ABSTRACT
Introduction
A decrease in weight velocity and feeding difficulties in infants may be caused by an inadequate caloric intake and underlying medical conditions.
Case description
By focusing on four clinical cases, this article illustrates the temporary use of a special infant formula in orally-fed and enterally-fed infants with unsatisfactory weight gain and special medical conditions such as gastrointestinal and neurological disorders. The formula was a nutritionally complete hypercaloric infant formula containing partially hydrolyzed whey protein. It was used after full consideration of all feeding options including breastfeeding.
Conclusion
Implementing appropriate feeding behaviors, adapted to age and potential comorbidities, is an essential prerequisite for therapeutic management. The use of a nutritionally complete hypercaloric infant formula can be helpful to manage unsatisfactory weight gain and feeding difficulties in infants.
1. Introduction
A decrease in weight velocity and feeding difficulties in infants and young children are a common cause of concern for parents, leading them to seek advice from a pediatrician. Such conditions should prompt the identification of the origin of the problem, which can be multifactorial and various. The cause might be inadequate caloric intake, including family food insecurity and underlying medical conditions. Importantly, continuous inadequate caloric intake can have a negative impact on the child’s height and head circumference, and impair development of cognitive skills or immune system in extreme cases [Citation1–4]. From this perspective, identifying and managing the underlying causes of inadequate caloric intake is vital. In most cases, outpatient management based on proper nutrition advise and family support ensures an appropriate weight and growth velocity [Citation1]. Breastfeeding is the best way of feeding an infant during the first 6 months of life and is preferred whenever possible [Citation5]. However, a temporary use of hypercaloric nutritional formulas can be considered in infants and young children with unsatisfactory weight gain and special medical conditions. Such hypercaloric and easily digested formulas are useful to support catch-up growth in young children with or at risk of failure to thrive or at risk of malnutrition resulting from congenital heart defects, pulmonary diseases, neurological disorders, congenital genetic defects, cancer and prematurity, especially when complicated by low body weight and/or gastrointestinal dysfunction [Citation6–10].
An observational study reported an improved stool consistency and gastrointestinal tolerability (less regurgitation, vomiting, constipation, diarrhea and abdominal distension) in 29 infants aged under 12 months after being fed with hypercaloric whey peptide-based infant formula for 12 weeks [Citation7,Citation8]. The use of this product also allowed a significant catch-up growth in terms of head circumference and length [Citation7,Citation8].
This article aims at presenting some of our experience in using infant hypercaloric formula () in clinical practice. It describes four clinical cases on the dietary management of infants and young children with feeding difficulties and unsatisfactory weight gain because of gastrointestinal or neurological disorders. These clinical cases illustrate the use of a nutritionally complete hypercaloric infant formula to support this goal both in orally-fed infants (Patient 2 and Patient 3) and enterally-fed patients (Patient 1 and Patient 4).
Table 1. Description of the hypercaloric whey peptide-based infant formula
2. Clinical cases
2.1. Patient 1 – a 5-month-old girl enterally-fed with polymeric feed intolerance
On 21 December 2018, a 5-month-old girl born at 37 weeks of gestation with malformation syndrome was transferred from the Children’s Memorial Health Institute Surgery Department following laparoscopic fundoplication and gastrostomy tube placement to plan a feeding regimen. Patient presented with history of sucking and swallowing disorders with unsuccessful attempts at breast- and bottle feeding and was enterally-fed since birth via a nasogastric tube (with breast milk and standard infant formula). Due to bolus feeding intolerance (restlessness, vomiting, tolerance of only low-volume servings) enteral feeding was administered intermittently, 7 times a day over 2 hours via feeding pump (at a rate of 30 mL/h). Feeding regimen was not meeting the child’s estimated energy requirements.
On admission to the Department of Gastroenterology, Hepatology, Feeding Disorders and Pediatrics (DGHFDP), the girl was exclusively fed with standard infant formula (maternal milk dried up) via gastrostomy. Given her gastrointestinal symptoms, the girl was continuously receiving omeprazole (5 mg twice daily). Due to an insufficient caloric supply and growth faltering the infant formula was replaced with a high energy polymeric formula () which allowed a gradual weight gain. Consequently, feeding tolerance relatively improved – only periodic retching was observed without vomiting or sialorrhea. The girl was referred for home enteral nutrition (HEN) and discharged home with the recommendation to continue the current feeding regimen.
