ABSTRACT
Objectives
Residual next-day effects of sleep-promoting drugs are common and an important safety issue. Lemborexant is a dual orexin receptor antagonist approved in the United States and Japan for treatment of insomnia in adults. We evaluated the potential of lemborexant for residual morning and next-day effects, including somnolence, based on lemborexant clinical study findings.
Methods
This paper reports findings from 9 lemborexant clinical studies that incorporated next-day assessments of residual drug effects, based on published findings and data on file. Results are reported for healthy subjects or subjects with insomnia disorder treated with lemborexant 5 mg/day or 10 mg/day, placebo, or active comparator before bedtime. Outcomes assessed included next-morning postural stability (body sway measured by ataxiameter), cognitive performance (Cognitive Performance Assessment Battery), impact on driving (standard deviation of lateral position during highway driving test), subjective sleepiness (sleep diary entries), and adverse events of somnolence.
Results
Change from baseline in postural stability the morning after lemborexant administration did not differ from placebo. Among 4 Cognitive Performance Assessment Battery measures, only power of attention declined significantly more with lemborexant treatment compared with placebo in 1 of 2 studies, whereas zolpidem differed from placebo on multiple measures. On the highway-driving test, lemborexant did not significantly impair driving performance versus placebo, however, zopiclone did differ. In large phase 3 trials, next-morning sleep diary ratings showed significantly greater alertness with lemborexant compared with placebo after up to 6 months of treatment. As expected, somnolence was the most common adverse event reported with lemborexant treatment. Somnolence was typically mild to moderate in severity and rarely caused discontinuation of study drug.
Conclusion
Across 9 clinical studies, lemborexant did not substantially impair next-day functioning among healthy subjects and subjects with insomnia.
1. Introduction
Dysregulation of sleep-wake regulatory mechanisms can lead to insomnia disorder, which is characterized by difficulty initiating and/or maintaining sleep and is associated with daytime impairment [Citation1]. Current treatments for insomnia include cognitive behavioral therapy for insomnia, benzodiazepines, nonbenzodiazepine hypnotics, orexin receptor antagonists, melatonin receptor agonists, and antidepressants [Citation1].
In addition to evaluating effectiveness of sleep-promoting drugs, it is important to assess the residual effects of these agents on morning and next-day functioning. Safety of these drugs is a direct concern, as drug-related somnolence is implicated in a large proportion of motor vehicle accidents [Citation2,Citation3]. Furthermore, patients taking medications for insomnia commonly report experiencing residual effects that interfere with work and home activities [Citation4].
Benzodiazepines, particularly those with longer half-lives, have been associated with next-day fatigue, impairment on psychomotor and performance tests, driving impairment, and possibly increased rates of motor vehicle accidents [Citation3,Citation5,Citation6]. Nonbenzodiazepines, including zolpidem, zaleplon, zopiclone, and eszopiclone, have been shown to cause next-day cognitive impairment, memory impairment, postural instability (measured by changes in body sway), reduced psychomotor test performance, and driving impairment, with these functions typically returning to normal as pharmacological effectiveness wears off [Citation3–7].
Among other medications, persistent daytime somnolence has also been reported with use of hypnotics [Citation3]. Sedating antidepressants, including tricyclics and tetracyclics, have been shown to cause next-day sleepiness and to impair daytime performance and driving test performance [Citation3]. Concerns have also been raised about the potential for next-day effects of the orexin receptor antagonist suvorexant, particularly its effects on next-day driving when the drug is taken at higher doses [Citation8,Citation9].
Lemborexant is a dual orexin receptor antagonist that has been approved by the US Food and Drug Administration (FDA) and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) for the treatment of insomnia in adults [Citation10] and is under investigation for the treatment of irregular sleep-wake rhythm disorder [Citation11].
At doses up to 10 mg, lemborexant displays linear pharmacokinetics [Citation12]. Lemborexant is absorbed rapidly after oral administration, with median time to reach maximum concentration after administration ranging from 1 to 3 h for doses of 2.5 to 25 mg and from 1 to 2 h at doses of 5 to 10 mg [Citation12]. The median effective half-life of lemborexant ranges from 17 to 19 h at doses of 5 to 10 mg [Citation10], the doses approved by the US FDA and PMDA for the treatment of patients with insomnia.
