ABSTRACT
Obesity is a chronic, relapsing metabolic disease, linked to a number of health risks and serious complications. Although highly prevalent in adults in the United States, it is underdiagnosed and untreated. Primary care providers (PCPs) are uniquely poised to diagnose and treat patients with obesity, using a selection of treatment strategies including lifestyle modifications and pharmacotherapies. As a physiological regulator of appetite and energy intake, the glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide 3.0 mg is approved for chronic weight management in individuals with overweight (pre-obesity) or obesity. In this review, we provide an overview of the clinical data supporting the use of liraglutide 3.0 mg, as well as practical advice for PCPs on the initiation and maintenance of treatment over the long term. This also covers the management of side effects and how to manage patient expectations over time.
1. Introduction
Obesity is a chronic, progressive, and relapsing metabolic disease caused by multiple factors resulting from a dysregulation of energy balance [Citation1]. Over time, excess adiposity develops, which is often linked to structural and functional abnormalities, which can in turn predispose affected individuals to greater health risks and serious adiposity-related complications [Citation1]. In the United States (US) and globally, obesity is an urgent public health epidemic [Citation1–3]. Despite an age-standardized prevalence of nearly 40% among US adults [Citation4], obesity remains both underdiagnosed and undertreated [Citation3]. Notably, although long-term treatment strategies can be implemented to manage and treat obesity, it cannot be cured [Citation5,Citation6].
Obesity is typically measured in a clinical setting using metrics such as body mass index (BMI), which, at a population level, can provide important guidance for clinicians on the likelihood of health- and mortality-related risks [Citation1]. For example, it has been shown that for a BMI above 30 kg/m2, life expectancy decreases as BMI increases [Citation7]. At a BMI of 40–45 kg/m2, median survival is reduced by 8–10 years [Citation7]. Mortality data have epidemiological relevance and provide an indication of the risk of death associated with higher BMI, but of course, they cannot be applied to individual patients. Furthermore, excess adiposity is associated with a variety of serious adiposity-related complications, including type 2 diabetes (T2D) [Citation6], certain cancers [Citation8,Citation9], cardiovascular disease [Citation10], hypertension [Citation11], nonalcoholic fatty liver disease [Citation12], sleep apnea [Citation13], asthma [Citation14], gallbladder disease [Citation15], lower-limb osteoarthritis [Citation16], depression and anxiety disorders [Citation17,Citation18], fertility problems (related to conception, miscarriage, and pregnancy) [Citation6], polycystic ovary syndrome, and reduced quality of life [Citation19]. Guidelines developed by the American Association of Clinical Endocrinologists and the American College of Endocrinology (AACE/ACE) recommend that individuals with BMI ≥25 kg/m2 (or ≥23 kg/m2 in South Asian, Southeast Asian, and East Asian adults) should be screened for the presence and severity of adiposity-related complications on an annual basis [Citation6].
Importantly, benefits of even a modest weight reduction (e.g. as little as 5–10%) may have a clinically meaningful impact on an individual’s health outcomes (including improvements in hyperlipidemia, hyperglycemia, hypertension, and sleep apnea) and significantly improve health-related quality of life [Citation20] (). Current treatment guidelines recommend a number of interventions for different severities of obesity to achieve weight reduction and mitigate long-term risk [Citation6,Citation21], similar in some ways to the different interventions for distinct levels of hypertension or dyslipidemia. Primary recommendations for obesity management include lifestyle modifications, such as nutritional changes and physical activity, with pharmacotherapy being considered in individuals with BMI ≥27 kg/m2 with related complications, and ≥30 kg/m2 for those without [Citation6,Citation21]. In the case of those with a BMI ≥35 kg/m2 and severe complications, bariatric surgery is often recommended. Unfortunately, weight reduction as a result of lifestyle intervention is often accompanied by slowing of resting metabolic rate, as the body attempts to balance the discrepancy between energy intake and expenditure [Citation22]. This process is known as metabolic adaptation [Citation22]. As a consequence, lifestyle modifications tend not to yield meaningful reductions in weight in the long term [Citation23,Citation24]. Even with intensive lifestyle interventions, such as those utilized in the Look AHEAD trial, 33% of individuals did not achieve a ≥5% weight-reduction target at 1 year [Citation25].