Table 2. Patient 1 – changes in the feeding regimen across time
On 13 February 2019 (first 2-month follow-up visit) at the age of 7 months, the parents reported problems with feeding tolerance as retching and vomiting became more frequent at 2-week post-hospital discharge. Given the unsatisfactory results of the nutritional treatment, continued therapy with omeprazole was recommended and the feeding regimen was changed by limiting the formula volume per portion and extending the delivery time (). Also, a second hospitalization was scheduled to perform a bowel transit time test on 28 February 2019.
After a 2-week observation, the parents reported a minor improvement in the severity of vomiting but retching and restlessness during feeding persisted, and weight gain remained unsatisfactory (+100 g; , red diamond). The bowel transit time test performed through the gastrostomy tube did not show any significant deviation. Due to the lack of indications for treatment modification and insufficient tolerance of enteral nutrition (EN), high-energy polymeric formula was replaced with hypercaloric whey peptide-based infant formula – (). Afterward, the girl appeared calmer during feeding, vomiting frequency decreased and a gradual weight gain was seen (, green diamond). The girl was discharged home with the recommendation to continue this feeding regimen and attempt to increase the servings volume during the day while limiting night supply.
Figure 1. Changes in the anthropometric measurements (A: weight, B: length and C: body mass index, BMI) using the World Health Organization (WHO) growth charts, before and after adding infasource® (nestlé health science, Biessenhofen, DE) to the feeding regimen. patient 1 (diamond); patient 2 (x mark); patient 3 (dots)
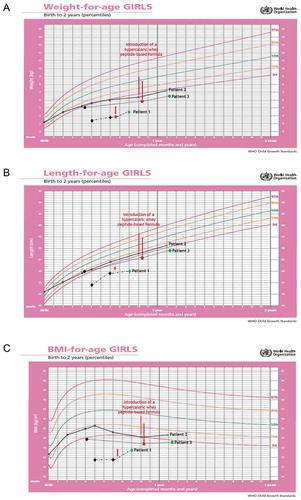
On 16 April 2019 (second 6-week follow-up visit) at the age of 9 months, the parents did not report any new feeding difficulties. On the contrary, they managed to gradually eliminate night time feeding and started the first attempts to oral feeding. Improvement in the nutritional status was satisfactory (). Retching persisted but was less troublesome than before. Parents were recommended to continue with the current feeding regimen, try to increase the servings volume, reduce the feeding time and the number of meals, and discontinue omeprazole. A follow-up visit was scheduled after 3 months.
The use of the hypercaloric whey peptide-based infant formula in this clinical case contributed to a reduction of gastrointestinal symptoms leading to improved tolerance of EN. This further contributed to an improved nutritional status () and helped calm down the negative emotions associated with oral feeding which led to developing new skills.
Table 3. Patient 1 – changes in the feeding regimen and resulting changes in the nutritional status during the total 4-month follow-up
2.2. Patient 2 – a healthy 10-month-old girl with feeding difficulties and unsatisfactory weight gain
A girl was born at 39 weeks of gestation by C-section with the following anthropometric measurements: weight: 3.2 kg/50th percentile as per WHO; length: 51 cm/50th-85th percentile as per WHO; and BMI: 12.3 kg/m2/15th-50th percentile as per WHO. Her Apgar score was 10.
At 10 months of life, the girl was referred for a nutritional consultation at the Department of Gastroenterology, Hepatology, Feeding Disorders and Pediatrics due to a decrease in the weight gain velocity and feeding difficulties. The parents reported that patient experienced feeding problems since 5 months of life when weaning started. Medical history revealed that the girl was always fed unconsciously as her parents forced her to eat or entertained her during feeding. She had no regular meal times – 8–10 daily feeding attempts were made. The girl’s diet was low in calories, unvaried, and inadequate for her age and level of eating skills. Her daily menu was based on foods she accepted, i.e. milk-based meals, such as infant formula and baby cereal with formula. According to the parents, all attempts to serve blended vegetables/fruits were unsuccessful. In parallel, the parents did not report any gastrointestinal symptoms.
Feeding difficulties led to a deterioration in the girl’s anthropometric measurements between birth and 10 months of life (, black and red X marks). Also, laboratory tests showed signs of anemia with hemoglobin level at 10.2 g/dL (normal range:10.9–13.0 g/dL).
