ABSTRACT
Objective
Globally, 5 serogroups (A, B, C, W, and Y) cause the majority of invasive meningococcal disease (IMD). Vaccines targeting these serogroups are currently part of the US adolescent immunization platform, which includes 1 + 1 dosing of a MenACWY vaccine routinely at ages 11 and 16 years and 2 doses of a MenB vaccine at age 16–23 years under shared clinical decision-making between the patient and healthcare provider. In 2018, MenACWY vaccination coverage was 86.6% for ≥1 dose and 50.8% for ≥2 doses, whereas MenB vaccination coverage was 17.2% for ≥1 dose and <50% for completion of the multidose series. A pentavalent MenABCWY vaccine could simplify immunization schedules and improve vaccination coverage. We estimated the public health impact of a pentavalent MenABCWY vaccine using a model that considers meningococcal carriage and vaccination coverage.
Methods
A population-based dynamic model estimated the 10-year reduction in IMD from implementing a MenABCWY vaccine within the existing US meningococcal immunization platform. Five vaccination schedules (4 new, 1 existing) were examined to estimate the impact of different recommendations on the overall reduction in the number of IMD cases. Sensitivity analyses were performed by varying vaccination coverage at age 16 years.
Results
The existing schedule and coverage of MenACWY and MenB vaccines (total 4 doses) could potentially avert 165 IMD cases over 10 years versus no vaccination. Assuming similar MenABCWY and MenACWY vaccination coverage rates at age 16 years, replacing 1 or more MenACWY and/or MenB doses with MenABCWY could avert more cases, ranging from 189 to 256. The most beneficial MenABCWY vaccine schedule was 2 doses at age 11 years and 1 dose at age 16 years.
Conclusions
Replacing one or more MenACWY/MenB vaccine doses with MenABCWY could reduce IMD caused by all 5 meningococcal serogroups among the US adolescent population, while also reducing the number of injections required.
1. Introduction
Invasive meningococcal disease (IMD) caused by Neisseria meningitidis is an uncommon, rapidly progressing, and potentially deadly infection with the highest incidence observed in infant and adolescent age groups [Citation1–3]. Serogroups A, B, C, W, and Y account for 94% of disease globally [Citation1,Citation4]; in the United States, most disease is caused by serogroups B, C, and Y [Citation3]. According to 2018 surveillance data from the United States, the incidence of IMD was 0.10 cases per 100,000 population [Citation3]. Serogroup B was predominant among US adolescents and young adults (62% of cases among 16- to 23-year-olds) and also across all age groups (36% of cases) [Citation3].
The US Advisory Committee on Immunization Practices (ACIP) currently recommends 2 types of meningococcal vaccines to help protect healthy adolescents against IMD. The quadrivalent meningococcal serogroups A, C, W, and Y (MenACWY) vaccine is routinely recommended as a primary dose at age 11 to 12 years and a booster dose at age 16 years [Citation5]. Meningococcal serogroup B (MenB) vaccination is recommended for adolescents and young adults aged 16 to 23 years (16–18 years preferred) based on shared clinical decision-making between the patient and healthcare provider [Citation6–8]. In 2018, the estimated MenACWY vaccination coverage for adolescents aged 13 to 17 years was 86.6% for ≥1 dose and 50.8% for ≥2 doses [Citation9]. In contrast, only 17.2% of 17-year-olds received ≥1 dose of a MenB vaccine, and fewer than 50% of these individuals completed the multidose vaccination series [Citation9,Citation10]. These data suggest that many adolescents in the United States are not fully protected against meningococcal disease.
A single vaccine that can help protect against meningococcal disease caused by all 5 serogroups (i.e., a MenABCWY pentavalent vaccine) instead of 2 separate MenB and MenACWY vaccines with different vaccination schedules could simplify immunization, reduce the number of injections required [Citation5–7], and potentially improve vaccination coverage [Citation9,Citation11,Citation12]. We developed a model to evaluate the public health impact of assorted meningococcal immunization programs using a pentavalent MenABCWY vaccine.
