ABSTRACT
Purpose: Drug therapy problems impact about one-third of US adults, and these issues are likely to continue to worsen as the population of aging Americans increases. The objective of this study is to assess the feasibility of a remotely delivered Comprehensive Medication Management (CMM) for primary practice patients who are polypharmatic and at high risk for drug therapy problems.
Methods: Using medical and prescription claims data, a list of Medicare Advantage beneficiaries at high risk for drug therapy problems was identified. Participants were enrolled in a 6-month CMM program from February – November 2020. In the program, their existing drug therapy was assessed by a pharmacist, Drug therapy problems were identified and resolved. A Collaborative Practice Agreement allowed the pharmacists to make prescription changes as needed.
Results: Eighty-three percent (202) of contacted individuals agreed to participate in the study. All participants were on five medications or more, and 71% were on more than eight. A clinical pharmacist found that 86% of participants had a drug therapy problem according to classification criteria. Seventy-nine percent of all drug therapy problems identified were resolved upon completion of the study.
Conclusion: The findings of this study suggest that engagement of a remote clinical pharmacist can contribute to efficient resolution of most drug therapy problems identified in a primary care population. A service model using remote pharmacist services may be an effective means of improving team-based primary care medication management for this population.
Introduction
Drug therapy problems (DTPs) are an issue for around one-third of US adults, occurring most commonly in individuals who are receiving treatments for multiple comorbid health conditions [Citation1]. DTPs are a well-established public health crisis resulting in increased morbidity, mortality, and healthcare expenditures worldwide [Citation1–3]. This crisis is of increasing concern as the US population continues to age, and the number of citizens taking multiple medications increases [Citation4]. The cost of morbidity and mortality associated with drug therapy problems is an estimated 528 USD billion annually in the US, accounting for nearly 15% of the total healthcare expenditures in 2018. Individual per patient costs associated with suboptimal medication use averages 2500 USD annually [Citation5,Citation6].
Drug therapy problems involve the use of unnecessary or ineffective medication, the absence of indicated medications, adverse drug reactions (ADRs), and/or medication non-adherence [Citation7]. Polypharmacy (the use of five or more medications) increases the likelihood of poor patient outcomes [Citation1–3,Citation8,Citation9]. An estimated 39% of adults aged 65+ take 5 or more medications [Citation4]. These risks are compounded by healthcare service fragmentation. Emergency department (ED) utilization patterns highlight the impact of drug therapy problems in a disjointed care system, as approximately 1 in 9 ED visits are due to adverse drug reactions and 68% of adverse drug reactions resulting in ED visits are preventable [Citation10].
One paradigm for optimizing medication use, improving clinical outcomes, and reducing healthcare expenditures is Comprehensive Medication Management (CMM). CMM is a standard of care that ensures medications are appropriate, effective at treating or preventing a designated condition, and safe to use [Citation11]. Ward and Zu compared the 9-month pre-intervention and 9-month post-intervention all-cause healthcare spending of Medicare patients who received telephonic CMM services. The study demonstrated a 20% reduction in total healthcare expenditures with this intervention, compared to a 2.3% post-intervention reduction in the control group [Citation12]. Health outcomes can also be improved by comprehensive medication management services. Hui et al. reported a 14% reduction in all-cause mortality and a 3% reduction in hospitalization for patients receiving CMM services compared to a control group [Citation13].
A collaborative practice agreement (CPA) can improve provider efficiency and clinical outcomes. CPAs can allow pharmacists to prescribe or modify medications, as well as order labs necessary for medication monitoring. McFarland et al. reported that 69% of patients engaged in services with a clinical pharmacist practicing under a collaborative practice agreement achieved a targeted A1c level, compared to only 36% in the control group [Citation14]. Additionally, Anaya and colleagues demonstrated a post-intervention reduction in average hospital costs of nearly 1800 USD for patients engaged in services from a pharmacist utilizing a CPA, compared to the average pre-intervention hospital cost without such services [Citation15].