In 2 pivotal phase 3 studies of lemborexant for insomnia disorder, Study E2006-G000-303 (Study 303; SUNRISE-2; NCT02952820) and Study E2006-G000-304 (Study 304; SUNRISE-1; NCT02783729), lemborexant treatment was associated with significantly greater improvements in sleep onset and sleep maintenance compared with placebo [Citation13,Citation14]. In the study with an active comparator (SUNRISE-1), lemborexant treatment was associated with significantly greater improvements in sleep onset and sleep maintenance compared with zolpidem tartrate extended release [Citation13].
The objective of this paper is to report evaluations of the potential for residual morning and next-day effects, including somnolence, of lemborexant and comparator drugs based on clinical study findings.
2. Methods
2.1 Design of analysis
This article reports findings from lemborexant clinical studies conducted by Eisai Inc. as part of the clinical development program for the registration of lemborexant for the treatment of insomnia. Findings from 9 studies are included (); these 9 studies included next-morning or across-the-day assessments of residual drug effects. Results are reported for subjects treated with lemborexant 5 mg/day or 10 mg/day (the doses studied in phase 3, based on a phase 2 dose-finding study) [Citation15], placebo, and comparator or active control treatments (zolpidem and zopiclone) before bedtime.
Table 1. Clinical studies investigating next-day effects of lemborexant treatment
For each study, the protocol, protocol amendments or revisions, and informed consent form were approved by a qualified institutional review board or independent ethics committee. Each study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice principles. All participants completed written informed consent before study participation, including consent to publish and anonymity. All studies were registered on clinicaltrials.gov ().
A brief description of each study’s methodology can be found in the Supplemental Methods. For Studies 106 [Citation16], 108 [Citation17], 201 [Citation15], 303 (SUNRISE-2) [Citation14], and 304 (SUNRISE-1) [Citation13], findings are reported from previous publications (where available) or from unpublished data on file (Eisai Inc., Woodcliff Lake, New Jersey, USA), and additional details of study methodology are available in those publications. For Studies 001 Part B, 002, 003, and 107, findings are reported from unpublished data on file (Eisai Inc., Woodcliff Lake, New Jersey, USA) or from clinicaltrials.gov records.
Findings are presented based on analyses originally specified in the protocol for each study and as presented in each study’s clinical study report. Results from different studies that used similar outcome measures were not pooled or compared statistically. Rather, findings from different studies that employed similar endpoints are presented alongside each other, and patterns of findings across studies are described qualitatively.
Administration of next-day outcome measures in each study is described in .
Table 2. Administration of outcome measures
2.2 Postural stability (body sway)
Postural stability was assessed in the laboratory using an apparatus similar to the Wright ataxiameter. This device measured directional trunk movements (i.e., body sway) through a cable placed around the subject’s waist and connected to the ataxiameter. The subject was instructed to stand as still as possible with eyes closed for 1 minute. Body sway was measured in units of 1/3° of the angle of arc. A higher number indicated more body sway (less postural stability).
In the Study 108 analysis, the repeated mixed-effects model contained factors for treatment, sequence, period, baseline (time-matched), time (hours post dose), treatment × time, baseline × time as fixed effects, and time with subject as a random effect and a repeated effect for time, with subject within sequence. The treatment-by-time interaction was used to construct the treatment comparisons at a specific time. A compound symmetry covariance matrix was used. Imputation for the body sway endpoint was based on all subjects (including subjects with incomplete data) using 2 multiple imputation methods to impute missing values.
In Study 304, a mean value for body sway was calculated over Days 2 and 3 and over Days 30 and 31. There were 2 subjects from 1 site whose recorded body sway values were well out of physiological range, hence results here are reported without the extreme values for those 2 subjects (receiving zolpidem and lemborexant 10 mg). However, conclusions remain the same as those based on analyses including all subjects. This analysis was based on a mixed-effect model repeated measurement analysis with factors of age group, region, treatment, visit (Days 2/3 and Days 30/31), and treatment-by-visit interaction as fixed effect, and the baseline posture stability of body sway as a covariate. Missing values were not imputed and assumed to be missing at random.