Table 1. Summary of the benefits of obesity management
Despite guideline recommendations, treatment rates for obesity (2.0–2.2% [Citation34,Citation35]) are significantly lower than those for other chronic diseases, such as hypertension (70.0–90.3% [Citation36,Citation37]) and hypercholesterolemia (with any cholesterol-lowering medication; 32.6–55.5% [Citation36,Citation38]), suggesting a poor diagnosis-to-treatment conversion rate and lack of a good shared decision-making process with patients. Furthermore, far fewer patients adhere to treatment with anti-obesity medications (AOMs) for 6 months or more than those using anti-hypertensive or cholesterol-lowering medications [Citation39–41]. Low rates of prescribing pharmacologic treatments by primary care providers (PCPs) may be due to lack of education on US Food & Drug Administration (FDA)-approved AOMs and their long-term safety and efficacy [Citation42,Citation43]. This in turn can only be fully appreciated if there is a thorough understanding of the physiological role of endogenous hormones such as glucagon-like peptide-1 (GLP-1) in the regulation of appetite and energy intake. Limited health insurance coverage of AOMs may further limit patient access to GLP-1 receptor agonist (GLP-1 RA) medications [Citation44]. It is also likely that there is an insufficient understanding among PCPs as well as patients themselves that obesity is akin to other complex, chronic metabolic diseases such as hypertension, and therefore requires a similar intensification and chronicity of treatment.
GLP-1 RAs are a class of therapeutic agents that mimic endogenous GLP-1 [Citation45,Citation46]. Originally developed to improve glycemic control, a number of GLP-1 RAs are approved for the treatment of type 2 diabetes [Citation47]. Of particular importance to this review, GLP-1 acts as a physiological regulator of appetite and energy intake [Citation45,Citation46]. Due to their multiple metabolic effects, they are positioned to play an important role as part of a multi-modal, long-term treatment strategy for obesity [Citation48]. Liraglutide 3.0 mg is the only drug of this class that is currently approved for chronic weight management [Citation49]. Other GLP-1 RAs such as semaglutide (e.g. NCT03548935) and dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA therapies such as tirzepatide (e.g. NCT03730662) remain under clinical development for chronic weight management.
The goal of this review is to provide PCPs with a brief overview of the clinical data supporting the use of, primarily, liraglutide 3.0 mg in patients with obesity. We emphasize the PCP’s role in the initiation and maintenance of treatment over the long term, describe the mitigation of gastrointestinal (GI) side effects, and discuss how to manage patients’ expectations. This working knowledge will help PCPs advise their patients on initiating treatment for weight management with liraglutide 3.0 mg and adherence to it; such knowledge may be translated to other treatments with GLP-1 RAs in development, once they have received approval.
1.1. Understanding GLP-1 and GLP-1 RAs
GLP-1 is a member of the incretin hormone family and is produced in the GI tract in response to food [Citation46,Citation50]. GLP-1 is primarily secreted from L-cells in the gut, but it is also secreted from neurons in the hindbrain. Endogenous GLP-1 has a short half-life (approximately 2 minutes) in the plasma, as it is rapidly degraded by the ubiquitous enzyme dipeptidyl peptidase-4 (DPP-4) and is not metabolized by the liver or kidney [Citation45,Citation50]. In individuals with a healthy weight, postprandial GLP-1 has been shown to play an important role in regulating appetite through activation of areas of the hypothalamus implicated in energy intake. Peripheral infusion of native GLP-1 has been shown to increase satiety and fullness while reducing hunger and energy intake.
Due to its short half-life, as a result of rapid degradation by DPP-4, native GLP-1 has limited therapeutic potential. However, GLP-1 RAs have been developed with longer half-lives, making them more robust against DPP-4 degradation, thereby rendering them more suitable as therapeutic agents [Citation45]. As a consequence of the broad distribution of GLP-1 receptors in body tissues, GLP-1 RAs have a variety of therapeutically desirable effects [Citation45,Citation51]. Most relevant for individuals with obesity are their stimulation of anorexigenic neurons and inhibition of orexigenic neurons [Citation52], resulting in increased satiety and fullness, with reduced hunger and prospective food consumption [Citation53]. In addition, GLP-1 RAs, particularly short-acting preparations, are associated with delayed gastric emptying, which may contribute to their efficacy in obesity as well as adverse GI effects [Citation54]. Liraglutide 3.0 mg, a long-acting formulation, was the first GLP-1 RA to be investigated and approved for weight management [Citation49,Citation55].
1.2. The clinical profile of liraglutide in obesity
The efficacy and safety of liraglutide 3.0 mg have been studied in the randomized, controlled, phase 3 SCALE trials enrolling a variety of populations with obesity or overweight, which informed its prescribing label [Citation49]. SCALE Obesity and Prediabetes enrolled individuals with prediabetes (but not T2D); all individuals had BMI ≥30 kg/m2 or BMI ≥27 kg/m2 and treated or untreated hypertension and/or dyslipidemia [Citation56,Citation57]. SCALE Diabetes enrolled individuals with T2D and BMI ≥27 kg/m2 [Citation58]. While SCALE Maintenance had a similar patient population to SCALE Obesity and Prediabetes [Citation59], individuals participating in SCALE Sleep Apnea had moderate or severe sleep apnea and BMI ≥30 kg/m2 () [Citation60].