To date, we are unaware of a study that explores a remote model for medication management involving both comprehensive medication management and a collaborative practice agreement in a highly complex patient population. The purpose of this study was to assess the feasibility of a remotely delivered CMM, including a collaborative practice agreement, for patients who are polypharmatic, at high risk for drug therapy problems, and cared for in a primary care practice.
Methods
Study design
This was a non-randomized, prospective, observational data collection study.
Participant identification and recruitment
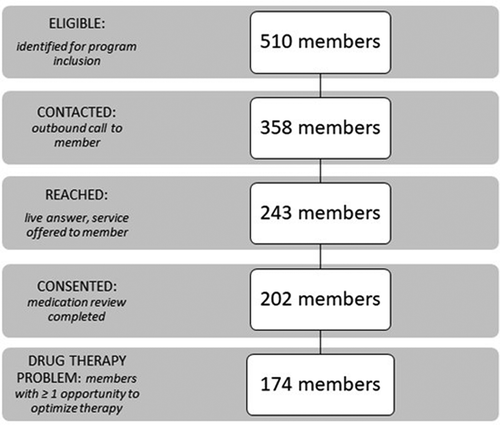
All participants were United HealthCare members who receive care at American Health Network (AHN), a part of OptumCare based in Indiana and Ohio. Using prior medical and prescription claim data, patients were identified as being at high risk of having drug therapy problems (see ). Patient outreach consisted of a mailer sent by Genoa Healthcare on behalf of the primary care providers of AHN. The letters were followed by an introductory message prerecorded by the Chief Medical Officer of AHN explaining the program to the list of prospective participants. These patients were then contacted via phone by a clinical pharmacist. Up to 3 calls were made to reach potential participants. Contacted potential participants were then provided information about the program and offered participation. Regulatory approval included a waiver of written consent & HIPAA authorization (i.e. approval for verbal consent). The study was exempt from the IRB approval process.
Table 1. Inclusion and exclusion criteria
Medication review and resolution
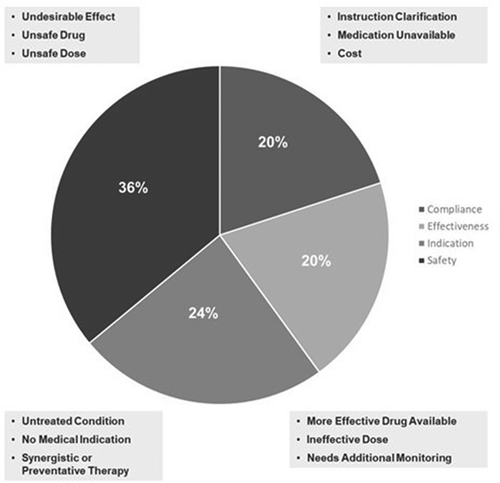
The 6-months of Comprehensive Medication Management services were delivered telephonically by a pharmacist between February and November 2020. The review consisted of obtaining a list of current medications, medication and medical history, and questions about medication adherence. Based on the review, the pharmacist identified possible DTPs, generated recommendations for resolution, and shared recommendations with the provider. Drug therapy problems were identified and categorized into the following 4 groups: Indication, Effectiveness, Safety, and Compliance () [Citation7]. Recommendations made to AHN providers were communicated through the electronic medical record (EMR) whereby the provider was able to [Citation1]: accept the recommendations [Citation2]; accept the recommendations with modification(s), or [Citation3]; reject the recommendations. Resolution of a drug therapy problem was determined by physician entry in the EMR resolving the DTP or communication with non-AHNI providers via fax or telephone, patient reports of medication change, and pharmacy data reflected in claims.
Table 2. Types of drug therapy problems
Collaborative practice agreement
A collaborative practice agreement was executed between AHN-Indiana (AHNI) and Genoa Healthcare clinical pharmacists in accordance with Indiana regulations to allow the pharmacist(s) to implement recommendations within the scope of the agreement. The physician only needed to acknowledge the medication changes that the pharmacist made per the collaborative practice agreement in the EMR. Medication recommendations outside of the scope of the CPA were reviewed and made by the physician directly. The pharmacist’s recommendations were communicated to AHNI physicians within their Electronic Medical Record also sent to the patient by mail. Physicians outside the AHNI network were contacted by mail and/or phone, if the patient was under the care of multiple physicians.