2.3 Cognitive performance
The Cognitive Performance Assessment Battery was administered by computer and comprised 9 tasks, including simple reaction time, choice reaction time, digit vigilance, immediate word recall, delayed word recall, word recognition, picture recognition, numeric working memory, and spatial working memory. Four composite domain factor scores were calculated by combining outcome variables from the various tests:
Quality of memory: Composite score combining the accuracy measures from the 2 tests of working memory and the 4 tests of episodic memory. Reflects the ability to store information in memory and subsequently retrieve it. Higher scores are better.
Speed of memory retrieval (msec): Composite score combining the reaction time scores from the 2 working memory tests and the 2 episodic recognition tests. Reflects time taken to retrieve information held in both working and episodic memory. Lower scores are better.
Continuity of attention: Composite score combining the accuracy scores from the tests of attention. Reflects the ability to sustain attention (vigilance). Higher scores are better.
Power of attention (msec): Composite score from the speed scores of 3 tests of attention. Reflects the ability to focus attention and process information. Lower scores are better.
In Study 304, a mean value for each Cognitive Performance Assessment Battery measure was calculated over Days 2 and 3 and over Days 30 and 31. Statistical analyses were similar to those for the body sway measure in both Study 108 and Study 304.
2.4 Impact on driving
To assess possible effects of medication on driving, a standardized 100-km driving test was administered on the highway in the morning. The endpoint of the assessment was standard deviation of lateral position (SDLP). An SDLP value > 2.4 cm versus placebo is equivalent to the effect of 0.05% blood alcohol content and was considered a clinically meaningful treatment difference. Zopiclone is known to impair driving performance [Citation18] and was used to verify assay sensitivity [Citation16].
In Study 106, SDLP was analyzed using repeated-measures analyses of variance for Days 2 and 9 of each treatment period. The model included fixed effects for age group, sequence, period, time, treatment and the interaction of treatment and time, and a repeated effect for time, with subject within period.
2.5 Objective sleepiness
The Digit Symbol Substitution Test (DSST) was used to measure attention, perceptual speed, motor speed, visual scanning, and memory. Subjects filled in digits corresponding to shapes as quickly as possible on the basis of a coding key. The key outcome parameter for the DSST was the number correct in 90 seconds.
The Psychomotor Vigilance Test (PVT) measures sustained attention, lapses in attention, and simple reaction time (RT) [Citation19]. Subjects were required to respond with a button press to a light signal. The signals were presented with randomly varying interstimulus intervals. When a subject failed to respond within 500 msec, this was recorded as a ‘lapse.’ The reciprocal of RT to each response (1000/reaction time, in msec, equivalent to speed of response) was recorded as reciprocal reaction time (RRT).
The Reaction Time Index required subjects to respond as quickly and accurately as possible to the appearance of a stimulus in a single-fixed location (simple RT) or a stimulus in 1 of 5 possible locations (5-choice RT).
Mean changes from time-matched baseline (0.25, 1, and 2 hours after waketime) are presented by treatment group for Days 2, 3, 14, and 15 in Study 002 and Study 003, and by presentation of means at Days 2/3 and Days 15/16 in Study 201. Findings at 4, 8, and 12 hours after waketime in Study 002 are presented separately.
2.6 Subjective sleepiness: Karolinska sleepiness scale
On the Karolinska Sleepiness Scale (KSS), subjects rated their sleepiness using a 9-point verbally anchored scale. The verbal anchors and corresponding scores were extremely alert, 1; very alert, 2; alert, 3; somewhat alert, 4; neither alert nor sleepy, 5; some signs of sleepiness, 6; sleepy–but no difficulty remaining awake, 7; sleepy–some effort to keep awake, 8; and extremely sleepy–fighting sleep, 9.
Mean changes from time-matched baseline (0.25, 1, and 2 hours after waketime) are presented by treatment group for Days 2, 3, 14, and 15 in Study 002 and Study 003, and by presentation of means at Days 2/3 and Days 15/16 in Study 201. Findings at 4, 8, and 12 hours after waketime in Study 002 are presented separately.