2. Methods
2.1. Model description
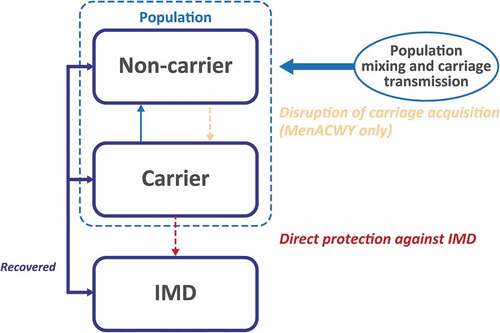
A population-based dynamic model was developed to estimate the expected number of IMD cases averted over a 10-year period in the United States (). The model structure is similar to what has been previously described in full elsewhere [Citation13]. The population was stratified into 101 single-year age bands, and individuals in each age band transitioned to the next age band in the following year. Meningococcal carriage was the principal source of infectious disease transmission and was the primary consideration in the model calculations [Citation13,Citation14]. Meningococcal carriage and transmission were modeled by stratifying the population into 10 mutually exclusive age groups (0–5 months, 6–12 months, 1 year, 2–4 years, 5–9 years, 10–14 years, 15–19 years, 20–24 years, 25–59 years, and ≥60 years) [Citation13]. Each age group was characterized by a proportion of individuals who were carriers of meningococcal serogroups A, B, C, W, and Y and had age-specific probabilities of developing IMD and transmitting the bacteria within their age group or to other age groups [Citation13,Citation15]. The proportion of meningococcal carriers in each of the 10 age groups for each year was calculated based on (1) carriage prevalence in the prior year; (2) bacterial transmission and mixing patterns within and among the age groups; (3) number of vaccinated individuals (vaccination coverage); and (4) vaccine efficacy against carriage acquisition. During each year of the 10-year time horizon within the model, proportions of individuals in targeted age groups were estimated to receive MenACWY, MenB, and/or MenABCWY vaccines under 4 different scheduling scenarios. For all vaccination scenarios, the model estimated that individuals who developed IMD either recovered with or without complications, or died [Citation13].
2.2. Model inputs
Average age-group–based IMD incidence rates for each serogroup were derived from the Centers for Disease Control and Prevention (CDC) Enhanced Meningococcal Disease Surveillance reports from 2015 to 2017 () [Citation16–18]. The MenABCWY vaccine was assumed to provide direct protection against serogroups A, B, C, W, and Y [Citation19–21].
Table 1. IMD incidence by serogroup and age.*
Vaccine efficacy assumptions against serogroup B in adolescents receiving 1 or 2 doses of the MenABCWY vaccine were based on a published clinical study for the MenB-FHbp vaccine (Trumenba®, bivalent rLP2086; Pfizer Inc, Philadelphia, PA). In this study, the percentage of subjects with serum bactericidal activity in assays using human complement (hSBA) titers ≥1:8 (standard correlate of protection is ≥1:4 [Citation22]) at 1 month postvaccination ranged from 23.8% to 67.6% and 69.1% to 100% after 1 or 2 doses of MenB-FHbp, respectively [Citation19]. Based on these data, estimates of 30% and 85% vaccine efficacy against serogroup B were assumed for adolescents receiving 1 or 2 doses of MenABCWY vaccine, respectively ().
Table 2. Vaccine efficacy assumptions.*
Vaccine efficacy assumptions against serogroups A, C, W, and Y were based on a review of published immunogenicity and efficacy data from clinical studies of the MenACWY-TT vaccine (Nimenrix®, Pfizer Ltd, Sandwich, UK) in which the percentage of subjects with serum bactericidal activity in assays using rabbit complement (rSBA) or hSBA titers ≥1:8 at 1 month postvaccination ranged from 81.9% to 97.4% after 1 dose of MenACWY-TT [Citation23–25]. Based on these data, an estimate of 95% vaccine efficacy was assumed against serogroups A, C, W, and Y ().
Indirect protection of nonvaccinated individuals due to reduction of carriage prevalence and transmission was assumed to be 0% for serogroup B and 36.2% for serogroups A, C, W, and Y, both derived from the published literature on MenB and MenACWY vaccines () [Citation26–28].