Table 2. Weight loss in randomized, placebo-controlled trials of liraglutide 3.0 mg*
For all these trials, dosing was initiated at 0.6 mg daily and increased each week to achieve the maximum of 3.0 mg dose [Citation56–60]. The rationale for this dosing was based on results from a previous phase 2 dose-ranging trial that identified liraglutide 3.0 mg as the optimal dose with which to maximize weight loss as compared with liraglutide 1.2 mg, 1.8 mg, and 2.4 mg, while also maintaining an acceptable tolerability. Clinical endpoints studied in these trials included safety/tolerability, weight reduction, and/or maintenance of weight reduction (after intensive lifestyle interventions) from baseline [Citation56,Citation58,Citation59,Citation61], delay in onset of T2D in individuals with obesity and pre-diabetes [Citation57], and improvements in obstructive sleep apnea [Citation60]. Appreciating what the available data have shown can help PCPs understand when it is appropriate to initiate treatment with liraglutide 3.0 mg and also help to set patients’ expectations.
1.2.1 How much weight reduction can patients expect with liraglutide 3.0 mg?
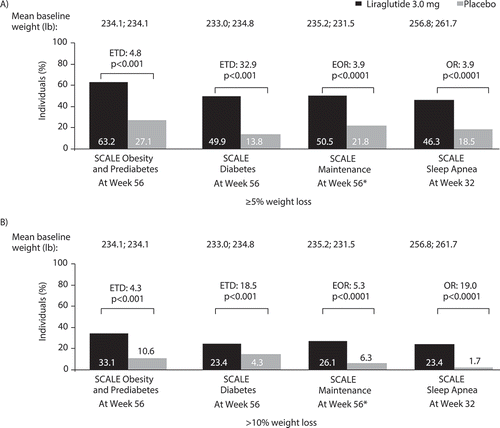
Among these four SCALE trials, weight reduction after liraglutide 3.0 mg treatment alongside lifestyle modification (nutrition and physical activity) ranged from 5.7% to 8.0% of baseline body weight compared with placebo [Citation56–60] (). Furthermore, it was shown that significantly more individuals with obesity taking liraglutide 3.0 mg lost ≥5% of baseline weight (46.3% to 63.2%) ()) and >10% of baseline weight (23.4% to 33.1%) ()) compared with placebo [Citation56–60]. In a randomized, three-arm, parallel-group trial, 150 individuals with obesity were randomized to intensive behavior therapy (IBT) alone, IBT + liraglutide 3.0 mg, or IBT + liraglutide 3.0 mg + low-calorie nutrition meal-replacement diet [Citation61]. Weight reduction in the liraglutide-treated groups was highly superior to IBT alone, with 44.0%, 70.0%, and 74.0% losing ≥5% of weight and 26.0%, 46.0%, and 72.0% losing ≥10%, respectively. Mean weight loss was 6.1% ± 1.3%, 11.5% ± 1.3%, and 11.8% ± 1.3%, respectively. Importantly, as demonstrated in the SCALE Maintenance trial, liraglutide 3.0 mg was shown to be associated with a greater likelihood of maintaining run-in weight reduction after 56 weeks compared with individuals taking placebo (81.4% vs 48.9%, estimated odds ratio (OR) 4.8 [3.0; 7.7]95% CI, p< 0.0001) [Citation59].
Figure 1. Proportion of individuals losing ≥5% (a) and >10% (b) body weight with liraglutide treatment in randomized, placebo-controlled, phase 3 trials
1.2.2. Is weight reduction with liraglutide 3.0 mg in real-world settings comparable to those seen in randomized trials?
Real-world clinical effectiveness of liraglutide 3.0 mg was reported in a Canadian cohort of 311 individuals [Citation62]. After 6 months, mean weight reduction was −16.1 lb (p< 0.001). Those who persisted with treatment for ≥6 months (n = 167) had a mean weight reduction of −17.6 lb (p< 0.001), representing a decrease of 7.1% [Citation62]. Among these individuals, nearly two-thirds (64.1%) lost ≥5%, and over one-third (34.5%) lost >10% body weight. Similar results were reported from an Italian cohort of 93 individuals treated with liraglutide 3.0 mg. In this population, patients who persisted with treatment for ≥6 months (n = 43) had a mean weight reduction of −17.6 lb (a decrease of 7.4%). Among these individuals, 69.8% lost ≥5% and 23.2% lost ≥10% of body weight [Citation54]. These trials, however, were in specialist care settings and so may not be directly applicable for most primary care settings.