2.7 Subjective sleepiness: sleep diary
In the electronic sleep diary measure, morning sleepiness was assessed by the question, ‘How alert/sleepy do you feel this morning?’ Subjects rated their sleepiness or alertness level on a 9-point Likert scale, with 1 being extremely sleepy and 9 being extremely alert.
The Study 303 analysis within 1 h of waketime was based on a mixed-effect model repeated measurement analysis with factors of age group, region, treatment, visit (first 7 mornings, Month 1, Month 2, Month 3, Month 4, Month 5, and Month 6), and treatment-by-visit interaction as fixed effect, and the study baseline mean rating on morning sleepiness as a covariate.
The Study 304 analysis within 1 h of waketime was based on a mixed-effect model repeated measurement analysis with factors of age group, region, treatment, visit (first 7 mornings and last 7 mornings of 1 month of study treatment), and treatment-by-visit interaction as fixed effect, and the baseline mean rating on morning sleepiness as a covariate. For the measure taken 90 minutes after waketime, the analysis was similar except data were from Days 2/3 and Days 30/31. In all analyses, missing values were not imputed and assumed to be missing at random.
2.8 Somnolence
An examination of somnolence, the most common treatment-emergent adverse event reported with lemborexant therapy, was conducted across lemborexant clinical trials of subjects with insomnia disorder. Findings are reported for the All Insomnia Pool, which includes subjects who received at least 1 dose of study drug in Studies 001 Part B, 107, 201, 303, and 304. For Studies 107 and 303, subjects who received different treatments during the treatment periods were counted under applicable treatment groups. In each study, somnolence was assessed as an adverse event (reported spontaneously by subjects as per clinical trial protocol) with verbatim descriptions classified into standardized medical terminology using the Medical Dictionary for Regulatory Activities (MedDRA). Findings are presented descriptively.
3. Results
3.1 Postural stability (body sway)
In Study 108 of healthy subjects, change in postural stability (body sway) the morning after 1 dose of lemborexant did not differ from placebo [Citation17] (). Patients receiving zolpidem had a significantly larger increase in body sway than those receiving placebo.
Figure 1. Postural stability: mean change from baseline in body sway at lights on. Lower values are better. Body sway is reported in units of 1/3° angle of arc movement of the ataxiameter. Baseline measures were taken in the morning, before initiation of study treatment. In Study 304, subjects with extreme values were excluded from this analysis. For baseline, Days 2/3 and Days 30/31, respectively, 1 subject had values 3084, 856, and 853, and the other had values 253, 1286, and 1293. CI = confidence interval; LEM5 = lemborexant 5 mg; LEM10 = lemborexant 10 mg; LSM = least squares mean; PBO = placebo; ZOL = zolpidem extended release 6.25 mg
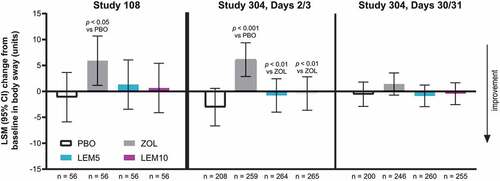
In subjects with insomnia disorder (Study 304), change from baseline in body sway with lemborexant treatment at Days 2/3 and Days 30/31 was small and did not differ from placebo. Zolpidem treatment was associated with a greater increase in body sway at Days 2/3 than treatment with lemborexant 5 or 10 mg or placebo (). No significant treatment differences were observed for change in body sway at Days 30/31.
