ABSTRACT
Treatment with icosapent ethyl 4 g/day, a highly purified and stable ethyl ester of eicosapentaenoic acid (EPA), demonstrated a significant reduction in atherosclerotic cardiovascular disease (ASCVD) events and death in REDUCE-IT. However, analyses of REDUCE-IT and meta-analyses have suggested that this clinical benefit is greater than can be achieved by triglyceride reduction alone. EPA therefore may have additional pleiotropic effects, including anti-inflammatory and anti-aggregatory mechanisms. EPA competes with arachidonic acid for cyclooxygenase and lipoxygenase, producing anti-inflammatory and anti-aggregatory metabolites rather than the more deleterious metabolites associated with arachidonic acid. Changing the EPA:arachidonic acid ratio may shift metabolic status from pro-inflammatory/pro-aggregatory to anti-inflammatory/anti-aggregatory. EPA also has antioxidant effects and increases synthesis of nitric oxide. Incorporation of EPA into phospholipid bilayers influences membrane structure and may help to prevent cardiac arrhythmias. Clinically, this may translate into improved vascular health, including regression of atherosclerotic plaque. Overall, EPA has a range of pleiotropic effects that contribute to a reduction in ASCVD.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide [Citation1] and is responsible for more than 800,000 deaths in the United States annually [Citation2]. This high rate of mortality occurs despite the broad use of statins to reduce atherogenic low-density lipoprotein cholesterol (LDL-C); statins have also had a significant impact on the incidence of ASCVD and cardiovascular-related death [Citation3,Citation4]. There is therefore a substantial persistent risk of ASCVD despite effective cholesterol-management strategies.
One risk factor for ASCVD, independent of LDL-C, is hypertriglyceridemia [Citation5–10]. Despite this, triglyceride-lowering therapies such as niacin and fibrates have failed to show any reduction in the risk of ASCVD events [Citation11–14]. However, results from two large CV outcome trials featuring high-purity formulations of eicosapentaenoic acid (EPA) have demonstrated a reduction in the risk of ASCVD events in patients with elevated triglyceride levels [Citation15,Citation16]. In the Japanese JELIS study, after 4.6 years of follow-up, 1.8 g/day of EPA plus statins in patients with LDL-C > 170 mg/dL was associated with significantly fewer major coronary events than patients treated with statins alone (P = 0.011; this was not a placebo-controlled study) [Citation15]. REDUCE-IT evaluated the use of icosapent ethyl, a highly purified, stable EPA ethyl ester, in patients on statin therapy with established CVD and/or diabetes plus at least one additional CV risk factor, compared to a mineral oil placebo [Citation16]. After an average of 4.9 years follow-up, 4 g/day icosapent ethyl treatment resulted in a 25% relative reduction in the composite primary endpoint of CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina (P < 0.001), as well as a 20% decrease in CV death (P = 0.03), 31% reduction in myocardial infarction (P < 0.001), and 28% reduction in stroke (P < 0.001) compared to statin alone. Some commentators have suggested that the positive results seen in this trial are due to the use of a mineral oil placebo, positing that mineral oil may raise levels of inflammatory markers and atherogenic lipoproteins, as well as interfering with drug absorption [Citation17,Citation18]. However, a recent comprehensive review found mineral oil to be biologically inert except when taken in high doses as a lubricant laxative, with no consistent evidence of any impact on lipid levels or inflammatory markers in the trials reviewed [Citation19]. EPA’s metabolite, docosapentaenoic acid (DPA), has been shown to be associated with lower C-reactive protein levels [Citation20,Citation21] and inhibit oxidation of small, dense LDL, but to a lesser extent than EPA [Citation22]. These data suggest DPA may exert beneficial anti-inflammatory and lipid effects. However, no DPA-related analyses were performed during JELIS or REDUCE-IT to determine the role of DPA in the CV outcomes observed; thus, further studies are needed to determine the effects of DPA on CV risk. Prior to REDUCE-IT, icosapent ethyl was FDA-approved as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia [Citation23]. Three global regulatory agencies have extensively reviewed the REDUCE-IT dataset and provided approval for the use of icosapent ethyl in at-risk patients to reduce CV risk. Based on the results of the REDUCE-IT trial, icosapent ethyl was granted an expanded FDA indication as an adjunct to maximally tolerated statins to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adults with elevated triglyceride levels (≥150 mg/dL) and established CVD or diabetes mellitus and two or more additional risk factors for CVD [Citation23]; the Canadian label also includes prevention of CV death [Citation24]. More recently, the European Medicines Agency approved icosapent ethyl for CV risk reduction and specifically stated in the approved label that changes in biomarkers such as triglycerides and LDL-C in the REDUCE-IT trial showed little to no correlation with CV effect [Citation25,Citation26]. By contrast, the recent STRENGTH trial failed to show any reduction in CVD in patients treated with a mixed EPA plus docosahexaenoic acid (DHA) compound, and was halted early due to a low probability of demonstrating clinical benefit and an increased risk of atrial fibrillation in the treatment arm [Citation27]. The populations in STRENGTH and REDUCE-IT included high-risk hypertriglyceridemic patients, with comparable triglyceride levels at baseline, baseline statin use, and entry criteria. However, a greater proportion of patients in REDUCE-IT had established ASCVD as compared to STRENGTH. One possible reason for the difference in outcomes reported in this trial compared to REDUCE-IT is the inclusion of DHA, which has differential effects on membrane stability, plaque formation, antioxidation, and lipids such as LDL-C [Citation28,Citation29].
The absolute triglyceride level reduction from baseline to the last clinic visit was 9% in JELIS and approximately 22% in REDUCE-IT [Citation15,Citation16]. Notably, in REDUCE-IT, the lowered risk of first and total ASCVD events occurred irrespective of baseline and achieved triglyceride levels. By contrast, prespecified analyses of baseline and post-baseline EPA levels in REDUCE-IT (REDUCE-IT EPA) revealed that tertiles of EPA levels correlated strongly with the primary endpoint, key secondary endpoint (CV death, myocardial infarction, or stroke), and the incidence of CV death, myocardial infarction, stroke, coronary revascularization, unstable angina, sudden cardiac death, cardiac arrest, new heart failure, and all-cause mortality [Citation30]. Therefore, changes in EPA levels, not levels of triglyceride and other biomarkers, accounted for the overwhelming majority of the relative risk reduction observed for the primary and secondary endpoints [Citation30]. JELIS was the first CV outcome trial utilizing purified EPA to demonstrate a positive relationship of on-treatment plasma EPA concentration and reduction of coronary event rates [Citation31]. The mean baseline plasma EPA levels in the EPA and control groups were, respectively, 97 μg/mL and 93 μg/mL; the on-treatment mean plasma EPA levels were, respectively, 170 μg/mL and 95 μg/mL. A mean on-treatment plasma EPA level of ≥150 μg/mL compared to an EPA level of <150 μg/mL in this study was associated with an 18% reduction in major coronary events (hazard ratio 0.82; 95% confidence interval 0.68–0.98; P = 0.032). In REDUCE-IT, the median serum EPA level at baseline was 26.1 μg/mL in both the icosapent ethyl and placebo groups [Citation16]. After 1 year of treatment, the median serum EPA level increased to 144 μg/mL in the icosapent ethyl group and decreased to 23.3 μg/mL in the placebo group. However, in STRENGTH, the plasma EPA level increased from 21.0 μg/mL to only 89.6 μg/mL in the EPA+DHA group at 1 year; this relatively modest response may have been a contributing factor to the negative outcome of the trial [Citation27]. Additional analyses (REDUCE-IT REVASC) revealed that icosapent ethyl reduced first and total revascularization events by 34% and 36%, respectively, making it the first non-LDL-C intervention in a major randomized trial to result in fewer coronary artery bypass grafting surgeries among statin-controlled patients [Citation32]. Taken together, this suggests that at least some of the ASCVD risk reduction in REDUCE-IT occurred independently of triglyceride-lowering effects.