After nutritional team’s consultation, the feeding regimen was changed to improve the nutritional value of the girl’s diet. The number of daily meals was limited to 5, including 2x high-energy polymeric formula (1 kcal/1 mL for a total of 250 kcal/250 mL), 2x baby cereal with formula, and 1x yogurt with blended fruit or blended vegetables with meat.
Parents were instructed not to entertain the girl while feeding and not to force feed. A meal could last up to 30 minutes if she showed an interest in eating. Only water was allowed in-between meals and at night.
A few days later, the parents reported that the recommended hypercaloric formula was poorly tolerated as the girl experienced flatulence and diarrhea. A gastrointestinal infection was ruled out based on a stool culture test. The team suggested changing the infant formula to the hypercaloric whey peptide-based infant formula described in and maintaining all other dietary recommendations. Until the next visit, the parents did not report any alarming symptoms.
At the 3-month follow-up visit, a satisfactory weight gain (, green X mark) and progress in introduction of solid foods was noticed. During that time, the girl was given 270 mL/day (+270 kcal) of the hypercaloric whey peptide-based infant formula additionally to the regular food. She ate her meals without being entertained with an increased interest in complementary foods, including solids. No significant deviation was seen in the lab test results; hemoglobin increased to 11.5 g/dL.
The team decided to remove one serving of the hypercaloric whey peptide-based infant formula and a new feeding regimen was recommended, including: 1xhypercaloric whey peptide-based infant formula (180 mL), 1x baby cereal with formula, 1x vegetable soup with meat, 1x finger foods, and 1x plain yogurt with added fruit. The next follow-up visit was scheduled after 3 months.
2.3. Patient 3 – a 12-month-old girl with trisomy of 21st pair of chromosomes and abnormal weight gain
A 12-month-old girl was admitted at the Department of Gastroenterology, Hepatology, Feeding Disorders and Pediatrics due to an unsatisfactory weight gain and persistent feeding difficulties. She had trisomy 21, was born at 33 weeks of gestation (prematurity) with a low birth weight (1.4 kg, depicted on the developmental grid of prematurely born girls according to T.R. Fenton (https://ucalgary.ca/fenton). The increasing feeding problems have been observed since introducing complementary foods.
On hospital admission, anthropometric measurements were alarming and deviation from previous measurements was observed (weight: 5.8 kg/<3rd percentile); length: 66 cm/<3rd percentile; and BMI: 13.3 kg/m2/<3rd percentile; , black and red dots). Measurements were plotted on the WHO standard growth charts as a common practice in Poland for children with Down syndrome and given the absence of guidelines recommending the use of any specific growth charts for this condition in the country. Due care was made for the correct interpretation of the girl’s growth parameters.
No significant deviations were found in the physical examination and laboratory tests. The bowel transit time test showed slightly delayed gastric emptying. During the hospitalization, the feeding disorders team (gastrologist, nutritionist/dietitian, speech-language therapist, and psychologist) identified the behavioral disorders as the main cause of feeding difficulties and unsatisfactory weight gain. The main identified abnormalities were conditional distraction while feeding, tolerance of meals only in pureed consistency, and giving infant water with infant bottle despite of much wider range of skills.
Since a longer stay was not possible in the clinic, feeding disorders therapy was initiated at home. Scheduled mealtimes and only conscious feeding were recommended. The meal plan was based on meals and textures currently accepted by the girl. To complement the diet with new flavors and consistencies, soft solid foods were given twice a day before regular meals. Due to the unsatisfactory nutritional status, two portions of regular infant milk were replaced with the hypercaloric whey peptide-based infant formula (1 kcal/1 mL; 2 × 180 mL/day).
At 3-month follow-up visit, anthropometric measurements had improved (weight:6.5 kg/<3rd percentile as per WHO); length: 69 cm/<3rd percentile as per WHO; and BMI: 13.65 kg/m2/<3rd percentile as per WHO; , green dot). The parents associated this progress with feeding disorders therapy. The diet was still based on pureed food, but the girl was fed only consciously. She was more interested in food and was more willing to touch and taste solid foods. Further therapy steps were discussed with parents, including the rules of biting training. The feeding regimen was modified by changing the one pureed-consistency meal into another consisting only of soft solid products. Given the ineffectiveness of the standard preparations, any improvement in nutritional status was considered a great success with the hypercaloric whey peptide-based infant formula. Accordingly, the supplementation with that formula was reduced to 1 × 180 mL/day. A follow-up visit was scheduled after 3 months.
