ABSTRACT
Accumulating studies on COVID-19 patients report high incidences of thrombotic complications, but guidance on the best diagnostic approach for suspected pulmonary embolism (PE) in COVID-19 patients is lacking. Diagnosing PE in these patients is challenging as signs and symptoms of PE and COVID-19 show wide overlap, D-dimer levels are often elevated in the absence of thrombosis and computed tomography pulmonary angiography (CTPA) may be unfeasible in the case of severe renal impairment and/or hemodynamic instability.
This narrative review discusses available literature and guidelines on current diagnostic algorithms for suspected PE in special patient populations, in particular COVID-19. A special focus is on reviewing the literature aimed at identifying symptoms with a high suspicion for PE and on the diagnostic performance of diagnostic algorithms for suspected PE in the setting of COVID-19.
Based on available literature, the index of suspicion for PE should be high in the case of unexplained abrupt worsening of respiratory status, typical symptoms of deep-vein thrombosis and/or acute unexplained right ventricular dysfunction. Despite the lack of prospective diagnostic management studies, we propose to adhere to current diagnostic algorithms applying assessment of pretest probability and D-dimer testing as available evidence suggests that these might be considered safe. Preferably, algorithms using adjusted D-dimer thresholds are recommended as it likely improves the yield of the clinical decision rule/D-dimer combination.
1. Introduction
Venous thromboembolism (VTE), manifesting as deep-vein thrombosis (DVT) or pulmonary embolism (PE), is a well-known complication in (hospitalized) patients with acute infections [Citation1–4]. This is related to strong thrombotic risk factors including inflammation, activation of the coagulation system, immobilization, and diffuse intravascular coagulation, the latter in patients with serious disease [Citation1,Citation2]. International guidelines recommend pharmacological thromboprophylaxis in patients with infectious diseases upon hospitalization [Citation5]. However, how often respiratory tract infections led to clinically relevant thrombotic disease was hitherto not well known. This has changed dramatically since the outbreak of the new coronavirus-induced severe acute respiratory disease (COVID-19). Early studies from Wuhan (China) already reported on coagulation abnormalities and coagulopathy in hospitalized patients with COVID-19 at the beginning of 2019 [Citation6–8]. This was followed by studies from Europe and America finding alarmingly high rates of VTE in patients with severe COVID-19 infection treated at Intensive Care Units (ICU) and wards [Citation9–15]. Incidences in these studies varied, but cumulative incidences of nearly 50% were reported in ICU patients and, although lower, between 5% and 10% in COVID-19 patients hospitalized on the general wards [Citation16–18].
Diagnosing VTE and specifically PE is long recognized to be difficult as signs and symptoms of PE are nonspecific and show a wide variety. Common signs of PE comprise chest pain, shortness of breath and hemoptysis [Citation3]. Yet, PE shares signs and symptoms with other conditions, including acute coronary syndrome, dissection of the thoracic aorta, pneumothorax and respiratory tract infections [Citation3]. As a consequence, many patients are investigated for PE and referred for diagnostic imaging, with a low proportion of confirmed cases [Citation19–21]. Diagnosing PE is thus already notoriously difficult and COVID-19 challenges our usual way to deal with VTE suspicion and management even more. As guidance on the best diagnostic approach in COVID-19 patients is lacking, we will focus in this review on the diagnosis of pulmonary embolism in patients presenting with (suspected) COVID-19.
2. Diagnostic approach of PE in general
As signs and symptoms are nonspecific, a diagnosis of VTE can only be established by means of imaging, of which computed tomography pulmonary angiography (CTPA) is the current standard for PE [Citation22,Citation23]. Unfortunately, imaging tests as CTPA are associated with radiation exposure, complications related to contrast material, and are also costly and time-consuming [Citation24]. Besides, overdiagnosis due to the identification of smaller (isolated), possibly irrelevant, subsegmental emboli is an increasing challenge due to current high-sensitive scanning techniques [Citation25,Citation26]. Nevertheless, these patients are often treated with anticoagulants and thus at risk for bleeding complications. Therefore, various diagnostic algorithms have been developed, with the aim of simplifying the diagnostic management of patients with suspected PE, and to reduce the number of required imaging tests. These algorithms start with a clinical decision rule (CDR), which combines different clinical factors to yield a score. These scores estimate the pretest probability of PE, and together with D-dimer testing, decide to refer patients for imaging or not [Citation21]. Following these algorithms, imaging must follow in the case of a high pretest probability and/or abnormal D-dimer [Citation21]. These algorithms have proven to be safe and efficient in the general population as PE can be ruled out based on CDR and D-dimer testing alone (without imaging) in approximately one-third of patients, and the proportion of these patients with symptomatic VTE during 3-months follow-up (the failure rate) is less than 1% [Citation3,Citation21,Citation27].