The 5-year duration of protection and a fixed 10% annual waning rate for the MenABCWY vaccine against serogroup B shown in were assumed based on considerations of (1) previous published health economic models [Citation13,Citation29–32] and (2) clinical data from a phase 3 extension study in adolescents that evaluated the persistence of the immune response elicited by the MenB-FHbp vaccine. Results from the clinical study indicated that response rates peaked after primary vaccination, declined over the subsequent 12 months, and then remained stable above baseline through 48 months [Citation33]. At 48 months after primary vaccination, 18.0% to 61.3% of subjects had hSBA titers greater than or equal to the lower limit of quantification (i.e., 1:16 or 1:8 depending on strain) across the 4 diverse serogroup B test strains used to assess the breadth of protection [Citation33].
For assessment of the protection of the MenABCWY vaccine against serogroups A, C, W and Y (), the 5-year duration of direct protection and 10% annual waning rate were conservative assumptions based on clinical data from studies that evaluated the persistence of the immune response elicited by MenACWY-TT in adolescents and adults aged 11 to 55 years through 10 years after primary vaccination [Citation34,Citation35]. At year 10, 70.2% to 90.7% of vaccinated subjects had rSBA titers ≥1:8 across serogroups A, C, W and Y compared with 99.7% to 100% at 1 month postvaccination [Citation34–36]. Currently, no data are available regarding the duration of indirect protection and waning rates for licensed MenACWY vaccines; the model therefore assumed waning rates for MenACWY were equal to MenABCWY.
2.3. Vaccination scenarios and sensitivity analyses
The vaccination scenarios were built based on the existing adolescent meningococcal vaccination platform (i.e., at ages 11 and 16 years) in the United States [Citation5–8]. Four primary vaccination schedules were examined and compared with the current schedule: (1) 1 dose of MenACWY vaccine at age 11 years and 2 doses of MenABCWY vaccine at age 16 years; (2) 1 dose of MenABCWY vaccine at age 11 years and 2 doses of MenABCWY vaccine at age 16 years; (3) 2 doses of MenABCWY vaccine at age 16 years only; and (4) 2 doses of MenABCWY vaccine at age 11 years and 1 dose at age 16 years.
The assumptions of vaccination coverage for each primary schedule were taken from observed adolescent vaccination coverage, vaccination age, and number of doses required as reported in the 2018 National Immunization Survey-Teen (NIS-Teen) [Citation9]. In line with the trends observed in the NIS-Teen survey, vaccination coverage among adolescents aged 11 years was assumed to be higher than at age 16 years, and the compliance (i.e., completion of the recommended dosing series) with a 2-dose series at age 11 years was assumed to be higher than compliance with a 2-dose series at age 16 years [Citation9].
For the base case analysis, vaccination coverage for the first dose at age 11 years was assumed to be the same as the overall coverage of the primary dose of MenACWY vaccine reported in 2018 (86.6%), and vaccination coverage for the first dose at age 16 years was assumed to be the same as the coverage of the MenACWY booster dose (50.8%) [Citation9]. It was assumed based on these data that 80% of adolescents aged 11 years who received the first dose of MenABCWY would complete the 2-dose series, whereas compliance with a 2-dose series at age 16 years was assumed to be 50% based on available information on MenB vaccine series completion [Citation10]. Sensitivity analyses were performed for each of the 4 meningococcal vaccine administration schedules using levels of adolescent vaccine coverage at age 16 years reported in 2018. The highest coverage assumed was the same as that for ≥1 dose of human papillomavirus (HPV) vaccine (68.1%) at age 11–12 years, and the lowest coverage assumed was the same as that for ≥1 dose of MenB vaccine (17.2%) at age 16 years [Citation9]. After considering the primary dosing schedules and vaccination coverage estimates, a total of 13 different scenarios were assessed.