2.4. Patient 4 – a 3-month-old girl with suspicion of cerebral palsy
A girl was born at 32 weeks of gestation by C-section due to threatened asphyxia, with pregnancy being complicated by malformation syndrome. The girl was graded 7-8-8-9 points on the Apgar scale and her birth weight was low at 1.8 kg (). She has been hospitalized in the Neonatology, Pathology and Intensive Care Clinic of the Newborn at Children’s Memorial Health Institute since 9th day of life due to a congenital aortic stenosis. The girl underwent a cardiac surgery on 11th day of life. On admission, the newborn was additionally found to have reduced muscle tone, low vitality and no sucking reflex. Based on these symptoms, cerebral palsy was suspected but not confirmed as the child was too young to undergo an unequivocal diagnosis. Therefore, and a regular care of a neurologist and physiotherapist was recommended.
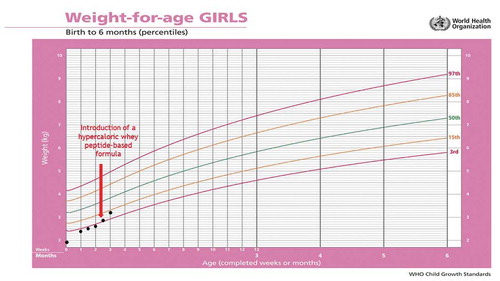
Figure 2. Patient 4 – changes in body weight during 3 months of follow-up using the World Health Organization (WHO) growth charts, before and after adding infasource® (nestlé health science, biessenhofen, DE) to the feeding regimen
As for her feeding problems, the girl was on exclusive enteral feeding through the nasogastric tube with mother’s milk since first day of life. Given an improved general condition at 33 days of life, the oral feeding was attempted, but no effective suckling and swallowing reflex was obtained. After speech and language therapist and gastroenterologist consultation, videofluoroscopy (VFSS) was recommended. VFSS showed lack of coordination of oropharyngeal reactions and aspiration of food into the respiratory tract, which was the basis for stopping the oral feeding attempts. In addition, during feeding through the nasogastric tube, regurgitations and vomiting were observed which made it difficult to achieve an expected weight gain. In the bowel transit time test no significant deviations (including anatomical defects) were found.
The girl was referred for a nutritional consultation at the Department of Gastroenterology, Hepatology, Feeding Disorders and Pediatrics to determine further feeding plan. Due to the medical history, available test results, observed gastrointestinal symptoms, the girl’s age and current body weight, she was qualified for surgical insertion of gastrostomy with Nissen fundoplication. The procedure was performed without any complications. Feeding via gastrostomy was started 6 hours after surgery, initially with a 20-mL serving (1/3 of the target serving). Due to the previously observed problems of small weight gain and lack of tolerance of recommended diet’s volume, breast milk was partially replaced with a high-energy polymeric formula (1 kcal/1 mL). Feeding by gastrostomy was recommended as per the following scheme: 4 × 50 mL of expressed breast milk plus 3 × 50 mL high-energy polymeric formula per day. Feeding plan provided approximately 280 kcal/day (130 kcal/kg body weight/day). Additionally, gastrostomy care competencies were discussed with parents.
A few days after introducing the high-energy polymeric formula, the nutrition team was sought for additional consultation due to poor tolerance of the recommended formula manifested by increased retching and vomiting. Therefore, the formula was replaced with a complete, hypercaloric whey peptide-based diet. The feeding method was also modified by extending each serving time to 30 minutes. Due to lactation suppression, breast milk was replaced with standard infant formula. Previously recommended servings were maintained.
After one week in the ward, a partial but satisfactory improvement was observed as vomiting subsided but retching persisted. The girl was qualified for HEN and discharged home with a recommendation to continue the established feeding method. A follow-up visit was scheduled after 6 weeks.
Two weeks after discharge, the parents came for unscheduled follow-up visit to the Nutrition Clinic due to girl’s severe vomiting. At that time, she was on enteral feeding in accordance with previous recommendations with infant formula and high-energy peptide diet. Due to ailments, parents reduced single-dose portions administered enterally to 45 mL. Any attempt to increase the portion was associated with increased vomiting. Due to the unsatisfactory tolerance of EN, the industrial diet was replaced with the hypercaloric whey peptide-based infant formula described in . Taking into account the volume of tolerated portions and the current energy requirements, it was recommended to feed the girl only with this formula at 8 × 45 mL (140 kcal/kg body weight/ day).