Clinical leadership at AHN identified the types of conditions and medications that would fall under the collaborative practice agreement. The CPA covered common chronic conditions, while conditions considered outside of the scope of the CPA were those related to behavioral and psychological disorders, pain management, cardio and peripheral vascular disease, stroke-related and urologic disorders. All controlled substances were out of scope of the CPA.
Measures
Primary outcome measure was [Citation1] drug therapy problems identified by a CMM pharmacist and the percentage of DTPs resolved, rejected, or unresolved. (subcategorized by type).
Secondary measurements included 1.) patient demographic information, 2.) number of patients eligible for the CMM program, 3.) overall recruitment metrics and number of patients that consented to undergo a medication review, 4.) number and type of DTPs for each patient, 5.) provider acknowledgment from AHN providers and non-AHN providers 6.) medication changes recommended for each patient by the reviewing pharmacist, and 7.) number of accepted the recommended medication changes by type of drug therapy problem.
Statistical analysis
To compare results for drug therapy problems falling under the collaborative practice agreement or outside the CPA, chi-square tests of independence were used. Statistical significance was determined if the alpha was <0.05.
Results
Enrollment and participation
Five hundred and ten potential participants met inclusion criteria. The pharmacists successfully reached 243 individuals out of an attempted 348. As enrollment was capped at 200 individuals, the remaining 162 individuals were not attempted for enrollment. The rate of consent for those reached was 83% (). The demographic and clinical characteristics of the 202 study participants are shown in .
Table 3. Participant characteristics
Disposition of drug therapy problems
Drug therapy problems were identified in 173/202 (86%) of participants. A total of 438 DTPs were identified, with an average of 2.53 DTPs per individual. Identified DTPs were categorized into those which could resolved by the pharmacist and those outside of the scope of the collaborative practice agreement. Of the identified drug therapy problems, 266 were outside the scope of the CPA. In these cases, the clinical pharmacist provided recommendations to the physician but did not make adjustments to the prescription within the EMR. Of the 266 DTPs not under collaborative practice agreement, pharmacist recommendations were rejected in 33 instances (12%). One hundred and ninety-nine (75%) were resolved and 34 (16.5%) were reviewed with no action or remain under review. Of the resolved drug therapy problems, 50% were resolved within two months and 68% were resolved within three months of the date of recommendation. Of the 266 DTPs outside the CPA, 75% were resolved either though a physician making the recommended drug change or patient action (see ).
Figure 2. Drug therapy problem waterfall: Initial engagement. Confirmation of resolution included provider communication via EMR, patient report and claims medication data
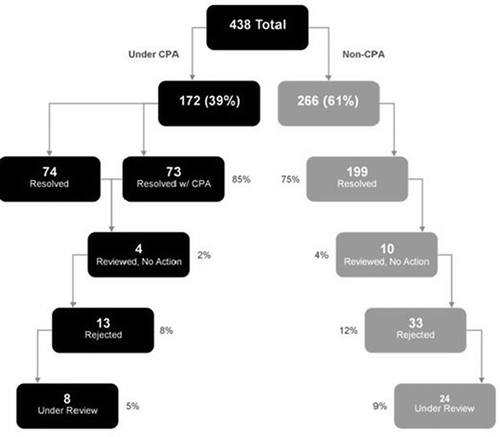
There were 172 drug therapy problems under the CPA, which comprised 39% of the total DTPs seen in the population. Of the 172 recommendations made, 13 (8%) were rejected. Of the 172 DTPs under the CPA, 85% were resolved either though a pharmacist or a physician making the recommended drug change. Four recommendations (2%) were reviewed and not acted upon or remain under review at the time of this report.