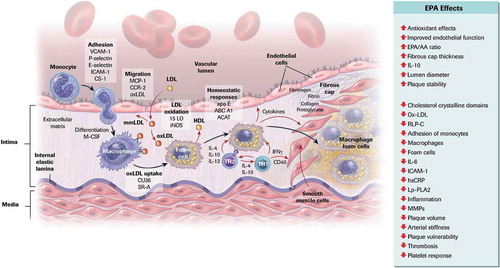
A recent meta-analysis indicated that ASCVD risk reduction associated with triglyceride lowering was relatively modest, and not statistically significant after the exclusion of REDUCE-IT [Citation33]. The authors suggested, therefore, that the benefits of high-dose EPA appear to exceed its lipid-lowering effects, further substantiating that EPA appears to have a myriad of pleiotropic effects [Citation34–36]. The objective of this review is to explore the possible pleiotropic mechanisms of EPA that may help to mitigate atherogenesis and reduce ASCVD risk beyond triglyceride lowering alone () [Citation37].
Figure 1. Cellular and Molecular Mechanisms of Atherosclerosis and the Role of EPA
2. LIPID METABOLISM
The most well-established effects of EPA are those on lipid metabolism and triglyceride lowering; these are summarized in . Clinical studies have demonstrated that high-dose EPA is associated with reduced circulating non-high-density lipoprotein cholesterol (non-HDL-C), very-low-density lipoprotein cholesterol (VLDL-C), and triglycerides [Citation38,Citation39]. EPA also reduces apolipoprotein B, apolipoprotein CIII, and remnant-like particle cholesterol levels [Citation40]. The mechanism of EPA-mediated triglyceride lowering is believed to be through reduced hepatic production of VLDL lipoproteins, including a reduction in both particle size and number [Citation41]. Consistent with this, a recent study using radio-labeled EPA showed that EPA slowed production of triglyceride-rich lipoproteins, including VLDL [Citation42].
Table 1. Effect of EPA on Lipid Markers, Inflammatory Markers, and Atherosclerotic Plaque
There are several other putative mechanisms for reduced VLDL synthesis. One such mechanism is the induction of enhanced β-oxidation of free fatty acids in peroxisomes and mitochondria, thus reducing the amount of substrate available for triglyceride and VLDL synthesis [Citation43]. EPA has also been shown to inhibit the enzymes phosphatidic acid phosphatase/phosphohydrolase and diacylglycerol acyltransferase, involved in triglyceride synthesis [Citation43]. EPA may also reduce triglyceride levels through increased clearance of the triglyceride-rich lipoproteins VLDL and chylomicrons via lipoprotein lipase activity, likely through increased gene expression [Citation41,Citation43]. This increased activity results in a higher post-prandial clearance rate of triglyceride-rich lipoproteins. These effects, in turn, may have an effect on LDL particle size, causing a shift from the more atherogenic small dense LDL to larger less atherogenic LDL particles [Citation44,Citation45]. However, Dunbar et al. showed that the reduction in triglyceride-rich lipoproteins was independent of the accompanying decrease in cholesterol-rich lipoproteins, including LDL [Citation42]. This would suggest, therefore, that EPA-induced increases in LDL clearance occurs via a separate mechanism.
Peroxisome proliferator-activated receptor α (PPARα) is a nuclear receptor activated by fibrates, fatty acids, and eicosanoids and is expressed in monocytes, macrophages, and foam cells, and may be affected by EPA. PPARα regulates genes controlling lipid and glucose metabolism, including the reverse cholesterol transport pathway [Citation46]. Activation of PPARα also helps regulate the conversion of free cholesterol to cholesteryl esters in macrophages, possibly leading to reduced cholesterol content in plaque. EPA has been shown to increase PPARα and acyl-coenzyme A:cholesterol acyltransferase 1 expression in macrophages, and may help to prevent foam cell formation [Citation47].
3. INFLAMMATION
It is well established that inflammation is central to the CV risk associated with dyslipidemia [Citation48]. Icosapent ethyl has been shown in a number of clinical trials to reduce key markers of acute inflammation, including high-sensitivity C-reactive protein () [Citation16,Citation49].
EPA plays a critical role in regulating inflammation through its role as a competitive inhibitor to arachidonic acid for cyclooxygenase and lipoxygenase enzymes () [Citation50,Citation51]. EPA-derived lipoxygenase metabolites are anti-inflammatory and include the resolvins RvE1–RvE3 () [Citation52–58]. These metabolites play an active role in the resolution of inflammation and regulation of the inflammatory response. Maresins and protectins have also been observed in vitro in cells incubated with EPA [Citation52]. In contrast, metabolites derived from arachidonic acid include lipoxins LXA4 and LXB4, which are both pro- and anti-inflammatory, as well as pro-inflammatory leukotrienes and prostaglandins [Citation59,Citation60]. Plasma levels of these pro-inflammatory eicosanoids are reduced during treatment with EPA, demonstrating the competitive nature of their metabolism [Citation61]. Changing the ratio of EPA to arachidonic acid by supplementation with EPA can help shift metabolism from a pro-inflammatory to an anti-inflammatory state, with a lower EPA:arachidonic acid ratio being pro-inflammatory and a higher ratio being anti-inflammatory [Citation61]. The EPA:arachidonic acid ratio has also been shown to be directly associated with diabetes [Citation62]. In a double-blind randomized trial in patients with type 2 diabetes and a body mass index of 25 to 30 kg/m2, EPA treatment was associated with significant decreases in fasting plasma glucose, HbA1c, and insulin resistance [Citation63]. Adiponectin, an anti-inflammatory and insulin sensitizing protein produced in adipose tissue, is increased by EPA through PPARγ-dependent and -independent pathways [Citation64,Citation65]. While the mechanism for this effect is unclear, EPA has been demonstrated in a mouse model to have a role in controlling insulin and glucose homeostasis, which has additional important implications for metabolic diseases [Citation66].
Figure 2. Bioactive Metabolites of EPA
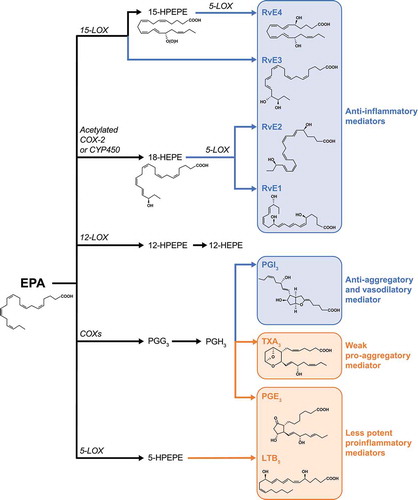
Table 2. EPA-Derived Bioactive Metabolites That Control Inflammation and Tissue Homeostasis
The production of resolvins affects cytokine-induced signal transduction and production of reactive oxygen species, which are directly responsible for activation of NFκB [Citation67,Citation68]. NFκB in turn controls expression of a number of pro-inflammatory and pro-atherogenic genes in macrophages, including those encoding for IL-1β, IL-6, IL-8, tumor necrosis factor alpha (TNFα), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, and cyclooxygenase-2. EPA has been shown to inhibit NFκB directly [Citation69,Citation70], and also indirectly through PPARγ [Citation71,Citation72]. Specifically, RvE1 has been shown to bind to the leukotriene B4 receptor, BLT1, on human polymorphonuclear leukocytes (PMNs) and to attenuate leukotriene B4-induced proinflammatory signals and PMN migration in vitro [Citation73]. RvE2 enhances phagocytosis of human macrophages and production of the anti-inflammatory cytokine IL-10 [Citation74]. Finally, RvE3 has potent inhibitory action on neutrophil chemotaxis both in vitro and in vivo [Citation75]. Studies in a mouse myocardial infarction model have further shown that EPA treatment results in a shift in the balance of macrophages from pro-inflammatory to anti-inflammatory macrophage infiltration in the left ventricle 28 days after myocardial infarction, reducing cardiac remodeling and leading to improved survival and reduced progression to heart failure [Citation76]. Chen et al. further showed that EPA prevented this cardiac remodeling by suppressing TGF-β1 [Citation77]. EPA and DHA activate cGMP/protein kinase G signaling, hence blocking profibrotic TGF-β1 signaling. In turn, EPA also reduced TGF-β1-induced nuclear translocation of phospho-Smad2 and phospho-Smad3, blocking TGF-β1-induced collagen synthesis and cardiac fibrosis.