3. Results
With the current vaccination schedule and reported vaccination coverage in 2018 (MenACWY, 86.6% at age 11 years and 50.8% at age 16 years; MenB, 17.2% at age 16 years [Citation9]), vaccination with 2 doses each of MenACWY and MenB vaccines, for a total of 4 injections between 11 and 16 years of age, could potentially avert 165 cases of IMD over the next 10 years compared with no meningococcal vaccination (, current scenario). Under this scenario, 19 serogroup B cases (11.5% of the total preventable IMD cases) are estimated to be prevented over the next 10 years. Replacing either MenACWY and/or MenB vaccines with a pentavalent MenABCWY vaccine would eliminate 1 or 2 injections, depending on the vaccination schedule, and potentially avert a higher number of IMD cases (, scenarios 1, 2, 4, 5, 7, 8, 10, and 11). This is assuming that MenABCWY vaccination coverage at 16 years of age remains similar to 2018 MenACWY vaccination coverage (50.8%; , scenarios 1, 4, 7, and 10) or perhaps rises to the slightly higher HPV vaccination coverage observed at age 11 to 12 years (68.1%; , scenarios 2, 5, 8, and 11) [Citation9].
Figure 2. Estimated number of IMD cases averted over 10 years in the United States under various vaccine administration strategies.*
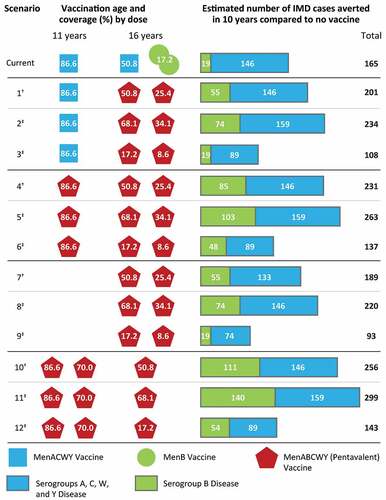
However, assuming MenABCWY vaccination coverage at age 16 years is the same as for the current 2-dose MenB vaccination schedule at age 16 years (17.2%) [Citation9], a MenABCWY regimen of 1 dose at age 11 years and 2 doses at age 16 years (i.e., similar to the current schedule) would prevent fewer IMD cases (n = 137) compared with the current vaccination schedule (, scenario 6). These results are mainly driven by the assumption of lower MenACWY vaccination coverage than is currently reported at age 16 years [Citation9], leading to a lower estimated number of serogroups A, C, W, and Y cases averted (n = 89; 65.0%) compared with the current schedule.
3.1. Base case vaccination coverage assumptions
In all base case vaccination scenarios (, scenarios 1, 4, 7, and 10), assuming MenABCWY vaccination coverage is the same as current coverage for the MenACWY vaccine at age 11 years (86.6%) or age 16 years (50.8%), replacing either the MenACWY and/or MenB vaccine with a pentavalent MenABCWY vaccine would avert a greater number of IMD cases than the current schedule (range, 189–256 IMD cases averted, depending on schedule). The higher number of total IMD cases averted compared with the current vaccination schedule is mainly driven by the greater number of serogroup B cases prevented (range, 55–111 serogroup B cases). Among all base case vaccination scenarios assessed, disease prevention would be maximized by administering 2 doses of MenABCWY vaccine at age 11 years and 1 dose at age 16 years (, scenario 10; 256 cases averted [111 serogroup B; 146 serogroups A, C, W, and Y]).
3.2. Sensitivity analysis based on alternative vaccination coverages
If MenABCWY vaccination coverage at age 16 years rises to the levels observed for the HPV vaccine at 11 to 12 years of age (68.1%) [Citation9], the greatest impact on meningococcal disease prevention would be provided by 2 doses of MenABCWY vaccine at age 11 and 1 dose at age 16 (, scenario 11; 299 total cases averted). This schedule would also prevent the greatest number and percentage of serogroup B cases out of the total preventable cases over a 10-year period (140 serogroup B cases averted [46.8%]). The next best regimen was 1 dose of MenABCWY vaccine at age 11 years and 2 doses of MenABCWY vaccine at age 16 years (, scenario 5; 263 cases averted; 103 serogroup B; 159 serogroups A, C, W, and Y). Additionally, a similar number of cases would be averted with or without including the currently recommended MenACWY dose at age 11 years (, scenarios 2 [234 cases averted; 74 serogroup B; 159 serogroups A, C, W, and Y] and 8 [220 cases averted; 74 serogroup B; 146 serogroups A, C, W, and Y]). In contrast, regimens where MenABCWY vaccination coverage was assumed to be the same as for the current 2-dose MenB vaccination schedule at age 16 years (17.2% for ≥1 dose) [Citation9] led to far fewer estimated IMD cases prevented, mainly due to fewer serogroup B cases averted (, scenarios 3, 6, 9, and 12). Under this vaccination coverage assumption, the lowest number of total IMD cases prevented would be through a regimen of 2 MenABCWY vaccines at age 16 years (, scenario 9, 93 total cases averted [19 serogroup B; 74 serogroups A, C, W, and Y]).