We analyzed the net effect of actions within and outside the CPA because both reflect the value of the pharmacist in optimizing medication use for patients within the care model. A total of 346 of 438 identified drug therapy problems (79%) were resolved by interaction of the clinical pharmacist with the primary care practice for these 173 patients. Notably, only 46 DTP recommendations were rejected (10.5%) at the time of this report and a total of 46 (10.5%) were reviewed with no action or are under review pending the next patient visit (). While the data suggest that more DTPs recommendations outside of the collaborative practice agreement were rejected (12% vs. 8%) and more DTPs were left unresolved in the non-CPA group than in those covered by the CPA (13% vs. 7%), neither reached statistical significance (p = 0.11 and p = 0.05, respectively). See for the breakdown of all drug therapy problems by type. Drug safety DTPs were the most common type constituting 36% of those identified while those of drug indication was second at 24%.
Discussion
The results of this feasibility study demonstrate the important role clinical pharmacists play in the healthcare team. Through individual engagement and assessment, pharmacists were able to optimize medication therapy of participating individuals. Our study is notable for an exceptional engagement rate. Our outbound call to reach rate was high at 68%. Subsequently, when members were reached by phone the consent rate was 83%. Importantly, common barriers to telephonic engagement such as incorrect, or out of service phone numbers were not experienced in this study. Initial phone numbers were provided by the insurance plan; however, if numbers were inoperable, we used the contact information available to the primary care team because of access to the EMR. This speaks to the importance of shared efforts on communication.
We found an average of 2.53 drug therapy problems per individual and 79% resolution rate. Additionally, the study demonstrated the value a collaborative practice agreement can play in improving physician efficiency by allowing the pharmacist to have prescriptive authority to resolve some DTPs. However, it also demonstrated the opportunity for further refinement of the conditions and medications encompassed within the CPA to fully leverage this capability.
These are important findings during a time where the practice of primary care continues to get more challenging. The primary care population is aging, and subsequently the proportion of complex patients who are taking many medications is increasing [Citation4,Citation16]. This greater complexity is occurring at a time when primary care providers have increasing time constraints, and in which multiple non-direct care tasks must be completed [Citation17]. These challenges add additional layers of demands to the primary care physician, who may lack the time to complete the tasks necessary to adequately manage medications for complex patients [Citation18]. In our study, drug safety and indication issues constituted 60% of drug therapy problems identified. While issues of effectiveness and compliance are also important, the fact that the majority of drug therapy problems (60%) were safety and indication-related further speaks to the priority of integrating pharmacist expertise into primary care.
It is common for complex patients to have their care divided between multiple specialists and the PCP. However, this approach can increase care fragmentation and is a source of dissatisfaction to patients [Citation19]. Sinsky and Bodenheimer offer the opinion that ‘primary care is underpowered,’ and propose that team-based models are needed. They argue that primary care teams are better suited to tackle separate aspects of the clinical workload, allowing each team member to add the greatest value to care. They also emphasize the opportunity cost of primary care physicians doing work that others could do, and advocate for ‘in room’ team members [Citation20]. Meyers et al. go further in their discussion of optimal workforce design, by suggesting that team-based care is central to improving primary care design [Citation21].
Studies by Bazemore et al. and Jabbarpour and colleagues also review team design and its likely importance for the complex patient panel [Citation21,Citation22]. Using survey data from 2014 to 2018 and the American Board of Family Medicine, these authors found that while 56% of respondents operate their practices with nurse practitioners, only 26% of family practice practices described including a behavioral health specialist or clinical pharmacist as part of the clinical team. The 2018 report by Meyers et al. adds additional insight about primary care team structure by considering team composition, as well as operational and cost models for four hypothetical patient panels (index, high geriatric, high social need, and rural). For each type of panel, the authors identify the importance of a clinical pharmacist as a team member, with the greatest need for pharmacist support in the high geriatric and high social needs panels. Meyers et al. go on to consider costs of each model and identify the challenges of cost and scaling such expert team models to small practices [Citation19].