3.2 Cognitive performance
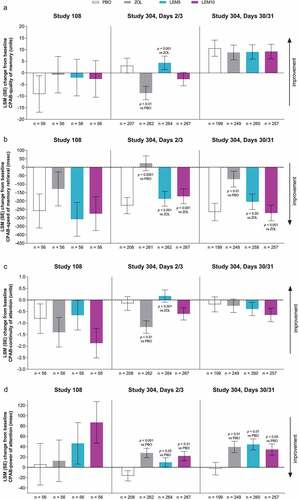
In Study 108, there were no statistically significant differences between lemborexant and placebo in 4 morning assessments of cognitive performance [Citation17] (). There were also no differences between zolpidem and lemborexant or placebo. In Study 304, there were no statistically significant differences between lemborexant and placebo for change in quality of memory, speed of memory retrieval, or continuity of attention at Days 2/3 and Days 30/31 (). At Days 2/3 and 30/31, change in power of attention was significantly greater (worse) for lemborexant 5 mg and 10 mg versus placebo (). In Study 304, zolpidem differed from placebo and lemborexant on multiple measures. Reduction in quality of memory and continuity of attention was significantly greater with zolpidem compared with placebo and with lemborexant 5 mg at Days 2/3 (). Change in speed of memory retrieval was statistically worse after zolpidem treatment compared with placebo, lemborexant 5 mg, and lemborexant 10 mg on Days 2/3 and Days 30/31 (). Finally, change in power of attention was significantly greater (worse) for zolpidem compared with placebo at Days 2/3 and 30/31 ().
Figure 2. Cognitive performance: mean change from baseline for Cognitive Performance Assessment Battery (CPAB) domains (a) quality of memory, (b) speed of memory retrieval, (c) continuity of attention, and (d) power of attention. For quality of memory and continuity of attention, higher scores are better. For speed of memory retrieval and power of attention, lower scores are better. LEM5 = lemborexant 5 mg; LEM10 = lemborexant 10 mg; LSM = least squares mean; PBO = placebo; SE = standard error; ZOL = zolpidem extended release 6.25 mg
3.3 Impact on driving
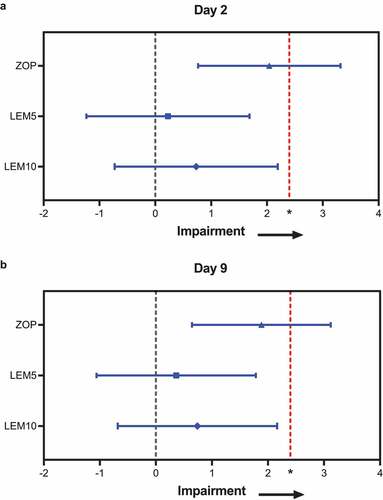
In Study 106, lemborexant 5 or 10 mg did not impair driving performance as measured by change in SDLP versus placebo at Day 2 or Day 9 () [Citation16]. The 95% confidence interval (CI) of the treatment difference for lemborexant 5 or 10 mg at Day 2 and Day 9 always included 0, and the upper limit of the CI fell below the threshold of 2.4 cm, indicating no statistically significant nor clinically meaningful differences between lemborexant and placebo. No drives were stopped or not started due to sleepiness after lemborexant or placebo treatment. Three drives were stopped following zopiclone treatment [Citation16]. Because the upper limit of the 95% CI for the treatment difference of zopiclone versus placebo exceeded 2.4 cm at both Days 2 and 9 (), validity of the study was confirmed [Citation16].
Figure 3. Impact on driving: least squares mean (95% confidence interval) drug minus placebo changes in standard deviation of lateral position at (a) Day 2 and (b) Day 9. Lower values are better. Measurements were taken in Study 106. *Standard deviation of lateral position > 2.4 cm versus placebo is an equivalent effect to that of 0.05% blood alcohol content. n = 48 in ZOP; n = 32 in each lemborexant group. LEM5 = lemborexant 5 mg; LEM10 = lemborexant 10 mg; ZOP, zopiclone 7.5 mg
3.4 Objective sleepiness
In healthy subjects (Study 002), performance on the DSST declined slightly from baseline at 1 h after waketime during early days of treatment with both placebo and lemborexant 10 mg treatment (Figure S1). At 2 hours after waketime and at later days of treatment, performance on the DSST generally improved compared with baseline in all treatment groups (Figure S1). In subjects with insomnia disorder (Study 201), scores on the DSST increased (improved) with successive administrations in all treatment groups, suggestive of a learning effect (Figure S1). In a statistical analysis (analysis of covariance [ANCOVA] with covariates of baseline value and treatment), there were no consistent effects of lemborexant compared with placebo on Days 2/3 or Days 15/16 (data not shown) [Citation15]. Based on the RRT measure on the PVT assessed in Study 002 and Study 003, reaction speed worsened slightly from baseline at all timepoints during the morning; this effect was true in the placebo and lemborexant groups (Figure S2).