EPA also reduces concentrations of inflammatory oxidized LDL by inhibiting the established CVD risk factor lipoprotein-associated phospholipase A2 [Citation38,Citation78]. In vitro studies in human endothelial coronary cells have further demonstrated that EPA attenuates expression of adhesion molecules vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and P-selectin induced by oxidized LDL-C and subsequent adhesion of monocytes through modulation of the protein kinase B intracellular signaling pathway [Citation79–81].
Increasing EPA concentrations, therefore, results in a reduction of pro-inflammatory compounds and inflammatory macrophages, leukocytes, and neutrophils. A possible underlying mechanism for the myriad anti-inflammatory effects of EPA is through inhibition of the leucine-rich repeat-containing protein-3 inflammasome. The inflammasome is a cytosolic protein complex central to regulating innate immunity and inflammation that includes a nucleotide-binding domain and leucine-rich repeat-containing proteins or AIM2, ASC, and caspase-1 [Citation82,Citation83]. The inflammasome promotes maturation and release of several pro-inflammatory cytokines [Citation82]. In preclinical studies using a type 2 diabetes mouse model, exposure to omega-3 fatty acids resulted in suppression of inflammation by reducing leucine-rich repeat-containing protein-3 inflammasome activation in macrophages [Citation83].
High mobility group box-1 protein, a non-histone DNA binding protein, can activate multiple inflammatory pathways, including NFκB, extracellular regulated kinase, p38, CD24, Toll-like receptor-2, Toll-like receptor-4, Toll-like receptor-9, and the advanced glycation end-product receptor [Citation84]. EPA has been shown to reduce high mobility group box-1 protein and TNFα by a PPARγ-dependent mechanism, and by a PPARγ-independent pathway inhibiting the glycation end-product and TLR9, which has shown to attenuate ischemic brain damage in ovariectomized rats [Citation85]. These anti-inflammatory effects of EPA may be contributing to the 28% (P = 0.01) relative risk reduction of fatal/nonfatal stroke in REDUCE-IT [Citation16], and the 20% (P = 0.047) reduction in secondary stroke in JELIS [Citation86].
4. Antioxidant
EPA has antioxidant properties, as well as anti-inflammatory properties, through upregulated expression and activity of the antioxidant enzymes paraoxonase 1 and 2 (PON1 and PON2), leading to reduced oxidative stress [Citation87,Citation88]. EPA has been shown to increase PON in patients with type 2 diabetes; EPA 2 g/day increased PON1 activity and levels over 8 weeks versus placebo [Citation87]. In humans PON1 inhibits oxidation of HDL-C and preserves its function; in vitro and in vivo models provide some mechanistic insight, as PON1 has been shown to decrease macrophage cholesterol synthesis and oxidative stress [Citation89–91]. In another study, after 8 weeks of treatment, EPA significantly increased gene expression of PON2 versus placebo [Citation88].
Inhibition of lipid/lipoprotein oxidation by EPA has also been demonstrated. In an in vitro study, EPA inhibited the oxidation of apolipoprotein B lipoproteins (LDL-C, small dense LDL, and VLDL-C) [Citation92]. Furthermore, in plasma samples from healthy volunteers, EPA inhibited the oxidation of HDL; however, the sustained antioxidant effects of EPA on HDL oxidation were not replicated by DHA [Citation93]. A similar study from the same group showed that EPA also inhibited oxidation of small dense LDL and model membranes, as well as blocking cholesterol crystal domain formation [Citation22].
5. Platelets and Thrombosis
The potential benefit of shifting the EPA:arachidonic acid ratio has long been recognized with regard to platelet aggregation [Citation94]. Whereas cyclooxygenase in platelets converts arachidonic acid to the pro-aggregatory thromboxane-A2, it converts EPA to the anti-aggregatory thromboxane-A3. Similarly, in the vessel wall arachidonic acid is converted to the pro-aggregatory prostacyclin PGI2 while EPA is converted to the anti-aggregatory prostacyclin PGI3 [Citation94]. Production of resolvins, notably resolvin E1, may also play a role in reducing platelet activation and thrombosis [Citation54]. RvE1 has been shown to regulate leukocyte extravasation via the cell surface adhesion molecule L-selectin, which has a role in leukocyte rolling and tethering, as well as reducing ADP and thromboxane-A2 receptor-stimulated platelet aggregation. These effects also extend to platelets, with RvE1 blocking platelet aggregation and reducing ADP-stimulated P-selectin expression and surface mobilization. This suggests that RvE1 may also block initial platelet–leukocyte interactions [Citation54,Citation95].
Combination EPA and aspirin therapy has been shown to have synergistic effects on the prevention of neointima formation due to their anti-inflammatory and anti-aggregatory effects [Citation96]. This effect appears to be due, at least in part, to inhibition of thromboxane-A2 and PGI2 synthesis by aspirin coupled with RvE1-mediated anti-inflammatory effects and polarization of anti-inflammatory M2 macrophages caused by EPA. The addition of aspirin to EPA therefore may help in tipping the scale of the EPA:arachidonic acid ratio further toward the anti-inflammatory and anti-aggregatory state.
6. Membrane Function and Stability
EPA and DHA can be incorporated into the phospholipid bilayer where they have the potential to affect cell membrane structure and function () [Citation51]; for instance, omega-3 fatty acids influence membrane organization, including raft formation and membrane fluidity [Citation97]. EPA helps to preserve membrane structure, promotes normal distribution of cholesterol, and inhibits lipid oxidation and related cholesterol crystal formation, as well as facilitating signal transduction [Citation28,Citation98,Citation99]. DHA, by contrast, increases membrane fluidity, promotes lipid domain changes, and reduces antioxidant activity due to lipid-disordering effects [Citation28,Citation98,Citation99]. Following incorporation into the phospholipid bilayer, the multiple double bonds of EPA also allow the molecule to act as an antioxidant by facilitating electron stabilization mechanisms that inhibit free radical propagation [Citation37,Citation97]. Consistent with this, EPA has been shown to attenuate LDL oxidation and glucose-induced lipid peroxidation in membranes [Citation100]. Stabilization of membrane structure by EPA may also affect endothelial function, vascular tone, cytokine expression, immunity, and recruitment and activation of inflammatory cells.