4. Discussion
This is the first study to our knowledge that models the impact of a pentavalent MenABCWY vaccine to protect against meningococcal disease caused by the 5 most prevalent disease-causing serogroups (i.e., serogroups A, B, C, W, and Y) in the context of the US adolescent meningococcal immunization platform. Globally, various monovalent, bivalent, or quadrivalent meningococcal vaccine formulations that target different combinations of these 5 serogroups are used to help protect against meningococcal disease [Citation37,Citation38]. In several countries, ongoing surveillance efforts have detected changes in circulating disease-causing meningococcal serogroups [Citation39–40]; these epidemiological shifts prompted changes to some national vaccination strategies to include MenACWY vaccines for comprehensive protection against IMD [Citation41–46]. Deploying a MenABCWY vaccine would potentially protect against the 94% of IMD cases worldwide estimated to be caused by these 5 serogroups [Citation4].
In the United States, MenACWY vaccination for adolescents has been recommended since 2005 [Citation47], and MenB vaccination recommendations were put into effect in 2015 [Citation6]. A single pentavalent MenABCWY vaccine could simplify immunization schedules by eliminating the need for multiple injections using 2 different vaccines at different ages [Citation5,Citation7,Citation8], potentially improving vaccination coverage [Citation9,Citation10] and enhancing protection against most prevalent disease-causing serogroups [Citation4]. Based on the current schedule and vaccination coverage for MenACWY and MenB vaccines in the United States, our model estimates that vaccination with both MenACWY and MenB vaccines could potentially avert 165 cases of IMD over 10 years compared with no vaccination. Assuming MenABCWY vaccination coverage is similar to current MenACWY vaccination coverage noted by the CDC NIS-Teen survey from 2018 [Citation9], our model estimates that replacing one or more MenACWY or MenB vaccine doses with a MenABCWY vaccine would lead to as many as 256 IMD cases averted among US adolescents while simultaneously reducing the recommended number of vaccine injections. In fact, most of the scenarios examined in this study demonstrate added benefit of a single MenABCWY vaccine in terms of greater number of cases averted compared with the current schedule.
Adolescent immunization delivery is challenging, in part because rates of preventative well visits – when immunizations typically occur – decline steadily after 16 years of age, and when combined with the transition from pediatricians to medical providers who are typically less involved in adolescent immunizations [Citation48], may contribute to suboptimal protection against disease in this age-group. An age-based platform for MenABCWY vaccination would support and catalyze adolescent immunization by enabling vaccine administration at ages where adolescents are more likely to receive and comply with multidose regimens and reduce the number of injections and visits. Importantly, immunization of adolescents with MenACWY conjugate vaccines [Citation21] and recombinant protein MenB vaccines [Citation33,Citation49,Citation50] both elicit protective immune responses following primary vaccination and robust responses following a booster dose. Together, these data not only support the existing US adolescent MenACWY and MenB immunization platform [Citation5–8], but also lend support to a flexible MenABCWY vaccination schedule where adolescents can start the vaccination series anywhere between the ages of 11 and 16 years and maintain protection throughout the period of highest risk.
Studies have shown that socioeconomic status, education, and race also play a role in vaccination awareness, access, and utilization, and series-completion rates [Citation51–56]. As such, a MenABCWY vaccine could help reduce these disparities by simplifying meningococcal vaccination recommendations, thereby reducing vaccine access issues, and eliminating confusion surrounding existing MenB and MenACWY vaccine recommendations [Citation54,Citation57,Citation58]. Moreover, combination vaccines in general have been shown to improve vaccination coverage among a variety of age groups [Citation9,Citation11,Citation12].