3. Diagnostic approach of PE in special patient populations
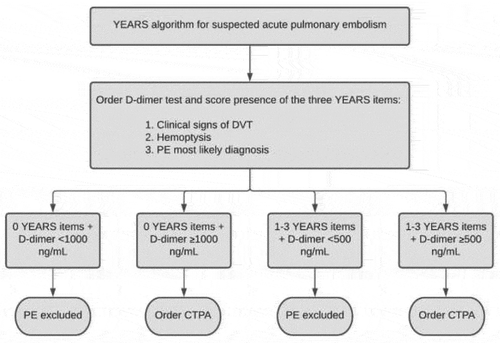
Special patient populations with a high risk for PE are for instance patients with cancer, elderly patients, patients who are hospitalized or patients with renal insufficiency [Citation3,Citation21]. As a result, the diagnosis of PE is frequently considered in these patients, yet the diagnostic approach is particularly challenging. Aging is for instance associated with an increasing prevalence of cardiac and pulmonary comorbidities, of which the symptoms can mimic the nonspecific symptoms of PE [Citation28]. It is recognized that CDRs and D-dimer tests may not be as safe and efficient in these patient subgroups. This is related to diverse variables included in the CDRs – where for instance the presence of cancer alone increases the pretest probability if using the commonly used Wells or Geneva CDRs [Citation29,Citation30] – but also D-dimer levels are often elevated in the absence of thrombosis due to low specificity of D-dimer testing. As a consequence, many patients are referred for imaging, with a low proportion of confirmed cases among those tested. To improve the yield of these diagnostic algorithms, age-adjusted D-dimer thresholds and D-dimer thresholds adapted to clinical probability were developed [Citation31–34]. The age-adjusted threshold of D-dimer is calculated as age ˣ 10 µg/L for patients above 50 years, while the clinical probability dependent threshold varies between 500 and 1000 ng/mL dependent on the pretest probability () [Citation31–33]. As such, the threshold of 1000 ng/mL is high and would only be reached in a patient of 100 years old when incorporating the age-adjusted D-dimer threshold. When applying these adjusted D-dimer thresholds, the number of patients requiring an imaging test has come down to 50–65% in the general population [Citation31–33,Citation35]. Even in these special patient populations, the adjusted D-dimer threshold doubles the number of patients that can be ruled out based on CDR and D-dimer testing alone [Citation32,Citation36]. Other important patient subgroups in which the CDR/D-dimer combination has been shown useful and safe are inpatients and patients with renal insufficiency [Citation37,Citation38].
4. Diagnostic approach of PE in COVID-19 patients
4.1 Evidence for thrombotic complications in COVID-19
COVID-19 patients show a wide spectrum of clinical presentation, from mild disease with flu-like symptoms to a critical care respiratory condition requiring ICU admission and mechanical ventilation [Citation39,Citation40]. This latter category of patients exhibits a high risk of VTE, despite pharmacological thromboprophylaxis [Citation9–13,Citation16]. This may be explained by a more intensive inflammatory state and resultant coagulation activation, combined with complete immobilization and resultant lower limb paralysis during mechanical ventilation, and the frequent use of central venous catheters [Citation40–43]. Moreover, the incidence of thrombotic complications in COVID-19 patients seems to be higher than reported in ICU patients hospitalized for other diseases including influenza [Citation14,Citation44]. Once the high incidence of thrombotic complications in hospitalized COVID-19 patients was elucidated, the threshold to suspect VTE and foremost PE among clinicians lowered. Nevertheless, COVID-19 challenges our usual way to deal with VTE suspicion and management and in particular guidance on the best diagnostic management approach of suspected PE is lacking.