It is noteworthy that real-world evidence also demonstrates that IBT does not provide lasting or significant weight reduction in individuals with obesity [Citation63,Citation64], compared with randomized clinical trials such as Look AHEAD [Citation25]. A retrospective longitudinal analysis of 177,743 individuals with a baseline BMI ≥30 kg/m2 found that weight regain and weight cycling (defined as not continuously losing, gaining, or maintaining weight throughout the 2-year observation period relative to its beginning) were common occurrences, following an initial 6-month intensive weight-reduction period [Citation63].
1.2.3 What other clinically important endpoints are associated with liraglutide 3.0 mg?
Treatment with liraglutide 3.0 mg has been shown to have numerous beneficial effects beyond weight reduction alone in individuals with obesity, including delaying the onset of T2D among individuals with prediabetes, improving the symptoms of obstructive sleep apnea, and improving various biomarkers of cardiovascular risk [Citation56–60]. In SCALE Obesity and Prediabetes, significantly fewer individuals in the liraglutide 3.0 mg group than in the placebo group were diagnosed with diabetes while on treatment (n = 26/1472; 2% and n = 46/738; 6%, respectively) [Citation57]. The time to diagnosis of T2D was 2.7 ([1.9; 3.9]95% CI, p< 0.0001) times longer with liraglutide than with placebo at 3 years (hazard ratio 0.21 [0.13; 0.34]95% CI) [Citation57].
Improvements in obstructive sleep apnea, a condition often accompanying obesity, have also been noted. In SCALE Sleep Apnea, individuals treated with liraglutide 3.0 mg had a greater mean reduction in the number of apnea events (or similar) per hour with liraglutide than placebo (−12.2 vs −6.1 events/h, estimated treatment difference: −6.1 events/h, p= 0.015) [Citation60]. The degree of weight reduction was also significantly correlated with the clinical improvement of obstructive sleep apnea (p< 0.01).
A post hoc analysis of SCALE data indicated that liraglutide 3.0 mg was not associated with excess cardiovascular risk [Citation65]. Furthermore, statistically significant improvements in various cardiometabolic and inflammatory parameters with liraglutide 3.0 mg versus placebo have been demonstrated across the SCALE trials (e.g. C-reactive protein [Citation56–58], plasminogen activator [Citation56,Citation58], adiponectin [Citation56], total cholesterol [Citation56,Citation57], very low-density lipoprotein cholesterol [Citation56,Citation57], high-density lipoprotein cholesterol [Citation56,Citation57], low-density lipoprotein cholesterol [Citation56], and free fatty acids [Citation56]). Statistically significant decreases in systolic blood pressure have also been reported [Citation56–59], and diastolic blood pressure to a lesser extent, with significant reductions reported in only one trial [Citation56].
1.2.4 Liraglutide and obesity: safety and tolerability
The clinical trial safety and tolerability data for liraglutide 3.0 mg utilized within the prescribing information included a total of 3384 individuals with overweight or obesity [Citation49], exposed to liraglutide for treatment periods of up to 56 weeks [Citation56,Citation58,Citation59], 52 weeks [Citation66], and 32 weeks [Citation60]. Across the trials supporting the prescribing information, liraglutide 3.0 mg was generally well tolerated, with 9.8% of those treated with liraglutide 3.0 mg prematurely discontinuing treatment due to adverse events, compared with 4.3% with placebo [Citation49].
GI adverse events (AEs) were the most common reason for discontinuation, including nausea (2.9% vs 0.2% for liraglutide 3.0 mg vs placebo, respectively), vomiting (1.7 vs <0.1%), and diarrhea (1.4 vs 0%) [Citation49]. GI AEs reported in ≥5% of liraglutide-treated individuals (and more frequently than placebo) are reported in [Citation49]. Higher incidences of events such as nausea are thought to be associated with delays in gastric emptying linked to liraglutide and other GLP-1 RAs, as well as activation of the centers involved in appetite regulation, satiety, and nausea during the peak of the GLP-1 effect [Citation67]. Liraglutide has been shown to have a more significant delay in gastric emptying at 5 weeks than at 16 weeks, and the incidence of nausea and degree of weight loss demonstrated a similar pattern [Citation68]. However, 5-h gastric emptying with liraglutide 3.0 mg is comparable with placebo, and as such, the role of gastric emptying on weight loss with liraglutide 3.0 mg requires further study [Citation53]. Suggestions on how to help mitigate GI AEs are provided in the next section.
