ABSTRACT
Objective
To evaluate the diagnostic and antibiotic treatment strategies for patients suspected of sepsis, in a tertiary hospital in Indonesia. This can identify areas for improvement in care provided, and inform diagnostic and antimicrobial stewardship activities within the hospital.
Methods
Retrospective review of medical records with regards to the diagnosis and management of adult patients with sepsis admitted to a tertiary hospital in Indonesia. We assessed the diagnostic process, and whether or not the antibiotic treatment provided was appropriate for the diagnosis. Appropriateness of antibiotic treatment was classified as being definite appropriate, probable appropriate, inappropriate, or unknown.
Results
The study included 535 adult patients, of whom 295 (55%) were diagnosed with a community-acquired sepsis, and 240 (45%) with a hospital-acquired sepsis. A specimen for culture and antimicrobial susceptibility testing was collected from three out of four patients (392/535). All but 10 patients had information on antibiotic treatment at the time of sepsis diagnosis. Of those, nearly 50% (257/525) of the patients received antibiotic treatment with unknown appropriateness because no cultures were taken (n = 141) or all cultures were negative (n = 116). Just 3.4% and 9.1% of the patients received definite or probable appropriate antibiotic treatment, respectively.
Conclusions
There is a clear need in encouraging attending physicians to obtain the much-required blood cultures, or cultures from the suspected source of infection before empirical antibiotic treatment is started. This will improve the use of appropriate antibiotic treatment strategies, and contribute to antimicrobial stewardship.
Introduction
Sepsis, defined as the presence (probable or documented) of an infection with systemic manifestations, is a potentially life-threatening condition, the incidence of which remains high worldwide [Citation1–5]. International guidelines on the management of sepsis (Surviving Sepsis Campaign [SSC]) advice is to start empirical broad-spectrum antibiotic therapy within one hour when sepsis is suspected[Citation6]. This timely start of antibiotic treatment is associated with a reduced mortality [Citation7,Citation8]. An adequate diagnosis of sepsis requires that routine microbiological cultures are obtained before the start of any antibiotic therapy[Citation6].
In Indonesia, the first ever guideline on the diagnosis and management of patients with sepsis issued by the Indonesian Ministry of Health in 2017 was based on the SSC guidance from 2012 (Sepsis-2 definition) which includes the central concept of the presence of the Systemic Inflammatory Response Syndrome (SIRS) in sepsis diagnosis[Citation9]. This guidance is still operational to-date, despite the SSC guidance being updated in 2016[Citation6]. The Indonesian guideline underlines the importance of obtaining appropriate microbiological cultures before the start of antibiotic therapy, and the rapid start of such therapy (antibiotics from at least two different classes) as soon as sepsis is suspected[Citation9].
We set out to evaluate the diagnostic and antibiotic treatment strategies for patients suspected of sepsis, employed by physicians in a tertiary hospital in Indonesia. The objective of the evaluation is to assess if i) the employed strategies are in line with the prevailing guidelines in Indonesia, and ii) the antibiotic treatment provided is appropriate for the diagnosis. The purpose of the evaluation is to identify areas for improvement in care provided and to inform diagnostic and antimicrobial stewardship activities within the hospital. Such an evaluation is deemed timely, given the continuous increase in the number of patients diagnosed with sepsis in the hospital in previous years (2011: 631, 2015: 731).
Using an evaluation approach differs from performing a clinical audit, where the evaluation aims to provide a judgment on the quality of the current service, while a clinical audit aims to measure clinical practise against pre-set targets[Citation10]. Although Indonesia provides guidelines for the diagnosis and management of sepsis, it does not define targets to define quality of care, precluding the clinical audit approach.
Methods
Ethics approval
The study was approved by the institutional ethical review boards of the Faculty of Medicine, Universitas of Sumatera Utara and H Adam Malik General Hospital, Medan, Sumatra (286/KOMET/FKUSU/2013). Given the use of data derived solely from the hospital administrative system, no individual informed consent was required.
Design and setting
This retrospective study is a review of medical records with regards to the diagnosis and management of adult patients with sepsis, admitted to Adam Malik Hospital in Medan, Indonesia. Adam Malik Hospital is a tertiary facility with 721 beds servicing mostly patients coming from North Sumatera Province. The hospital admits around 55,000 patients every year.
Patient population and data collection
The identification of patients to be included in the review followed a hierarchical approach. We extracted all medical records belonging to patients with a discharge diagnosis of sepsis in the Hospital Information System for the complete year of 2016. From these, we selected those records that used the International Classification of Diseases-tenth revision (ICD-10) code for sepsis, after which we selected those records compatible with the Sepsis-2 definition of sepsis. The final selection step limited the study population to adults (17 years of age or older). We hand-searched the selected medical records for information with regards to demographic characteristics, underlying condition (Charlson Comorbidity Index), results of cultures and antimicrobial susceptibility tests, length of hospital stay, source of infection leading to sepsis, and antibiotic treatment received. Information on culture and AST was extracted from the Hospital Information System if not present in the medical record, which might have happened if the patient died or was discharged before these results were received from the laboratory. The extracted information followed a pre-defined protocol and was reviewed by two independent physicians.