4.2 Difficulties encountered in the diagnostic evaluation of suspected PE in COVID-19
The diagnostic approach of suspected PE in COVID-19 patients is hampered by the wide overlap between symptoms associated with COVID-19 and symptoms associated with pulmonary embolism [Citation3]. Patients with COVID-19 typically present to the emergency department with respiratory complaints, but chest pain and hemoptysis can also be present [Citation39]. This raises the question when to suspect PE in a COVID-19 patient. Subsequently, the yield of applying a diagnostic algorithm, including D-dimer test, in COVID-19 patients is unknown. This is especially relevant since elevated D-dimer levels are one of the most consistent findings across studies in hospitalized COVID-19 patients [Citation39,Citation40], which presumes that the D-dimer test is of less value in these patients. As a consequence of the unknown safety and efficacy of these diagnostic algorithms in patients with (suspected) COVID-19 and the presumed futility of D-dimer as a diagnostic test, clinicians may often directly order CTPA when suspecting PE. Consequently, patients are potentially overexposed to the risks of CTPA. In addition, renal function can be compromised in patients with severe COVID-19 infection which limits imaging requiring contrast material. Finally, performing CTPA is not always feasible in the critically ill instable ICU admitted COVID-19 patient.
4.3 Identifying who to suspect for concomitant PE
Despite the known high incidence of thrombotic complications in COVID-19 patients, routine investigation for PE, in the absence of clinical manifestations of PE or other supporting information, is not warranted [Citation45–47]. Guidance on the diagnostic approach of suspected PE in COVID-19 patients thus begins by correctly identifying who to investigate for concomitant acute PE. Recent studies have shed light on reasons for performing diagnostic tests for suspected VTE in COVID-19 patients [Citation48]. Importantly, an international survey among clinicians was performed, on current practice patterns about prevention, diagnosis and treatment of VTE in COVID-19 patients [Citation49]. Characteristics including abrupt worsening of respiratory status, hemodynamic instability (hypotension and/or tachycardia), unilateral limb swelling, signs of right-heart strain on electrocardiogram (ECG) or bedside echocardiogram, increasing D-dimer levels over time, IV-line malfunction or increase in ventilated to perfused lung areas (dead space) alarmed clinicians to suspect VTE in (suspected) COVID-19 patients [Citation48–50]. Indeed, higher D-dimer levels were found to be associated with thrombotic complications in a previously published systematic review and meta-analysis [Citation16]. Following the European Society of Cardiology recommendations, acute PE should be considered in patients with COVID-19 infection in the setting of unexpected respiratory worsening, new/unexplained tachycardia, a fall in blood pressure not attributable to tachyarrhythmia, hypovolemia or sepsis, (new-onset) ECG changes suggestive of PE, and signs of deep-vein thrombosis of the extremities [Citation51].
4.4 Diagnostic algorithms for suspected PE in COVID-19
Diagnostic algorithms are the cornerstone of the diagnostic management of suspected PE, also in special patient populations [Citation52]. However, the safety and efficacy of these diagnostic algorithms in (suspected) COVID-19 patients is unknown, as prospective diagnostic management studies are lacking. Recent literature on hospitalized COVID-19 patients showed that (highly) elevated D-dimer levels are common, even in the absence of thrombosis [Citation39,Citation40,Citation53–56]. Importantly, these studies used the fixed D-dimer threshold of 500 ng/mL and a considerable number – varying between 18% and 53% – of patients in these studies had D-dimer values below 1000 ng/mL [Citation53,Citation56–58]. This is crucial since adjusted D-dimer thresholds, up to 1000 ng/mL, have been validated in the general population and could still be useful in the setting of COVID-19 () [Citation31–33].
Up to now only a limited number of retrospective cohort studies studied the performance of the CDR/D-dimer combination in COVID-19 patients. One study examined the performance of the Wells score with a fixed D-dimer threshold of 500 ng/mL for diagnosing PE in (suspected) COVID-19 patients [Citation59]. 74% of the patients in this study had a Wells score indicating an unlikely probability for PE, but this had a limited role in excluding PE since only 2% of the patients had a negative D-dimer [Citation59]. As expected, this study is limited by the use of the fixed D-dimer threshold of 500 ng/mL. Another study in 71 non-ICU patients investigated the negative predictive value of a D-dimer level below 1000 ng/mL, taking into account the latest available D-dimer value prior to VTE diagnosis [Citation60]. The negative predictive value in this study was 95% and 100% for VTE and PE, respectively [Citation60]. Several studies have tried to retrospectively determine an optimal D-dimer threshold in patients with COVID-19 infection and clinically suspected VTE [Citation56,Citation61–65]. Importantly, these studies are limited by small sample size and lack of prospective validation. Moreover, these studies included patients based on performed imaging tests and identified the last available D-dimer values in these patients, which were subsequently used for the analyses. This selection bias limits the ability to study the performance of D-dimer as patients who were ruled out from having VTE based on CDR/D-dimer alone (thus without imaging) were not included.