Table 3. Gastrointestinal adverse events reported in ≥5% of individuals treated with liraglutide 3.0 mg (and more frequently than placebo)
It should be noted that in rodents, liraglutide has been shown to cause dose- and duration-dependent thyroid C-cell tumors, although the relevance of these findings in humans is yet to be determined [Citation49]. Some clarification here is important. Clinicians frequently encounter thyroid nodules during physical examination or during procedures such as ultrasonography of the neck to examine the carotid arteries. Most of these are benign, although a small portion (5–10%) are not. Of the latter, most (80–85%) are papillary thyroid cancers (PTCs) arising from follicular cells in the thyroid; they have an indolent nature and are associated with long-term survival [Citation69]. These PTCs should not be confused with the much less common medullary thyroid carcinomas (MTCs), which originate from thyroid C-cells and have been associated with liraglutide exposure in the rodent models. Until further causality is established, liraglutide 3.0 mg is contraindicated in individuals with a personal or family history of MTC or multiple endocrine neoplasia syndrome type 2 (MEN2) [Citation49]. In the trials of liraglutide 3.0 mg reviewed here, there were no cases of MTC, but some have been reported post-marketing [Citation49].
Acute pancreatitis is another precaution noted within the prescribing information [Citation49]. A total of 15 cases of pancreatitis were reported in 3291 patients exposed to liraglutide 3.0 mg versus two cases in placebo-treated patients [Citation49,Citation55]. Particularly for rare events with long timelines to onset, such as pancreatic and thyroid cancers, longer observational self-reported surveillance data will be required to monitor the long-term safety of liraglutide at the 3.0 mg dose.
Although not specifically metabolized in the kidneys, patients treated with GLP-1 RAs, including liraglutide 3.0 mg, have been shown to experience acute renal failure and worsening of chronic renal failure, sometimes requiring hemodialysis [Citation49]. Some of these events were reported in patients without known underlying renal disease. It should be noted that the majority of the reported events occurred in patients experiencing dehydration as a result of nausea, vomiting, or diarrhea [Citation49]. Altered renal function has been reversed in many of the reported cases through discontinuation of liraglutide 3.0 mg or another causative agent being taken concomitantly [Citation49]. Caution should be taken when escalating the dose of liraglutide 3.0 mg in patients with preexisting renal impairment.
Substantial or rapid weight reduction, such as that observed with liraglutide 3.0 mg, has also been linked to an increased risk of gallbladder problems [Citation49]. Furthermore, the incidence of acute gallbladder disease was greater in liraglutide-treated than in placebo-treated patients even after accounting for the degree of weight reduction. Liraglutide 3.0 mg was associated with a higher incidence of cholelithiasis and cholecystitis compared to placebo (2.2% vs 0.8%, and 0.8% vs 0.4%, respectively) [Citation49]. To prevent the development of gallbladder complications, patients should be monitored regularly, with ultrasound for documenting gallstone disease performed on those with symptoms. For patients with known stone disease, counseling on precautions to limit the worsening of symptoms and further stone formation (e.g. amount of fat in the diet, use of ursodiol) should be used. The majority of patients with AEs of cholelithiasis and cholecystitis required cholecystectomy [Citation49].
Because liraglutide 3.0 mg stimulates insulin secretion in a glucose-dependent manner, the likelihood of a hypoglycemic episode occurring is low when used as a monotherapy [Citation49]. The risk for serious hypoglycemia is increased when liraglutide 3.0 mg is used in combination with insulin and insulin secretagogues, such as sulfonylureas, in patients with T2D [Citation49]. Patients may therefore require a lower dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin in this setting [Citation49]. Educating patients with T2D on the signs and symptoms of hypoglycemia may help to mitigate the risk of serious AEs [Citation49]. Importantly, for patients who do not have T2D, regular blood glucose monitoring is not required. Mean increases in resting heart rate of 2 to 3 beats per minute (bpm) have been observed with liraglutide 3.0 mg use during routine clinical monitoring. In a clinical pharmacology trial that monitored heart rate continuously for 24 hours, liraglutide 3.0 mg treatment was shown to increase resting heart rate by 4 to 9 bpm higher than that observed with placebo. Heart rate should be monitored at regular intervals, consistent with usual clinical practice. Patients should inform healthcare providers of palpitations or feelings of a racing heartbeat while at rest during treatment with liraglutide 3.0 mg. For patients who experience a sustained increase in resting heart rates while on treatment, liraglutide 3.0 mg should be discontinued.
