IS, et al. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax. 2015;70(7):668–676.
- Disse B, Speck GA, Rominger KL, et al. Tiotropium (Spiriva™): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999;64(6–7):457–464.
- Billington CK, Penn RB, Hall IP. β2 agonists. Handb Exp Pharmacol. 2017;237:23–40.
- Lipworth BJ. Antagonism of long-acting beta2-adrenoceptor agonism. Br J Clin Pharmacol. 2002;54(3):231–245.
- Blais CM, Davis BE, Cockcroft DW, “Duration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmatics”. [Respiratory Medicine. 118 (September 2016) 96–101]. Respir Med. 2017;132:268.
- Anzalone G, Gagliardo R, Bucchieri F, et al. IL-17A induces chromatin remodeling promoting IL-8 release in bronchial epithelial cells: effect of tiotropium. Life Sci. 2016;152:107–116.
- Toumpanakis D, Loverdos K, Tzouda V, et al. Tiotropium bromide exerts anti-inflammatory effects during resistive breathing, an experimental model of severe airway obstruction. Int J Chron Obstruct Pulmon Dis. 2017;12:2207–2220.
- Neri T, Scalise V, Passalacqua I, et al. Tiotropium inhibits proinflammatory microparticle generation by human bronchial and endothelial cells. Sci Rep. 2019;9(1):11631.
- Wang J, Diao X, Zhu H, et al. Effect of tiotropium bromide on airway inflammation and programmed cell death 5 in a mouse model of ovalbumin-induced allergic asthma. Can Respir J. 2019;2019.
- Bateman E, Rennard S, Barnes P, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009;22(6):533–542.
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
- Peters SP, Jones CA, Haselkorn T, et al. Real-world evaluation of asthma control and treatment (REACT): findings from a national web-based survey. J Allergy Clin Immunol. 2007;119(6):1454–1461.
- Demoly P, Paggiaro P, Plaza V, et al. Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev. 2009;18(112):105–112.
- Global Initiative for Asthma [Internet]. Global strategy for asthma management and prevention, 2020 What’s new in GINA 2020. [ cited 2020 Apr 10]. Available from: https://ginasthma.org/gina-reports/
- Federal registrar [Internet]. National Asthma Education and Prevention Program (NAEPP) guidelines 2020 working draft. [ cited 2020 Feb 21]. Available from: https://www.federalregister.gov/documents/2019/12/02/2019-26017/request-for-information-on-update-on-selected-topics-in-asthma-management-2020-a-report-from-the.
- National Heart, Lung, and Blood Institute [Internet]. National Asthma Education and Prevention Program (NAEPP) guidelines for the diagnosis and management of asthma (EPR-3). [ cited 2020 Feb 21]. Available from: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma.
- Albertson TE, Chenoweth JA, Adams JY, et al. Muscarinic antagonists in early stage clinical development for the treatment of asthma. Expert Opin Investig Drugs. 2017;26(1):35–49.
- Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367–376.
- Kerstjens HA, Disse B, Schröder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128(2):308–314.
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–1207.
- Bateman ED, Kornmann O, Schmidt P, et al. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128(2):315–322.
- Beeh KM, Kirsten AM, Dusser D, et al. Pharmacodynamics and pharmacokinetics following once-daily and twice-daily dosing of tiotropium Respimat® in asthma using standardized sample-contamination avoidance. J Aerosol Med Pulm Drug Deliv. 2016;29(5):406–415.
- Beeh KM, Moroni-Zentgraf P, Ablinger O, et al. Tiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res. 2014;15:61.
- Timmer W, Moroni-Zentgraf P, Cornelissen P, et al. Once-daily tiotropium Respimat® 5 µg is an efficacious 24-h bronchodilator in adults with symptomatic asthma. Respir Med. 2015;109(3):329–338.
- Paggiaro P, Halpin DM, Buhl R, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(1):104–113.e2.
- Ohta K, Ichinose M, Tohda Y, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One. 2015;10(4):e0124109.
- Vogelberg C, Engel M, Laki I, et al. Tiotropium add-on therapy improves lung function in children with symptomatic moderate asthma. J Allergy Clin Immunol Pract. 2018;6(6):2160–2162.e9.
- Vogelberg C, Engel M, Moroni-Zentgraf P, et al. Tiotropium in asthmatic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging study. Respir Med. 2014;108(9):1268–1276.
- Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. 2015;16(1):20.
- Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441–450.e8.
- Hamelmann E, Bernstein JA, Vandewalker M, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49(1).
- Szefler SJ, Murphy K, Harper T 3rd, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140(5):1277–1287.
- Vrijlandt E, El Azzi G, Vandewalker M, et al. Safety and efficacy of tiotropium in children aged 1-5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):127–137.
- Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715–1726.
- Spiriva® Respimat® prescribing information [ cited 2019 Dec 31]. Available from https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf
- Rodrigo GJ, Castro-Rodríguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2015;115(3):211–216.
- Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28(6):573–578.
- Vandewalker ML, Hamelmann E, Engel M, et al. Once-daily tiotropium Respimat® add-on therapy has a safety profile comparable with placebo in children and adolescents. J Allergy Clin Immunol. 2017;139(2):AB94.