Figure 3. Proposed Molecular Mechanisms of Cardioprotection by Omega-3 Fatty Acids
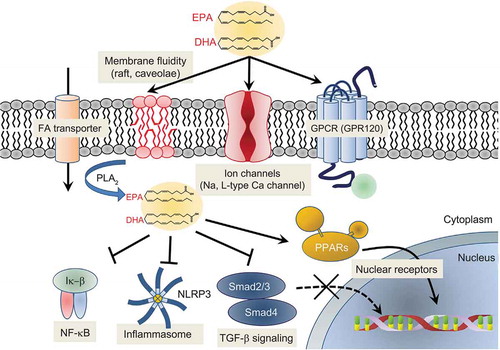
Membrane EPA may neutralize the deleterious effects of excessive cholesterol accumulation in the membrane, including effects on ion transport and signal transduction mechanisms [Citation101]. EPA inhibits the formation of cholesterol domains in membranes in the presence and absence of oxidative stress and in the presence of elevated cholesterol, indicating that it has a direct effect on membrane lipid structure under disease-like conditions [Citation102]. Studies have shown that exposure to omega-3 fatty acids results in increased sodium and potassium ion channel expression and activity in cardiomyocytes and atrial myocytes, reducing arrhythmia and risk of atrial fibrillation through reduction in membrane cholesterol content [Citation103,Citation104]. EPA and DHA have also been shown to modulate the activity of L-type calcium channels. Still, the authors concluded that this was not due to physical changes in the cell membrane as there was no difference in response between myocytes incubated for 3 to 5 days in their presence compared to acutely bathing them in culture medium; rather, they suggested that omega-3 fatty acids in the membrane may act as a local reservoir for these important bioactive molecules [Citation105]. EPA and DHA, therefore, may have anti-arrhythmic effects since arrhythmias are related to poorly functioning sodium and L-type calcium channels. In the clinic, omega-3 fatty acid supplementation has been shown to reduce the risk of ventricular arrhythmia-related events in post-myocardial infarction patients with diabetes [Citation106]. In a rat model, EPA – specifically icosapent ethyl – significantly reduced the occurrence of fatal arrhythmias following myocardial infarction, possibly due to icosapent ethyl’s ability to attenuate reactive oxygen species by activating G protein-coupled receptor 120 and increasing connexin 43 phosphorylation following infarction [Citation107].
7. Vascular Health
Clinical evidence for a role for EPA in vascular health comes from a series of studies in which EPA treatment significantly improved endothelial function in patients with hyperlipidemia, diabetes, and/or ASCVD [Citation108–111]. As already discussed, EPA appears to have numerous vascular benefits, including reduced inflammation, lower oxidative stress levels, improved endothelial function, and decreased plaque growth () [Citation112]. Another potential vascular health benefit of EPA is its effects on nitric oxide [Citation113], which acts as a vasodilator and is an established mediator of vascular health [Citation114]. Nitric oxide has a role in maintaining normal endothelial function through modulation of vascular dilator tone by decreasing endothelin-1, regulation of vascular permeability, platelet aggregation and adherence, and recruitment and adherence of immune cells circulating in the blood. Production or bioavailability of nitric oxide has been found to be decreased in patients with hypertension, hypercholesterolemia, history of smoking, diabetes, and heart failure, all of which are risk factors for atherosclerosis.
Figure 4. Lipid Interactions of EPA and Impact on Vascular Health
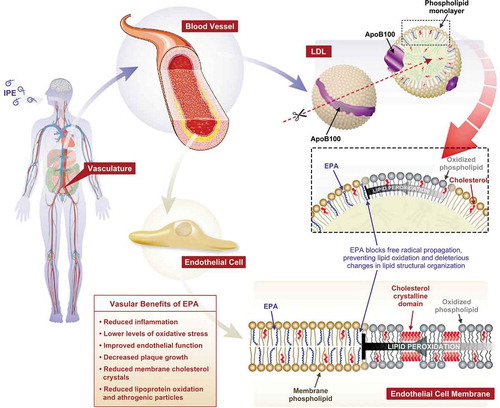
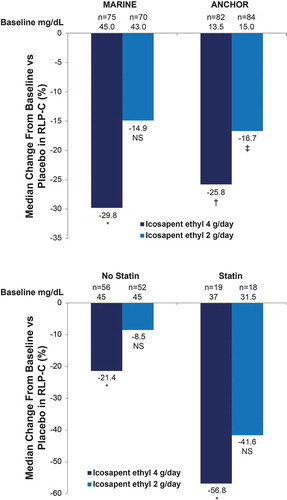
Nitric oxide is synthesized in endothelial cells from L-arginine by the enzyme nitric oxide synthase (eNOS) [Citation114]. In vitro experiments have shown that EPA induces reversal of endothelial dysfunction parameters by increasing the ratio of nitric oxide to peroxynitrite; these effects are enhanced in combination with a statin [Citation113]. The effect of EPA was independent of eNOS expression, indicating that its mechanism relates to improving the efficiency of eNOS activity. Another possible mechanism for the effects of EPA on nitric oxide is via its influence on remnant-like particle cholesterol. In the MARINE and ANCHOR studies, remnant-like particle cholesterol was decreased by 29.8% (P = 0.004) and 25.8% (P = 0.0001), respectively, following 12 weeks of icosapent ethyl 4 g/day () [Citation40]. Remnant-like particle cholesterol impairs endothelial function through direct and indirect effects on eNOS, including activation of focal adhesion kinase and downstream effects on phosphatidyl kinase/protein kinase B (PI3K/Akt), followed by eNOS inactivation due to induction of intracellular oxidative stress in endothelial cells [Citation115].
Figure 5. Icosapent Ethyl Effects on RLP-C From the MARINE and ANCHOR Studies
EPA has also been shown to have a role in the prevention of arterial calcification. Vascular calcification, thought to occur secondary to inflammation, is highly prevalent in patients with atherosclerosis, diabetes, and chronic kidney disease [Citation116]. In a preclinical model using klotho mutant mice that have increased susceptibility to development of arterial calcification, EPA intake prevented arterial calcification, likely through decreased expression of NADPH oxidase-4 [Citation117]. This effect was mediated through interaction of EPA with G protein-coupled receptor 120, which has been shown to mediate the anti-inflammatory effects of omega-3 fatty acids [Citation117,Citation118]. EPA has also been shown to reduce vascular smooth muscle dysfunction and calcification through RvE1’s role as an inhibitory ligand on ChemR23, resulting in reduced BMP-2 gene expression and ultimately reduced calcification [Citation119].
Several other mechanisms related to the possible vascular benefits of EPA have been proposed. In vitro studies have shown a potential role for EPA and DHA in improving the function of endothelial progenitor cells [Citation120], which have shown to predict CV morbidity and mortality in patients with coronary artery disease [Citation120,Citation121]. The number of these cells present has further been shown to inversely correlate with high ASCVD risk. High-risk subjects have fewer endothelial progenitor cells, and these become senescent more rapidly than in low-risk subjects [Citation122,Citation123]. Incubation of endothelial progenitor cells with EPA and DHA increases their numbers, migratory capacity, adhesive properties, and incorporation into tubules [Citation120].
In other studies, HDL-C enriched with EPA was high in PON1 activity, promoted endothelial repair, caused cholesterol efflux from macrophages, attenuated vascular cell adhesion molecule 1 expression, and promoted endothelial cell migration in patients with dyslipidemia [Citation124]. EPA has also been shown to abrogate endothelial cell apoptosis [Citation125].
8. Coronary Health and Atherosclerotic Plaque
A number of studies have provided both preclinical and clinical evidence that EPA helps to stabilize and even regress atherosclerotic plaque () [Citation37,Citation126–129]. A retrospective study of 193 patients without known coronary artery disease who underwent coronary computed tomography angiography showed that high-risk plaque was an independent risk factor of CV events, including early revascularization. This study also demonstrated that a low EPA:arachidonic acid ratio was associated with the presence of high-risk plaque [Citation130].