2. Practical advice for implementing treatment recommendations
2.1. Initiating liraglutide 3.0 mg
When initiating liraglutide in patients with obesity, it is recommended that the daily dose is escalated slowly (week 1: 0.6 mg, week 2: 1.2 mg, week 3: 1.8 mg, week 4: 2.4 mg, week 5, and onward: 3.0 mg) [Citation49]. From our clinical experience, utilizing the 10 ‘micro-clicks’ between each dose can help to titrate slowly (i.e. titrating up 1–2 micro-clicks as tolerated), although this recommendation is off-label. If nausea develops at any time, reducing down to, and remaining at, a lower tolerated dose for a sustained period may be useful before trying to up-titrate. Remaining on a lower dose for longer periods of time may be of benefit to patients who are struggling with continued nausea-related side effects. Although GI AEs were the most common reason for withdrawal from the SCALE trials [Citation57], it may be helpful to remind patients that most (~93%) of these AEs tend to be transient and mild in nature and do not lead to discontinuation of treatment [Citation49]. PCPs should inform patients that dietary modifications such as eating smaller, frequent meals (~1/3 typical portion size, 3–5 times daily) consisting of protein with vegetables and/or fruits and avoiding fatty foods may minimize GI AEs and may ease their concerns, and that anti-emetics may be prescribed if necessary. Over time, meal size and frequency may be adjusted accordingly.
Some patients experience an increase in gastroesophageal reflux and constipation during the initiation phase. We have found that discussing reflux precautions (eating smaller meals, not eating 2 hours before bedtime) and/or adding a fiber supplement to help prevent constipation may be helpful, especially if the patient had these conditions prior to liraglutide 3.0 mg initiation. Constipation can occur when patients eat less volume, as they naturally consume less fiber. Recommending a fiber supplement routinely is highly encouraged. Fiber has also been shown to have satiety effects and may also aid in weight reduction. Over-the-counter magnesium may also help to alleviate related symptoms. In patients experiencing GI AEs, including nausea, vomiting, or diarrhea, it is important that hydration is maintained to prevent possible kidney damage. As natural hydration through food consumption may be limited due to GLP-1 RA usage, it is recommended that patients seek additional hydration through other means (e.g. increased water consumption or over-the-counter oral rehydration treatments).
To reassure patients as they start on this treatment, it is useful to discuss steps to follow in the event of missed doses, and possible reductions in dosing ahead of further attempts to increase to the 3.0 mg recommended target. If a dose is missed, the once-daily regimen should be resumed as prescribed in the next scheduled dose. If more than 3 days have elapsed since the previous dose of liraglutide 3.0 mg, patients should reinitiate at 0.6 mg daily and escalate slowly to their tolerated dose. Further details of the recommended escalation schedule can be found in the prescribing information [Citation49]. Patients should be made aware that the pen does not need to be refrigerated between uses. Eliminating worries about access to refrigeration can help to increase compliance and ease the fear of traveling with the medication. Recommending that patients store their pen in a place that they will remember to take it, such as the bathroom or their bedside drawer, and to set a reminder alarm on their phone can also help to boost compliance.
It is helpful to provide training on using pens, especially if the individual is new to using a treatment that requires injections. This includes demonstrating proper injection technique (including appropriate administration sites and injection-site rotation) during an office visit, dose-setting, and needle handling and disposal. Reminding the patient that it is only an injection because the medication cannot be delivered orally (due to absorption in the GI tract) can help to ease the anxiety around taking an injectable medication. Discussing and showing that the small size of the needle (30–32 gauge and 4–8 mm in length) compared to a noticeably larger phlebotomy needle (~21 gauge), and that only a small amount of solution is injected, meaning that administration is virtually painless, may also help to ease concerns. Finally, patients may be under the impression that injectable medications are more serious or associate them with being in poorer health. Confirmation that this is not the case can help in gaining agreement to try the medication. If you as the treating healthcare provider do not have time to undertake all these steps with your patient, it may be worthwhile training a medical assistant (MA) or another member of your team to perform such demonstrations and to answer any questions.
It is also important to be mindful of the discontinuation recommendations, which (in the US) indicate that liraglutide 3.0 mg should be discontinued if individuals have not achieved a 4% reduction in weight after 16 weeks of treatment [Citation55], since it is unlikely that they will achieve and sustain clinically meaningful weight reduction with continued treatment.
When considering therapeutic options, it is important to note that certain insurance/employer-dependent policies may not cover FDA-approved AOMs. For individuals who have coverage, co-pay support plans exist, details of which can be found on the AACE website (http://prescriptionhelp.aace.com/), and pharmacists can help patients access such programs if needed.
Practical recommendations for the initiation of liraglutide 3.0 mg are summarized in .