Notably, while the reports of Meyers et al. and Jabbarpour et al. are recent, the COVID-19 pandemic has changed how care will be delivered [Citation19,Citation22]. Telemedicine and virtual care models appear to be here to stay. Reeves emphasizes the importance of interprofessional practice models and argues that interprofessional practice can be effectively achieved through ‘networks’ rather than requiring in-person teams [Citation24]. The appropriateness of remote, interprofessional network approaches is determined by the type of problem that needs to be solved, its predictability, its urgency, and its complexity [Citation16].
Our investigation was the practical instantiation of the ideas framed by previous studies [Citation23,Citation20,Citation21,Citation22,Citation24,Citation16]. We began with the identification of polypharmacy as a priority issue in primary care practice. Secondly, we believed that the time burden of medication management could be largely addressed by a pharmacist rather than the primary care physician. We worked with the assumptions that a clinical pharmacist might possess greater expertise and be more current in their knowledge of medication management, and that the nature of medication management met the criteria required to be effectively provided in an asynchronous ‘network’ model. We also speculated that the asynchronous model, using remote pharmacists as a shared resource, might be a means to address the cost and scalability challenge faced by smaller practices [Citation12,Citation14,Citation15].
The present study pushed the boundaries of team care with the implementation of a CPA. In our investigation, the collaborative practice agreement authorized pharmacists to initiate, continue, modify and discontinue drug therapy for the following conditions: asthma, COPD, diabetes, dyslipidemia, GERD, gout, heart failure, hypertension and tobacco cessation. It also authorized pharmacists to order vaccines and laboratory tests relevant to the monitoring of drug therapy to treat the above conditions. All drug therapy for conditions outside of the above as well as controlled substances were excluded from the CPA. It was also limited to medication therapy initially prescribed by the AHNI primary care providers. Of the DTPs identified, the majority fell outside the CPA, and of those within the collaborative practice agreement, the pharmacist directly resolved only a fraction. This is not unexpected, due to the conservative nature of our CPA approach, and since the practice had never implemented this model of care. Further, drug therapy problems which could be resolved by the pharmacist’s scope of practice without a CPA were considered non-CPA drug therapy problems. These include recommendations to start, modify or stop nonprescription/over-the-counter medications, education to improve administration techniques and addressing barriers to non-adherence. Nevertheless, 79% of DTPs were resolved as a result of pharmacist input and only 46 of 438 (10.5%) drug therapy problem recommendations were rejected by the physician staff. While our CPA agreement did not accomplish as much as we hoped it is our assessment that CPAs add value for both the provider and the patient and will be an important part of health service design as medication complexity increases.
There were limitations to this study which should be considered. Due to the nature of recruitment and the target population, it is possible that selection bias is represented in the final sample of participants. Specifically, patients who expressed interest in participating may have been at higher likelihood, or history, of medication-related problems.
Secondly, while a small percentage, 10% of the drug therapy problems identified have not been resolved at the time of this report. Most unresolved cases (7%) are awaiting physician review. As the study was completed during the COVID-19 pandemic, in-person appointments proved particularly challenging and infrequent compared to practice function pre-pandemic.
Third, at the time of this report we cannot determine whether the DTP resolutions were ‘sticky.’ To this end, the medication management program will run for 6 months for each participant after the initial set of interventions by the clinical pharmacist. During this period, the project will continue to address any new DTPs through ongoing review of patient status by EMR surveillance to determine if the drug therapy changes made were sustained or if there was a reversion to the medication profile.
Fourth, the collaborative practice agreement itself only contributed to a fraction of the drug therapy problem resolution by direct change of medication by the pharmacist, and most of the DTPs were outside the scope of the CPA. The value of the pharmacist intervention is demonstrated in the 79% resolution rate overall, so we expect over time, as physicians become comfortable with a clinical pharmacist as a care member that the category of ‘resolved under CPA’ can increase.