In conclusion, the safety and efficiency of these diagnostic algorithms is not prospectively validated in the setting of COVID-19 thus far, although there are no reasons to doubt the sensitivity of the algorithm. Importantly, as COVID-19 patients who are hospitalized are at increased risk for developing PE, due to the infection itself and other risk factors such as immobility, these patients remain at risk to develop new (de novo) thrombotic events during follow-up. This can ‘artificially’ increase the failure rate, yet the same accounts for patients in whom imaging was performed, as CTPA itself was demonstrated previously to not be ‘failure rate free’ as well [Citation20,Citation66]. Although diagnostic algorithms might be considered safe, efficiency could be diminished in the setting of COVID-19, especially when applying a fixed D-dimer threshold. In conclusion, the use of such diagnostic algorithms for suspected PE is supported by the current international consensus documents on this topic [Citation51,Citation67].
4.5 Performing CTPA in COVID-19
In accordance with non-COVID-19 patients, the current golden standard for diagnosing PE in patients with COVID-19 is CTPA. Of note, relative contraindications for CTPA and iodinated contrast agents are acute kidney injury or an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2 in patients who are not undergoing maintenance dialysis. In these patients, prophylaxis with intravenous normal saline is indicated, if there are no contraindications (i.e. heart failure). Prophylaxis can also be considered in patients with an eGFR of 30–44 mL/min/1.73 m2 at the discretion of the treating physician [Citation68]. Pre-medication may be recommended in patients with previous hypersensitivity (allergic) reactions [Citation69]. Finally, the ventilation-perfusion (VQ) scan may be considered as an alternative imaging method. Performing imaging tests is not always feasible in critically ill instable ICU admitted patients. It could be an option in these instable ICU patients to perform a bilateral compression ultrasonography of the legs (CUS), as a positive CUS provides an indication for anticoagulant therapy and imaging of the chest is not necessary anymore [Citation61,Citation70]. Such a strategy is also recommended by the current consensus documents on COVID-19 and suspected PE [Citation45–47,Citation67]. Yet, the yield of this approach differs between studies and above all is reported to be low in ward admitted patients without symptoms of DVT [Citation61,Citation70]. Nevertheless, in patients admitted to the ICU, screening with CUS revealed high percentages of DVT of the legs [Citation62,Citation71–74]. Finally, empiric anticoagulation in patients in whom imaging is absolute impossible should be considered in patients with a high clinical suspicion of PE, balancing the risk of untreated PE with the risk of recurrent (fatal) PE and bleeding associated with anticoagulation [Citation67]. In the case of empiric anticoagulation, performing CTPA at a later stage when the clinical condition of the patient is improved is nevertheless recommended. If a patient presents with clinically suspected PE with hemodynamic instability, an (point-of care) echocardiogram may be performed which can show central pulmonary emboli and/or right ventricular dysfunction, although a normal echocardiogram does not exclude PE [Citation45,Citation47,Citation67].
5. Conclusion
Diagnosing PE is particularly challenging in patients with COVID-19. Despite the known higher risk of thrombotic complications in the setting of COVID-19, screening investigation for acute PE in all COVID-19 patients is not warranted. Identifying who to investigate for concomitant PE is difficult, as signs and symptoms of PE and COVID-19 show wide overlap and elevated D-dimer levels are a common finding in patients with COVID-19. The index of suspicion for PE should be high in the setting of unexpected respiratory worsening, new/unexplained tachycardia, a fall in blood pressure not attributable to tachyarrhythmia, hypovolemia or sepsis, (new-onset) ECG changes suggestive of PE, and signs of deep-vein thrombosis of the extremities. Also, increasing D-dimer levels over time could be helpful in recognizing patients at risk for PE. Imaging tests are often ordered at a low threshold, due to the unknown safety and efficiency of diagnostic algorithms for suspected PE in the setting of COVID-19. Despite the lack of prospective diagnostic management studies, available evidence suggests that diagnostic algorithms for ruling out PE might be considered safe in patients with COVID-19, although efficiency could be diminished. This advice is being supported by current international consensus documents and guidelines on this topic. As D-dimer levels are often elevated in COVID-19 patients, even in the absence of thrombosis, applying a fixed D-dimer threshold limits the ability to exclude PE without CTPA. Therefore, we propose to use algorithms with age-adjusted or pretest probability adjusted D-dimer thresholds since this likely increases the diagnostic yield of the CDR/D-dimer combination. The diagnostic standard for diagnosing PE is CTPA. Performing CTPA may be contra-indicated because of high-risk in hemodynamically instable ICU admitted patients. As confirming the diagnosis is preferred over empirical anticoagulation therapy, bilateral CUS for the presence of DVT or echocardiogram for signs of right ventricular overload may be considered. If positive this may justify the start of anticoagulation therapy.