Modeling studies can shape our understanding and guide policy decisions relating to vaccine-preventable diseases but also have inherent limitations. A potential limitation of this analysis is that assumptions for MenABCWY vaccination coverage rely on historical data for individual MenB and MenACWY vaccines [Citation9,Citation10,Citation13,Citation19–21,Citation23,Citation26,Citation28–36,Citation59]. There are some hSBA data showing that sera from MenB-vaccinated adolescents have bactericidal activity against non-serogroup B meningococcal strains [Citation60]; however, the majority of the benefit from a MenABCWY vaccine would likely stem from the highly efficacious protection provided by the MenACWY conjugate component [Citation61–63]. Definitive estimates of vaccine efficacy await clinical trials and real-world data.
5. Conclusions
Invasive meningococcal disease is a rare and potentially life-threatening disease, with adolescents and young adults being at substantial risk of infection [Citation1–3]. Results from our modeling analysis demonstrate that introduction of a pentavalent MenABCWY vaccine into the US meningococcal immunization platform would reduce the number of meningococcal disease cases, reduce complexity in vaccination schedules, and reduce the number of injections and medical visits required. This would likely help improve compliance [Citation9,Citation11,Citation12] and contribute to alleviating disparities in vaccine access and receipt. Overall, a MenABCWY vaccine could potentially simplify public health responses to individual IMD cases.
Declaration of interest
LH, AS, and PB are employees of Pfizer and may hold stock or stock options. SJS is an employee of Pharmerit – an OPEN Health Company, which is a paid consultant to Pfizer.
Declaration of financial/other relationships
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial/medical writing support was provided by Emily Stackpole, PhD, and Srividya Ramachandran, PhD, of ICON plc (North Wales, PA, USA) and was funded by Pfizer Inc.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study.
Additional information
Funding
References
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(suppl 2):S3–S11.
- Martinon-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2 suppl):S12–20.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2018 [Internet]. 2019 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf
- Purmohamad A, Abasi E, Azimi T, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;134:103571.
- Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the advisory committee on immunization practices (ACIP) [Internet]. MMWR Recomm Rep. 2013 Mar 22 [[cited 2020 Jul 2]]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm
- MacNeil JR, Rubin L, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–1176.
- Ahmed F, Centers for Disease Control and Prevention. U.S. Advisory Committee on Immunization Practices (ACIP) handbook for developing evidence-based recommendations. Version 1.2 [Internet]. 2013 November 1 [cited 2020 Jul 2]. Available from: http://www.cdc.gov/vaccines/acip/recs/GRADE/downloads/handbook.pdf.
- Advisory Committee on Immunization Practices. Summary report, June 26-27, 2019 [Internet]. 2019 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-06-508.pdf
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723.
- Advisory Committee on Immunization Practices. Summary report, February 27-28, 2019 [Internet]. 2019 February 27-28 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-02-508.pdf
- Happe LE, Lunacsek OE, Marshall GS, et al. Combination vaccine use and vaccination quality in a managed care population. Am J Manag Care. 2007;13(9):506–512.
- Kurosky SK, Davis KL, Galindo CM. Effect of combination vaccines on hepatitis B vaccine compliance in children in the United States. Pediatr Infect Dis J. 2017;36(7):e189–e96.
- Breton MC, Huang L, Snedecor SJ, et al. Cost-effectiveness of alternative strategies for vaccination of adolescents against serogroup B IMD with the MenB-FHbp vaccine in Canada. Can J Public Health. 2020;111(2):182–192.
- Balmer P, Burman C, Serra L, et al. Impact of meningococcal vaccination on carriage and disease transmission: a review of the literature. Hum Vaccin Immunother. 2018;14(5):1118–1130.
- Trotter CL, Gay NJ, Edmunds WJ. The natural history of meningococcal carriage and disease. Epidemiol Infect. 2006;134(3):556–566.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2017 [Internet]. 2017 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2015 [Internet]. 2015 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2016 [Internet]. 2016 [cited 2020 Jul 2]. Available from: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf
- Vesikari T, Ostergaard L, Diez-Domingo J, et al. Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J Pediatric Infect Dis Soc. 2016;5(2):152–160.