Finally, and most importantly, we do not provide clinical or economic outcomes associated with the program. We certainly expect that identifying and resolving drug therapy problems in an older, chronically ill population on a large number of medications will improve outcomes and reduce costs, however, a 6-month study may not be sufficient to demonstrate that. A strong argument can be made that resolving drug therapy problems in a highly complex population is desirable; however, it is necessary to demonstrate a justification for adding the cost of a clinical pharmacist to the care team will need to be demonstrated.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2013;13(1):57–65.
- Chiatti C, Bustacchini S, Furneri G, et al. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf. 2012;35(S1):73–87.
- Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN Electron J. 2012. DOI:10.2139/ssrn.2222541
- Charlesworth CJ, Smit E, Lee DSH, et al. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J Gerontol Ser A. 2015;70(8):989–995.
- Watanabe JH, Mcinnis T, Hirsch JD. Cost of prescription drug–related morbidity and mortality. Ann Pharmacother. 2018;52(9):829–837.
- Center for Medicare and Medicaid Services. NHE fact sheet. [ cited 2020 Aug 10]. https://www.cms.gov/files/document/highlights.pdf
- Cipolle RJ, Strand L, Morley P. Pharmaceutical care practice: the patient-centered approach to medication management. 3rd ed. New York, USA: McGraw-Hill Companies, Inc.; 2012.
- Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1). DOI:10.1186/s12877-017-0621-2
- Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–1386.
- Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. Can Med Assoc J. 2008;178(12):1563–1569.
- McInnis T, Webb E, Strand L, Patient-Centered Primary Care Collaborative (PCPCC). The patient-centered medical home: integrating comprehensive medication management to optimize patient outcomes resource guide. 2nd ed. Washington, DC: PCPCC; 2012. Available from: www.pcpcc.org/sites/default/files/media/medmanagement.pdf
- Ward MA, Zu Y. Pharmacist-provided telephonic medication therapy in an MAPD plan. Am J Manag Care. 2011;17(10): e399–e409.
- Hui RL, Yamada BD, Spence MM, et al. Impact of a Medicare MTM program: evaluating clinical and economic outcomes. Am J Manag Care. 2014;20(2):e43–e51.
- McFarland M, Davis K, Wallace J, et al. Use of home telehealth monitoring with active medication therapy management by clinical pharmacists in veterans with poorly controlled type 2 diabetes mellitus. Pharmacother J Human Pharmacol Drug Ther. 2012;32(5):420–426.
- Anaya JP, Rivera JO, Lawson K, et al. Evaluation of pharmacist-managed diabetes mellitus under a collaborative drug therapy agreement. Am J Health Syst Pharm. 2008;65(19):1841–1845.
- Nobili A, Marengoni A, Tettamanti M, et al. Association between clusters of diseases and polypharmacy in hospitalized elderly patients: results from the REPOSI study. Eur J Intern Med. 2011;22(6):597–602.
- Abbo ED, Zhang Q, Zelder M, et al. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- Ong M-S, Olson KL, Chadwick L, et al. The impact of provider networks on the co-prescriptions of interacting drugs: a claims-based analysis. Drug Saf. 2016;40(3):263–272.
- Meyers D, Leroy L, Bailit M, et al. Workforce configurations to provide high-quality, comprehensive primary care: a mixed-method exploration of staffing for four types of primary care practices. J Gen Intern Med. 2018;33(10):1774–1779.
- Sinsky CA, Bodenheimer T. Powering-up primary care teams: advanced team care with in-room support. Anna Family Med. 2019;17(4):367–371.
- Bazemore A, Wingrove P, Peterson L, et al. The diversity of providers on the family medicine team. J Am Board Fam Med. 2016;29(1):8–9.
- Jabbarpour Y, Jetty A, Dai M, et al. The evolving family medicine team. J Am Board Family Med. 2020;33(4):499–501.
- Reeves S, Xyrichis A, Zwarenstein M. Teamwork, collaboration, coordination, and networking: Why we need to distinguish between different types of interprofessional practice. J Interprof Care. 2018;32(1):1–3.
- Dow AW, Zhu X, Sewell D, et al. Teamwork on the rocks: rethinking interprofessional practice as networking. J Interprof Care. 2017;31(6):677–678.