Declaration of financial/other relationships
FK reports research grants from Bayer, Bristol–Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi–Sankyo, Actelion, the Dutch thrombosis association, the Netherlands Organisation for Health Research and Development and the Dutch Heart foundation.
MH reports receiving research grants from ZonMW, Boehringer Ingelheim, Bayer Health Care and Pfizer-Bristol-Myers Squibb. He has received consultancy and lecture fees from Pfizer-Bristol-Myers Squibb, Boehringer Ingelheim, Bayer Health Care, and Aspen.
The other authors have nothing to disclose.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Smeeth L, Cook C, Thomas S, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006 Apr 1;367(9516):1075–1079.
- Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012 May;107(5):827–837.
- Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018 May;17(4):18028.
- Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers. 2015 May;7(1):15006.
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2Suppl):e195S–e226S.
- Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Bmj. 2003 Jun 21;326(7403):1358–1362.
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr;18(4):844–847.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus–infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
- Klok FA, Kruip M, Njm VDM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–147.
- Klok FA, Kruip M, Njm VDM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020 Jul;191:148–150.
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 Jun;46(6):1089–1098.
- Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020 Jul;191:9–14.
- Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020 Jul 14;142(2):184–186.
- Stals MAM, Grootenboers MJH, Van Guldener C, et al. Risk of thrombotic complications in influenza versus COVID-19 hospitalized patients. Res Pract Thromb Haemost. 2021. Feb;5(3): 412-420.
- Kaptein FHJ, Stals MAM, Grootenboers M; Dutch COVID & Thrombosis Coalition. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb Res. 2021;199:143–148.
- Nopp S, Moik F, Jilma B, et al. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020 Sep 25;4(7):1178–1191.
- Di Minno A, Ambrosino P, Calcaterra I, et al. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin Thromb Hemost. 2020 Oct;46(7):763-771.
- Roncon L, Zuin M, Barco S, et al. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020 Dec;82:29–37.
- Le Gal G, Bounameaux H. Diagnosing pulmonary embolism: running after the decreasing prevalence of cases among suspected patients. J Thromb Haemost. 2004 Aug;2(8):1244–1246.
- Dronkers CEA, Van Der Hulle T, Le Gal G, et al. Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: communication from the SSC of the ISTH. J Thromb Haemost. 2017 May;15(5):1040–1043.
- Van Es N, Van Der Hulle T, Van Es J, et al. Wells rule and d-dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016 Aug 16;165(4):253–261.
- Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013 Mar;11(3):412–422.
- Huisman MV, Klok FA. How I diagnose acute pulmonary embolism. Blood. 2013 May 30;121(22):4443–4448.
- Kooiman J, Klok FA, Mos IC, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010 Feb;8(2):409–411.
- Carrier M, Klok FA. Symptomatic subsegmental pulmonary embolism: to treat or not to treat? Hematol Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):237–241.
- Den Exter PL, Van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013 Aug 15;122(7):1144–1149. quiz 1329.
- Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010 Apr;125(4):e123–7.
- Righini M, Le Gal G, Perrier A, et al. The challenge of diagnosing pulmonary embolism in elderly patients: influence of age on commonly used diagnostic tests and strategies. J Am Geriatr Soc. 2005 Jun;53(6):1039–1045.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001 Jul 17;135(2):98–107.
- Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006 Feb 7;144(3):165–171.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. Jama. 2014 Mar 19;311(11):1117–1124.
- Van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390(10091):289–297.
- Kearon C, De Wit K, Parpia S, et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med. 2019 Nov 28;381(22):2125–2134.
- Van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019 Mar 21;380(12):1139–1149.
- Douma RA, Le Gal G, Söhne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475.
- Wilts IT, Le Gal G, Den Exter PL, et al. Performance of the age-adjusted cut-off for D-dimer in patients with cancer and suspected pulmonary embolism. Thromb Res. 2017 Apr;152:49–51.