Table 4. Summary of practical recommendations for the initiation and maintenance of liraglutide 3.0 mg treatment in patients with obesity*
2.2. Maintaining adherence to liraglutide 3.0 mg
When discussing the importance of adherence to liraglutide 3.0 mg, it is essential to remind patients that weight-reduction pharmacotherapies are not a ‘quick fix’ but a long-term option for managing obesity as a chronic and complex condition. Describing the mode of action of liraglutide 3.0 mg, and how this relates to its efficacy, may help to encourage patients to continue with their prescribed dose. It may also be worthwhile to remind patients that a 5–10% reduction in weight and management of obesity have many health benefits, such as prevention of T2D in those with prediabetes (). Furthermore, liraglutide 3.0 mg has been shown not to increase cardiovascular risk in adults with overweight or obesity and at least one weight‐related comorbidity [Citation70], and to improve associated risk factors (i.e. blood pressure and lipid levels) [Citation56–60]. Liraglutide 1.8 mg has also been shown to provide cardio-renal benefits to individuals with T2D [Citation71–73]. Many patients with obesity respond well upon understanding that weight reduction is not the only advantage of taking the medication.
With regards to weight reduction, patients typically desire more than a 5–10% target. Managing this expectation is a critical component of obesity treatment. Reminding patients of their success and celebrating their accomplishment are of vital importance to maintain weight reduction. Showing patients a graph of their weight reduction over time, and improvement in metabolic markers such as glycated hemoglobin in those with T2D or prediabetes, or balance in lipid levels can be motivating for patient adherence to treatment. Furthermore, reflecting on improvements to their adiposity-related conditions and overall health-related quality of life (i.e. improvements in mobility, reductions in body pain) can help showing them how far they have come. Using evidence-based examples may also help them to maintain adherence to the medication. Reminding individuals, using visual aids if necessary, that when medication is stopped, most patients tend to regain weight over time can be helpful in promoting adherence to long-term medication management in conjunction with lifestyle changes. Discussing that all weight reduction reaches a plateau eventually due to metabolic adaptation, and if more loss is needed/desired then further interventions may be required can help individuals to accept the level of weight reduction that has been achieved or motivate them to find ways to make further adjustments in treatment. However, it should be noted that further studies are needed to identify the best strategies to address a weight-loss plateau in patients on liraglutide, and therefore, personal experience should guide the healthcare provider at this stage.
Practical recommendations for the maintenance of liraglutide 3.0 mg are summarized in .
3. Conclusion
Obesity is a chronic disease that can be managed but not cured, and can significantly impact the health and well-being of those affected. Due to its long-term efficacy and safety profile, supported by clinical and real-world data, liraglutide 3.0 mg is an attractive treatment option for patients with obesity, both in terms of significant weight reductions and improvements of other adiposity-related conditions. PCPs play an essential role in supporting patients with the initiation and maintenance of liraglutide, minimizing the risk of unwanted AEs, and ensuring the best overall health outcomes.
Declaration of financial/other relationships
Angela Fitch reports that she acts on the advisory board of SetPoint Health, Gelesis, and Phenomix Sciences and has previously consulted for Novo Nordisk. Amy Ingersoll has previously consulted for Novo Nordisk.
Declaration of interest
No potential conflict of interest was reported by the authors.
Author contributions
Both authors contributed to the design and scope of this review article, reviewed and commented on several drafts of the manuscript, and approved the final version for publication. Both authors take responsibility for the integrity of the work.
Acknowledgments
Writing and editing support was provided by Sasha Walton and Izabel James, from Watermeadow Medical, an Ashfield company, funded by Novo Nordisk Inc.
References
- Jastreboff AM, Kotz CM, Kahan S, et al. Obesity as a disease: the obesity society 2018 position statement. Obesity (Silver Spring). 2019;27:7–9.
- American Medical Association. 2012 Resolutions. 2012. Cited Jul 2020. Available from: https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/hod/a12-resolutions_0.pdf.
- Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the national action study. Obesity (Silver Spring). 2018;26:61–69.
- Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319:1723–1725.
- Food and Drug Administration. Guidance for industry developing products for weight management. 2007. Cited Jul 2020. Available from: https://www.fda.gov/media/71252/download.
- Garvey WT, Mechanick JI, Brett EM, et al. AACE/ACE comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract. 2016;22:842–884.
- Collaboration PS, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096.
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798.
- Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2017;66:1052–1058.
- Caleyachetty R, Thomas GN, Toulis KA, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70:1429–1437.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486.
- Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030.
- Li C, Ford ES, Zhao G, et al. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: national health and nutrition examination survey, 2005-2006. Prev Med. 2010;51:18–23.