In Study 201, changes in simple RT and 5-choice RT on the Reaction Time Index measure were small, and the direction of change (positive or negative) was not consistent (Figure S3) [Citation16]. In a statistical analysis (ANCOVA with covariates of baseline value and treatment), there were no significant differences between lemborexant dose and placebo at any time point for either simple or 5-choice RT (data not shown).
Overall, changes in all measures of objective sleepiness were small and differed little between lemborexant and placebo.
3.5 Subjective sleepiness: Karolinska sleepiness scale
In Study 002, subjective sleepiness measured by the KSS was somewhat greater during the first 2 hours after waketime compared with baseline in both the placebo and lemborexant groups (Figure S4). In Study 003, sleepiness was somewhat greater than at baseline at 1–2 hours after waketime among white subjects receiving lemborexant 10 mg (Figure S4). Statistical testing was not performed in these studies. In subjects with insomnia (Study 201), changes from baseline on the KSS were modest (Figure S4), and there were no significant differences between lemborexant 5 or 10 mg and placebo in a statistical analysis (ANCOVA with covariates of baseline value and treatment; data not shown) [Citation15].
3.6 Sleepiness findings 4–12 h after waketime
At 4–12 h after waketime in Study 002, performance on the DSST, an objective measure of sleepiness, generally improved compared with baseline in all treatment groups (Figure S5A, S5B, and S5C). Based on the RRT measure on the PVT, reaction speed (an objective measure) worsened slightly from baseline at most of these timepoints (Figure S5D, S5E, and S5F). At these later timepoints during the day, KSS scores, representing subjective sleepiness, were slightly larger than at baseline in all treatment groups (Figure S5G, S5H, and S5I). All of these effects were seen in both placebo and lemborexant treatment groups.
3.7 Subjective sleepiness: sleep diary
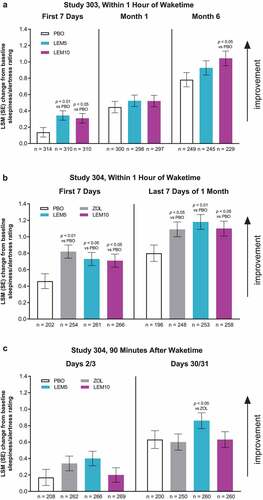
In Study 303, morning alertness increased from baseline in all treatment groups [Citation14] (). This increase was statistically greater for lemborexant 5 mg compared with placebo in the first 7 days of treatment, and for lemborexant 10 mg versus placebo in the first 7 days and at Month 3 and Month 6 (data for Month 3 not shown). In Study 304, the increase from baseline in morning alertness within 1 h of waketime was statistically greater for active treatment (lemborexant or zolpidem) compared with placebo in the first 7 days and last 7 days of treatment (). This pattern was not found at 90 min after waketime, where there were no significant differences between active treatment and placebo (). In both studies, there were no significant differences between the lemborexant treatment groups and the zolpidem group, except in Study 304 at Days 30/31, 90 min after waketime, when the increase from baseline in morning alertness was greater with lemborexant 5 mg than with zolpidem ().
Figure 4. Subjective sleepiness/alertness: mean change from baseline in morning sleepiness/alertness assessed using an electronic sleep diary (a) within an hour of waketime in Study 303, (b) within an hour of waketime in Study 304, and (c) 90 minutes after waketime in Study 304. Sleepiness/alertness was measured from 1 to 9 (higher values indicate greater alertness). LEM5 = lemborexant 5 mg; LEM10 = lemborexant 10 mg; LSM = least squares mean; PBO = placebo; SE = standard error; ZOL = zolpidem extended release 6.25 mg
3.8 Somnolence
Somnolence was the most common adverse event reported among all subjects treated with lemborexant in the clinical studies (All Insomnia Pool; Studies 001 Part B, 107, 201, 303, and 304), occurring in 6.3% of subjects receiving lemborexant 5 mg and 10.6% of subjects receiving lemborexant 10 mg, compared with 1.4% receiving placebo (). This finding suggests an apparent dose-related incidence of somnolence with lemborexant treatment.