- Van der Hulle T, Den Exter PL, Mos IC, et al. Optimization of the diagnostic management of clinically suspected pulmonary embolism in hospitalized patients. Br J Haematol. 2014 Dec;167(5):681–686.
- Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med. 2009 Nov;122(11):1050–1053.
- Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062.
- Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015 Aug 18;19(1):287.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Cannegieter SC, Klok FACOVID-19. associated coagulopathy and thromboembolic disease: commentary on an interim expert guidance. Res Pract Thromb Haemost. 2020 May;4(4):439–445.
- Smilowitz NR, Subashchandran V, Yuriditsky E, et al. Thrombosis in hospitalized patients with viral respiratory infections versus COVID-19. Am Heart J. 2020 Nov 10;231:93–95.
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–2973.
- Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020 Sep;158(3):1143–1163.
- Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 Aug;18(8):1859–1865.
- Koleilat I, Galen B, Choinski K, et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2021;9(1):36-46.
- Rosovsky RP, Sanfilippo KM, Wang TF, et al. Anticoagulation practice patterns in COVID-19: a global survey. Res Pract Thromb Haemost. 2020 Jul 9;4(6):969–983.
- Garcia-Olivé I, Sintes H, Radua J, et al. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. 2020 Aug;169:106023.
- The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. [ cited 2020 jun 10]. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESCCOVID-19-Guidance
- Stals MAM, Klok FA, Huisman MV. Diagnostic management of acute pulmonary embolism in special populations. Expert Rev Respir Med. 2020 Jul;14(7):729–736.
- Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020 Jul 23;136(4):489–500.
- Gervaise A, Bouzad C, Peroux E, et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020;9:1–8.
- Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 Jun;18(6):1421–1424.
- Choi JJ, Wehmeyer GT, Li HA, et al. D-dimer cut-off points and risk of venous thromboembolism in adult hospitalized patients with COVID-19. Thromb Res. 2020 Sep 17;196:318–321.
- Stoneham SM, Milne KM, Nuttall E, et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med (Lond). 2020 Jul;20(4):e76–e81.
- Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med. 2020 Sep;48(9):e805–e808.
- Whyte MB, Kelly PA, Gonzalez E, et al. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020 Jul 10;195:95–99.
- Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020 Jul;50(1):211–216.
- Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020 Aug;192:23–26.
- Gibson CJ, Alqunaibit D, Smith KE, et al. Probative value of the D-dimer assay for diagnosis of deep venous thrombosis in the coronavirus disease 2019 syndrome. Crit Care Med. 2020 Sep 15;48:e1322–e1326.
- Cho ES, McClelland PH, Cheng O, et al. Utility of d-dimer for diagnosis of deep vein thrombosis in coronavirus disease-19 infection. J Vasc Surg Venous Lymphat Disord. 2020 Jul 30;9:47–53.
- Ventura-Díaz S, Quintana-Pérez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: a retrospective study. Emerg Radiol. 2020;6:1–11.
- Zermatten MG, Pantet O, Gomez F, et al. Utility of D-dimers and intermediate-dose prophylaxis for venous thromboembolism in critically ill patients with COVID-19. Thromb Res. 2020 Aug 21;196:222–226.
- Van der Hulle T, Van Es N, Den Exter PL, et al. Is a normal computed tomography pulmonary angiography safe to rule out acute pulmonary embolism in patients with a likely clinical probability? A patient-level meta-analysis. Thromb Haemost. 2017 Jul 26;117(8):1622–1629.
- Lee AH, deSancho MT, Pai M, et al. COVID-19 and pulmonary embolism: frequently asked questions [Webpage]. American Society of Hematology2020 [cited2020 Nov 30; updated 2020 11-12-2020].
- Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Kidney Medi. 2020;2(1):85–93.
- Torres M, Trautmann A, Hm I, et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. 2020. doi:10.1111/all.14656.
- Longchamp A, Longchamp J, Manzocchi-Besson S, et al. Venous thromboembolism in critically Ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020 Jul;4(5):842–847.
- Voicu S, Bonnin P, Stépanian A, et al. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020 Jul 28;76(4):480–482.
- Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020 Jul 14;142(2):181–183.
- Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 Jul;18(7):1743–1746.
- Nahum J, Morichau-Beauchant T, Daviaud F, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Network Open. 2020;3(5):e2010478–e2010478.