Outcome
The outcome of interest was the appropriateness of antibiotic treatment at the time of sepsis diagnosis, which we classified as being definite appropriate, probable appropriate, inappropriate, or unknown. Definite appropriate antibiotic treatment is the administration of antibiotics for which all micro-organisms identified in blood cultures are susceptible. Antibiotic treatment is classified as probable appropriate if, in the absence of a positive blood culture, all micro-organisms identified in cultures of any other patient specimens are susceptible for these antibiotics. Inappropriate antibiotic treatment is the administration of at least one antibiotic to which none of the micro-organism cultured from blood or other specimens (in the absence of blood culture) is susceptible. Appropriateness of antibiotic treatment is unknown if there is no positive culture, or no cultures are performed.
In addition, we compared whether or not the antibiotic treatment at sepsis diagnosis differed from the empirical antibiotic treatment given during admission, and if this empirical treatment was in line with the Indonesian treatment guidelines.
Antibiotic susceptibility testing (AST) was performed in the hospital microbiology laboratory using the Vitek2 Compact platform (Biomerieux, France), and following CLSI guidelines. AST results were interpreted using CLSI-defined breakpoints (version 2016), in which an intermediate test result was considered resistant.
Analyses were stratified for patients admitted to the intensive care unit (ICU) or non-ICU wards, and the sepsis being community-acquired or hospital-acquired, the latter being defined as a sepsis developed at least 48 hours after hospital admission. Patients who did not receive any antibiotics were excluded from the analysis of appropriateness.
Statistical analysis
Data on antibiotic use, performance of cultures, and appropriateness of treatment are summarized as frequencies and percentages. This study being a process evaluation, we did not perform any hypothesis testing. Analyses were performed using STATA version 12 (College Station, Texas, USA).
Results
Study population
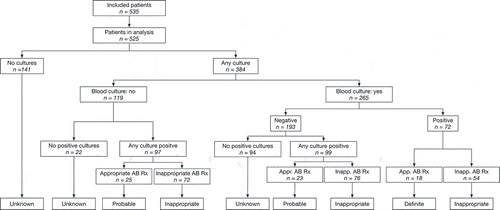
The Hospital Information System recorded 1,200 patients with a discharge diagnosis of sepsis in 2016. Of these records, 950 (79%) had an ICD-10 code for the diagnosis, of which 636 (67%) were Sepsis-2 compatible (). The main reason for not having a Sepsis-2 compatible diagnosis was the absence of any reference to sepsis in the medical record, other than the discharge note. The selection procedure identified 535 medical records of adult patients, the characteristics of whom are reported in . Of these, 295 (55%) patients were diagnosed with a community-acquired sepsis, and 240 (45%) patients with a hospital-acquired sepsis.
Figure 1. Flow chart for patient inclusion
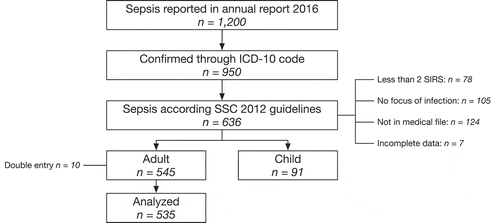
Table 1. Patient characteristics
Alignment with Indonesian practise guidelines
A specimen for culture and antimicrobial susceptibility testing (AST) was collected from on average three out of four patients (392/535; 73%), although this was less often for patients with a community-acquired sepsis (187/295; 63%, ). Blood cultures were performed for around half of the patients (269/535), with a slightly higher frequency in ICU (87/150; 58%) versus non-ICU wards (182/385; 47%), and hospital-acquired sepsis (127/240; 53%) versus community-acquired sepsis (142/295; 48%, ).
Table 2. Management and appropriateness of antibiotic treatment of patients with sepsis
All but four patients started on empirical antibiotic treatment during admission (), although in around one-third of the patients (147/521), the empirical antibiotic treatment provided consisted of antibiotics from a single class (). Empirical treatment according to the Indonesian sepsis guidelines was provided for 101/150 (67.3%) patients in the ICU, 256/385 (66.5%) patients on non-ICU wards, 154/240 (64.2%) patients with a hospital-acquired sepsis, and 203/295 (68.9%) patients with a community-acquired sepsis. The preferred combination was a cephalosporin with a fluoroquinolone (n = 125, 35% of combination antibiotic treatment), followed by the combination of cephalosporin, fluoroquinolone, and metronidazole (n = 29, 8.1% of combination antibiotic treatment). Cephalosporins were by far the most preferred antibiotics in any of the settings (70–80%), followed by the quinolones (30–50%). Meropenem was used in almost half the number of patients with sepsis in the ICU.