One critical anti-inflammatory mechanism of EPA is promoting the thickening of the fibrous cap of vulnerable plaques. In one study of 31 patients with untreated dyslipidemia and thin-cap fibroatheroma diagnosed by optical coherence tomography, concomitant use of EPA and rosuvastatin resulted in a greater degree of plaque stabilization than statin alone [Citation127]. A preclinical study using an LDL-receptor knockout mouse model attributed EPA-associated regression of atherosclerotic lesions to its anti-inflammatory effects on dendritic cells and a decrease in the number of T-lymphocytes [Citation131]. EPA treatment also results in reduced expression of pentraxin-3, a biomarker of cardiac and arterial inflammation produced by macrophages and vascular smooth muscle cells in the region of atherosclerotic plaque, causing fibrous cap thickening and stabilization [Citation126,Citation127].
Further evidence for the role of EPA in stabilizing plaque is that patients with cholesterol crystals in culprit atherosclerotic lesions have a lower EPA:arachidonic acid ratio than those without, and have worse 1-year clinical outcomes [Citation132]. EPA has also been shown to increase cholesterol efflux from HDL-C, indicating increased transport of cholesterol from peripheral tissues to the liver for biliary excretion. For example, increasing the EPA phosphatidylcholine content of reconstituted HDL-C was demonstrated to increase cholesterol efflux from HDL-C particles [Citation133]. In another in vitro study using multilamellar vesicles, EPA inhibited glucose-induced membrane cholesterol crystalline domain formation [Citation100]. EPA and DPA also inhibited oxidation of membrane cholesterol domains in this model [Citation22].
There is ample evidence from clinical intervention studies for a role for EPA in stabilizing atherosclerotic plaque (). In the CHERRY study, which enrolled patients with stable angina pectoris and acute coronary syndrome who had undergone successful percutaneous coronary intervention, intravascular ultrasound showed that treatment with EPA plus pitavastatin resulted in greater reductions in coronary plaque, including lipid plaque volume, than pitavastatin alone [Citation134]. Similar results were seen in a retrospective analysis of 121 patients with coronary artery disease who had undergone percutaneous coronary intervention; 109 patients received no EPA and 12 received EPA for at least 1 month [Citation135]. Optical frequency domain imaging indicated that EPA therapy was associated with decreased plaque instability in this population, as evidenced by lower lipid burden, higher fibrous cap thickness, and less macrophage accumulation. This improvement in coronary plaque stability has been associated with a reduction in the lipid content of plaques and a decrease in markers of inflammation including pentraxin-3 and MCP-1, as well as a reduction in inflammatory cytokines in patients with angina pectoris scheduled to receive a coronary stent and who were taking EPA in combination with high-intensity statin, compared to patients not receiving EPA [Citation136]. Results from the EVAPORATE study in 80 patients with coronary atherosclerosis taking a statin, and who were randomized to receive icosapent ethyl or placebo, showed that after 18 months, icosapent ethyl significantly reduced low-attenuation plaque compared with placebo (−0.3 ± 1.5 vs 0.9 ± 1.7 mm3, respectively, P = 0.006) [Citation137]. Icosapent ethyl also significantly reduced total plaque (−9% vs 11%, P = 0.002), total non-calcified plaque (−19% vs 9%, P = 0.0005), fibrofatty plaque (−34% vs 32%, P = 0.0002), and fibrous plaque (−20% vs 1%, P = 0.003) compared with placebo. Calcified plaque reduction was higher in the icosapent ethyl group versus placebo, though the difference was not significant (−1% vs 15%, P = 0.053). In light of the previous comments regarding mineral oil as a placebo, it was decided to compare the rates of progression of total plaque and total non-calcified plaque on coronary computed tomography angiography in the mineral oil placebo group from EVAPORATE with the non-mineral oil placebo group (per 100 mg: microcrystalline cellulose 89.83 mg, hydroxypropyl cellulose 10.07 mg, and caramel 0.10 mg) from the Garlic 5 study [Citation138]. This comparison found no significant differences in progression of log total plaque volume (β: 0.03 ± 0.13; P = 0.84) or log total non-calcified plaque volume (β: 0.01 ± 0.16; P = 0.94) between the mineral oil and non-mineral oil placebo groups. Conversely, the HEARTS trial, which investigated the effects of a mixed EPA plus DHA product on plaque, failed to show a significant difference in the change from baseline of non-calcified plaque, fibrous plaque, fatty plaque, calcified plaque, and total plaque when compared with placebo at 30 months [Citation139].
9. Innate and Adaptive Immunity
EPA has significant effects on both the innate and adaptive immune systems. The key early steps in inflammation include the adhesion of PMNs and monocytes to the endothelium, and their transendothelial migration. RvE1, which we have shown is regulated by EPA, in turn decreases CD18 expression on PMNs and monocytes [Citation54], which may be of interest in hypertriglyceridemia as CD11/CD18 is upregulated on monocytes with enhanced adhesion to vascular cell adhesion molecule 1 during hypertriglyceridemia [Citation140]. RvE1 has also been shown in vivo to inhibit human neutrophil transendothelial migration [Citation141].
Regulatory T cells secrete anti-inflammatory cytokines such as IL-10, induce anti-inflammatory macrophages, inhibit foam cell formation and suppress the differentiation and function of effector T cells [Citation142]. EPA and its metabolite 5-HEPE enhance macrophage-mediated regulatory T cell induction in mice [Citation143].
Although not typically found in normal coronary arteries, CD4+ and CD8+ T lymphocytes are found in atherosclerotic plaque [Citation144]. In a study of atopic dermatitis in mice, RvE1 reduced IL-4 and IFN-γ driven production of activated CD4+ cells and suppressed the infiltration of CD4+, CD8+, and mast cells [Citation145]. IL-23 is another interleukin affected by RvE1 and is associated with multiple chronic diseases. The interleukin drives a pathologic T-cell population (a subset of activated CD4+ cells), characterized by production of IL-17, IL-17F, IL-6, and TNF, and induces autoimmune inflammation [Citation146,Citation147]. In an experimental model of asthma, RvE1 decreased IL-23, IL-6, and IL-17 and increased production of IFN-γ and the specialized pro-resolving mediator LXA4 [Citation148].
10. Infectious Disease and COVID-19
It is anticipated that the use of EPA and icosapent ethyl will likely extend to other clinical areas outside of CVD. One area of interest is managing clinical sequelae related to infectious disease. EPA has been shown to have bacteriostatic and bactericidal properties in preclinical models [Citation149–152]. More specifically, EPA’s anti-inflammatory properties are hypothesized to resolve tissue damage following inflammatory responses to infection [Citation153,Citation154]. Given the current COVID-19 (severe acute respiratory syndrome coronavirus 2) pandemic, pilot trials of EPA (NCT04335032) and icosapent ethyl (NCT04412018, NCT04460651, and NCT04505098) are in early stages and results are eagerly awaited. Importantly, preliminary data with EPA reported inhibition of the SARS-CoV-2 spike protein attachment to the ACE2 receptor, hence blocking viral cell entry [Citation155]. MITIGATE is an open-label clinical trial evaluating the use of icosapent ethyl 4 g/day in preventing moderate to severe upper respiratory infection and subsequent clinical sequelae in 16,500 adults with established ASCVD [Citation156]. However, some preliminary data are available. A pilot trial conducted in 100 patients hospitalized with COVID-19 found a trend toward reduced risk of death in those with higher serum omega-3 fatty acids [Citation157]. Another trial, VASCEPA COVID-19 CardioLink-9 (NCT04412018), showed that icosapent ethyl 4 g twice daily for 3 days followed by 2 g twice daily for 11 days in patients with a positive SARS-CoV-2 test and upper respiratory infection symptoms reduced high-sensitivity C-reactive protein levels by 25% compared with placebo, consistent with previous results seen in patients with elevated triglyceride levels at high risk of CVD [Citation158].