Table 3. Somnolence: occurrence, severity, and event rates of somnolence adverse events among all subjects with insomnia disorder enrolled in lemborexant clinical studies
The onset of most somnolence treatment-emergent adverse events was within the first few days of lemborexant administration. Events of somnolence were primarily mild to moderate in severity and occurred at a similar rate per subject-year of drug exposure among patients treated with lemborexant compared with zolpidem. Few subjects (1.0% of subjects treated with lemborexant 5 mg and 2.0% of those treated with lemborexant 10 mg) discontinued study drug as a result of somnolence ().
4. Discussion
For safety and optimal functioning, it is critical for sleep-promoting drugs to balance efficacy with minimal next-day or residual effects [Citation2,Citation3]. Many medications used to treat insomnia have been associated with next-day fatigue, somnolence, cognitive impairment, and driving impairment [Citation3–7].
Lemborexant is a dual orexin receptor antagonist now approved for treatment of insomnia. As reviewed in this paper, lemborexant 5 mg/day or 10 mg/day did not demonstrate clinically significant latent or morning effects over multiple measures across 9 studies.
In 2 studies, change from baseline in postural stability (body sway) the morning after lemborexant administration did not differ from placebo. Among 4 measures of next-day cognitive performance assessed in 2 studies, only power of attention declined significantly more with lemborexant treatment compared with placebo in 1 study, whereas zolpidem differed from placebo and lemborexant on multiple measures. In a next-morning highway driving test, lemborexant did not significantly impair driving performance versus placebo, whereas zopiclone did impair performance as expected based on its role as the active control.
Over 4 objective measures and 1 subjective measure (KSS) of next-day sleepiness administered in 3 studies, changes from baseline were small and differed little between lemborexant and placebo. Statistical analyses, which were conducted in 1 of these studies, found no consistent differences between lemborexant and placebo.
In a large phase 3 trial (Study 304), next-morning sleep diary ratings showed significantly greater alertness with active treatment (lemborexant or zolpidem) compared with placebo. This pattern was also found with lemborexant 10 mg compared with placebo after 6 months of treatment in Study 303. These findings highlight a positive outcome of lemborexant treatment for insomnia: an increase in alertness the next day. It is possible that improvements in next-day alertness are a result of improvements in sleep parameters (e.g., total sleep time) observed with lemborexant treatment [Citation13,Citation14], but our data have not been analyzed to draw this causal connection.
Although mean morning alertness improved with lemborexant compared with placebo, a small proportion of subjects experienced sleepiness at some point during the day. Not surprisingly for a sleep-promoting agent, somnolence was the most common adverse event reported with lemborexant treatment, and it was experienced by more subjects receiving lemborexant than placebo or zolpidem. Overall, 6.3% of subjects treated with lemborexant 5 mg and 10.6% of subjects receiving lemborexant 10 mg experienced somnolence at some point. The onset of somnolence adverse events most commonly occurred within a few days of lemborexant administration, was mild to moderate in severity, and rarely caused discontinuation of study drug. The difference in rates of somnolence between lemborexant and zolpidem may be related to the longer effective half-life of lemborexant [Citation12].
Taken together, findings of multiple clinical studies demonstrate minimal effects on postural stability, cognitive performance, driving, sleep propensity, and other objective measures of sleepiness the morning after lemborexant administration, compared with placebo. Sensitivity of study measures was demonstrated by negative next-day effects found with zolpidem and zopiclone. Lemborexant treatment was also associated with significant benefits in the form of increased morning alertness.
In addition to demonstrating the minimal effects of lemborexant on next-day performance, this series of studies also highlights the critical importance of assessing next-day effects of sleep-promoting agents in early-phase dose-selection studies, so that optimal doses are carried forward into pivotal trials. Regulators are rightly concerned with the safety of these agents, and they carefully examine data on next-day safety issues. Without thorough evaluation and consideration of next-day effects, sleep-promoting agents may encounter regulatory challenges for approval at doses the sponsor believes are most effective.