Two weeks later, the parents informed the nutrition team that their girl is tolerating her new feeding scheme better than the previous one. Vomiting subsided, retching was clearly reduced and the girl’s body weight improved (). The initial date of the follow-up visit in the Nutrition Clinic was maintained.
Four weeks later, the girl was followed-up in the Nutrition Clinic. At that time, parents did not report any gastrointestinal complaints. Diet tolerance was good and the serving volumes were increased to 55 mL/meal. The girl’s body weight increased at 3.8 kg (). It was recommended to continue the current feeding plan, trying to increase the volume of servings and reduce the number of meals.
3. Discussion
These four cases focused on the dietary management of infants and young children with feeding difficulties and unsatisfactory weight gain caused by gastrointestinal or neurological disorders.
They showed that feeding difficulties required the care of a specialized multidisciplinary team, including a gastrologist, a dietitian, a speech and language therapist and a psychologist. Moreover, a proper and early assessment of the child’s anthropometric measurements, nutritional status, current diet and type of feeding difficulty was necessary to choose the best diagnostic and therapeutic approach. As such, feeding regimen was modified and the correct feeding behaviors were shaped taking into account the child’s current nutritional needs as well as their food intake abilities. Also, a temporary use of a high-energy, nutritionally complete formula was considered in infants and young children with unsatisfactory weight gain, whether they were enterally-fed (Patient 1 and Patient 4) or orally-fed (Patient 2 and Patient 3). The clinical observations suggest that in case of intolerance to a hypercaloric polymeric formula, the physician and/or dietician may consider a change of the latter with a hypercaloric infant formula containing (partially or fully) hydrolyzed whey protein [Citation11–13] and adapt the form of administration and dosage of the nutritional formula.
Particularly, Patient 1 and Patient 4 illustrated how the formula containing partially hydrolyzed whey protein contributed to a reduction of gastrointestinal symptoms leading to improved tolerance of EN. Despite being the preferred method of alternative feeding, one of the most common complications of EN is feeding intolerance. The most common symptoms of feeding intolerance include constipation, gastric residuals (tolerance of low-volume servings inappropriate for the age), retching and vomiting, diarrhea, bloating or restlessness during feeding [Citation14,Citation15]. Feeding intolerance is often manifested by a combination of these symptoms, making the patient unable to intake a sufficient amount of feed and/or properly digest/absorb the feed, which leads to a deterioration in the patient’s nutritional status/malnutrition. Individual interpretation and acting on the symptoms of intolerance is the best diagnostic and therapeutic approach [Citation16]. Experience shows that feeding intolerance in enterally-fed patients is usually due to a very rapid buildup of feed delivery; delivery of an excessive bolus volume; delivery of the feed at too high or too low temperatures; excessive osmolarity of the feed; fluid deficiency; and/or, insufficient supply of dietary fiber. If the abovementioned factors are elucidated/excluded, the formula should be changed [Citation17].
Accordingly, a change of the enteral formula may be required to improve EN efficiency. Challenges in choosing the right formula are usually faced in infants whose digestive tract and digestion and absorption mechanisms are still being developed. Likewise oral feeding, breast milk is the optimal and best-tolerated way of feeding for EN of infants in the first 6 months of life. As per the recommendations of the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) on choosing an EN preparation for infants, polymeric formulas containing 1 kcal/mL are appropriate in most cases of malnutrition/tolerance of low-volume servings. Formulas containing protein hydrolyzate are well indicated in case of intolerance/insufficient tolerance to the standard polymeric diet [Citation18,Citation19]. However, clinical evidence on the use of semi-elemental or even elemental formulas in intolerant and GI impaired young age patients are still scarce. Based on real-life experience with cases commonly seen in clinical practice, this paper describes the possible benefits of using a nutritionally complete hypercaloric whey peptide-based infant formula in both orally and enteral-fed children with special needs.