Omega-3 fatty acids may have a role in preventing severe outcomes of COVID-19 in certain populations. A case series of three patients with severe COVID-19 treated with icosapent ethyl gives some preliminary support to this contention [Citation159]. In these patients, inflammatory markers decreased substantially after administration of icosapent ethyl and was associated with clinical improvement. Resolvins, protectins, and maresins derived from EPA and DHA have been shown to have antiviral activity in influenza, and have a role in resolution of inflammation [Citation160]. However, resolvins have been shown to be reduced in patients with obesity, an important independent risk factor for morbidity and mortality due to SARS-CoV-2 infection [Citation160]. As a result, it has been speculated that patients with obesity and a low level of pro-resolving mediators may be at higher risk for adverse outcomes. In addition, genetic variations in fatty acid desaturase and elongase genes, more frequent in African American and Hispanics, results in more efficient endogenous synthesis of arachidonic acid, shifting the EPA:arachidonic acid ratio toward a proinflammatory state and possibly greater susceptibility to COVID-19 [Citation161]. Additional research is required to establish such a link between omega-3 fatty acid status and risk of severe COVID-19.
11. CONCLUSIONS
There is increasing evidence that EPA has pleiotropic actions beyond triglyceride lowering alone. These physiologic mechanisms of EPA have a range of overlapping benefits across the spectrum of CV disease, including enhanced lipid metabolism, anti-inflammatory and antithrombotic effects, cell membrane stabilization, and plaque stabilization, resulting in improved vascular and coronary health. Given its anti-inflammatory effects in particular, EPA has the potential to ameliorate any disease process that involves inflammation, though well-controlled studies are required in conditions beyond CV disease. In terms of its demonstrated ability to reduce ASCVD risk and ASCVD events, these pleiotropic benefits of EPA may be more important than its direct effects on triglyceride levels.
Transparency
Declaration of funding
This article was funded by Amarin Pharma, Inc., Bridgewater, NJ.
Declaration of financial/other relationships
JRN serves as a speakers bureau member, consultant, and advisor to Amarin Pharma, Inc., from which he has received honoraria.
MJB has served as a speaker for Amarin Pharma, Inc., and has received grant/research funding from Amarin Pharma, Inc.
ORW has served as a speaker for Amarin Pharma, Inc., and Amgen.
VL has served as a speaker for Amarin Pharma, Inc., and has received grant/research funding from Amarin Pharma, Inc., both paid to institution.
DKP has served as a speaker for Amarin Pharma, Inc., AstraZeneca, Boehringer Ingelheim, Dexcom, Lilly, Merck, Novo Nordisk, Xeris and Zealand, and as a consultant to Amarin Pharma, Inc., Bayer, Dexcom, Lilly, Insulet and Sanofi.
RLN has served as an unpaid consultant for Amarin Pharma, Inc.
AN reports no conflicts of interest.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
Medical writing assistance was provided by James Street, Rohan Shah, and Jim Wood of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ and was funded by Amarin Pharma, Inc.
References
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: a Report From the American Heart Association. Circulation. 2018;137(12):e67–e492.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350.
- Fruchart JC, Davignon J, Hermans MP, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. 2014;13(1):26.
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219.
- Patel A, Barzi F, Jamrozik K, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110(17):2678–2686.
- Kasai T, Miyauchi K, Yanagisawa N, et al. Mortality risk of triglyceride levels in patients with coronary artery disease. Heart. 2013;99(1):22–29.
- Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458.
- Varbo A, Benn M, Tybjaerg-Hansen A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427–436.
- Toth PP, Philip S, Hull M, et al. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin-treated patients. Mayo Clin Proc. 2019;94(9):1670–1680.
- AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011;161(3):538–543.
- The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212.
- The ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362(17):1563–1574.
- The FIELD Study Investigators, Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366(9500):1849–1861.
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098.
- Bhatt DL, Steg G, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22.
- Kastelein JJP, Stroes ESG. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med. 2019;380(1):89–90.
- Jo SH, Han SH, Kim SH, et al. Cardiovascular effects of omega-3 fatty acids: hope or hype? Atherosclerosis. 2021;322:15–23.
- Olshansky B, Chung MK, Budoff MJ, et al. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur Heart J Suppl. 2020;22(suppl J):J32–J48.
- Reinders I, Virtanen JK, Brouwer IA, et al. Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr. 2012;66(6):736–741.
- Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009;63(9):1154–1156.
- Sherratt SCR, Juliano RA, Mason RP. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochimica Et Biophysica Acta Biomembranes. 2020;1862(7):183254.
- Vascepa [package insert]. Bridgewater, NJ: Amarin Pharma Inc.; 2019.
- HLS Therapeutics announces Health Canada approval for Vascepa® to reduce the risk of cardiovascular events [press release] [Internet]. Toronto, Canada: HLS Therapeutics; 2019 [2020 Jan 29]. Available from: http://hlstherapeutics.investorroom.com/2019-12-31-HLS-Therapeutics-Announces-Health-Canada-Approval-for-Vascepa-R-to-Reduce-the-Risk-of-Cardiovascular-Events.
- Committee for Medicinal Products for Human Use. Summary of opinion: vazkepa [Internet]. European Medicines Agency; 2021 [2021 Mar 4]. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/vazkepa.
- Vazkepa [summary of product characteristics]. Dublin, Ireland: Amarin Pharmaceuticals Ireland; 2021.
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268–2280.
- Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40(5):1135–1147.
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75(3):645–662.
- Bhatt DL, Miller M, Steg G, et al. EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl-intervention trial [oral presentation]. Chicago, IL. 2020March28–30;Annual Scientific Session of the American College of Cardiology.
- Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99–107.
- Peterson BE, Bhatt DL, Steg G, et al. Reduction of revascularization in patients with hypertriglyceridemia with icosapent ethyl: insights from REDUCE-IT REVAS [oral presentation]. Annual Scientific Sessions of the Society for Cardiovascular Angiography and Interventions. 2020 Apr 28-May 28-May 1. Chicago, IL
- Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308–1317.
- Nemiroff RL. Addressing cardiovascular risk: anti-inflammatory properties and pleiotropic effects of eicosapentaenoic acid. Contemp Ob Gyn. 2016;(suppl(3):1–8.
- Nelson JR, True WS, Le V, et al. Can pleiotropic effects of eicosapentaenoic acid (EPA) impact residual cardiovascular risk? Postgrad Med. 2017;129(8):822–827.
- Le V, Nelson JR. Eicosapentaenoic acid: pleiotrope extraordinaire? LipidSpin. 2017;15(1): 12–14. 35.
- Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357–366.
- Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–690.
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–992.
- Ballantyne CM, Bays HE, Philip S, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies Atherosclerosis. 2016;253:81–87.
- Oscarsson J, Hurt-Camejo E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: a review. Lipids Health Dis. 2017;16(1):149.
- Dunbar RL, Copland C, Jiao L, et al. Icosapent ethyl mitigates dyslipidemia by both slowing triglyceride-rich lipoprotein production and hastening LDL clearance [poster 222]. In: Annual Vascular Discovery: from Genes to Medicine Scientific Sessions. Chicago, IL; 2020May5–7.
- Bays HE, Tighe AP, Sadovsky R, et al. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6(3):391–409.