- Bermal N, Huang LM, Dubey A, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7(2):239–247.
- Baxter R, Baine Y, Kolhe D, et al. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J. 2015;34(11):1236–1243.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–1326.
- Nimenrix (meningococcal group A, C, W-135 and Y conjugate vaccine). Summary of Product Characteristics. Pfizer Ltd: Sandwich, UK; 2019.
- Rivera L, Schwarz TF, Kim KH, et al. Immunogenicity and safety of the quadrivalent meningococcal vaccine MenACWY-TT co-administered with a combined diphtheria-tetanus-acellular pertussis vaccine versus their separate administration in adolescents and young adults: a phase III, randomized study. Vaccine. 2018;36(31):4750–4758.
- Quiambao BP, Bavdekar A, Dubey AP, et al. Antibody persistence up to 5 y after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in adolescents. Hum Vaccin Immunother. 2017;13(3):636–644.
- Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–2131.
- Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–327.
- McMillan M, Walters L, Sullivan T, et al. Impact of meningococcal B (4CMenB) vaccine on pharyngeal Neisseria meningitidis carriage density and persistence in adolescents. Clin Infect Dis. 2020. DOI:10.1093/cid/ciaa610. [Epub ahead of print].
- Christensen H, Trotter CL, Hickman M, et al. Re-evaluating cost-effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014;349:g5725–g5725.
- Hanquet G, Christensen H, Agnew E, et al. A quadrivalent vaccine against serogroup B meningococcal disease: a cost-effectiveness study [Internet]. Belgian Healthcare Knowledge Centre; 2014 [cited 2020 Jul 2]. Available from: https://kce.fgov.be/sites/default/files/atoms/files/KCE_231S_Meningococcal%20disease_Supplement_1.pdf
- Lecocq H, Parent Du Chatelet I, Taha MK, et al. Epidemiological impact and cost-effectiveness of introducing vaccination against serogroup B meningococcal disease in France. Vaccine. 2016;34(19):2240–2250.
- Boccalini S, Bechini A, Sartor G, et al. [Health Technology Assessment of meningococcal B vaccine (Trumenba®) in adolescent in Italy]. J Prev Med Hyg. 2019;60(3 suppl 2):E1–E94.
- Vesikari T, Ostergaard L, Beeeslaar J, et al. Persistence and 4-year boosting of the bactericidal response elicited by two- and three-dose schedules of MenB-FHbp: a phase 3 extension study in adolescents. Vaccine. 2019;37(12):1710–1719.
- European Medicines Agency. Nimenrix®: EPAR - product information [Internet]. 2020 March 3 [cited 2020 Jul 6]. Available from: https://www.ema.europa.eu/en/documents/variation-report/nimenrix-h-c-2226-ii-0096-epar-assessment-report-variation_en.pdf
- Borja-Tabora CFC, Peyrani P, Webber C, et al. A phase 2b/3b MenACWY-TT study of long-term antibody persistence after primary vaccination and immunogenicity and safety of a booster dose in individuals aged 11 through 55 years. BMC Infect Dis. 2020;20(1):426.
- Borja-Tabora C, Montalban C, Memish ZA, et al. Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open, randomised, controlled study. BMC Infect Dis. 2013;13:116.
- Burman C, Serra L, Nuttens C, et al. Meningococcal disease in adolescents and young adults: a review of the rationale for prevention through vaccination. Hum Vaccin Immunother. 2019;15(2):459–469.
- MENHIBRIX® (Hib-MenCY-TT). Full Prescribing Information [package insert]. Rixensart, Belgium: GlaxoSmithKline; 2013.
- Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–585.
- Stefanelli P, Miglietta A, Pezzotti P, et al. Increased incidence of invasive meningococcal disease of serogroup C/clonal complex 11, Tuscany, Italy, 2015 to 2016. Euro Surveill. 2016;21(12). DOI:10.2807/1560-7917.ES.2016.21.12.30176
- Knol MJ, Ruijs WL, Antonise-Kamp L, et al. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Euro Surveill. 2018;23(16). DOI:10.2807/1560-7917
- Villena R, Valenzuela MT, Bastias M, et al. Meningococcal invasive disease by serogroup W and use of ACWY conjugate vaccines as control strategy in Chile. Vaccine. 2019;37(46):6915–6921.