Furthermore, Patient 4 illustrated that intolerance of commerical polymeric diet is common complication of EN in infants with neurological disorders, such as cerebral palsy. Appropriate selection of formula and the supply method are key to the correct implementation of nutritional treatment in this group of patients. Improving the nutritional status through the implementation of an appropriate nutrition plan has a huge impact on the patients’ development, course of the disease, and the quality of life of the patients and their whole family/caregivers. Patient 4 is an example where expected outcomes were not achieved with a polymeric diet; it was not sufficiently well tolerated, preventing effective implementation of the assumed goals of EN. Changing the formula to a hypercaloric whey peptide-based infant formula was beneficial as gastrointestinal symptoms were significantly reduced, feeding tolerance improved overall, and satisfactory weight gain was achieved. In summary, such hypercaloric formula, containing partially hydrolyzed whey protein and a special fatty acid profile with human milk structured lipid analogues (palmitic acid positioned at the sn-2 position as indicated in ), may contribute to better tolerance of EN in infants and children with neurological and co-occurring gastrological disorders [Citation2,Citation12,Citation20].
Implementing the appropriate methods of nutritional support in infants and young children with feeding difficulties and unsatisfactory weight gain is necessary to ensure long-term improvement of nutritional status and to develop proper eating habits. A properly adjusted nutritional support is known to have beneficial influence on the treatment outcome and prognosis in chronic diseases. It is also associated with the overall reduction of treatment costs. Access to special formulas for oral and/or enteral supply and to HEN may significantly improve the quality and effectiveness of the nutritional support that can be offered to infants and young children. Unlike older children, a variety of commercial diets is not available for infants and choosing a preparation that meets the infant’s needs is sometimes difficult. In everyday practice, youngest patients with different health conditions experience symptoms of intolerance to regular commercial formula, most often resulting from immaturity and/or disturbances in the functioning of the digestive tract.
Currently, there are no specific recommendations on the type of hypercaloric formulas that should be used in infants and young children with special nutritional needs arising from different chronic medical conditions. According to the ESPGHAN guidelines, polymeric diets are indicated as a standard diet in these situations, while diets containing hydrolyzed proteins are recommended when polymeric formulas are not well tolerated or in case of established cow’s milk protein allergy. In practice, it is difficult to conduct high-quality and large prospective clinical trials comparing the efficacy and/or tolerance of different special formulas in infants and young children with different clinical conditions. Therefore, retrospective studies and case reports can provide significant knowledge in this area. Indeed, case studies describe practical real-world clinical data and are still indispensable for broadening medical knowledge, despite being assigned limited level of evidence and their inability to deliver quantitative data [Citation21]. They provide an in-depth understanding of each individual case as opposed to large research studies with their ‘nomothetic approach’ [Citation22]. Additionally, a case report is the only way to describe in details any unusual observations regarding symptoms, medical history, clinical findings, course of disease, pharmacological and non-pharmacological interventions and their tolerability [Citation21]. Consequently, a case report is a major educational tool that can inform readers on new observations, generate hypotheses, and facilitate detection of similar or identical cases. Ultimately, the present cases illustrated the possible benefits of using a nutritional complete hypercaloric whey peptide-based infant formula in infants with special needs. These benefits included improved catch-up growth, nutritional status, and quality of life although not measured with any validated instrument. Such evidence may guide the physicians and dietitians to choose the method of nutritional support on a case-by-case basis.
4. Conclusion
The present four clinical cases further document the safety and the possible benefits of using a nutritionally complete hypercaloric whey peptide-based infant formula in both orally and enterally-fed infants with special needs. Our experience provide learnings in nutritional management of infants various conditions such as those born prematurely or infants with chronic cardiac or neurological conditions who have intolerance to regular polymeric formulas. As a result, the quality of care and treatment outcomes in these groups of children may improve in terms of catch-up growth and nutritional status. Ultimately, these case reports may be also a basis to plan larger observational studies to further assess the benefits of using such formula in various medical conditions of the infant. The results of such analyzes will also ensure a better guidance for the treatment and feeding of young children with special nutritional needs.
Transparency
Declaration of Funding
Content Ed Net Switzerland provided editorial and medical writing assistance for the preparation of this manuscript; this assistance was funded by Nestlé Health Science S.A. The views and opinions expressed are those of the authors. The authors retained the editorial process, including the discussion at all times. There was no financial reward associated with writing the paper.