4.1 Study limitations
Studies in this analysis included different populations (healthy subjects and patients with insomnia), as well as different drug dosing regimens, both of which should be taken into consideration regarding the conclusions. Some studies enrolled few subjects and may not have included statistical comparisons between groups. The number of studies included (9) is limited, the outcome measures and timepoints for analysis are heterogeneous, and for some outcome measures only 1 study is reported. Strengths of the analysis include the range of study populations and measures, as well as the inclusion of placebo and/or active comparator groups across study. Other strengths include the use of standardized and validated instruments (e.g., DSST, KSS) for the measures assessed.
5. Conclusion
This analysis of 9 studies and multiple heterogeneous measures showed that single and chronic dosing with lemborexant does not appear to lead to negative consequences in next-day functioning among healthy subjects and patients with insomnia disorder.
Abbreviations
Declaration of interest
This study was sponsored by Eisai Inc. (Woodcliff Lake, NJ), the owner and manufacturer of lemborexant. The study sponsor participated in the study design, data collection/analysis, data interpretation, and development and approval of the submitted manuscript. Jane Yardley and Kate Pinner are employees of Eisai Ltd., United Kingdom. Margaret Moline, Dinesh Kumar, Carlos Perdomo and Jocelyn Y. Cheng are employees of Eisai Inc., United States. Gary Zammit is an employee and shareholder of Clinilabs Drug Development Corporation; has ownership interest in the Sleep Disorders Institute and Home Sleep and Respiratory Care; has served as a consultant for Eisai Inc., Janssen Pharmaceutical Purdue, and Takeda; and has served on the speakers’ bureau for Merck. A reviewer on this manuscript has disclosed that they are an advisor on the speakers bureau for Dayvigo. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download ()Acknowledgments
We would like to acknowledge Lisa Baker, CMPP, PhD, with the support of Rebecca Jarvis, PhD, CMPP from ProScribe – part of the Envision Pharma Group (both funded by Eisai Inc.) for their editorial assistance and help in writing the article.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Riemann D, Nissen C, Palagini L, et al. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558.
- US Food and Drug Administration. Evaluating drug effects on the ability to operate a motor vehicle: guidance for industry 2017. [cited 2019 Aug 9]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluating-drug-effects-ability-operate-motor-vehicle
- Pagel JF. Drug-induced hypersomnolence. Sleep Med Clin. 2017;12(3):383–393.
- Fitzgerald T, Vietri J. Residual effects of sleep medications are commonly reported and associated with impaired patient-reported outcomes among insomnia patients in the United States. Sleep Disord. 2015; 2015607148.
- Asnis GM, Thomas M, Henderson MA. Pharmacotherapy treatment options for insomnia: a primer for clinicians. Int J Mol Sci. 2015;17(1):50.
- Roth T, Eklov SD, Drake CL, et al. Meta-analysis of on-the-road experimental studies of hypnotics: effects of time after intake, dose, and half-life. Traffic Inj Prev. 2014;15(5):439–445.
- Gunja N. In the Zzz zone: the effects of Z-drugs on human performance and driving. J Med Toxicol. 2013;9(2):163–171.
- Sutton EL. Profile of suvorexant in the management of insomnia. Drug Des Devel Ther. 2015;9:6035–6042.
- Vermeeren A, Sun H, Vuurman EF, et al. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep. 2015;38(11):1803–1813.
- Eisai Inc. DAYVIGO (lemborexant) [Prescribing Information]. 2019. Woodcliff Lake, NJ: Eisai Inc.
- Beuckmann CT, Suzuki M, Ueno T, et al. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295.
- Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lenborexant: findings from single-dose and multiple-ascending dose Phase 1 studies in healthy adults [published online ahead of print, 2020 May 28]. Clin Pharmacol Drug Dev. 2020;10.1002/cpdd.817.
- Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Network Open. 2019;2(12):e1918254.
- Kärppä M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2 [published online ahead of print, 2020 Jun 26]. Sleep. 2020;43(9):zsaa123.
- Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299.
- Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):zsy260.
- Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765–773.
- Vermeeren A, Vuurman EF, Leufkens TR, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep. 2014;37(3):489–496.
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322.