Table 3. Antibiotic treatment, empirical and at sepsis diagnosis
Appropriateness of antibiotic treatment
Appropriateness of antibiotic treatment was based on the regimen received at the time that sepsis was diagnosed, defined as the time sepsis was first reported in the medical record. Ten patients were excluded from this analysis as they had died before antibiotics could be started.
One in two patients, 257/525 (49.9%) received antibiotic treatment with unknown appropriateness, of whom 141/257 (54.9%) because no cultures were taken, and 116/257 (45.1%) because all cultures were negative.
The frequency of inappropriate antibiotic treatment was high, reaching 38.5% of all patients admitted to the ICU (n = 148) or a non-ICU ward (n = 377). More than half of the patients (56.2%) treated for hospital-acquired sepsis (n = 233) received inappropriate antibiotic treatment ().
Consequently, appropriateness of antibiotic treatment at the time of sepsis diagnosis was low, with 18/525 (3.4%) patients receiving definite appropriate antibiotic treatment, and 48/525 (9.1%) patients receiving probable appropriate antibiotic treatment ().
Of all patients receiving definite or probable appropriate antibiotic treatment at the time of sepsis diagnosis, 49/66 (74.2%) were using their initial empirical treatment regimen. However, of all patients receiving inappropriate antibiotic treatment at the time of sepsis diagnosis, 46/202 (22.8%) were on a changed treatment regimen.
For 114/525 (21.7%) patients, the antibiotic treatment at the time of sepsis diagnosis was different from the initial empirical antibiotic treatment received. This was clearly more frequent in patients with at least one culture taken (101/384; 26.3%) compared to patients with no cultures taken (13/141; 9.2%).
Discussion
The current study shows that patients with sepsis frequently receive inappropriate antibiotic treatment in a tertiary health facility in Medan, Indonesia, and that appropriateness could often not be assessed due to the absence of any microbiological culture, or available cultures being negative.
Inappropriate antibiotic treatment in patients with severe sepsis or septic shock has been linked to increased mortality. A systematic review and meta-analysis of this association including 70 studies reported a doubling in odds for 30-day all-cause mortality when antibiotic treatment in the first 48 hours was inappropriate[Citation11].
Not only the antibiotic therapy at sepsis diagnosis was often inappropriate, also the empirical treatment given during admission in this patient group was. The received empirical treatment differed in less than 30% of the patients from the (often inadequate) antibiotic treatment at sepsis diagnosis, and the empirical treatment followed prevailing Indonesian treatment guidelines in less than 70% of the patients (antibiotics from at least two different classes).
Because we used routine data for the study, we cannot know for certain why there is a high frequency of inappropriate antibiotic treatment for patients with sepsis. However, some potential reasons could be envisioned.
Firstly, it may be due to logistical problems when results of antimicrobial sensitivity testing do not reach the treating physician in a timely manner for appropriate treatment to start or initial inappropriate treatment to change. A second reason may be a mismatch between the prevailing antimicrobial resistance (AMR) prevalence in the hospital and treatment guidelines. Indonesia has its own guidelines for the treatment of infectious diseases, including sepsis [Citation9].
Its strong reliance on the use of cephalosporins and fluoroquinolones is in line with the guidance provided in several syndrome-specific antibiotic treatment guidelines from the Infectious Disease Society of America, but clearly does not consider the high prevalence of AMR for these drugs reported in the country.
This situation will be compounded if actual knowledge of the prevalence of AMR is absent. This can occur when there are no proper data on AMR patterns, and/or if this information does not reach the treating physician.
Low- and middle-income countries often lack information on the prevalence of AMR due to the absence of surveillance networks, and limited laboratory capacity[Citation12]. This also holds for Indonesia where information on AMR is infrequently derived from a limited number of university laboratories[Citation13]. We know from our earlier work that AMR prevalence is high in Indonesia, and can differ markedly between settings and even hospital wards [Citation14,Citation15].
There is a clear need for strategies that can provide rapid and locally relevant information on AMR to guide empirical antibiotic treatment choices in a variety of settings. We showed earlier that this is possible by assessing whether the prevalence of AMR is above a clinically relevant level that precludes the use of an antibiotic, rather than attempting to assess a precise AMR prevalence[Citation15].