- Hamelmann E, Szefler SJ. Efficacy and safety of tiotropium in children and adolescents. Drugs. 2018;78(3):327–338.
- Abadoglu O, Berk S. Tiotropium may improve asthma symptoms and lung function in asthmatic patients with irreversible airway obstruction: the real-life data. Clin Respir J. 2016;10(4):421–427.
- Price D, Kaplan A, Jones R, et al. Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy. 2015;8:1–13.
- Dusser D, Ducharme FM. Safety of tiotropium in patients with asthma. Ther Adv Respir Dis. 2019;13:1753466618824010.
- Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15(8):1115–1124.
- Gregory MD, Mersfelder TL, Jamieson T. Accidental overdose of tiotropium in a patient with atrial fibrillation. Ann Pharmacother. 2010;44(2):391–393.
- Tashkin DP, Leimer I, Metzdorf N, et al. Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res. 2015;16(1):65.
- Hohlfeld JM, Furtwaengler A, Könen-Bergmann M, et al. Cardiac safety of tiotropium in patients with COPD: a combined analysis of Holter-ECG data from four randomised clinical trials. Int J Clin Pract. 2015;69(1):72–80.
- Dahl R, Engel M, Dusser D, et al. Safety and tolerability of once-daily tiotropium Respimat® as add-on to at least inhaled corticosteroids in adult patients with symptomatic asthma: a pooled safety analysis. Respir Med. 2016;118:102–111.
- Zafari Z, Sadatsafavi M, Mark FitzGerald J, et al. Cost-effectiveness of tiotropium versus omalizumab for uncontrolled allergic asthma in US. Cost Eff Resour Alloc. 2018;16:3.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118(2):220–225.
- Willson J, Bateman ED, Pavord I, et al. Erratum to: cost effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting β-agonists. Appl Health Econ Health Policy. 2016;14(1):119–125.
- Willson J, Bateman ED, Pavord I, et al. Cost effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting β-agonists. Appl Health Econ Health Policy. 2014;12(4):447–459.
- Echave M, Ojanguren ME, Elías I, et al. Cost-effectiveness of tiotropium in the treatment of patients with asthma. Value Health. 2015;18(7):A501–A502.
- Silva Miguel L, Manaças M, Pinheiro B. Economic evaluation of tiotropium for severe persistent asthma in Portugal. Value Health. 2015;18(7):A502.
- Pawlik M, Walczak J, Pieniazek I. Economic evaluation of tiotropium administrated through the Respimat inhaler as add-on therapy in patients with uncontrolled severe asthma in Poland. Value Health. 2015;18(7):A502.
- Silva D, Jacinto T. Inhaled β2-agonists in asthma management: an evolving story. Breathe (Sheff). 2016;12(4):375–377.
- Kwda A, Gldc P, Baui B, et al. Effect of long term inhaled corticosteroid therapy on adrenal suppression, growth and bone health in children with asthma. BMC Pediatr. 2019;19(1):411.
- Hossny E, Rosario N, Lee BW, et al. The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organ J. 2016;9:26.
- Smith RW, Downey K, Gordon M, et al. Prevalence of hypothalamic-pituitary-adrenal axis suppression in children treated for asthma with inhaled corticosteroid. Paediatr Child Health. 2012;17(5):e34–e39.
- Masoli M, Weatherall M, Holt S, et al. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60(9):730–734.
- Buhl R, FitzGerald JM, Busse WW. Tiotropium add-on to inhaled corticosteroids versus addition of long-acting β(2)-agonists for adults with asthma. Respir Med. 2018;143:82–90.
- Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30.
- Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4).
- Ducharme FM. Inhaled glucocorticoids versus leukotriene receptor antagonists as single agent asthma treatment: systematic review of current evidence. BMJ. 2003;326(7390):621.
- Kaplan A, Fitzgerald JM, El Azzi G, et al. Tiotropium provides greater improvements in FEV1 than leukotriene receptor antagonists as add-on to ICS in adults with asthma. A systematic review. Eur Respir J. 2018;52(suppl 62):PA1033.
- Vogelberg C, Goldstein S, Graham L, et al. A comparison of tiotropium, long-acting β2-agonists and leukotriene receptor antagonists on lung function and exacerbations in paediatric patients with asthma. Respir Res. 2020;21(1):19.
- Darveaux J, Busse WW. Biologics in asthma–the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;32:152–160.
- Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium Respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2018;6(3):923–935.e9.
- Park HW, Yang MS, Park CS, et al. Additive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009;64(5):778–783.
- Price D, Bjermer L, Bergin DA, et al. Asthma referrals: a key component of asthma management that needs to be addressed. J Asthma Allergy. 2017;10:209–223.
- Hochrainer D, Hölz H, Kreher C, et al. Comparison of the aerosol velocity and spray duration of Respimat soft mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282.
- Wachtel H, Kattenbeck S, Dunne S, et al. The Respimat® development story: patient-centered innovation. Pulm Ther. 2017;3(1):19–30.
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1417.