- Tani S, Nagao K, Matsumoto M, et al. Highly purified eicosapentaenoic acid may increase low-density lipoprotein particle size by improving triglyceride metabolism in patients with hypertriglyceridemia. Circ J. 2013;77(9):2349–2357.
- Sala-Vila A, Cofan M, Mateo-Gallego R, et al. Eicosapentaenoic acid in serum phospholipids relates to a less atherogenic lipoprotein profile in subjects with familial hypercholesterolemia. J Nutr Biochem. 2013;24(9):1604–1608.
- Chinetti G, Lestavel S, Fruchart JC, et al. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res. 2003;92(2):212–217.
- Reza JZ, Doosti M, Salehipour M, et al. Modulation peroxisome proliferators activated receptor alpha (PPAR alpha) and acyl coenzyme A: cholesterol acyltransferase1 (ACAT1) gene expression by fatty acids in foam cell. Lipids Health Dis. 2009;8(1):38.
- Ruparelia N, Chai JT, Fisher EA, et al. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14(3):133–144.
- Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13(1):37–46.
- Nelson JR, Raskin S. The eicosapentaenoic acid: arachidonicacid ratio and its clinical utility in cardiovascular disease. Postgrad Med. 2019;131(4):268–277.
- Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol. 2016;67(1):22–27.
- Gdula-Argasinska J, Czepiel J, Wozniakiewicz A, et al. n-3 Fatty acids as resolvents of inflammation in the A549 cells. Pharmacol Rep. 2015;67(3):610–615.
- Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722.
- Dona M, Fredman G, Schwab JM, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112(3):848–855.
- Hasturk H, Abdallah R, Kantarci A, et al. Resolvin E1 attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35(5):1123–1133.
- Keyes KT, Ye Y, Lin Y, et al. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299(1):H153–164.
- Salic K, Morrison MC, Verschuren L, et al. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016;250:158–165.
- Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31(9):559–567.
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111(10):5922–5943.
- Zarate R, Jaber‐Vazdekis N, Tejera N, et al. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. 2017;6(1):25.
- Lee A, Kinninger A, Jayawardena E, et al. Relationship between lipid levels and coronary atherosclerotic plaque scores by coronary computed tomography angiography (CTA) in subjects with elevated triglycerides [abstract 265]. J Clin Lipidol. 2019;13(3):e27.
- Nelson JR, Mehan MR, Alexander LE, et al. Eicosapentaenoic acid and the eicosapentaenoic acid/arachidonic acid ratio is associated with prevalent diabetes [poster]. Annual Scientific Sessions of the American Heart Association, November 11–15, 2017; Anaheim, CA.
- Sarbolouki S, Javanbakht MH, Derakhshanian H, et al. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: a double-blind randomised clinical trial. Singapore Med J. 2013;54(7):387–390.
- Tishinsky JM, Ma DW, Robinson LE. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARγ-dependent manner in human adipocytes. Obesity (Silver Spring, Md). 2011;19(2):262–268.
- Lefils-Lacourtablaise J, Socorro M, Geloen A, et al. The eicosapentaenoic acid metabolite 15-deoxy-delta(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARgamma-dependent mechanism. PLoS One. 2013;8(5):e63997.
- Pal A, Al-Shaer AE, Guesdon W, et al. Resolvin E1 derived from eicosapentaenoic acid prevents hyperinsulinemia and hyperglycemia in a host genetic manner. FASEB J. 2020;34(8):10640–10656.
- Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. 2010;21(5):444–450.
- Massaro M, Scoditti E, Carluccio MA, et al. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):109–115.
- Allam-Ndoul B, Guenard F, Barbier O, et al. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016;15(1):69.
- Zhao Y, Joshi-Barve S, Barve S, et al. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23(1):71–78.
- Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82.
- Takata Y, Kitami Y, Yang ZH, et al. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91(5):427–433.
- Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178(6):3912–3917.
- Oh SF, Dona M, Fredman G, et al. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188(9):4527–4534.
- Isobe Y, Arita M, Matsueda S, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287(13):10525–10534.
- Takamura M, Kurokawa K, Ootsuji H, et al. Long-Term Administration of Eicosapentaenoic Acid Improves Post-Myocardial Infarction Cardiac Remodeling in Mice by Regulating Macrophage Polarization. J Am Heart Assoc. 2017;6(2):2.
- Chen J, Shearer GC, Chen Q, et al. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123(6):584–593.
- Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol. 2008;101(12A):41F–50F.
- Chen H, Li D, Chen J, et al. EPA and DHA attenuate ox-LDL-induced expression of adhesion molecules in human coronary artery endothelial cells via protein kinase B pathway. J Mol Cell Cardiol. 2003;35(7):769–775.
- Huang CY, Sheu WH, Chiang AN. Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms. Mol Nutr Food Res. 2015;59(4):751–762.
- Yamada H, Yoshida M, Nakano Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28(12):2173–2179.
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27(1):229–265.
- Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–1163.
- Gonelevue S, Bandyopadhyay A, Bhagat S, et al. Sterile inflammatory role of high mobility group box 1 protein: biological functions and involvement in disease. J Vasc Res. 2018;55(4):244–254.
- Sumiyoshi M, Satomi J, Kitazato KT, et al. PPARγ-dependent and -independent inhibition of the HMGB1/TLR9 pathway by eicosapentaenoic acid attenuates ischemic brain damage in ovariectomized rats. J Stroke Cerebrovasc Dis. 2015;24(6):1187–1195.
- Tanaka K, Ishikawa Y, Yokoyama M, et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008;39(7):2052–2058.
- Golzari MH, Hosseini S, Koohdani F, et al. The effect of eicosapentaenoic acid on the serum levels and enzymatic activity of paraoxonase 1 in the patients with type 2 diabetes mellitus. Acta Med Iran. 2017;55(8):486–495.
- Golzari MH, Javanbakht MH, Ghaedi E, et al. Effect of eicosapentaenoic acid supplementation on paraoxonase 2 gene expression in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Clin Nutr Res. 2019;8(1):17–27.
- Aviram M, Rosenblat M, Bisgaier CL, et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101(8):1581–1590.
- Rozenberg O, Shih DM, Aviram M. Human serum paraoxonase 1 decreases macrophage cholesterol biosynthesis: possible role for its phospholipase-A2-like activity and lysophosphatidylcholine formation. Arterioscler Thromb Vasc Biol. 2003;23(3):461–467.
- Rozenberg O, Shih DM, Paraoxonase AM. 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 2005;181(1):9–18.
- Mason RP, Sherratt SCR, Jacob RF. Eicosapentaenoic acid inhibits oxidation of apoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J Cardiovasc Pharmacol. 2016;68(1):33–40.
- Sherratt SCR, Mason RP. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun. 2018;496(2):335–338.
- Dyerberg J, Bang HO, Stoffersen E, et al. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2(8081):117–119.
- Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30(10):2005–2013.
- Gong Y, Lin M, Piao L, et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br J Pharmacol. 2015;172(23):5647–5660.
- Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21(1):2.
- Williams JA, Batten SE, Harris M, et al. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103(2):228–237.
- Leng X, Kinnun JJ, Cavazos AT, et al. All n-3 PUFA are not the same: MD simulations reveal differences in membrane organization for EPA, DHA and DPA. Biochimica Et Biophysica Acta Biomembranes. 2018;1860(5):1125–1134.
- Mason RP, Jacob RF. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim Biophys Acta. 2015;1848(2):502–509.
- Mason RP, Jacob RF. Membrane microdomains and vascular biology: emerging role in atherogenesis. Circulation. 2003;107(17):2270–2273.
- Mason RP, Jacob RF, Shrivastava S, et al. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858(12):3131–3140.