- Australian Government Department of Health. Invasive meningococcal disease national surveillance report with a focus on menW [Internet]. 2018 [cited 2020 Jul 2]. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/1Jan-31Mar2018-Consol-Invasive-Men-W.pdf
- Presa J, Findlow J, Vojicic J, et al. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8(3):307–333.
- Public Health England. Meningococcal ACWY conjugate vaccination (MenACWY) [Internet]. 2015 [cited 2020 Jul 2]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/437901/150622_ACWY_bipartite_letter.pdf
- Signorelli C, Guerra R, Siliquini R, et al. Italy’s response to vaccine hesitancy: an innovative and cost effective National Immunization Plan based on scientific evidence. Vaccine. 2017;35(33):4057–4059.
- Centers for Disease Control and Prevention. Revised recommendations of the advisory committee on immunization practices to vaccinate all persons aged 11–18 years with meningococcal conjugate vaccine. MMWR Wkly. 2007;56(31):794–795.
- Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18(2s):S72–S78.
- Nolan T, Santolaya ME, de Looze F, et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine. 2019;37(9):1209–1218.
- Vesikari T, Ostergaard L, Beeslaar J, et al. Antibody persistence up to 26 months after booster dosing in adolescents 4 years following a 2- and 3-dose primary vaccination series of Menb-FHbp. Paper presented at: European Society for Paediatric Infectious Diseases; 2019 May 6-11; Llubjiana, Slovenia.
- Gallagher KE, Kadokura E, Eckert LO, et al. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health. 2016;16:172.
- Khan F, Swerdlow DL, York L, et al. 2455. Is category B working? Uptake patterns of meningococcal group B vaccine among US adolescents and young adults. Open Forum Infect Dis. 2018 Nov;5(Suppl 1):S735.
- Watkins E, Feemster K. 2460. Factors associated with uptake of meningococcus B vaccination after an ACIP category B recommendation. Open Forum Infect Dis. 2018;5(suppl 1):S737.
- Huang L, Goren A, Lee LK, et al. Disparities in healthcare providers’ interpretations and implementations of ACIP’s meningococcal vaccine recommendations. Hum Vaccin Immunother. 2020;16(4):933–944.
- Basta NE, Becker AB, Li Q, et al. Parental awareness of meningococcal B vaccines and willingness to vaccinate their teens. Vaccine. 2019;37(4):670–676.
- Srivastava A, Dempsey A, Galitsky A, et al. Parental awareness and utilization of meningococcal serogroup B vaccines in the United States. BMC Public Health. 2020;20(1):1109.
- Kempe A, Allison MA, MacNeil JR, et al. Adoption of serogroup B meningococcal vaccine recommendations. Pediatrics. 2018;142(3):e20180344.
- Kempe A, Allison MA, MacNeil JR, et al. Knowledge and attitudes regarding category B ACIP recommendations among primary care providers for children. Acad Pediatr. 2018;18(7):763–768.
- Marshall HS, Richmond PC, Nissen MD, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine. 2013;31(12):1569–1575.
- Harris SL, Tan C, Andrew L, et al. The bivalent factor H binding protein meningococcal serogroup B vaccine elicits bactericidal antibodies against representative non-serogroup B meningococci. Vaccine. 2018;36(45):6867–6874.
- Knol M, Hahne S, Ruijis W, et al. Invasive meningococcal serogroup W disease in the Netherlands after a MenACWY vaccination campaign. Poster presented at: European Society for Paediatric Infectious Diseases 2020 Oct 26-29; Virtual meeting
- Mbaeyi S, Pondo T, Blain A, et al. Incidence of meningococcal disease before and after implementation of quadrivalent meningococcal conjugate vaccine in the United States. JAMA Pediatr. 2020;174(9):843–851.
- Cohn AC, MacNeil JR, Harrison LH, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193.