- Hjellvik V, Tverdal A, Furu K. Body mass index as predictor for asthma: a cohort study of 118,723 males and females. Eur Respir J. 2010;35:1235–1242.
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
- Messier SP, Resnik AE, Beavers DP, et al. Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res (Hoboken). 2018;70:1569–1575.
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830.
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229.
- Hopman WM, Berger C, Joseph L, et al. The association between body mass index and health-related quality of life: data from CaMos, a stratified population study. Qual Life Res. 2007;16:1595–1603.
- Wright F, Boyle S, Baxter K, et al. Understanding the relationship between weight loss, emotional well-being and health-related quality of life in patients attending a specialist obesity weight management service. J Health Psychol. 2013;18:574–586.
- He Mw B, Christensen S, Seger J, et al. Obesity algorithm slides, presented by the obesity medicine association. 2019. Cited Jul 2020. Available from: https://obesitymedicine.org/obesity-algorithm/.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612–1619.
- Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond). 2005;29:1168–1174.
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604.
- Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34:2152–2157.
- Feldman AL, Griffin SJ, Ahern AL, et al. Impact of weight maintenance and loss on diabetes risk and burden: a population-based study in 33,184 participants. BMC Public Health. 2017;17:170.
- Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10:e0121993.
- Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing diabetes prevention study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–480.
- Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308.
- Ghaemi A, Taleban FA, Hekmatdoost A, et al. How much weight loss is effective on nonalcoholic fatty liver disease? Hepat Mon. 2013;13:e15227.
- Morales E, Praga M. The effect of weight loss in obesity and chronic kidney disease. Curr Hypertens Rep. 2012;14:170–176.
- Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–649A.
- Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–171.
- Xia Y, Kelton CM, Guo JJ, et al. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring). 2015;23:1721–1728.
- Samaranayake NR, Ong KL, Leung RY, et al. Management of obesity in the National Health and Nutrition Examination Survey (NHANES), 2007-2008. Ann Epidemiol. 2012;22:349–353.
- Kalra R, Parcha V, Patel N, et al. Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005–2016. Eur J Prev Cardiol. 2020.Doi: 10.1177/2047487320905190.
- Wang D, Hatahet M, Wang Y, et al. Multivariate analysis of hypertension in general US adults based on the 2017 ACC/AHA guideline: data from the national health and nutrition examination survey 1999 to 2016. Blood Press. 2019;28:191–198.
- Mercado C, DeSimone AK, Odom E, et al. Prevalence of cholesterol treatment eligibility and medication use among adults—United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2015;64:1305–1311.
- Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract. 2018;143:348–356.
- Wogen J, Kreilick CA, Livornese RC, et al. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Manag Care Pharm. 2003;9:424–429.
- Colantonio LD, Rosenson RS, Deng L, et al. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376.
- Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy. 2013;33:1299–1307.
- Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130:173–182.
- Jannah N, Hild J, Gallagher C, et al. Coverage for obesity prevention and treatment services: analysis of medicaid and state employee health insurance programs. Obesity. 2018;26:1834–1840.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157.
- Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124:4223–4226.
- Gentilella R, Pechtner V, Corcos A, et al. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35:e3070.
- Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37.
- Novo Nordisk. SAXENDA® (liraglutide) injection, for subcutaneous use. 2018. [cited 2020 Oct]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf.
- Gupta V. Glucagon-like peptide-1 analogues: an overview. Indian J Endocrinol Metab. 2013;17:413–421.
- Ryan D, Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring). 2015;23:1119–1129.
- Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–4488.
- van Can J, Sloth B, Jensen CB, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38:784–793.
- Maselli DB, Camilleri M. Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol. 2021;1307:171-192.
- Novo Nordisk. Saxenda 6 mg/mL solution for injection in pre-filled pen: summary of product characteristics. 2019. Cited Oct 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
- le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. JAMA. 2015;314:687–699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443–1451.
- Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310–1319.
- Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity (Silver Spring). 2019;27:75–86.
- Wharton S, Liu A, Pakseresht A, et al. Real-world clinical effectiveness of liraglutide 3.0 mg for weight management in Canada. Obesity (Silver Spring). 2019;27:917–924.
- DerSarkissian M, Bhak RH, Huang J, et al. Maintenance of weight loss or stability in subjects with obesity: a retrospective longitudinal analysis of a real-world population. Curr Med Res Opin. 2017;33:1105–1110.
- Mann T, Tomiyama AJ, Westling E, et al. Medicare’s search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220–233.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461–2498.
- Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843–854.
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202–230.
- Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890–899.
- Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65:125–137.
- Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20:734–739.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322.
- Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138:2908–2918.
- Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848.