- Moreno C, De La Cruz A, Oliveras A, et al. Marine n-3 PUFAs modulate IKs gating, channel expression, and location in membrane microdomains. Cardiovasc Res. 2015;105(2):223–232.
- Li GR, Sun HY, Zhang XH, et al. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc Res. 2009;81(2):286–293.
- Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci U S A. 1992;89(5):1760–1764.
- Kromhout D, Geleijnse JM, De GJ, et al. n-3 Fatty acids, ventricular arrhythmia-related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care. 2011;34(12):2515–2520.
- Chen WT, Chen SY, Wu DW, et al. Effect of icosapent ethyl on susceptibility to ventricular arrhythmias in postinfarcted rat hearts: role of GPR120-mediated connexin43 phosphorylation. J Cell Mol Med. 2020;24(16):9267–9279.
- Sasaki J, Miwa T, Odawara M. Administration of highly purified eicosapentaenoic acid to statin-treated diabetic patients further improves vascular function. Endocr J. 2012;59(4):297–304.
- Toyama K, Nishioka T, Isshiki A, et al. Eicosapentaenoic acid combined with optimal statin therapy improves endothelial dysfunction in patients with coronary artery disease. Cardiovasc Drugs Ther. 2014;28(1):53–59.
- Yamakawa K, Shimabukuro M, Higa N, et al. Eicosapentaenoic acid supplementation changes fatty acid composition and corrects endothelial dysfunction in hyperlipidemic patients. Cardiol Res Pract. 2012;2012:754181.
- Takaki A, Umemoto S, Ono K, et al. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: a randomized controlled study. J Atheroscler Thromb. 2011;18(10):857–866.
- O’Connell TD, Mason RP, Budoff MJ, et al. Mechanistic insights into cardiovascular protection for omega-3 fatty acids and their bioactive lipid metabolites. Eur Heart J Suppl. 2020;22(suppl J):J3–J20.
- Mason RP, Dawoud H, Jacob RF, et al. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother. 2018;103:1231–1237.
- Tousoulis D, Kampoli AM, Tentolouris C, et al. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10(1):4–18.
- Zheng XY, Liu L. Remnant-like lipoprotein particles impair endothelial function: direct and indirect effects on nitric oxide synthase. J Lipid Res. 2007;48(8):1673–1680.
- Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34(4):715–723.
- Nakamura K, Miura D, Saito Y, et al. Eicosapentaenoic acid prevents arterial calcification in klotho mutant mice. PLoS One. 2017;12(8):e0181009.
- Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698.
- MacRitchie N, Resolvin MP. E1 for reducing vascular calcification. Cardiovasc Res. 2019;115(10):1457–1459.
- Devaraj S, Chien A, Rao B, et al. Modulation of endothelial progenitor cell number and function with n-3 polyunsaturated fatty acids. Atherosclerosis. 2013;228(1):94–97.
- Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007.
- Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600.
- Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26(2):257–266.
- Tanaka N, Ishida T, Nagao M, et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis. 2014;237(2):577–583.
- Tithof PK, Elgayyar M, Schuller HM, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone, a nicotine derivative, induces apoptosis of endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281(5):H1946–H1954.
- Nelson JR, Wani O, May HT, et al. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul Pharmacol. 2017;91:1–9.
- Nishio R, Shinke T, Otake H, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234(1):114–119.
- Sakakura K, Nakano M, Otsuka F, et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22(6):399–411.
- Uehara H, Miyagi N, Shimajiri M, et al. The additional effect of eicosapentanoic acid on coronary plaque stability in stable angina patients with statin use by optical coherence tomography analysis [abstract P5495]. Eur Heart J. 2013;34(suppl 1):1011.
- Nagahara Y, Motoyama S, Sarai M, et al. Eicosapentaenoic acid to arachidonic acid (EPA/AA) ratio as an associated factor of high risk plaque on coronary computed tomography in patients without coronary artery disease. Atherosclerosis. 2016;250:30–37.
- Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(9):1963–1972.
- Fujiyoshi K, Minami Y, Ishida K, et al. Incidence, factors, and clinical significance of cholesterol crystals in coronary plaque: an optical coherence tomography study. Atherosclerosis. 2019;283:79–84.
- Tanaka N, Irino Y, Shinohara M, et al. Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J. 2018;82(2):596–601.
- Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70(6):537–544.
- Konishi T, Sunaga D, Funayama N, et al. Eicosapentaenoic acid therapy is associated with decreased coronary plaque instability assessed using optical frequency domain imaging. Clin Cardiol. 2019;42(6):618–628.
- Niki T, Wakatsuki T, Yamaguchi K, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. 2016;80(2):450–460.
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925–3932.
- Lakshmanan S, Shekar C, Kinninger A, et al. Comparison of mineral oil and non-mineral oil placebo on coronary plaque progression by coronary computed tomography angiography. Cardiovasc Res. 2020;116(3):479–482.
- Alfaddagh A, Elajami TK, Ashfaque H, et al. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6(12):12.
- Gower RM, Wu H, Foster GA, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31(1):160–166.
- Serhan CN, Clish CB, Brannon J, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204.
- Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015;35(2):280–287.
- Onodera T, Fukuhara A, Shin J, et al. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci Rep. 2017;7(1):4560.
- Jonasson L, Holm J, Skalli O, et al. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6(2):131–138.
- Kim TH, Kim GD, Jin YH, et al. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol. 2012;14(4):384–391.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240.
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587.
- Haworth O, Cernadas M, Yang R, et al. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–879.
- Le PNT, Desbois AP. Antibacterial effect of eicosapentaenoic acid against bacillus cereus and staphylococcus aureus: killing kinetics, selection for resistance, and potential cellular target. Mar Drugs. 2017;15(11):334.
- Shin SY, Bajpai VK, Kim HR, et al. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int J Food Microbiol. 2007;113(2):233–236.
- Cheng CL, Huang SJ, Wu CL, et al. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J Biomed Sci. 2015;22(1):103.
- Desbois AP, Lawlor KC. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar Drugs. 2013;11(11):4544–4557.
- D’Elia RV, Harrison K, Oyston PC, et al. Targeting the. “cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20(3):319–327.
- Mo Z, Tang C, Li H, et al. Eicosapentaenoic acid prevents inflammation induced by acute cerebral infarction through inhibition of NLRP3 inflammasome activation. Life Sci. 2020;242:117133.
- Goc A, Niedzwiecki A, Polyunsaturated RM. ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. 2021;11(1):5207.
- Ambrosy AP, Malik UI, Thomas RC, et al. Rationale and design of the pragmatic randomized trial of icosapent ethyl for high cardiovascular risk adults (MITIGATE). Am Heart J. 2021;235:54–64.
- Asher A, Tintle NL, Myers M, et al. Blood omega-3 fatty acids and death from COVID-19: a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2021;166:102250.
- Kosmopoulos A, Verma S, Meglis G, et al. VASCEPA COVID-19 CardioLink-9: first human trial of a loading dose of icosapent ethyl in patients with COVID-19 [presentation]. Annual Scientific Sessions of the National Lipid Association, December 10–12, 2020 ( virtual).
- Suh W, Urits I, Viswanath O, et al. Three cases of COVID-19 pneumonia that responded to icosapent ethyl supportive treatment. Am J Case Reps. 2020;21:e928422.
- Pal A, Gowdy KM, Oestreich KJ, et al. Obesity-driven deficiencies of specialized pro-resolving mediators may drive adverse outcomes during SARS-CoV-2 infection. Front Immunol. 2020;11:1997.
- Simopoulos AP, Serhan CN, Bazinet RP. The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol Aspects Med. 2021;77:100943.