Declaration of financial/other relationships
Małgorzata Matuszczyk - lecturer fee, Nestle has received personal fees for lectures from Nutricia, HIPP, Nestlé, and Takeda. Paulina Mika-Stępkowska has received personal fees for lectures from Nestlé, HIPP, and Nutricia; and research grants from Nutricia foundation. Marcin Szary has received personal fees for lectures from Nestlé. Miroslaw Perlinski is an employee at Nestlé Health Science S.A., Poland. Jarosław Kierkuś - lecturer fee, Nestle has received personal fees for lectures from Nestlé and Nutricia. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Author contributions
MM, PM-S, AS, MS, and JK were involved in the writing of the clinical cases, discussion and conclusion. They also contributed to the infants’ care. MP coordinated the development of the cases and provided support to the doctors for the use of the product. All the authors reviewed and approved the final article.
Ethical considerations
Informed consent was obtained from the parents/legal representative of the four infants for the publication of the present case reports.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
The authors acknowledge the infants and their families, and the healthcare professionals at the affiliation center.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Drewett RF, Corbett SS, Wright CM. Cognitive and educational attainments at school age of children who failed to thrive in infancy: a population-based study. J Child Psychol Psychiatry. 1999;40(4):551–561.
- Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1–5.
- Heinonen K, Räikkönen K, Pesonen AK, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121(5):e1325–e1333.
- Kar BR, Rao SL, Chandramouli BA. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct. 2008;4(1):31.
- World Health Organization. Fifty-Fifth World Health Assembly. Infant and Young Child Nutrition. 16 April 2002. [cited 2020 Aug 19]. Available from: http://apps.who.int/gb/archive/pdf_files/WHA55/ea5515.pdf
- Leonard M, Caldari D, Mas E, et al. Experience of using a semielemental formula for home enteral nutrition in children: a multicenter cross-sectional study. J Pediatr Gastroenterol Nutr. 2019;68(4):585–590.
- Lama More RA, Moralis Lopez A, Ruiz Bartolomé H, et al. Nutritional support in infants with or at risk of malnutrition. [19th LatinoAmerican and 10th Iberoamerican Congress of Pediatric Gastroenterology, Hepatology and Nutrition. Centro de Convenções de Nata. RN. Brasil 26-29 March 2014].
- Morais A, Ruiz H, Magallares L, et al. Utility of a hypercaloric and partially hydrolyzed formula in the treatment of infants with malnutrition or nutritional risk. Journal of the Spanish Society for Research in Nutrition and Diet in Paediatrics (Sociedad Espanola de Investigacion en Nutricion y Alimentacion en Pediatria). May-June 2014. Vol 70. [21st Congress of the Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition. Pamplona, 22-24 May 2014].
- Florendo KN, Bellflower B, van Zwol A, et al. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. J Perinatol. 2009;29(2):106–111.
- Vandenplas Y, Alarcon P, Fleischer D, et al. Should partial hydrolysates be used as starter infant formula? A Working Group Consensus. J Pediatr Gastroenterol Nutr. 2016;62(1):22–35.
- Kerzner B, Milano K, Wc M Jr, et al. A practical approach to classifying and managing feeding difficulties. Pediatrics. 2015;135(2):344‐353.
- Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastr Nutr. 2008;46:99–110.
- Koletzko B, Goulet O. Nutritional support in infants, children and adolescents. In: Sobotka L, editor. Basics in clinical nutrition. Prague: Gelen; 2011. p. 625–653.
- Büyükçoban S, Akan M, Koca U, et al. Comparison of two different enteral nutrition protocol in critically ill patients. Turk J Anaesthesiol Reanim. 2016;44(5):265–269.
- Zanetti M Approach to oral and enteral nutrition in adults. Topic 8. [online] ESPEN LLL Programme 2016. [cited 2020 Aug 19]. Available from: https://www.testlllnutrition.com/mod_lll/TOPIC8/m81.pdf
- Fanaro S. Feeding intolerance in the preterm infant. Early Hum Dev. 2013;89(Suppl 2):S13–S20.
- Löser C, Aschl G, Hébuterne X, et al. Consensus statement. ESPEN guidelines on artificial enteral nutrition - percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005;24:848–861.
- Braegger C, Decsi T, Amil Dias J, et al. [ESPGHAN committee on nutrition]. practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;51(1):110–122.
- Romano C, van Wynckel M, Hulst J, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. 2017;65(2):242–264.
- Andrew MJ, Parr JR, Sullivan PB. Feeding difficulties in children with cerebral palsy. Arch Dis Child Educ Pract Ed. 2012;97(6):222–229.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7(1):264. PMID: 24758689; PMCID: PMC4001358
- Williams DD. In defence of the case report. Br J Psychiatry. 2004;184(1):84. PMID: 14702235