The clinical care of patients with suspected sepsis requires a careful balance between speed and accuracy in which the severity of the clinical condition of the patient plays a crucial role. But designing an appropriate antibiotic treatment strategy requires all efforts to obtain culture specimens. Our data show that this careful balance was not met, as cultures from blood or the suspected focus of infection were obtained infrequently. When taken, cultures frequently did not show any growth. This latter observation is likely due to the known high frequency of self-medication with readily available antibiotics, or generous antibiotic prescription practises by physicians, including in tertiary care settings in Indonesia[Citation16]. Combining the absence of cultures with the observation that a change in antibiotic treatment could still lead to an inappropriate antibiotic treatment regimen raises the question whether or not the results of cultures and AST are an integral part of clinical decision-making. Diagnostic and antimicrobial stewardship go hand-in-hand. There is a clear role for the microbiology laboratory in antimicrobial stewardship programs [Citation17], while continuous education of care providers on antimicrobial resistance and its stewardship is warranted for curbing AMR[Citation18].
The study included just 45% of patients who had a discharge diagnosis of sepsis recorded in the hospital administration system. This points towards poor record keeping and administration on one hand, and insufficient quality control on the other hand. The latter refers to the possibility that a sepsis diagnosis can be recorded in the system without a matching ICD-10 code available. The former refers to issuing a discharge note of sepsis by the attending physician, while the medical file does not provide adequate proof for the diagnosis. The resulting mismatch of diagnosis and administrative information is worrisome, as automated medical record systems are usually the primary source to assess a wide variety of indices related to hospital care.
We believe our study sample can be considered sufficiently representative for this study as it consists of a homogenous patient group for which sepsis treatment was indicated.
In conclusion, diagnosis and management of patients with suspected sepsis in a tertiary referral hospital in Indonesia can be improved. There is a clear need in encouraging attending physicians to obtain the much-required blood cultures, or cultures from the suspected source of infection before empirical antibiotic treatment is started. This will improve the use of appropriate antibiotic treatment strategies, and potentially reduce the high mortality currently seen in this patient group. In parallel, the hospital should review its empirical treatment guidelines in line with the observed spectrum of sepsis-associated micro-organisms and their antimicrobial susceptibility profile with a special attention to multidrug resistance. Relying on global treatment guidelines in a setting known for its high prevalence of antimicrobial resistance is no option. This intervention fits well with the recommendations of antimicrobial stewardship activities in sepsis management[Citation19].
Declaration of interest
The contents of the paper and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication.
Additional information
Funding
References
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174.
- Suarez De La Rica A, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Ann Transl Med. 2016;4(17):325.
- Vincent J-L, Lefrant J-Y, Kotfis K, et al. Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med. 2018;44(3):337–344.
- Vincent J-L, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353.
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377.
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755.
- Liu VX, Fielding-Singh V, Greene JD, et al. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863.
- Moeloek NF Keputusan menteri kesehatan republik indonesia nomor hk.01.07/menkes/342/2017 tentang pedoman nasional pelayanan kedokteran tata laksana sepsis. 2017.
- Twycross A, Shorten A. Service evaluation, audit and research: what is the difference? Evid Based Nurs. 2014;17(3):65.
- Paul M, Shani V, Muchtar E, et al. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54(11):4851–4863.
- Vernet G, Mary C, Altmann DM, et al. Surveillance for Antimicrobial Drug Resistance in Under-Resourced Countries. Emerg Infect Dis. 2014;20(3):434–441.
- Parathon H, Kuntaman K, Widiastoety TH, et al. Progress towards antimicrobial resistance containment and control in Indonesia. BMJ. 2017;358(Suppl1):31–35.
- Sugianli AK, Ginting F, Kusumawati RL, et al. Antimicrobial resistance in uropathogens and appropriateness of empirical treatment: a population-based surveillance study in Indonesia. J Antimicrob Chemother. 2017;72(5):1469–1477.
- Ginting F, Sugianli AK, Bijl G, et al. Rethinking Antimicrobial Resistance Surveillance: A Role for Lot Quality Assurance Sampling. Am J Epidemiol. 2019;188(4):734–742.
- Puspitasari HP, Faturrohmah A, Hermansyah A. Do Indonesian community pharmacy workers respond to antibiotics requests appropriately? Trop Med Int Health. 2011;16(7):840–846.
- Avdic E, Carroll KC. The role of the microbiology laboratory in antimicrobial stewardship programs. Infect Dis Clin North Am. 2014;28(2):215–235.
- Nand P, Wilson MD, Cohen SH, et al. Curbing antimicrobial resistance: do physicians receive adequate education about antibiograms? J Infect. 2016;72(1):127–129.
- De Waele JJ, Dhaese S. Antibiotic stewardship in sepsis management: toward a balanced use of antibiotics for the severely ill patient. Expert Rev Anti Infect Ther. 2019;17(2):89–97.